Abstract
Nucleotide excision repair (NER) clears genomes of DNA adducts formed by UV light, environmental agents, and antitumor drugs. Gene mutations that lead to defects in the core NER reaction cause the skin cancer-prone disease xeroderma pigmentosum. In NER, DNA lesions are excised within an oligonucleotide of 25–30 residues via a complex, multi-step reaction that is regulated by protein-protein interactions. These interactions were first characterized in the 1990s using pull-down, co-IP and yeast two-hybrid assays. More recently, high-resolution structures and detailed functional studies have started to yield detailed pictures of the progression along the NER reaction coordinate. In this review, we highlight how the study of interactions among proteins by structural and/or functional studies have provided insights into the mechanisms by which the NER machinery recognizes and excises DNA lesions. Furthermore, we identify reported, but poorly characterized or unsubstantiated interactions in need of further validation.
Keywords: DNA repair, nucleotide excision repair, xeroderma pigmentosum, protein-protein interactions
Introduction
The nucleotide excision repair (NER) pathway is an essential mechanism for the removal of bulky DNA adducts [1]. NER operates through the sequential action of more than 30 proteins that assemble as a dynamic series of multi-protein complexes at DNA lesions and excise them as part of an oligonucleotide [2–4]. NER is versatile in its ability to remove a range of structurally diverse lesions such as UV induced cyclobutane–pyrimidine dimers (CPDs) and (6–4) pyrimidine–pyrimidone photoproducts ((6–4) PPs) or intrastrand crosslinks caused by anticancer drugs such as cisplatin [1, 5]. Hereditary mutations in NER genes are associated with several disorders, including xeroderma pigmentosum (XP), a prototypical DNA repair disorder characterized by an extreme hypersensitivity to sunlight and more than 2000-fold increase in skin cancer. NER gene mutations are additionally associated with Cockayne syndrome, trichothiodystrophy, and UV sensitive syndrome, which display more complex pathologies including developmental and neurological abnormalities as well as premature aging, due to effects on factors outside of the core NER pathway [6, 7]. NER can be initiated through two damage recognition sub-pathways: global-genome NER (GG-NER) and transcription-coupled NER (TC-NER). In GG-NER, lesions are detected across the entire genome, while TC-NER is responsible for the accelerated detection of lesions in the transcribed strands of active genes (Figure 1). GG-NER is initiated by the recognition of bulky lesions by XPC-RAD23B-CETN2 [8–10], but for non-distorting lesions and in the context of chromatin, UV-DDB (DDB1-DDB2) and its associated ubiquitin ligase are needed for damage detection [11]. Additionally, numerous factors are involved in facilitating NER in the context of chromatin (reviewed in [12]). XPC-RAD23B-CETN2 recruits the 10-subunit TFIIH to the damage, which then uses its helicase subunits, XPB and XPD, to open the DNA duplex and verify the presence of the lesion [13–15]. TC-NER is initiated when an RNA polymerase II (RNAPII) stalls at the lesion site. Several proteins, including CSB, CSA, and UVSSA then assemble at the stalled RNAPII and together with ELOF1, which is part of the RNAPII complex, facilitate the engagement of TFIIH with the lesion site [16, 17]. In both GG-NER and TC-NER, the opening of the DNA by TFIIH generates a bubble with which XPA and RPA scaffold proteins engage to guide incision and removal of the damaged strand by the two endonucleases [18–20]; the first incision 5’ to the lesion is made by ERCC1-XPF, followed by the 3’ incision by XPG [21]. Filling of the resulting gap and sealing of the nick complete the NER process [22, 23].
Figure 1.
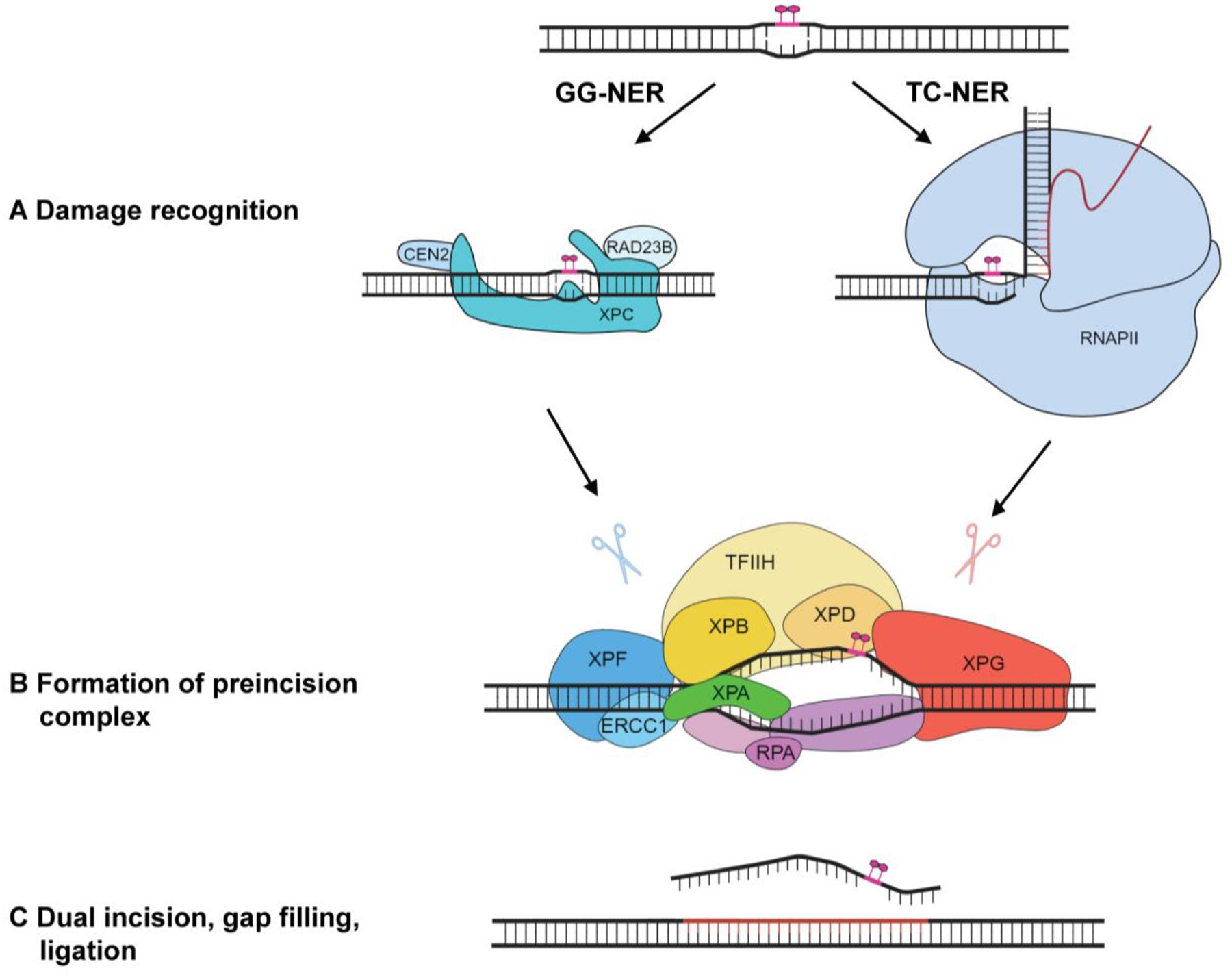
Illustration of the steps of global genome (GG-) and transcription coupled (TC-) nucleotide excision repair (NER). GG-NER and TC-NER differ at the damage recognition stage but converge upon formation of the pre-incision complex and subsequent dual incision.
Many excellent reviews on NER are available (including in the “cutting edge perspectives in genome maintenance series” in DNA Repair, [24–26]). This review is concerned with a central driving force of the NER pathway: protein-protein interactions among the six core NER factors - XPC-RAD23B-CETN2, TFIIH, XPA, RPA, XPG, and ERCC1-XPF. We discuss recent advances, formulate open questions, and importantly, provide a guide for the reader as to which of the many reported interactions among NER proteins we consider to be important, and which ones have not been held up to the scrutiny of vigorous experimentation after their initial reports. To keep our discussion focused, we refer the reader to recent reviews that discuss interactions specific to the TC-NER initiation complex [16, 17] or recognition of lesions in chromatin by the UV-DDB-CRL4 complex [12, 27].
Stage 1: Damage recognition by XPC-RAD23B-CETN2 and verification by TFIIH and XPA
GG-NER is initiated by XPC, which senses lesions through the thermodynamic destabilization induced in DNA [28]. High-resolution structures, first of the yeast ortholog Rad4 [29] and subsequently of the human XPC protein [30], show that the transglutaminase domain (TGD) and the first β-hairpin domain (BHD1) bind DNA independently of damage and sequence, while two additional β-hairpin domains BHD2 and BHD3 domains interact with the damaged site, encircling the DNA opposite the lesion (see Figure 2A, B for domain map and schematic of the complex). XPC forms a constitutive heterotrimer with RAD23B [31, 32] and Centrin 2 (CETN2) [10, 33] (Table 1, Figure 2B, C). A ~55 amino acid helical domain in RAD23B (amino acids 275–332) binds yeast Rad4/XPC via its N-terminal helix in the TGD domain [29, 33, 34] (Table 1). RAD23B has been shown to stabilize XPC and promote its binding to damaged DNA in biochemical and cellular experiments [10, 32, 35]. The C-terminal domain of XPC interacts with CETN2, a small EF-hand calcium-binding protein homologous to calmodulin, through residues 847 to 866, which are part of the long C-terminal helix of XPC that links it to XPB [30] (Table 1, Figure 2C, D). Cellular and biochemical studies using XPC mutants that cannot physically interact with CETN2 showed that the interaction between CETN2 and XPC had a stimulatory effect on the NER incision reaction, and contributed to resistance of cells to UV irradiation, although the interaction is not required for NER activity [10]. Interactions with RAD23B and CETN2 increase the cellular stability of XPC and its binding to DNA. They do not directly bind DNA or as far as is currently known, other NER proteins. It is worth noting that the interaction domains among XPC, RAD23B, and CETN2 determined in early biochemical studies were confirmed in structures of the larger NER recognition complexes (Table 1) [30]. Cellular studies have suggested that RAD23B dissociates from XPC following binding of XPC to UV lesions, raising the possibility of the dissociation of XPC-RAD23B-CETN2 prior to assembly of the NER pre-incision complex [35]. The cryo-EM structures of XPC and TFIIH bound to damaged DNA show the presence of both RAD23B and CETN2 suggesting that XPC-RAD23B-CETN2 might disassemble later, likely upon its displacement from the lesion and the NER complex [30].
Figure 2.

Interactions between XPC and TFIIH coordinate damage recognition in NER. (A) Domain map of XPC and TFIIH with their interacting regions highlighted. Overlapping domains are shaded as white stripes. HEL denotes the helicase domain, FeS the iron-sulfur cluster domain, and Zn indicates a Zn finger domain. (B) A schematic model for the interaction of XPC and TFIIH complexes in the presence of a DNA substrate. (C) Structure of the CETN2-XPC-TFIIH/XPB complex showing the interaction required for TFIIH recruitment (PDB: 8EBS). (D) CENT2-XPC interaction with residues L848/L851/L855 of XPC in the CETN2 binding pocket highlighted in red. (E) Solution structure of the AS motif of XPC interacting with the PH domain in p62 of TFIIH (PDB: 2RVB). Residue W133 and V136 of XPC in the binding pocket are highlighted in red.
Table 1.
List of NER protein-protein interactions verified by structural and functional studies.
| Protein | Interacting NER factors | Interaction domain (residues) | Methods | PDB ID | Mutations | UV sensitivity | UV repair | NER activity | Reference |
|---|---|---|---|---|---|---|---|---|---|
| XPC | RAD23B | 200–220 | EM | 8EBS, 8EBV | N/D | [29, 30] | |||
| Centrin2 | 847–866 | X-ray, ITC, Mut, EM | 2OBH, 8EBS, 8EBV, 8EBT | W848A/L851A/L855A | + | ++ | + | [10, 30, 80] | |
| p62/TFIIH | 124–137, 165–175 | NMR, ITC, Mut | 2RVB, 8EBS | W133A | ++ | +++ | N/D | [30, 42] | |
| XPB/TFIIH | 870–940, 849–910 | PD, EM | 8EBS, 8EBV, 8EBT | N/D | [30, 37] | ||||
| TFIIH | XPC | p62: 1–106, 176–186 | NMR, ITC | 2RVB, 8EBS | N/D | [30, 42] | |||
| XPB: 680–720 | EM | 8EBS, 8EBV, 8EBT | N/D | [30] | |||||
| XPA | p8: 1–15 | EM | 8EBY | Deletion of 1–15 of p8 | N/D | N/D | +++ | [30, 46] | |
| p52: 413–459 | EM | 8EBY | N/D | [30] | |||||
| XPB: 705–722 | EM | 8EBY | N/D | [30] | |||||
| XPA | TFIIH (XPB, p8, p52) | XPB: 229–241 | EM | 8EBY | N/D | [30, 45] | |||
| p52: 247–270 | EM | 8EBY | N/D | [30, 45] | |||||
| p8: 241–273 | EM | 8EBY | H244R | ++ | ++ | ++ | [30, 45, 49] | ||
| RPA | RPA32: 29–46 | NMR, X-ray, ITC, SAXS | 1DPU, PDBDEV : 124, 128 | R30E/K31E/R32E/R34E/R39E/R42E | ++ | ++ | +++ | [50, 57] | |
| RPA70: 98–129 | ITC, SAXS, Mut | PDBDEV : 039, 040, 124, 128 | D101N/E106K/K110E/E111K/F112A/D114N | ++ | ++ | +++ | [51, 57] | ||
| ERCC1 | 67–90 | X-ray, Mut | 2A1I | F75A, Deletion of G73, G74 | N/D | N/D | +++ | [70, 81] | |
| RPA | XPA | RPA32: 218–270 | NMR, X-ray, ITC, SAXS | 1DPU, PDBDEV: 124, 128 | N/D | [50, 57] | |||
| RPA70: 192–389 | Mut, NMR, SAXS | PDBDEV : 039, 040, 124, 128 | N/D | [51, 57] | |||||
| XPG | Active site | 1–77, 791–812 | X-ray | 6TUW, 6TUX | E791A D812A |
+++ | N/D | +++ | [60, 61] |
| XPF | ERCC1 | 848–905 | EM | 6SXA,6SXB | N/D | [73, 75] | |||
| Active site | 656–813 | EM | 6SXB | D687A D731A |
N/D | N/D | +++ | [76] | |
| ERCC1 | XPF | 220–297 | EM, X-ray | 6SXA, 6SXB,2A1J | N/D | [73, 75] | |||
| XPA | 110–152 | Mut, NMR | 2JNW | N110A, Y145A | ++ | ++ | ++ | [69, 71] |
EM: Cryogenic Electron Microscopy; FA: Fluorescence Anisotropy; ITC: Isothermal Calorimetry; Mut: Site-directed/deletion mutagenesis; NMR: Nuclear Magnetic Resonance Spectroscopy; PD: Pull-down assay; SAXS: Small Angle X-ray Scattering; X-ray: X-ray crystallography.
After initial damage recognition, XPC-RAD23B-CETN2 recruits TFIIH to the DNA around the lesion. TFIIH contains 10 subunits that are organized into the core (XPB, XPD, p62, p52, p44, p34, p8) and the CAK complex (CDK7, Cyclin H, and MAT1). The CAK complex dissociates from TFIIH once it is engaged in NER; the core alone is fully competent in NER [36]. The first interaction between XPC and TFIIH was shown to be with XPB through the C-terminal domain of XPC (Table 1) [37, 38]. Although this interaction was also seen in cryo-EM structures of NER complexes (Figure 2C) [30, 39], its functional importance for NER has not been thoroughly examined. XPC also interacts with TFIIH through its N-terminus, specifically through its “acidic string (AS)” motif (amino acids 124–137). This motif is rich in acidic residues interspersed with the hydrophobic residues W133 and V136 and interacts with the pleckstrin homology (PH) domain of p62 [40] (Figure 2E, Table 1). This interaction was also observed, albeit not at high resolution, in the cryo-EM structure of the yeast TFIIH/Rad4-Rad23-Rad33 complex on damaged DNA [39]. The PH domain of p62 interacts with many proteins, including XPC, XPG (see below), UVSSA, p53, and VP16 to regulate the function of TFIIH in various pathways [40–42]. With respect to XPC, a W133A mutation in the AS motif of XPC sensitizes the cells to UV and greatly reduces (6–4) PP repair, showing the importance of the AS motif in the assembly of the NER complex (Table 1) [40]. Interestingly, the PH domain of p62 is not visible in the cryo-EM structure of the human NER damage recognition complex [30], suggesting that it is flexible within TFIIH to aid its role in interacting with diverse proteins.
Once TFIIH engages with the XPC-lesion-containing DNA complex, its role is to open the helix, verify the lesion and provide a platform for the assembly of additional factors required to complete the NER incision complex. Central to this role is the XPB translocase, which is believed to open the DNA helix by binding 5’ to the lesion and induce negative supercoiling. The second ATPase of TFIIH, XPD, is a 5’->3’ helicase that engages with the open DNA and tracks along the DNA until it stalls at the bulky lesion [43]. To engage in this process, TFIIH undergoes a significant configurational change, brought about by the departure of the CAK complex. The key to this transition is the loss of the long α-helix of MAT1 that keeps XPB and XPD at a fixed distance. Upon the departure of MAT1 and association with the XPC-lesion-containing DNA complex, core TFIIH opens to assume a horseshoe shape [44] with XPB bound to duplex DNA and XPD placed away from the DNA but ready to engage and exert its activity in lesion scanning. Upon binding of XPA, core TFIIH rearranges to a closed σ-shape [45]. In this state, XPA is bound to DNA and TFIIH, forming an elongated arch over the DNA that bridges XPB and XPD (Figure 3A, 3B) [45]. Once engaged, the XPD helicase scans the damaged strand in the 5’ to 3’ direction and verifies the lesion. The disordered C-terminal domain of XPA engages with three different subunits of TFIIH (p8, p52, and XPB) [30] (Table 1, Figure 3C, 3D). The N-terminus (residues 1–15) of p8, the smallest subunit of TFIIH, has been shown to be essential for the association of XPA with NER complexes [46] (Table 1, Figure 2A). This interaction was clearly visualized in cryo-EM structures as a β-strand that interacts with the p8/p52 dimer [30, 45]. Conversely, a motif containing two conserved His (242, 244) and two conserved Cys (261, 264) residues in the C-terminal domain of XPA has long been known to interact with TFIIH (Table 1) [47, 48]. The H244R allele of XPA, which is found in patients with a mild XP phenotype, but neurological abnormalities, is in this interaction interface and has been shown to reduce NER activity and inhibit the interaction of XPA with TFIIH after UV irradiation [49] (Table 1, Figure 2F). Intriguingly, the H244R mutation exhibits a more pronounced defect in TC-NER than GG-NER, pointing to a possible difference in the interaction of XPA and TFIIH in the two sub-pathways.
Figure 3.
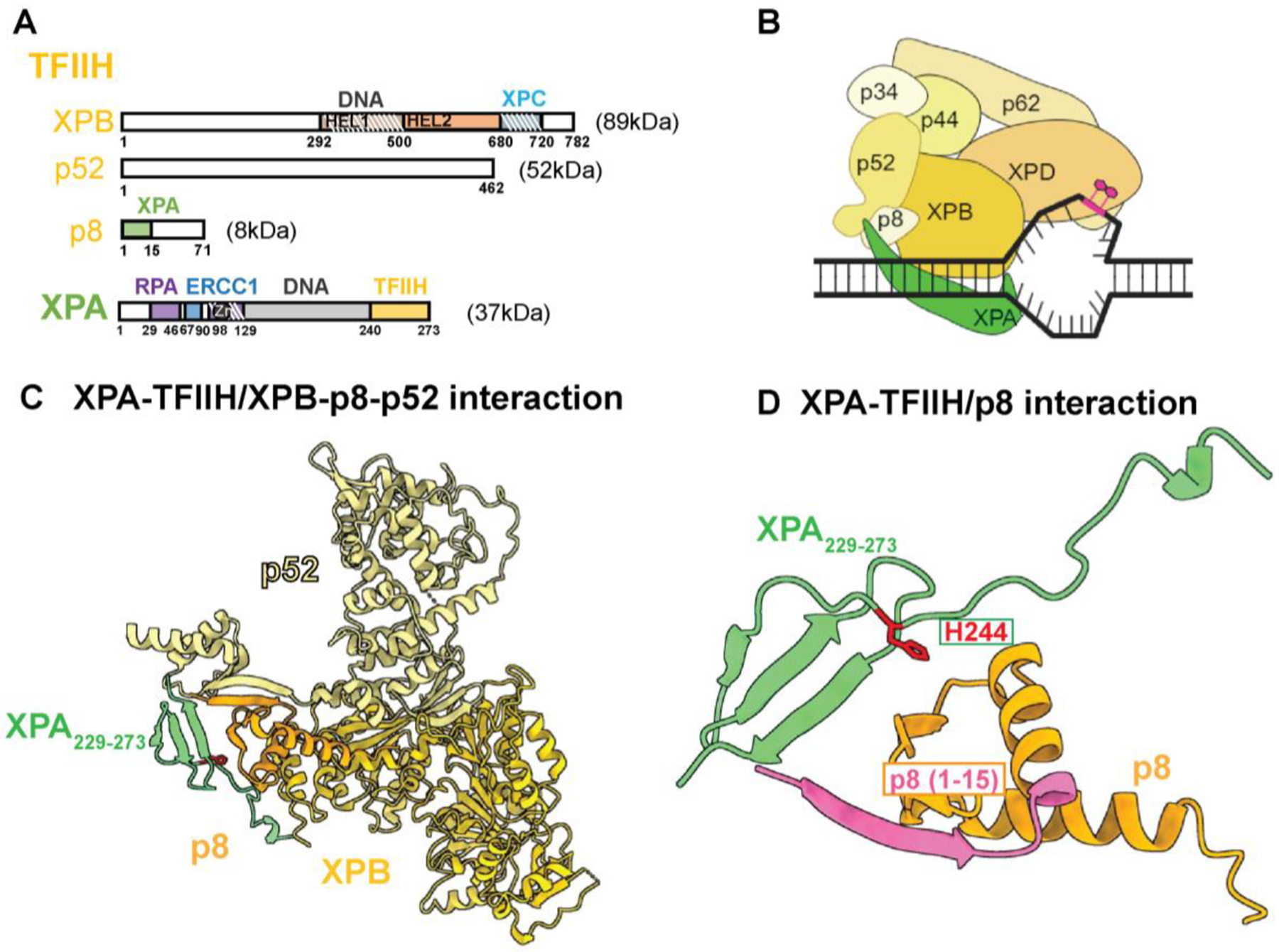
Interactions between TFIIH and XPA coordinate damage verification in NER. (A) Domain map of TFIIH and XPA with their interacting regions highlighted. (B) A schematic model of TFIIH as it undergoes major configurational rearrangement to interact with XPA and DNA in order to perform the lesion verification step. (C) Structure of the interaction of XPA with XPB-p8-p52 subunits of TFIIH (PDB: 8EBY). (D) Deletion of residues 1–15 of p8 (highlighted in magenta) disrupts interaction with XPA.
Stage 2: Formation of pre-incision complex by XPA-RPA mediated scaffolding of NER factors
Following damage verification, the pre-incision complex (PIC) is formed and stabilized by the addition of XPA, RPA, and XPG. The cryo-EM structures of TFIIH-XPA-DNA suggest that XPA is likely recruited first by TFIIH. Here, XPA is positioned at the ss-ds DNA junction of the NER bubble, to set the stage for proper opening of the DNA bubble and allow for damage verification by the XPD helicase. Subsequently, XPA serves important scaffolding functions in assembling the PIC and excising the damaged strand. Despite its small size of only 273 residues, XPA interacts with multiple NER proteins in the NER pathway: TFIIH, RPA, ERCC1-XPF, DDB2, and PCNA (Table 1) [48, 50–54]. In its function as NER scaffolding, XPA works in coordination with the ubiquitous ssDNA binding protein RPA, which occupies the undamaged strand. XPA and RPA together guide the accurate positioning of other NER proteins, especially the two endonucleases XPG and ERCC1-XPF [55].
XPA has a central globular DNA binding domain (DBD) spanning residues 98 to 239 with a zinc binding motif and disordered, flexible N-terminal (1–98) and C-terminal (240–273) domains. RPA is a heterotrimer consisting of the RPA70, RPA32, and RPA14 subunits. Its ssDNA binding apparatus consists of 4 OB-fold domains (RPA70A, RPA70B, RPA70C, and RPA32D) that can bind up to 30 nucleotides of ssDNA with a defined 5’ to 3’ polarity [56] (Table 1, Figure 4A). XPA and RPA interact through two distinct binding motifs: a primary site that involves the XPA N-terminal domain (residues 29–46) and the RPA32C winged-helix recruitment domain [49], and a secondary interaction involving the DNA binding domains (DBD) RPA70AB and XPA DBD [51] (Table 1, Figure 4B, 4C). The XPA DBD–RPA70AB interaction is crucial as it helps the positioning of XPA and RPA on the NER bubble [51]. A series of XPA variants were characterized that disrupted the interaction with the RPA32 and RPA70 subunits and showed that the two interaction surfaces synergistically contribute to NER (Table 1) [57]. Mutations in the RPA32 interacting domain abolished XPA localization to DNA damage, whereas mutations in the RPA70 interacting domain led to proper localization, but delayed departure of XPA from UV damaged sites. These results suggest that the stronger contact with RPA32C is responsible for XPA recruitment and localization to damage, whereas the weaker contact with RPA70AB is indispensable for the completion of NER. Based on SAXS data supported by computational modeling, it was observed that XPA and RPA can engage the two interactions simultaneously and that the XPA-RPA70 interaction localizes the two ss-dsDNA junctions in close proximity to facilitate the dual incision (Figure 4B) [57].
Figure 4.

Interactions in the NER pre-incision complex. (A) Domain map of XPA, RPA, XPG, XPF-ERCC1 with their interacting regions highlighted. Overlapping domains are identified with white stripes. NUC denotes nuclease domains. (B) A schematic model of the NER preincision complex with interactions among the component proteins indicated. (C) Structures of the dual interaction of XPA-RPA through two distinct binding motifs: XPA29–46/RPA32C (PDB: 1DPU) and XPA DBD/RPA70AB (PDB: PDBDEV_00000039) show the essential scaffolding role in NER. Mutation of residues in XPA that disrupt the RPA32 interaction (R30, K31, R32, R34, R39, R42) and the RPA70 interaction (D101, E106, K110, E111, F112, D114) are highlighted in red. (D) Structure of the acidic region of Rad2/XPG interacting with the pH domain of TFIIH-Tfb1/p62 (PDB: 2LOX), which is the same interaction interface as XPC/TFIIH-p62. This interaction interface has not been characterized using functional studies. (E) Structure showing how ERCC1-XPF is recruited to the pre-incision complex through an interaction between XPA and ERCC1 (PDB: 2JNW). Mutation of residues that disrupt the interaction - G72-G74, F75 of XPA are highlighted in magenta and N110, Y145 of ERCC1 are highlighted in red.
With TFIIH, XPA, and RPA bound at the lesion, the stage is set for the two endonucleases to engage. Although cellular experiments have shown that the two endonucleases can localize to the site of damage independently [9], it is likely that XPG engages with the complex first due to its tight interaction with TFIIH (Table 2) [58, 59], and observations showing that XPG needs to be present (although not catalytically active) for ERCC1-XPF to make the first incision [60, 61].
Table 2.
List of NER protein-protein interactions that are missing or need further validation.
| Protein | Interacting NER factors | Interaction domain (residues) | Methods | Reference |
|---|---|---|---|---|
| XPC | XPA * | 154–334 | PD | [33] |
| TFIIH | XPG | XPB, XPD, p62, and p44 | PD | [82] |
| XPF | XPB RPA32: 4–29; | Mut | [77] | |
| XPA | RPA * | RPA70: 153–176 aa | PD, Mut | [52, 83, 84] |
| RPA | XPG | N/D | co-IP | [85] |
| XPF | RPA70 | YH | [86] | |
| XPG | TFIIH | N/D | PD | [82] |
| RPA | 668–747 | co-IP | [85] | |
| XPF | TFIIH | N/D | Mut | [77] |
| RPA | N/D | YH | [86] |
CoIP: Co-Immunoprecipitation; Mut: Site directed/deletion mutagenesis, PD: pull-down assay, YH: Yeast two-hybrid assay.
Interaction interfaces not supported by current experimental structures and functional studies
XPG is a member of the FEN-1 (flap endonuclease) family of nucleases with a split active site consisting of the highly conserved N-terminal (N)- and internal (I)-nuclease regions separated by a large spacer region of 680 residues [62]. Its extended C-terminus is unique to XPG compared to other proteins in the FEN-1 family [63]. The spacer region in XPG is highly acidic and does not contain any known structural or functional motifs. The spacer region is known to interact with TFIIH (Table 2) [64, 65], although the molecular basis of the interactions between XPG and the subunits of TFIIH remains to be elucidated. In yeast, two acidic regions of Rad2/XPG (residues 359–383 and 642–690) were found to interact with the PH domain of Tfb1/p62 [65, 66] (Table 2, Figure 4D). This binding mode is similar to the interaction of Rad4/XPC with Tfb1/p62 and NMR titrations show that Rad2/XPG can outcompete Rad4/XPC to bind to the same interaction interface in Tfb1/p62. Since experimental evidence suggests that XPC and XPG do not co-exist in the NER complexes [19], it is tempting to speculate that the common interaction of XPC and XPG with p62 are key to a handover mechanism for the replacement of XPC with XPG. Cross-linking mass spectrometry provided additional information on the interaction interface of XPG with TFIIH (Table 2) [45]. The N-terminal region of XPG was localized near the FeS cluster and Arch domains of XPD and the C-terminal region of XPG is positioned near XPB and p52 subunits. It has been shown that XPG can stimulate the helicase and translocase activities of XPD and conversely that XPD can influence XPG cleavage activity [45, 68]. Further studies will be needed to fully define the architecture and functionality of XPG-TFIIH interactions.
Stage 3: Dual incision by ERCC1-XPF and XPG endonucleases
The active incision complex requires the second endonuclease ERCC1-XPF. The TFIIH-XPA-RPA-XPG complex provides the necessary preorganization to enable recruitment of ERCC1-XPF, which occurs through a direct interaction of XPA with ERCC1 (Table 1) [69–71]. ERCC1 engages a short 14 residue motif in the XPA N-terminal domain (residues 67–80) (Table 1). The XPA motif undergoes a disorder-to-order transition that is formed as a loop containing G72-G74 and F75 is inserted into an XPA binding pocket in ERCC1 (Table 1, Figure 4E). Deletion of the conserved glycines or mutation of F75 to A inhibits NER activity in vitro (Table 1) [71]. Mutation of two conserved residues (N110A/Y145A) in the XPA binding pocket of ERCC1 dramatically reduced NER activity in vitro and in vivo as ERCC1-XPF could not be recruited due to the weakened interaction with XPA (Table 1) [69].
ERCC1 and XPF form an obligate heterodimer through two evolutionarily related regions [72]. The two helix-hairpin-helix (HhH) domains at the C-terminus of XPF and ERCC1 form a tight heterodimer and bind the DNA at the junction [73–75]. Similarly, the central regions of ERCC1 and XPF dimerize, with this region in XPF making up the active site of the nuclease [76] and providing the XPA interaction domain in ERCC1 (Table 1) [71]. Less is known about the function of the helicase-like domain (HLD) of XPF in NER, although this HLD is likely to contribute to junction binding and/or protein-protein interactions. High resolution cryo-EM structures were determined for both DNA-free and DNA-bound ERCC1-XPF and show that the XPF HLD regulates XPF’s catalytic activity by forming an extensive interface with the ERCC1 HhH domain and blocking the catalytic site [75]. This autoinhibitory conformation of XPF needs to be released for XPF to bind to the DNA junction and perform 5’ incision. One mode of XPF activation could be the interaction of XPF HLD with the XPB subunit of TFIIH. S751 in XPB is a crucial residue in the C terminal region of the protein and its phosphorylation inhibits NER in vivo and regulates 5′ incision by the ERCC1- XPF (Table 2) [77]. The interaction between XPB and XPF seems ripe for structural and functional studies.
With the engagement of ERCC1-XPF, the stage is set for dual incision to occur with the first incision believed to be by ERCC1-XPF 5’ to the lesion [21]. Evidence for this order was provided by experiments showing that incision by ERCC1-XPF and initiation of repair synthesis requires the presence but not the catalytic function of XPG. Based on existing structural and biochemical evidence and in support of distinct structural and catalytic roles of XPG, the protein is likely to undergo a structural rearrangement from inactive to an active configuration to perform incision [78]. It has furthermore been shown that the incision activity of XPG can be stimulated by XPD if once it is stalled at the lesion and its ATPase activity is blocked [68]. Incision by ERCC1-XPF produces a free 3’-OH that can be used by the replication machinery to initiate repair synthesis [21]. After initiation of DNA repair synthesis, XPG incises 3’ to the lesion and marks the transition from the incision to the repair synthesis steps. While we focused on interactions that regulate formation of the incision complex, specific protein-protein interactions between NER and replication factors are certain to be involved in the later steps in NER. These are needed to provide a tight coordination of the two phases of NER and thereby avoid the formation of long-lived and toxic gaps upon removal of the oligonucleotide containing the damage [79].
Conclusion
Characterization of interactions among the constituent proteins using a broad range of biochemical, structural, and biophysical techniques is important to completely understand the inner workings of the complex NER machinery. Conversely, it is imperative to test the functional importance of the models derived from these results in biochemical and cellular NER assays. Through the years many interactions among NER proteins were reported based on yeast two-hybrid, co-IP, and pull-down studies (Table 2). Some of these reports lacked proper structural and functional characterization and were not validated in subsequent studies. In the main body of this review, we discussed the interactions that we consider to be properly validated by functional and/or structural studies. These interactions were critical to gain insight into the complex NER mechanisms. For completion, in Table 2 we include the list of interactions that were reported between NER proteins that we consider to be lacking proper validation using structural or functional studies or have been proven to be artefactual in subsequent studies. Figure 4 shows a summary of the interactions between the NER core factors discussed in this review (solid arrows) and partially characterized or expected interactions (dotted arrow) in the pre-incision complex. The latter await functional and structural characterization and once available, will provide further insight into how multiple proteins shape the pre-incision complex to license the incision reaction (Table 2). With advances in high-resolution cryo-EM structural studies and the development of deep-learning algorithms to predict structures of multi-protein complexes, we are now at a point in time where we can expect to gain a more complete understanding of the dynamic protein-protein interactions in NER that seemed out of reach just a few years ago.
Figure 5.

Summary of protein-protein interactions between core NER factors. Interactions validated using structural and functional studies are depicted as straight solid lines. Less well characterized and potential interactions are depicted as curved dotted lines.
Highlights.
NER is dynamic multistep pathway for the repair of bulky DNA lesions
Protein-protein interaction regulate the steps in the NER reaction
Structural and functional studies have elucidated the roles of protein-protein interactions in NER
This review provides a guide to the protein-protein interactions in NER
ACKNOWLEDGEMENTS
We thank current and past members of the Schärer and Chazin laboratories for their contributions to the research discussed in this review and for helpful discussions. The schematic models were created with a licensed version of BioRender.com. This work was supported by grants from the US NCI (R01 CA218315 and P01 CA092584 to ODS and WJC) and the Korean Institute for Basic Science (IBS-R022-A1 to ODS). MK was supported in part by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2023-00274772).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS
The authors declare no potential conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- [1].Gillet LC, Schärer OD, Molecular mechanisms of mammalian global genome nucleotide excision repair, Chem Rev, 106 (2006) 253–276. [DOI] [PubMed] [Google Scholar]
- [2].Schärer OD, Nucleotide excision repair in eukaryotes, Cold Spring Harb Perspect Biol, 5 (2013) a012609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].D’Souza A, Blee AM, Chazin WJ, Mechanism of action of nucleotide excision repair machinery, Biochem Soc Trans, 50 (2022) 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marteijn JA, Lans H, Vermeulen W, Hoeijmakers JH, Understanding nucleotide excision repair and its roles in cancer and ageing, Nat Rev Mol Cell Biol, 15 (2014) 465–481. [DOI] [PubMed] [Google Scholar]
- [5].Friedberg EC, Walker GC, Siede W, Wood RD, DNA repair and mutagenesis, American Society for Microbiology Press, 2005. [Google Scholar]
- [6].Cleaver JE, Lam ET, Revet I, Disorders of nucleotide excision repair: the genetic and molecular basis of heterogeneity, Nature Rev Genet, 10 (2009) 756–768. [DOI] [PubMed] [Google Scholar]
- [7].Kraemer KH, Patronas NJ, Schiffmann R, Brooks BP, Tamura D, DiGiovanna JJ, Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: a complex genotype–phenotype relationship, Neuroscience, 145 (2007) 1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sugasawa K, Ng JM, Masutani C, Iwai S, van der Spek PJ, Eker AP, Hanaoka F, Bootsma D, Hoeijmakers JH, Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair, Mol Cell, 2 (1998) 223–232. [DOI] [PubMed] [Google Scholar]
- [9].Volker M, Moné MJ, Karmakar P, van Hoffen A, Schul W, Vermeulen W, Hoeijmakers JH, van Driel R, van Zeeland AA, Mullenders LH, Sequential assembly of the nucleotide excision repair factors in vivo, Mol Cell, 8 (2001) 213–224. [DOI] [PubMed] [Google Scholar]
- [10].Nishi R, Okuda Y, Watanabe E, Mori T, Iwai S, Masutani C, Sugasawa K, Hanaoka F, Centrin 2 stimulates nucleotide excision repair by interacting with xeroderma pigmentosum group C protein, Mol Cell Biol, 25 (2005) 5664–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sugasawa K, Okuda Y, Saijo M, Nishi R, Matsuda N, Chu G, Mori T, Iwai S, Tanaka K, Tanaka K, Hanaoka F, UV-induced ubiquitylation of XPC protein mediated by UV-DDB-ubiquitin ligase complex, Cell, 121 (2005) 387–400. [DOI] [PubMed] [Google Scholar]
- [12].Apelt K, Lans H, Schärer OD, Luijsterburg MS, Nucleotide excision repair leaves a mark on chromatin: DNA damage detection in nucleosomes, Cell Mol Life Sci, (2021) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mathieu N, Kaczmarek N, Naegeli H, Strand-and site-specific DNA lesion demarcation by the xeroderma pigmentosum group D helicase, Proc Natl Acad Sci U S A, 107 (2010) 17545–17550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sugasawa K, Akagi J.-i., Nishi R, Iwai S, Hanaoka F, Two-step recognition of DNA damage for mammalian nucleotide excision repair: Directional binding of the XPC complex and DNA strand scanning, Mol Cell, 36 (2009) 642–653. [DOI] [PubMed] [Google Scholar]
- [15].Kuper J, Hove T, Maidl S, Neitz H, Sauer F, Kempf M, Schroeder T, Greiter E, Höbartner C, Kisker C, XPD stalled on cross-linked DNA provides insight into damage verification, Nat Struct Mol Biol, (2024) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nieto Moreno N, Olthof AM, Svejstrup JQ, Transcription-coupled nucleotide excision repair and the transcriptional response to UV-induced DNA damage, Ann Rev Biochem, 92 (2023) 81–113. [DOI] [PubMed] [Google Scholar]
- [17].van den Heuvel D, van der Weegen Y, Boer DE, Ogi T, Luijsterburg MS, Transcription-coupled DNA repair: from mechanism to human disorder, Trends Cell Biol, 31 (2021) 359–371. [DOI] [PubMed] [Google Scholar]
- [18].Evans E, Moggs JG, Hwang JR, Egly JM, Wood RD, Mechanism of open complex and dual incision formation by human nucleotide excision repair factors, EMBO J, 16 (1997) 6559–6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wakasugi M, Sancar A, Assembly, subunit composition, and footprint of human DNA repair excision nuclease, Proc Natl Acad Sci U S A, 95 (1998) 6669–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Riedl T, Hanaoka F, Egly JM, The comings and goings of nucleotide excision repair factors on damaged DNA, EMBO J, 22 (2003) 5293–5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Staresincic L, Fagbemi AF, Enzlin JH, Gourdin AM, Wijgers N, Dunand-Sauthier I, Giglia-Mari G, Clarkson SG, Vermeulen W, Schärer OD, Coordination of dual incision and repair synthesis in human nucleotide excision repair, EMBO J, 28 (2009) 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Araújo SJ, Tirode F, Coin F, Pospiech H, Syväoja JE, Stucki M, Hübscher U, Egly JM, Wood RD, Nucleotide excision repair of DNA with recombinant human proteins: definition of the minimal set of factors, active forms of TFIIH, and modulation by CAK, Genes Dev, 14 (2000) 349–359. [PMC free article] [PubMed] [Google Scholar]
- [23].Ogi T, Limsirichaikul S, Overmeer RM, Volker M, Takenaka K, Cloney R, Nakazawa Y, Niimi A, Miki Y, Jaspers NG, Three DNA polymerases, recruited by different mechanisms, carry out NER repair synthesis in human cells, Mol Cell, 37 (2010) 714–727. [DOI] [PubMed] [Google Scholar]
- [24].Theil AF, Häckes D, Lans H, TFIIH central activity in nucleotide excision repair to prevent disease, DNA Repair (Amst), (2023) 103568. [DOI] [PubMed] [Google Scholar]
- [25].Cohen Y, Adar S, Novel insights into bulky DNA damage formation and nucleotide excision repair from high-resolution genomics, DNA Repair (Amst), 130 (2023) 103549. [DOI] [PubMed] [Google Scholar]
- [26].Kuper J, Kisker C, Three targets in one complex: A molecular perspective of TFIIH in cancer therapy, DNA Repair (Amst), 105 (2021) 103143. [DOI] [PubMed] [Google Scholar]
- [27].Sugasawa K, Molecular mechanisms of DNA damage recognition for mammalian nucleotide excision repair, DNA Repair (Amst), 44 (2016) 110–117. [DOI] [PubMed] [Google Scholar]
- [28].Gunz D, Hess MT, Naegeli H, Recognition of DNA adducts by human nucleotide excision repair: evidence for a thermodynamic probing mechanism, J Biol Chem, 271 (1996) 25089–25098. [DOI] [PubMed] [Google Scholar]
- [29].Min J-H, Pavletich NP, Recognition of DNA damage by the Rad4 nucleotide excision repair protein, Nature, 449 (2007) 570–575. [DOI] [PubMed] [Google Scholar]
- [30].Kim J, Li C-L, Chen X, Cui Y, Golebiowski FM, Wang H, Hanaoka F, Sugasawa K, Yang W, Lesion recognition by XPC, TFIIH and XPA in DNA excision repair, Nature, 617 (2023) 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Masutani C, Sugasawa K, Yanagisawa J, Sonoyama T, Ui M, Enomoto T, Takio K, Tanaka K, Van der Spek P, Bootsma D, Purification and cloning of a nucleotide excision repair complex involving the xeroderma pigmentosum group C protein and a human homologue of yeast RAD23, EMBO J, 13 (1994) 1831–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sugasawa K, Masutani C, Uchida A, Maekawa T, van der Spek PJ, Bootsma D, Hoeijmakers JH, Hanaoka F, HHR23B, a human Rad23 homolog, stimulates XPC protein in nucleotide excision repair in vitro, Mol Cell Biol, 16 (1996), 4852–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bunick CG, Miller MR, Fuller BE, Fanning E, Chazin WJ, Biochemical and structural domain analysis of xeroderma pigmentosum complementation group C protein, Biochemistry, 45 (2006) 14965–14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kim B, Ryu KS, Kim HJ, Cho SJ, Choi BS, Solution structure and backbone dynamics of the XPC-binding domain of the human DNA repair protein hHR23B, The FEBS journal, 272 (2005) 2467–2476. [DOI] [PubMed] [Google Scholar]
- [35].Bergink S, Toussaint W, Luijsterburg MS, Dinant C, Alekseev S, Hoeijmakers JH, Dantuma NP, Houtsmuller AB, Vermeulen W, Recognition of DNA damage by XPC coincides with disruption of the XPC–RAD23 complex, J Cell Biol, 196 (2012) 681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Coin F, Oksenych V, Mocquet V, Groh S, Blattner C, Egly JM, Nucleotide excision repair driven by the dissociation of CAK from TFIIH, Mol Cell, 31 (2008) 9–20. [DOI] [PubMed] [Google Scholar]
- [37].Yokoi M, Masutani C, Maekawa T, Sugasawa K, Ohkuma Y, Hanaoka F, The xeroderma pigmentosum group C protein complex XPC-HR23B plays an important role in the recruitment of transcription factor IIH to damaged DNA, J Biol Chem, 275 (2000) 9870–9875. [DOI] [PubMed] [Google Scholar]
- [38].Uchida A, Sugasawa K, Masutani C, Dohmae N, Araki M, Yokoi M, Ohkuma Y, Hanaoka F, The carboxy-terminal domain of the XPC protein plays a crucial role in nucleotide excision repair through interactions with transcription factor IIH, DNA Repair (Amst), 1 (2002) 449–461. [DOI] [PubMed] [Google Scholar]
- [39].van Eeuwen T, Shim Y, Kim HJ, Zhao T, Basu S, Garcia BA, Kaplan CD, Min J-H, Murakami K, Cryo-EM structure of TFIIH/Rad4–Rad23–Rad33 in damaged DNA opening in nucleotide excision repair, Nature Commun, 12 (2021) 3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Okuda M, Kinoshita M, Kakumu E, Sugasawa K, Nishimura Y, Structural insight into the mechanism of TFIIH recognition by the acidic string of the nucleotide excision repair factor XPC, Structure, 23 (2015) 1827–1837. [DOI] [PubMed] [Google Scholar]
- [41].Gervais V, Lamour V, Jawhari A, Frindel F, Wasielewski E, Dubaele S, Egly J-M, Thierry J-C, Kieffer B, Poterszman A, TFIIH contains a PH domain involved in DNA nucleotide excision repair, Nat Struct Mol Biol, 11 (2004) 616–622. [DOI] [PubMed] [Google Scholar]
- [42].Okuda M, Nakazawa Y, Guo C, Ogi T, Nishimura Y, Common TFIIH recruitment mechanism in global genome and transcription-coupled repair subpathways, Nucleic Acids Res, 45 (2017) 13043–13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tsutakawa SE, Tsai C-L, Yan C, Bralić A, Chazin WJ, Hamdan SM, Schärer OD, Ivanov I, Tainer JA, Envisioning how the prototypic molecular machine TFIIH functions in transcription initiation and DNA repair, DNA Repair (Amst), 96 (2020) 102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Greber BJ, Toso DB, Fang J, Nogales E, The complete structure of the human TFIIH core complex, Elife, 8 (2019) e44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kokic G, Chernev A, Tegunov D, Dienemann C, Urlaub H, Cramer P, Structural basis of TFIIH activation for nucleotide excision repair, Nat Commun, 10 (2019) 2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ziani S, Nagy Z, Alekseev S, Soutoglou E, Egly JM, Coin F, Sequential and ordered assembly of a large DNA repair complex on undamaged chromatin, J Cell Biol, 206 (2014) 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Park CH, Mu D, Reardon JT, Sancar A, The general transcription-repair factor TFIIH is recruited to the excision repair complex by the XPA protein independent of the TFIIE transcription factor, J Biol Chem, 270 (1995) 4896–4902. [DOI] [PubMed] [Google Scholar]
- [48].Nocentini S, Coin F, Saijo M, Tanaka K, Egly JM, DNA damage recognition by XPA protein promotes efficient recruitment of transcription factor II H, J Biol Chem, 272 (1997) 22991–22994. [DOI] [PubMed] [Google Scholar]
- [49].van den Heuvel D, Kim M, Wondergem AP, van der Meer PJ, Witkamp M, Lambregtse F, Kim H-S, Kan F, Apelt K, Kragten A, A disease-associated XPA allele interferes with TFIIH binding and primarily affects transcription-coupled nucleotide excision repair, Proc Natl Acad Sci U S A, 120 (2023) e2208860120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mer G, Bochkarev A, Gupta R, Bochkareva E, Frappier L, Ingles CJ, Edwards AM, Chazin WJ, Structural basis for the recognition of DNA repair proteins UNG2, XPA, and RAD52 by replication factor RPA, Cell, 103 (2000) 449–456. [DOI] [PubMed] [Google Scholar]
- [51].Topolska-Woś AM, Sugitani N, Cordoba JJ, Le Meur KV, Le Meur RA, Kim HS, Yeo JE, Rosenberg D, Hammel M, Schärer OD, Chazin WJ, A key interaction with RPA orients XPA in NER complexes, Nucleic Acids Res, 48 (2020) 2173–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Li L, Lu X, Peterson CA, Legerski RJ, An interaction between the DNA repair factor XPA and replication protein A appears essential for nucleotide excision repair, Mol Cell Biol, 15 (1995) 5396–5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wakasugi M, Kasashima H, Fukase Y, Imura M, Imai R, Yamada S, Cleaver JE, Matsunaga T, Physical and functional interaction between DDB and XPA in nucleotide excision repair, Nucleic Acids Res, 37 (2009) 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gilljam KM, Müller R, Liabakk NB, Otterlei M, Nucleotide excision repair is associated with the replisome and its efficiency depends on a direct interaction between XPA and PCNA, PLoS one, 7 (2012) e49199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].De Laat WL, Appeldoorn E, Sugasawa K, Weterings E, Jaspers NG, Hoeijmakers JH, DNA-binding polarity of human replication protein A positions nucleases in nucleotide excision repair, Genes Dev, 12 (1998) 2598–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Brosey CA, Yan C, Tsutakawa SE, Heller WT, Rambo RP, Tainer JA, Ivanov I, Chazin WJ, A new structural framework for integrating replication protein A into DNA processing machinery, Nucleic Acids Res, 41 (2013) 2313–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kim M, Kim H-S, D’Souza A, Gallagher K, Jeong E, Topolska-Woś A, Ogorodnik Le Meur K, Tsai C-L, Tsai M-S, Kee M, Two interaction surfaces between XPA and RPA organize the preincision complex in nucleotide excision repair, Proc Natl Acad Sci U S A, 119 (2022) e2207408119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ito S, Kuraoka I, Chymkowitch P, Compe E, Takedachi A, Ishigami C, Coin F, Egly J-M, Tanaka K, XPG stabilizes TFIIH, allowing transactivation of nuclear receptors: implications for Cockayne syndrome in XP-G/CS patients, Mol Cell, 26 (2007) 231–243. [DOI] [PubMed] [Google Scholar]
- [59].Araujo SJ, Nigg EA, Wood RD, Strong functional interactions of TFIIH with XPC and XPG in human DNA nucleotide excision repair, without a preassembled repairosome, Mol Cell Biol, 21 (2001) 2281–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wakasugi M, Reardon JT, Sancar A, The non-catalytic function of XPG protein during dual incision in human nucleotide excision repair, J Biol Chem, 272 (1997) 16030–16034. [DOI] [PubMed] [Google Scholar]
- [61].Constantinou A, Gunz D, Evans E, Lalle P, Bates PA, Wood RD, Clarkson SG, Conserved residues of human XPG protein important for nuclease activity and function in nucleotide excision repair, J Biol Chem, 274 (1999) 5637–5648. [DOI] [PubMed] [Google Scholar]
- [62].Scherly D, Nouspikel T, Corlet J, Ucla C, Bairoch A, Clarkson SG, Complementation of the DNA repair defect in xeroderma pigmentosum group G cells by a human cDNA related to yeast RAD2, Nature, 363 (1993) 182–185. [DOI] [PubMed] [Google Scholar]
- [63].Muniesa-Vargas A, Theil AF, Ribeiro-Silva C, Vermeulen W, Lans H, XPG: a multitasking genome caretaker, Cell Mol Life Sci, 79 (2022) 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Thorel F, Constantinou A, Dunand-Sauthier I, Nouspikel T, Lalle P, Raams A, Jaspers NG, Vermeulen W, Shivji MK, Wood RD, Definition of a short region of XPG necessary for TFIIH interaction and stable recruitment to sites of UV damage, Mol Cell Biol, 24 (2004) 10670–10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Dunand-Sauthier I, Hohl M, Thorel F, Jaquier-Gubler P, Clarkson SG, Schärer OD, The spacer region of XPG mediates recruitment to nucleotide excision repair complexes and determines substrate specificity, J Biol Chem, 280 (2005) 7030–7037. [DOI] [PubMed] [Google Scholar]
- [66].Lafrance-Vanasse J, Arseneault G, Cappadocia L, Legault P, Omichinski JG, Structural and functional evidence that Rad4 competes with Rad2 for binding to the Tfb1 subunit of TFIIH in NER, Nucleic Acids Res, 41 (2013) 2736–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gong W, Holmberg H, Lu C, Huang M, Li S, Interplay of the Tfb1 pleckstrin homology domain with Rad2 and Rad4 in transcription coupled and global genomic nucleotide excision repair, Nucleic Acids Res, 52 (2024), 6333–6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bralić A, Tehseen M, Sobhy MA, Tsai C-L, Alhudhali L, Yi G, Yu J, Yan C, Ivanov I, Tsutakawa SE, A scanning-to-incision switch in TFIIH-XPG induced by DNA damage licenses nucleotide excision repair, Nucleic Acids Res, 51 (2023) 1019–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Orelli B, McClendon TB, Tsodikov OV, Ellenberger T, Niedernhofer LJ, Scharer OD, The XPA-binding domain of ERCC1 is required for nucleotide excision repair but not other DNA repair pathways, J Biol Chem, 285 (2010) 3705–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Li L, Elledge SJ, Peterson CA, Bales ES, Legerski RJ, Specific association between the human DNA repair proteins XPA and ERCC1, Proc Natl Acad Sci U S A, 91 (1994) 5012–5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tsodikov OV, Ivanov D, Orelli B, Staresincic L, Shoshani I, Oberman R, Schärer OD, Wagner G, Ellenberger T, Structural basis for the recruitment of ERCC1-XPF to nucleotide excision repair complexes by XPA, EMBO J, 26 (2007) 4768–4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sgouros J, Gaillard P-HL, Wood RD, A relationship between a DNA-repair/recombination nuclease family and archaeal helicases, Trends Biochem Sci, 24 (1999) 95–97. [DOI] [PubMed] [Google Scholar]
- [73].Tsodikov OV, Enzlin JH, Schärer OD, Ellenberger T, Crystal structure and DNA binding functions of ERCC1, a subunit of the DNA structure-specific endonuclease XPF–ERCC1, Proc Natl Acad Sci U S A, 102 (2005) 11236–11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Su Y, Orelli B, Madireddy A, Niedernhofer LJ, Schärer OD, Multiple DNA binding domains mediate the function of the ERCC1-XPF protein in nucleotide excision repair, J Biol Chem, 287 (2012) 21846–21855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Jones M, Beuron F, Borg A, Nans A, Earl CP, Briggs DC, Snijders AP, Bowles M, Morris EP, Linch M, Cryo-EM structures of the XPF-ERCC1 endonuclease reveal how DNA-junction engagement disrupts an auto-inhibited conformation, Nature Commun, 11 (2020) 1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Enzlin JH, Schärer OD, The active site of the DNA repair endonuclease XPF–ERCC1 forms a highly conserved nuclease motif, EMBO J, 21 (2002) 2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Coin F, Auriol J, Tapias A, Clivio P, Vermeulen W, Egly JM, Phosphorylation of XPB helicase regulates TFIIH nucleotide excision repair activity, EMBO J, 23 (2004) 4835–4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hohl M, Thorel F, Clarkson SG, Schärer OD, Structural determinants for substrate binding and catalysis by the structure-specific endonuclease XPG, J Biol Chem, 278 (2003) 19500–19508. [DOI] [PubMed] [Google Scholar]
- [79].Fagbemi AF, Orelli B, Schärer OD, Regulation of endonuclease activity in human nucleotide excision repair, DNA Repair (Amst), 10 (2011) 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Charbonnier J-B, Renaud E, Miron S, Le Du MH, Blouquit Y, Duchambon P, Christova P, Shosheva A, Rose T, Angulo JF, Structural, thermodynamic, and cellular characterization of human centrin 2 interaction with xeroderma pigmentosum group C protein, J Mol Biol, 373 (2007) 1032–1046. [DOI] [PubMed] [Google Scholar]
- [81].Tsodikov OV, Ivanov D, Orelli B, Staresincic L, Shoshani I, Oberman R, Schärer OD, Wagner G, Ellenberger T, Structural basis for the recruitment of ERCC1-XPF to nucleotide excision repair complexes by XPA, EMBO J, 26 (2007) 4768–4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Iyer N, Reagan MS, Wu K-J, Canagarajah B, Friedberg EC, Interactions involving the human RNA polymerase II transcription/nucleotide excision repair complex TFIIH, the nucleotide excision repair protein XPG, and Cockayne syndrome group B (CSB) protein, Biochemistry, 35 (1996) 2157–2167. [DOI] [PubMed] [Google Scholar]
- [83].Saijo M, Kuraoka I, Masutani C, Hanaoka F, Tanaka K, Sequential binding of DNA repair proteins RPA and ERCC1 to XPA in vitro, Nucleic Acids Res, 24 (1996) 4719–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Saijo M, Takedachi A, Tanaka K, Nucleotide excision repair by mutant xeroderma pigmentosum group A (XPA) proteins with deficiency in interaction with RPA, J Biol Chem, 286 (2011) 5476–5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].He Z, Henricksen LA, Wold MS, Ingles CJ, RPA involvement in the damage-recognition and incision steps of nucleotide excision repair, Nature, 374 (1995) 566–569. [DOI] [PubMed] [Google Scholar]
- [86].Fisher LA, Bessho M, Wakasugi M, Matsunaga T, Bessho T, Role of interaction of XPF with RPA in nucleotide excision repair, J Mol Biol, 413 (2011) 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]


