Abstract
The perinatal maternal environment is important for the normal development of the fetus. Epigenetic modifications that influence developmental control genes and signalling pathways for proper fetal development have been associated with maternal illnesses brought on by viruses, bacteria, or even parasitic protozoa. It is crucial to provide details on the onset, length, and timing of the mother's fever because these factors may influence the kind of certain abnormalities. Although fever is a primarily benign disease, it has been linked to negative health outcomes in children and has occasionally resulted in a substantial referral to critical care. This case report presents a 15-year-old female patient with repaired cleft palate and tetralogy of Fallot (TOF) who approached for esthetic rehabilitation of lower anterior teeth. The teeth (31, 32, 43) were tender on percussion. Radiographic evaluation showed the presence of periapical radiolucency. The root canal procedure was performed under local anaesthesia, and the supernumerary maxillary teeth were extracted. After cleaning and disinfecting, these teeth were used as biologic posts with respect to 32 and 33. A follow-up examination was performed after 12 months. The results of this case indicate that using autologous heterodontic biologic posts can lead to a favourable outcome.
Keywords: case report, genetics, pregnancy, fever, biologic post
Introduction
Fever during pregnancy is common, with approximately 20% of women reporting at least one febrile episode [1]. Fever related to urinary tract infection (UTI) was found to be the third most frequent cause. Although there were no maternal deaths, there were unfavourable fetal outcomes, such as preterm deliveries and low birth weight newborns. This indicates the necessity of increasing the use of bacterial sensitivity-guided antibiotic usage in pregnant women with UTIs and of urine cultures as a screening tool for asymptomatic bacteriuria [2]. Pregnant women have a reported 8% incidence of UTI [3]. Despite being a relatively benign condition, fever was associated with several neonatal affections and adverse health affections in offspring, leading sometimes to a significant referral to intensive care [1].
The development of a functioning circulatory system is necessary for the embryo to survive, and it starts as early as 28 days after conception [4]. The most prevalent cyanotic congenital heart condition, tetralogy of Fallot (TOF), affects around 1 in 3600 births and accounts for 7-10% of all congenital cardiac abnormalities [5]. Palatogenesis begins in the sixth week and is completed by palatal fusion at 12 weeks of gestation [6]. Failure to fusion of palatal shelves leads to CP and has a high prevalence of dental anomalies, such as dental agenesis, supernumerary teeth and morphologic irregularities [7]. The mandibular second premolar (3.4%) and maxillary lateral incisor (2.2%) are the most often congenitally missing permanent teeth, excluding third molars. A survey on congenitally missing teeth revealed that the incidence of congenitally missing permanent canines was 0.1% in the maxilla and 0.02% in the mandible [8]. We emphasize that the present case with dental anomalies may be because of partial expression of PAX and MSX gene family due to pathogenic infection during pregnancy. After one year of successful dental treatment, we could not find any recurrence of the lesion. The patient was asymptomatic and the teeth were functional.
Case presentation
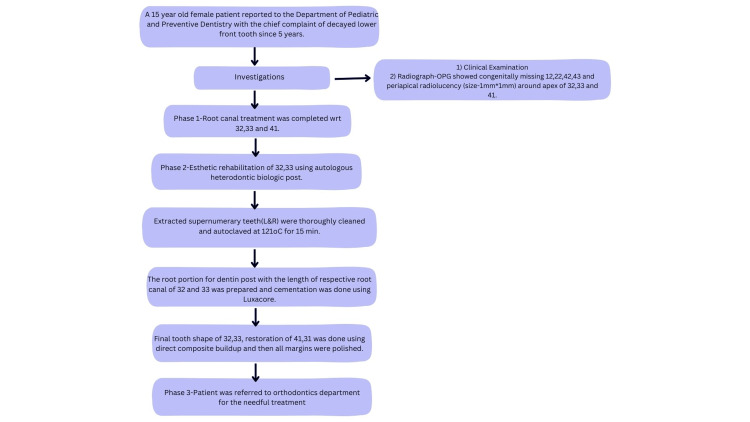
A 15-year-old female patient (student of class X) reported to the Department of Pediatric and Preventive Dentistry with a chief complaint of a decayed lower front tooth for five years (Figure 1).
Figure 1. Sequential timeline diagram emphasizes the events and their depiction.
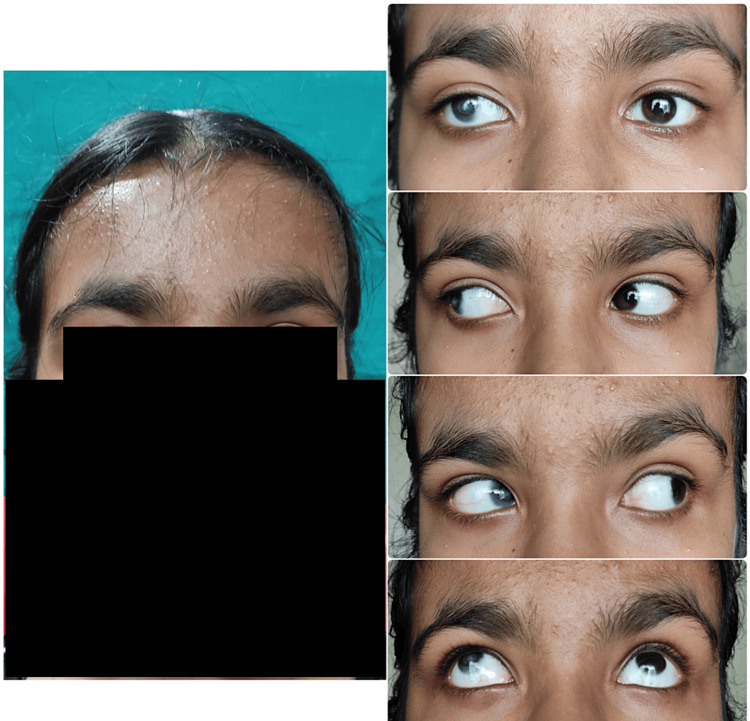
On extraoral examination, the patient had a broad forehead with a high hairline and sparse hair. She had bilateral corneal opacity (Figure 2).
Figure 2. Extraoral examination of the patient showing bilateral corneal opacity.
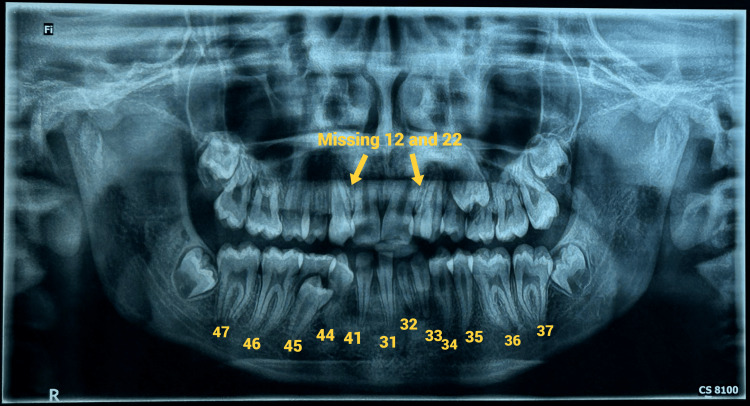
Intraoral examination revealed a repaired cleft palate with clinically missing teeth 12 and 22, a crossbite with respect to tooth 13, a rotated tooth 23, a scissor bite with respect to tooth 26, and labially placed bilateral maxillary supernumerary teeth with a shift in the midline. She also had difficulty pronouncing words starting with "s" due to carious mandibular anterior teeth. The radiograph (orthopantomogram, or OPG) revealed a full set of permanent teeth with congenitally missing teeth 12, 22, 42, and 43, as well as periapical radiolucency (size: 1 mm x 1 mm) around the apex of teeth 32, 33, and 41 (Figure 3).
Figure 3. Pretreatment panoramic radiograph.
Medical history
On detailed enquiring about medical history, the mother revealed that during the late first trimester, she suffered from high-grade fever with UTI. During a routine ultrasound scan in the early second trimester by a gynaecologist and ultrasonologist, the parents were asked to abort the child (fetal age: 16 weeks) due to fetal abnormality, but the parents denied it because she was their first child and they were ready to take the risk. The preterm (7.5 weeks) child was delivered by C-section with bilateral corneal opacity, TOF and cleft palate. At the age of 10 months, there was a diminution of vision (the right eye was more affected than the left eye). The ophthalmologist advised cornea transplantation but parents did not opt for surgery due to financial difficulties. The child was operated on at the age of 18 months for TOF and cleft palate repair at two years of age. A proper medical record of previous investigation and treatment could not be obtained as they were not properly maintained. Parents were recommended to undergo karyotyping; however, due to financial constraints, they were unable to proceed with the procedure.
The patient was presented with all available treatment options for teeth 32, 33, and 43 including extraction and replacement, root canal treatment followed by biological posts and restoration. The pros and cons of each option were explained in detail. The second option was ultimately chosen due to the patient’s desire and obtained written informed consent from the patient.
The treatment plan was done phasewise. In phase 1, root canal treatment was completed with respect to teeth 32, 33, and 41 after nonsurgical healing of periapical lesions. In phase 2, esthetic rehabilitation of teeth 32 and 33 was done using autologous heterodontic biologic post. For dentin post and core fabrication, the extracted supernumerary teeth (right and left) were thoroughly cleaned and autoclaved at 121 degrees Celsius for 15 minutes. The root portion for the dentin posts, matching the length of the respective root canals of teeth 32 and 33, was prepared, and cementation was performed using LuxaCore (DMG America, Englewood, USA). The final tooth shape of teeth 32 and 33 and the restoration of tooth 41 were accomplished using direct composite buildup, followed by polishing all margins (Figure 4).
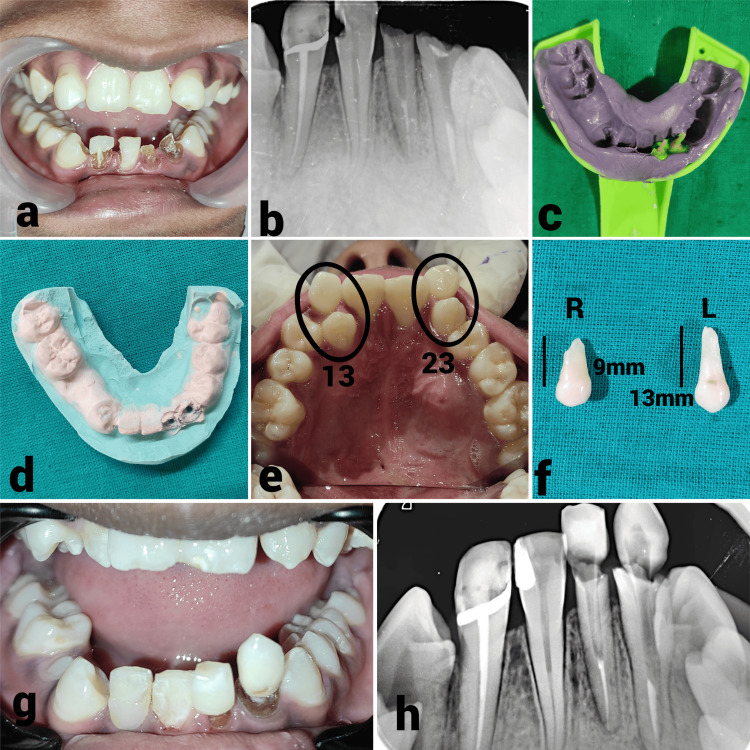
Figure 4. Sequential stages of endodontic intervention of lower anterior teeth.
(a) Preoperative photograph revealed grossly decayed teeth 32, 33, and 41; (b) Obturation of teeth 32, 33, and 41 done using gutta-percha and coated with AHPlus sealer (Dentsply Sirona, York, USA); (c) Impression of the post space of teeth 32 and 33 made with a custom-made self-cure post and medium-bodied elastomeric impression material; (d) Impression was poured with die stone to create a model, which served as a guide during the fabrication of the dentin post; (e) Extraction of both maxillary supernumerary teeth (left and right) was performed; (f) Extracted teeth were thoroughly cleaned and autoclaved at 121°C for 15 minutes; (g) Each tooth was trimmed circumferentially along the root portion to match the length of the respective root canal of teeth 32 and 33; (h) The model served as a reference for orienting the shape, length, and thickness of the dentin post, which was radiographically verified.
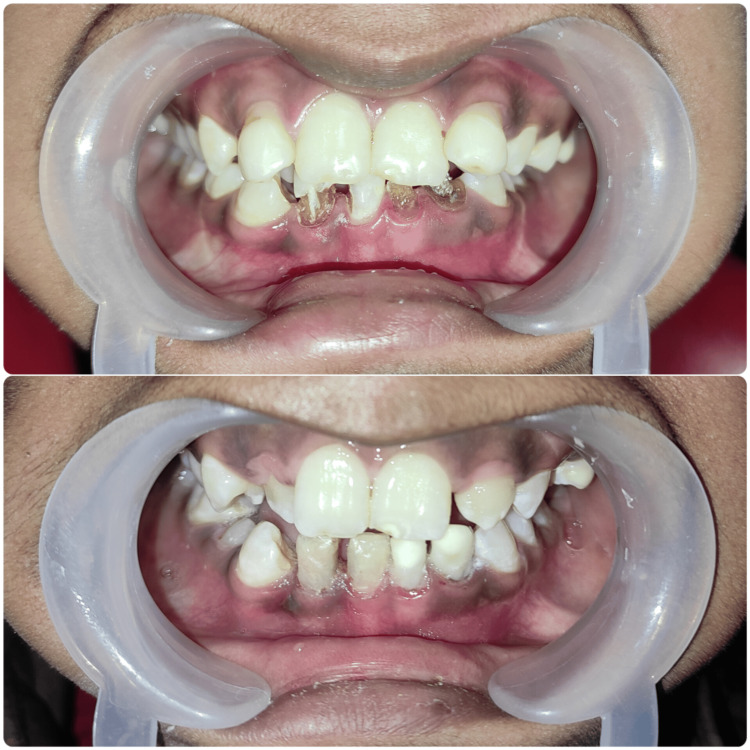
In phase 3 (future prospective plan), the patient was referred to the orthodontics department for necessary treatment. However, since the patient was in class 10 and appearing for board exams, her parents decided to defer further orthodontic treatment at this time. The patient was recalled for follow-ups at the end of three, six, and 12 months. The patient remained asymptomatic during the three-month, six-month, and 12-month follow-ups. On clinical examination, the teeth were nontender on percussion (Figure 5). The patient was satisfied with the treatment.
Figure 5. Before and after treatment photographs.
Discussion
Cellular processes and molecular events are highly regulated throughout organogenesis. The fetal immune system is incompetent and more vulnerable to infections spread vertically. Changes in the ratio of pro- to anti-inflammatory genes have been seen in abnormal inflammatory cytokine release as a result of maternal infection [9].
The PAX gene family, also known as the paired box (PAX) genes, codes for certain DNA-binding transcription factors that usually have a paired-type homeodomain and a PAX domain. There are nine genes in the mammalian PAX gene family that encode transcriptional regulatory proteins that bind DNA [10]. PAX gene mutations have been related to a variety of fetal abnormalities. All members of the PAX gene family influence cell fate, apoptosis, and differentiation, which all contribute to the formation of organs during embryogenesis [11].
One of the transcription factor family's genes, PAX9, is found on chromosome 14 (14q12-q13). These genes are crucial for the early stages of development in a number of multicellular animals [12]. PAX9 is essential for the development of the cardiovascular system because it causes a variety of cardiovascular abnormalities in newborns and embryos. In people with cardiovascular abnormalities and other disorders, gene mutations and chromosomal deletions, including PAX9, have been found [13]. TOF is also linked to new potential candidate genes, including CLDN9, CCDC168, SN2, L1TD, TACC3, CCDC36, and TTC5 [14]. The homeobox gene locus PAX6 is responsible for the transcription of several genes and growth factors involved in the development of the eye. Cells of the eye field express a set of eye field transcription factors (EFTFs) that are highly conserved throughout vertebrates. In mammals, the EFTFs include PAX6, RAX, SIX3, and LHX2 [15]. The transcription factors, PAX7 and PAX9, control the growth and differentiation of cranial neural crest cells, which in turn affects the creation of the craniofacial area. The interruption of cranial neural crest cell development resulting from dysfunction and impairment causes craniofacial deformities, such as orofacial clefts [16].
The genetic processes controlling the development of the human dentition are revealed via heritable tooth agenesis. The incidence of spontaneous agenesis, which often results in one to three missing teeth, may be impacted by hereditary or local circumstances. Based on cellular, molecular and genetic studies, genes PAX9, MSX1, and Bmp4 (bone morphogenetic protein-4) are responsible for tooth development. Oligodontia and hypodontia have been linked to mutation of PAX9 and MSX1 gene. Tooth agenesis is associated with several genes; however, non-syndromic human tooth agenesis has been associated with mutations in AXIN2, EDA, PAX9, MSX1, and PAX9 [17]. Unusual tooth agenesis is represented by nonsyndromic agenesis of permanent mandibular canines. Despite the fact that no particular genetic pattern or aberration has been linked to the agenesis of a single permanent lower canine.
A sporadic case is characterised by a novel mutation that arises spontaneously for no apparent cause in individuals without a positive family history of the condition. Since there was no positive family history, the present case is an illustration of a sporadic mutation. The patient suffered from a mild form of TOF associated with bilateral corneal opacity, incomplete cleft palate, bilateral maxillary supernumerary teeth and agenesis of teeth 12,22,42,43. Based on the literature and clinical findings of the present case, it can be suggested that there might be a case of mutation in the PAX and MSX gene family (Tables 1-2).
Table 1. Genes associated with tooth agenesis in humans.
Reference: [18]
| Genes involved | Mutations of genes | Defect |
| PAX9 | K114X, L21P, R26W, R28P, G51S, K91E, G73fsX316, V265fsX316 & R59fsX177 | Molar hypodontia, oligodontia, peg-shaped laterals |
| MSX1 | M61K, S105X, Q187X, R196P & S202X | Hypodontia, oligodontia |
| AXIN2 | Arg656Stop, 1994- 1995insG | Incisor agenesis |
| LTBP3 | Y774X | Oligodontia |
| EDA | Thr338Met | Hypodontia |
Table 2. Developmental roles of PAX genes on different human tissue types.
Reference: [19]
| Developmental role on human tissue types | Master regulator gene | |
| Group I | Teeth | PAX 9 |
| Thymus, parathyroid | PAX 1 | |
| Skeleton | PAX 1, PAX9 | |
| Group II | Thyroid | PAX 8 |
| B-cells | PAX 5 | |
| Kidneys | PAX 2, PAX 8 | |
| Group III | Skeletal muscle | PAX 3, PAX 7 |
| Neural crest | PAX 3, PAX 7 | |
| Group IV | Eye | PAX 6 |
| GI endocrine cells | PAX 4, PAX 6 | |
| Central nervous system | PAX 2, PAX 3, PAX 5, PAX 6, PAX 7, PAX 8 |
A phenotype-driven molecular genetic test was recommended to rule out genetic abnormality. Congenital abnormalities rank among the top 20 worldwide sources of illness burden. It is known that pregnancy-related hyperthermia is teratogenic. When protein synthesis is disrupted by hyperthermia, cell death, membrane and vascular disruption and placental infarction may result. This may result in fatalities or serious fetal deformities. As the first trimester of pregnancy is when any teratogenic influence on the fetus is most likely to occur, we concentrated on investigating the relationship between fever in pregnancy and fetal congenital abnormalities.
Conclusions
Cytogenetic testing provides an opportunity to enhance the prevention of congenital disorders. Parents can receive counselling regarding expectations for a specific congenital disorder, assess fetal and maternal risks, and make informed decisions regarding pregnancy continuation. The risks associated with terminating a pregnancy increase without an early cytogenetic diagnosis. Our data suggests that fever itself or other physiological changes linked to numerous infections are correlated with certain birth defects. Women who are pregnant or planning to become pregnant may want to consider speaking with their healthcare provider about the best ways to avoid infections that may cause fever and guidance on how to treat fevers during pregnancy.
Acknowledgments
We acknowledge the patient and parents for their full cooperation and support.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Sriparna De, Anshul Gangwar
Acquisition, analysis, or interpretation of data: Sriparna De
Drafting of the manuscript: Sriparna De, Anshul Gangwar
Critical review of the manuscript for important intellectual content: Sriparna De, Anshul Gangwar
Supervision: Anshul Gangwar
References
- 1.Fever during pregnancy as a risk factor for neurodevelopmental disorders: results from a systematic review and meta-analysis. Antoun S, Ellul P, Peyre H, Rosenzwajg M, Gressens P, Klatzmann D, Delorme R. Mol Autism. 2021;12:60. doi: 10.1186/s13229-021-00464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fever during pregnancy: etiology and fetomaternal outcomes. Brar R, Suri V, Suri V, Singh MP, Biswal M, Sikka P. J Obstet Gynaecol India. 2022;72:102–108. doi: 10.1007/s13224-021-01562-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urinary tract infections in pregnancy. Loh K, Sivalingam N. https://pubmed.ncbi.nlm.nih.gov/25606081/ Malays Fam Physician. 2007;2:54–57. [PMC free article] [PubMed] [Google Scholar]
- 4.A pictorial account of the human embryonic heart between 3.5 and 8 weeks of development. Hikspoors JP, Kruepunga N, Mommen GM, Köhler SE, Anderson RH, Lamers WH. Commun Biol. 2022;5:226. doi: 10.1038/s42003-022-03153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risk factors and outcomes of tetralogy of Fallot: from fetal to neonatal life. Silva JA, Neves AL, Flor-de-Lima F, Soares P, Guimarães H. Pediatr Cardiol. 2020;41:155–164. doi: 10.1007/s00246-019-02239-4. [DOI] [PubMed] [Google Scholar]
- 6.Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Bush JO, Jiang R. Development. 2012;139:231–243. doi: 10.1242/dev.067082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dental anomalies in cleft lip and palate: a case-control comparison of total and outside the cleft prevalence. Herrera-Atoche JR, Huerta-García NA, Escoffié-Ramírez M, et al. Medicine (Baltimore) 2022;101:0. doi: 10.1097/MD.0000000000029383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilateral mandibular permanent canine agenesis: a rare case report. Gnanabagyan JT, Rekha CV, Annamalai S, Baghkomeh PN. https://aimdrjournal.com/wp-content/uploads/2021/08/DE4_CR_Trophimus.pdf Ann Int Med Dent Res. 2016;3:10–12. [Google Scholar]
- 9.Maternal factors that induce epigenetic changes contribute to neurological disorders in offspring. Banik A, Kandilya D, Ramya S, Stünkel W, Chong YS, Dheen ST. Genes (Basel) 2017;8:150. doi: 10.3390/genes8060150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maternal transmission effects of the PAX genes among cleft case-parent trios from four populations. Sull JW, Liang KY, Hetmanski JB, et al. Eur J Hum Genet. 2009;17:831–839. doi: 10.1038/ejhg.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.PAX genes in cardiovascular development. Steele RE, Sanders R, Phillips HM, Bamforth SD. Int J Mol Sci. 2022;23:7713. doi: 10.3390/ijms23147713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genetic basis of non-syndromic anomalies of human tooth number. Galluccio G, Castellano M, La Monaca C. Arch Oral Biol. 2012;57:918–930. doi: 10.1016/j.archoralbio.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Pax9 is required for cardiovascular development and interacts with Tbx1 in the pharyngeal endoderm to control 4th pharyngeal arch artery morphogenesis. Phillips HM, Stothard CA, Shaikh Qureshi WM, et al. Development. 2019;146 doi: 10.1242/dev.177618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genes and pathways implicated in tetralogy of Fallot revealed by ultra-rare variant burden analysis in 231 genome sequences. Manshaei R, Merico D, Reuter MS, et al. Front Genet. 2020;11:957. doi: 10.3389/fgene.2020.00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eye development and retinogenesis. Heavner W, Pevny L. Cold Spring Harb Perspect Biol. 2012;4:0. doi: 10.1101/cshperspect.a008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.PAX7, PAX9 and RYK expression in cleft affected tissue. Vaivads M, Akota I, Pilmane M. Medicina (Kaunas) 2021;57:1075. doi: 10.3390/medicina57101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pathogenic mechanisms of tooth agenesis linked to paired domain mutations in human PAX9. Wang Y, Groppe JC, Wu J, Ogawa T, Mues G, D'Souza RN, Kapadia H. Hum Mol Genet. 2009;18:2863–2874. doi: 10.1093/hmg/ddp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genetic basis of dental agenesis - molecular genetics patterning clinical dentistry. Chhabra N, Goswami M, Chhabra A. Med Oral Patol Oral Cir Bucal. 2014;19:0–19. doi: 10.4317/medoral.19158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The PAX genes: roles in development, cancer, and other diseases. Shaw T, Barr FG, Üren A. Cancers (Basel) 2024;16:2–26. doi: 10.3390/cancers16051022. [DOI] [PMC free article] [PubMed] [Google Scholar]