Abstract
Antibodies raised against a 52 kDa rat liver microsomal glucose-transport protein were used to screen a rat liver cDNA library. Six positive clones were isolated. Two clones were found to be identical with the liver plasma-membrane glucose-transport protein termed GLUT 2. The sequence of the four remaining clones indicates that they encode a unique microsomal facilitative glucose-transport protein which we have termed GLUT 7. Sequence analysis revealed that the largest GLUT 7 clone was 2161 bp in length and encodes a protein of 528 amino acids. The deduced amino acid sequence of GLUT 7 shows 68% identity with the deduced amino acid sequence of rat liver GLUT 2. The GLUT 7 sequence is six amino acids longer than rat liver GLUT 2, and the extra six amino acids at the C-terminal end contain a consensus motif for retention of membrane-spanning proteins in the endoplasmic reticulum. When the largest GLUT 7 clone was transfected into COS 7 cells the expressed protein was found in the endoplasmic reticulum and nuclear membrane, but not in the plasma membrane. Microsomes isolated from the transfected COS 7 cells demonstrated an increase in their microsomal glucose-transport capacity, demonstrating that the GLUT 7 clone encodes a functional endoplasmic-reticulum glucose-transport protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Kayano T., Buse J. B., Burant C. F., Takeda J., Lin D., Fukumoto H., Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990 Mar;13(3):198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- Burchell A. Molecular pathology of glucose-6-phosphatase. FASEB J. 1990 Sep;4(12):2978–2988. doi: 10.1096/fasebj.4.12.2168325. [DOI] [PubMed] [Google Scholar]
- Burchell A., Waddell I. D. The molecular basis of the hepatic microsomal glucose-6-phosphatase system. Biochim Biophys Acta. 1991 Apr 17;1092(2):129–137. doi: 10.1016/0167-4889(91)90146-o. [DOI] [PubMed] [Google Scholar]
- Ferrannini E., Smith J. D., Cobelli C., Toffolo G., Pilo A., DeFronzo R. A. Effect of insulin on the distribution and disposition of glucose in man. J Clin Invest. 1985 Jul;76(1):357–364. doi: 10.1172/JCI111969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Bell G. I. Facilitative glucose transporters: an expanding family. Trends Biochem Sci. 1990 Jan;15(1):18–23. doi: 10.1016/0968-0004(90)90125-u. [DOI] [PubMed] [Google Scholar]
- Harding D., Wilson S. M., Jackson M. R., Burchell B., Green M. D., Tephly T. R. Nucleotide and deduced amino acid sequence of rat liver 17 beta-hydroxysteroid UDP-glucuronosyltransferase. Nucleic Acids Res. 1987 May 11;15(9):3936–3936. doi: 10.1093/nar/15.9.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. R., Nilsson T., Peterson P. A. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990 Oct;9(10):3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. H., Newgard C. B., Milburn J. L., Lodish H. F., Thorens B. The high Km glucose transporter of islets of Langerhans is functionally similar to the low affinity transporter of liver and has an identical primary sequence. J Biol Chem. 1990 Apr 25;265(12):6548–6551. [PubMed] [Google Scholar]
- Kayano T., Burant C. F., Fukumoto H., Gould G. W., Fan Y. S., Eddy R. L., Byers M. G., Shows T. B., Seino S., Bell G. I. Human facilitative glucose transporters. Isolation, functional characterization, and gene localization of cDNAs encoding an isoform (GLUT5) expressed in small intestine, kidney, muscle, and adipose tissue and an unusual glucose transporter pseudogene-like sequence (GLUT6). J Biol Chem. 1990 Aug 5;265(22):13276–13282. [PubMed] [Google Scholar]
- Kayano T., Fukumoto H., Eddy R. L., Fan Y. S., Byers M. G., Shows T. B., Bell G. I. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J Biol Chem. 1988 Oct 25;263(30):15245–15248. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lubon H., Ghazal P., Hennighausen L., Reynolds-Kohler C., Lockshin C., Nelson J. Cell-specific activity of the modulator region in the human cytomegalovirus major immediate-early gene. Mol Cell Biol. 1989 Mar;9(3):1342–1345. doi: 10.1128/mcb.9.3.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Kidd S., Kelley M. R. An improved filamentous helper phage for generating single-stranded plasmid DNA. Gene. 1986;45(3):333–338. doi: 10.1016/0378-1119(86)90032-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson I. A., Cushman S. W. Hormonal regulation of mammalian glucose transport. Annu Rev Biochem. 1986;55:1059–1089. doi: 10.1146/annurev.bi.55.070186.005211. [DOI] [PubMed] [Google Scholar]
- Thorens B., Charron M. J., Lodish H. F. Molecular physiology of glucose transporters. Diabetes Care. 1990 Mar;13(3):209–218. doi: 10.2337/diacare.13.3.209. [DOI] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Waddell I. D., Burchell A. Transverse topology of glucose-6-phosphatase in rat hepatic endoplasmic reticulum. Biochem J. 1991 Apr 1;275(Pt 1):133–137. doi: 10.1042/bj2750133. [DOI] [PMC free article] [PubMed] [Google Scholar]
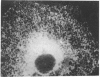
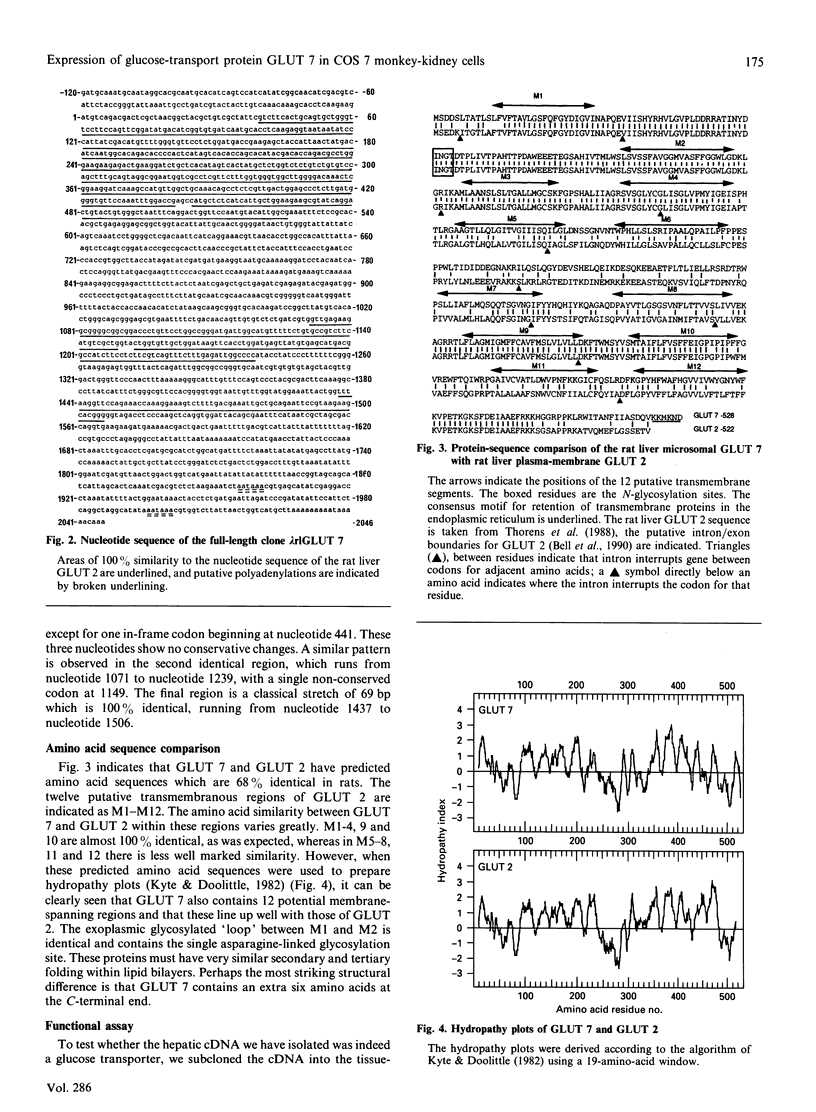
- Waddell I. D., Scott H., Grant A., Burchell A. Identification and characterization of a hepatic microsomal glucose transport protein. T3 of the glucose-6-phosphatase system? Biochem J. 1991 Apr 15;275(Pt 2):363–367. doi: 10.1042/bj2750363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T. J., Hinkle P. C. The glucose transporter of mammalian cells. Annu Rev Physiol. 1985;47:503–517. doi: 10.1146/annurev.ph.47.030185.002443. [DOI] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]