Abstract
The major phenobarbital-inducible rat hepatic cytochromes P-450, CYP2B1 and CYP2B2, are the paradigmatic members of a cytochrome P-450 gene subfamily that contains at least seven additional members. Specific oligonucleotide probes for these genomic members of the CYP2B subfamily were used to assess their tissue-specific expression. In Northern-blot analysis a probe specific to gene 4 (which is designated now as CYP2B12) hybridized to a single mRNA present in the preputial gland, an organ which is used as a model for sebaceous glands, but did not hybridize to mRNA isolated from the liver or from five other tissues of untreated or Aroclor 1254-treated rats. The cDNA sequence for the CYP2B12 RNA was determined from overlapping cDNA clones and contained a long open reading frame of 1476 bp. The nucleotide sequence of the CYP2B12 cDNA was 85% similar to the sequence of the CYP2B1 cDNA in its coding region and was different from any CYP2B cDNA characterized until now. The cDNA-derived primary structure of the CYP2B12 protein contains a signal sequence for its insertion into the endoplasmic reticulum and the putative haem-binding site characteristic of cytochromes P-450. A part of the potential haem pocket of CYP2B12 was identical with a similar structure in a bacterial protocatechuate dioxygenase. In immunoblot analysis of preputial-gland microsomes, antibodies against CYP2B1 recognized a single abundant protein with a lower apparent molecular mass than that of CYP2B1. Our results demonstrate that the CYP2B12 protein has the potential to be enzymically active and are the first demonstration that a member of the CYP2B subfamily is expressed exclusively and at high levels in an extrahepatic organ.
Full text
PDF







Images in this article
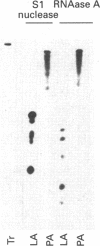
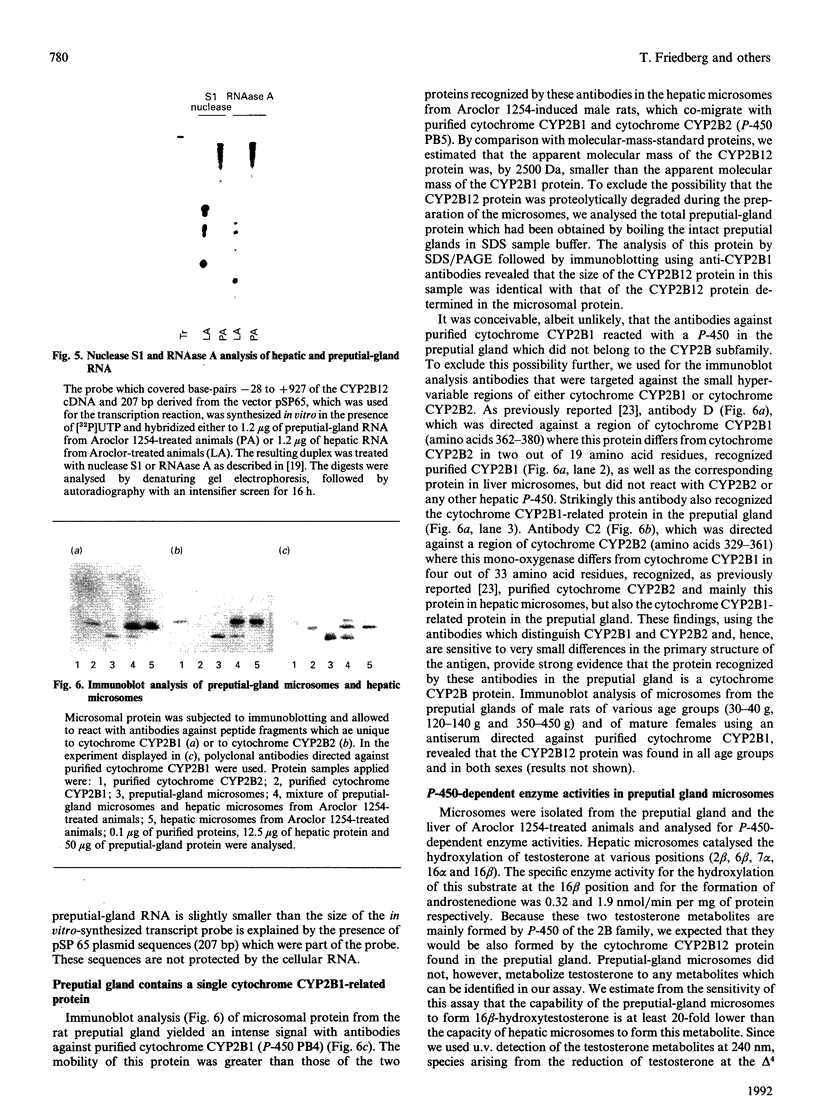
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Atchison M. Genes for cytochrome P-450 and their regulation. CRC Crit Rev Biochem. 1986;19(3):247–305. doi: 10.3109/10409238609084657. [DOI] [PubMed] [Google Scholar]
- Aoyama T., Korzekwa K., Nagata K., Adesnik M., Reiss A., Lapenson D. P., Gillette J., Gelboin H. V., Waxman D. J., Gonzalez F. J. Sequence requirements for cytochrome P-450IIB1 catalytic activity. Alteration of the stereospecificity and regioselectivity of steroid hydroxylation by a simultaneous change of two hydrophobic amino acid residues to phenylalanine. J Biol Chem. 1989 Dec 15;264(35):21327–21333. [PubMed] [Google Scholar]
- Atchison M., Adesnik M. Gene conversion in a cytochrome P-450 gene family. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2300–2304. doi: 10.1073/pnas.83.8.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brind J. L., Marinescu D., Gomez E. C., Wheatley V. R., Orenteich N. In-vitro testosterone metabolism in the mouse preputial gland: intercellular co-operation and changes with cell maturation. J Endocrinol. 1984 Mar;100(3):377–388. doi: 10.1677/joe.0.1000377. [DOI] [PubMed] [Google Scholar]
- Burke M. D., Thompson S., Elcombe C. R., Halpert J., Haaparanta T., Mayer R. T. Ethoxy-, pentoxy- and benzyloxyphenoxazones and homologues: a series of substrates to distinguish between different induced cytochromes P-450. Biochem Pharmacol. 1985 Sep 15;34(18):3337–3345. doi: 10.1016/0006-2952(85)90355-7. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clarke L., Waxman D. J. Oxidative metabolism of cyclophosphamide: identification of the hepatic monooxygenase catalysts of drug activation. Cancer Res. 1989 May 1;49(9):2344–2350. [PubMed] [Google Scholar]
- Doehmer J., Dogra S., Friedberg T., Monier S., Adesnik M., Glatt H., Oesch F. Stable expression of rat cytochrome P-450IIB1 cDNA in Chinese hamster cells (V79) and metabolic activation of aflatoxin B1. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5769–5773. doi: 10.1073/pnas.85.16.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis P. D., Zemmour J., Salter R. D., Parham P. Rapid cloning of HLA-A,B cDNA by using the polymerase chain reaction: frequency and nature of errors produced in amplification. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2833–2837. doi: 10.1073/pnas.87.7.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg T., Grassow M. A., Oesch F. Selective detection of mRNA forms encoding the major phenobarbital inducible cytochromes P450 and other members of the P450IIB family by the RNAse A protection assay. Arch Biochem Biophys. 1990 May 15;279(1):167–173. doi: 10.1016/0003-9861(90)90477-g. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachelli C. M., Lin-Jones J., Omiecinski C. J. Isolation and characterization of rat cytochrome P-450IIB gene family members. Use of the polymerase chain reaction to detect expression of a novel P-450 mRNA. J Biol Chem. 1989 Apr 25;264(12):7046–7053. [PubMed] [Google Scholar]
- Giachelli C. M., Omiecinski C. J. Developmental regulation of cytochrome P-450 genes in the rat. Mol Pharmacol. 1987 May;31(5):477–484. [PubMed] [Google Scholar]
- Guengerich F. P., Dannan G. A., Wright S. T., Martin M. V., Kaminsky L. S. Purification and characterization of microsomal cytochrome P-450s. Xenobiotica. 1982 Nov;12(11):701–716. doi: 10.3109/00498258209038945. [DOI] [PubMed] [Google Scholar]
- Gustafsson J. A., Stenberg A. Irreversible androgenic programming at birth of microsomal and soluble rat liver enzymes active on androstene-3,17-dione and 5alpha-androstane-3alpha,17beta-diol. J Biol Chem. 1974 Feb 10;249(3):711–718. [PubMed] [Google Scholar]
- Hartnett C., Neidle E. L., Ngai K. L., Ornston L. N. DNA sequences of genes encoding Acinetobacter calcoaceticus protocatechuate 3,4-dioxygenase: evidence indicating shuffling of genes and of DNA sequences within genes during their evolutionary divergence. J Bacteriol. 1990 Feb;172(2):956–966. doi: 10.1128/jb.172.2.956-966.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haymerle H., Herz J., Bressan G. M., Frank R., Stanley K. K. Efficient construction of cDNA libraries in plasmid expression vectors using an adaptor strategy. Nucleic Acids Res. 1986 Nov 11;14(21):8615–8624. doi: 10.1093/nar/14.21.8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Iwasaki M., Juvonen R., Lindberg R., Negishi M. Alteration of high and low spin equilibrium by a single mutation of amino acid 209 in mouse cytochromes P450. J Biol Chem. 1991 Feb 25;266(6):3380–3382. [PubMed] [Google Scholar]
- Juvonen R. O., Iwasaki M., Negishi M. Structural function of residue-209 in coumarin 7-hydroxylase (P450coh). Enzyme-kinetic studies and site-directed mutagenesis. J Biol Chem. 1991 Sep 5;266(25):16431–16435. [PubMed] [Google Scholar]
- Labbé D., Jean A., Anderson A. A constitutive member of the rat cytochrome P450IIB subfamily: full-length coding sequence of the P450IIB3 cDNA. DNA. 1988 May;7(4):253–260. doi: 10.1089/dna.1988.7.253. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg R. L., Negishi M. Alteration of mouse cytochrome P450coh substrate specificity by mutation of a single amino-acid residue. Nature. 1989 Jun 22;339(6226):632–634. doi: 10.1038/339632a0. [DOI] [PubMed] [Google Scholar]
- Monier S., Van Luc P., Kreibich G., Sabatini D. D., Adesnik M. Signals for the incorporation and orientation of cytochrome P450 in the endoplasmic reticulum membrane. J Cell Biol. 1988 Aug;107(2):457–470. doi: 10.1083/jcb.107.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Coon M. J., Estabrook R. W., Feyereisen R., Fujii-Kuriyama Y., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991 Jan-Feb;10(1):1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- Nef P., Heldman J., Lazard D., Margalit T., Jaye M., Hanukoglu I., Lancet D. Olfactory-specific cytochrome P-450. cDNA cloning of a novel neuroepithelial enzyme possibly involved in chemoreception. J Biol Chem. 1989 Apr 25;264(12):6780–6785. doi: 10.1016/S0021-9258(18)83497-4. [DOI] [PubMed] [Google Scholar]
- Oesch F., Waxman D. J., Morrissey J. J., Honscha W., Kissel W., Friedberg T. Antibodies targeted against hypervariable and constant regions of cytochromes P450IIB1 and P450IIB2. Arch Biochem Biophys. 1989 Apr;270(1):23–32. doi: 10.1016/0003-9861(89)90003-9. [DOI] [PubMed] [Google Scholar]
- Rampersaud A., Walz F. G., Jr At least six forms of extremely homologous cytochromes P-450 in rat liver are encoded at two closely linked genetic loci. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6542–6546. doi: 10.1073/pnas.80.21.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D. E., Thomas P. E., Reik L. M., Levin W. Purification, characterization and regulation of five rat hepatic microsomal cytochrome P-450 isozymes. Xenobiotica. 1982 Nov;12(11):727–744. doi: 10.3109/00498258209038947. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesna-Skorupa E., Browne N., Mead D., Kemper B. Positive charges at the NH2 terminus convert the membrane-anchor signal peptide of cytochrome P-450 to a secretory signal peptide. Proc Natl Acad Sci U S A. 1988 Feb;85(3):738–742. doi: 10.1073/pnas.85.3.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Ko A., Walsh C. Regioselectivity and stereoselectivity of androgen hydroxylations catalyzed by cytochrome P-450 isozymes purified from phenobarbital-induced rat liver. J Biol Chem. 1983 Oct 10;258(19):11937–11947. [PubMed] [Google Scholar]
- Waxman D. J., Walsh C. Phenobarbital-induced rat liver cytochrome P-450. Purification and characterization of two closely related isozymic forms. J Biol Chem. 1982 Sep 10;257(17):10446–10457. [PubMed] [Google Scholar]
- Wood A. W., Ryan D. E., Thomas P. E., Levin W. Regio- and stereoselective metabolism of two C19 steroids by five highly purified and reconstituted rat hepatic cytochrome P-450 isozymes. J Biol Chem. 1983 Jul 25;258(14):8839–8847. [PubMed] [Google Scholar]
- van der Hoeven T. Assay of hepatic microsomal testosterone hydroxylases by high-performance liquid chromatography. Anal Biochem. 1984 Apr;138(1):57–65. doi: 10.1016/0003-2697(84)90768-1. [DOI] [PubMed] [Google Scholar]