Graphical abstract

Keywords: Nanomedicine, Programmed cell death, Tumor, Immunotherapy
Highlights
-
•
This article focuses on immunotherapy, a new treatment for tumors with low side effects, and suggests the direction of improvement.
-
•
Programmed cell death (apoptosis, necroptosis, ferroptosis, pyroptosis and autophagy) induced by nanomedicine was reviewed in this paper.
-
•
This paper focuses on the role of nanomedicine-induced programmed cell death in cancer therapy.
-
•
This article reviews the improvement of the limitations of immunotherapy with nanomedicine-induced PCD.
-
•
In this paper, the development prospect of tumor therapy and immunotherapy of PCD mediated by nanomedical drugs is prospected.
Abstract
Background
There has been widespread concern about the high cancer mortality rate and the shortcomings of conventional cancer treatments. Immunotherapy is a novel oncology therapy with high efficiency and low side effects, which is a revolutionary direction for clinical oncology treatment. However, its clinical effectiveness is uneven. Based on the redefinition and reclassification of programmed cell death (PCD) (divided into necroptosis, ferroptosis, pyroptosis, and autophagy), the role of nanomedicine-induced PCD in cancer therapy has also received significant attention. Clinical and preclinical studies have begun to combine PCD with immunotherapy.
Aim of review
In this article, we present recent research in tumor immunotherapy, provide an overview of how nanomedicine-induced PCD is involved in tumor therapy, and review how nanomedicine-induced PCD can improve the limitations of immunotherapy to enhance tumor immunotherapy. The future development of nanomedicine-mediated PCD tumor therapy and tumor immunotherapy is also proposed Key scientific concepts of overview Nanomedicine-induced PCD is a prospective method of tumor immunotherapy. Nanomedicines increase tumor site penetration and targeting ability, and nanomedicine-mediated PCD activation can stimulate powerful anti-tumor immune effects, which has a good contribution to immunotherapy of tumors.
Introduction
The incidence and mortality rates of cancer are high worldwide and are increasing year by year [1]. The existing traditional treatment methods are surgery, chemotherapy, and radiation. However, the heterogeneity and metabolic plasticity of tumor microenvironment (TME) largely contribute to the unsatisfactory effect of tumor treatment and the transient effect of efficacy [2], [3].
In addition to traditional treatment strategy, cancer immunotherapy has aroused wide interest as a new treatment among scientists [4], [5]. Novel strategies that try to reprogram the immune system to target anti-tumor targets are gaining attention. This has placed immunotherapy at the forefront of cancer research, and developing new approaches with clinical impact is rather challenging [6], [7]. The principle of immunotherapy can be understood as reactivating the immune cycle (Fig. 1). During the cancer immune cycle, many immune-related cells and molecules recognize and destroy cancer cells [8]. Cancer immune surveillance is based on lymphocytes [9], is synergistic with interferon-gamma (IFN-γ) produced by dendritic cells (DCs) and induces natural killer (NK) cell effect mechanisms [10]. Checkpoint proteins can be targeted for tumor therapy because tumors rely on the immune checkpoint pathway as a means of surviving and proliferating [11]. Various engineered cell therapies can be used to effectively treat hematological malignancies [12]. In addition, the development and testing of cancer vaccines also made remarkable progress [13]. These techniques have the potential to solve contemporary problems in tumor therapy.
Fig. 1.
Immune cycle and targets of immunotherapy. The principle of immunotherapy against tumors can be understood as an immune cycle. Tumors can disrupt this cycle through various negative feedback immunomodulatory pathways that are increasingly becoming targets for cancer immunotherapy: immune checkpoint blockade inhibitor (ICB) therapy can block tumor immune escape generated by cancer using the immune checkpoint pathway; adoptive cell therapy technology (ACT) technology triggers an effective antigen-specific immune response directly through the administration of isolated engineered and expanded immune cells; cancer vaccines induce T cells of the immune system to recognize and kill tumor cells in cancer patients. I) Release of cancer cell antigens; II) Antigen is delivered to APCs, which are taken up and processed; III) APCs present antigens to naive T cells, generating cytotoxic T cells; IV) Activated cytotoxic T cells; V) Cytotoxic T cells infiltrate tumors under the influence of chemokines; VI) Cytotoxic T cells specifically recognize and kill cancer cells; VII) Tumor-associated antigens (TAAs) released by lysed cancer cells will promote a new round of immune cascades, leading to enhanced immune responses.
Tumor cells are malignant and can avoid cell death. The imbalance between cell death and proliferation is one of the main reasons for the proliferation of tumor cells [14]. The Nomenclature Committee on Cell Death (NCCD) classified cell death into accidental cell death (ACD) and regulated cell death (RCD). The physiological type of RCD is the one that is most frequently referred to as programmed cell death (PCD). Depending on the mode of action, PCD can be divided into autophagy, apoptosis, necrotic cell death, necroptosis, ferroptosis, pyroptosis, and anoikis. A variety of PCD pathways, except the apoptotic pathway, have been gradually identified and developed [15]. Abnormal regulation of PCD may be associated with several diseases, especially cancer. Moreover, PCD is essential for tissue homeostasis and can be controlled by medications and genetic factors [16].
Nanomedicine has been created to allow therapeutic medications or diagnostics to be encapsulated and subsequently adsorbed or covalently attached to nanoparticle (NP) surfaces, allowing NPs to deliver pharmaceuticals, genes, or vaccinations to specific tissues or cells by targeted delivery or systemic channels [17], [18]. The nanoscale range is 1–1000 nm in viruses or bacteria but larger than in atoms or small molecules [19]. Nanodrug delivery systems seem promising for targeted therapies, delaying drug release, improving drug solubility and availability, reducing drug side effects, and overcoming obstacles in the body [17]. Many nanocarriers targeting tumors to deliver drugs can promote PCD. By utilizing diverse physicochemical qualities to lessen toxicity and improve the circulation and residence time of the cells in the body, nanotechnology has the potential to completely transform the way that cancer is detected and treated. New nanoscale targeting techniques made possible by developments in materials science may give cancer patients new methods [19], [20]. Due to their size following endocytosis into specific target cells, nanodrugs for cancer have enhanced enhanced permeability and retention effects (EPR) as well as intracellular targeting [21].
In this article, we focus on nanomaterials that promote PCD therapy through targeted delivery, enhancing the efficacy of immunotherapy. Also, we reviewed different immunotherapies, types of nano-mediated PCD, and their mechanisms of action in tumor therapy. Next, we assessed how nano-mediated PCDs modulate key advances and updates in immunotherapy. Finally, it points out the development prospect and challenges in this field.
Immunotherapy
The innate immune system is the cells and molecules responsible for triggering and coordinating inflammation. TME recruits innate immune cells, releases cytokines, and exhibits characteristics, such as angiogenesis and tissue remodeling, driving the establishment of “tumor intrinsic” inflammation, and serving as a hallmark of cancer [22]. The body’s innate immune-induced inflammation serves as a host defense mechanism. The interleukin-1 family of cytokines, for instance, can activate innate immunity. Inflammation is brought on by bacteria, microbial products, viruses, nucleic acids, and damage-associated molecular patterns (DAMPs) through toll-like receptors (TLRs), which can also control acquired immunity [23]. The adaptive immune system aids humans in defens ing against any invasion of pathogens, and PCD plays a critical role in various immunological environments [24].
Immunocheckpoint inhibitor therapy
In recent years, immunocheckpoint blockade (ICB) has become the most prominent new immunotherapy with remarkable achievements in clinical application [25]. Co-stimulatory and inhibitory ligand-receptor interactions between T cells and antigen-presenting cells (APCs) are essential for their activation and functionality. Immune checkpoints are receptor-ligand combinations that are utilized to control the immune system [26]. The immune system can use these immune checkpoints to distinguish between external infections and its own cells, reducing the negative consequences on the body [4], however, these immune checkpoint pathways can be used by cancers to evade immunity [27]. Thus, an immune response to tumors can be triggered by blocking checkpoint-associated proteins produced by T cells and particular types of cancer cells [11], [26].
To balance the ratio of inhibitory and activating signals, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and CD28 compete bind to two recognized ligands [28], [29]. A popular CTLA-4 antibody called ipilimumab increases overall survival in people with advanced melanoma [30]. Another major targeted immune checkpoint is programmed cell death protein 1 (PD-1)/ programmed cell death protein ligand 1 (PD-L1) [26], predominantly found on activated T cells and transmits inhibitory signals to T cells by binding to PD-L1 or PD-L2 in TME [31]. The activation of PD-1 upregulates its expression levels in T, B, natural killer (NK), NKT cells, DCs, and macrophages. The cytotoxic activity mediated by T cells in vivo is blocked by PD-1 binding to PD-L1 [4]. PD-1 limits effector T cell activity but causes Treg proliferation, leading to immunosuppression when activated within the tumor. Pembrolizumab, nivolumab [32], atezolizumab, and other PD-1/PD-L1 inhibitors have a high remission capacity for several cancers (e.g., advanced melanoma, non-small cell lung cancer, squamous cell carcinoma of the head and neck) [26].
Cancer vaccines
Vaccination began to develop as a powerful cancer immunotherapy strategy [33], [34]. By a deeper comprehension of the research and development of tumor-associated antigen (TAA), immune response, and antigen delivery technologies is the direction of therapeutic vaccines for cancer [35]. Tumor antigens can be administered as entire cells, peptides, or nucleic acids in the four common cancer vaccines [34], [35]. Genetic vaccines based on deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) as well as subunit vaccines (containing bioengineered proteins, protein fragments, and peptides) are being developed [36]. Both genetic and subunit vaccines play important roles in the activation of CTL immunity, mainly including: antigen presentation and processing, activation of immune cells, and CTL functionality. DCs are critical targets for vaccine design because they can control immune tolerance and immunity [37], [38]. The tumor immune response relies heavily on the random contact between antigens and host APCs [39]. Inappropriate cross-presentation may lead to immune silencing [40]. Tumor-specific immunogenic antigen selection is a major hurdle [37]. Early therapeutic vaccines often use TAAs that lack tumor specificity and poor immunogenicity [41]. Neoantigens enhanced the anti-cancer immune response. Neoantigens, also known as new autoantigen epitopes, are produced by mutations in tumor cells, which are expressed only by tumor cells, have a higher affinity to bind to T cell receptor (TCR), and induce a robust T cell response. These can avoid “off-target” damage to non-malignant tissues and are less likely to be autoimmune targets [26], [37], [41].
Adoptive cell transfer (ACT)
Utilizing in vitro produced and expanded immune cells, ACT directly triggers an immunological response to an antigen [42]. In vivo cytotoxicity mediated by cytotoxic T cells is enhanced by chimeric antigen receptor (CAR) T therapy [26]. T cells from the patient are taken out and genetically altered with a viral vector to express CAR before being reinfused into the original patient. Due to the physical barrier formed by the immunosuppressive microenvironment and tumor stroma, this therapy initially demonstrated limited efficacy in other hematological malignancies and solid tumors [43]. Recent research showed that this CAR-T therapy could be more effective in treating B-cell malignancies and expanding its application to solid tumors [44]. Based on CAR-T therapy, several immune cell and tissue-based activities are elaborated in preclinical investigations or clinical trials, such as NK cells, macrophages, chondrocytes, cord blood, DCs, fibroblasts, keratinocytes, and thymus [42], [45], [46].
Among them, NK cells have a non-human leukocyte antigen (HLA)-restricted ability to detect and eliminate “stress cells” (such as tumor cells) without previous sensitization [47]. NK cells from allogeneic donors can be employed directly. Adoptive transfer of NK cells activates their anticancer characteristics through the secretion of cytolytic granules or the expression of tumor necrosis factor-associated apoptosis-inducing ligand (TRAIL), which is independent of major major histocompatibility complex (MHC). Additionally, NK cells release pro-inflammatory cytokines and chemokines that support anti-tumor innate and adaptive immune responses. CAR-NK therapy also overcomes the limitation that NK cells are unable to deliver targeted cancer immunotherapy because they lack cell-specific receptors [45]. Several CAR-NK cell lines successfully combat tumors in preclinical and clinical studies [43], [47], [48].
Deficiency of immunotherapy
The tumor's immunogenicity and the nature of the tumor immune microenvironment (TIME) have a significant impact on the efficacy of ICB therapy [49]. TIME dynamics and complexity facilitate a clinical response to treatment in only a limited percentage of patients [32], [50]. Immune checkpoint inhibitors (ICI) work better on inflammatory tumors or “hot” tumors, whereas immunologically “cold” tumors display modest mutation loads, few infiltrating T cells, or a phenotype in which T cells may be present but excluded from the tumor core [49], [51], [52], [53]. Recent clinical studies have demonstrated that intra-tumor invasion of CTL is positively correlated with the response rate to ICB therapy [54]. Nonetheless, the majority of human tumors are immunogenic cold [55], and hence, techniques that convert “cold” cancers into “hot” tumors and increase T-cell infiltration in TME are required to improve response rates to ICB therapy. In addition, highly inflammatory tumors and drug resistance in the clinical treatment of ICBs are major obstacles [26], [55].
Vaccine-induced immune responses against tumor-forming cells must have specific properties, including precise immune recognition, potent cytotoxic T cell activation, improved tumor infiltration of cytotoxic T cells, and modified TME. Tumors are formed by uncontrollable cells and evade most immune checks [36]. Vaccine adjuvants and vectors can also increase the immune response of antigens and produce long-lasting immunity [56]. Traditional vaccine carrier systems cannot produce a large number of T cells that can recognize antigens on cancer cells; hence, specialized APCs (such as DCs) are required to present exogenous antigens to CTLs. Maximizing cross-presentation effectiveness may therefore be the first crucial step in the successful creation of therapeutic cancer vaccines [57]. ACT has been successful in hematologic malignancies, but its effectiveness against solid tumors remains limited. Additionally, the clinical applicability of cell surface anchoring is severely constrained by its instability in vivo. TME immunosuppression can potentially lessen the effectiveness of NK cell defense [45]. Cytokine release syndrome (CR) and immunoeffector cell-associated neurotoxic syndrome (ICANS) pose clinical obstacles to CAR-T therapy [43].
Single immunotherapy has a good effect in only a small percentage of patients, and the overall clinical response to solid tumor treatment is significantly reduced [13], [54], [58]. Therefore, it is crucial to create novel tumor combination medicines that will boost effectiveness across a wide range of patients without escalating side effects [25]. Many nanodelivery systems that encapsulate immunomodulators have been developed, increasing the accumulation of drugs in tissues and reducing immune-related toxicity [59]. The combination of immunotherapy and other approaches is an important research direction at this stage.
Nanomedicine-induced PCD for tumor therapy
Recent research has identified a new process by which CD8+ T lymphocytes prevent the spread of tumors through ferroptosis and pyroptosis, that encouraged the investigation into the relationship between the mechanism of tumor cell death and immune system activation [60]. PCD causes a variety of immunological responses, such as the activation of innate and adaptive immune cells, conditioning or phagocytosis of dying cancer cells, and maturation of DCs by causing the produced DAMPs to bind to receptors in different immune cells [60], [61]. This drives tissue inflammation and further activation of the PCD pathway. PCD and inflammation also induce each other [62].
The nanotechnology-enhanced EPR effect can be used for passive targeted drug delivery, and the suitable surface operation of a nano-drug delivery system can be used for active targeted drug delivery to increase the therapeutic efficacy of pharmaceuticals at the location of the target [63]. Numerous nanodiagnostics and nanotherapeutics have been used in clinical settings or are being developed into products [64]. The nanomaterial delivery of drug-mediated PCD pathway has unlimited possibilities in tumor therapy.
Nanomedicine-induced apoptosis
Caspase is a cysteine protease that orchestrates apoptosis and inflammatory responses [65], [66]. The pathways triggered by apoptosis can be divided into extrinsic (death receptor-dependent pathway) and intrinsic (mitochondria-dependent pathway). The former is mediated by certain TNF receptor (TRAIL-R) superfamily transmembrane receptors. Death ligands bind to their respective death receptors and form dynamic multiprotein death-inducing signaling complexes (DISCs) with conformational changes, which activates and dimerizes caspase-8, ultimately leading to apoptosis. The endogenous apoptotic pathway is upregulated and activated by pro-apoptotic proteins of the B-cell lymphoma 2 (Bcl-2) family through DNA damage. Bax and Bak are prevented from working by the anti-apoptotic proteins. The complex known as an apoptosome, which is formed when multiple apoptotic components are released into the cytoplasm, activates caspase-9 and caspase-3, which in turn cause apoptosis [16], [67].
Several studies have shown that nano-mediated apoptosis increases the effectiveness of immunotherapy. Novel carbon NPs such as single-walled carbon nanohorns induce apoptosis via lysosomal damage [68]. Zheng et al. developed a metal-ligated nanomide that induces apoptosis in combination with tumor therapy [69]. ENG-Apt/mIP-10-LP NPs can increase tumor-cell apoptosis while reducing tumor cell growth and angiogenesis [70]. Zhou et al. changed the number and length of fluoroalkyl lipids and customized fluorine-containing dendrite molecules to form NPs with different properties and constructed mixed lipid-polymer NPs for siRNA delivery, effectively inducing cell apoptosis and inhibiting cell growth [71].
Nanomedicine-induced necroptosis
According to accumulating data, when apoptosis is prevented, necroptosis, an alternative cell death mechanism, is started [60], [72]. It is activated and is initiated by death receptor activation [52], whose role in cancer is to regulate adaptive functions in response to stress failure, is negatively regulated by caspase-8, and controlled by three main kinases: receptor-interacting protein kinase (RIPK)1, RIPK3, and mixed lineage kinase-like protein (MLKL) [16], [73]. Because they are discharged without coming into contact with the severe conditions that induce oxidation and protein hydrolysis, pro-inflammatory chemicals, including DAMPs, do not significantly suffer during necroptosis, in contrast to apoptosis [74].
RIPK1 is recruited to activate the dopamine receptor (DR), leading to nuclear factor (NF)-κB-dependent gene expression. Inhibitory FLICE-like inhibitory protein (cFLIP)/caspase-8 heterodimers are created by the NF-B signaling pathway, blocking caspase-8 activation and subsequent external apoptosis. Phosphorylated MLKL forms a complex with a heptameric pore that inserts into the cell membrane, allowing cation entry and subsequent influx of extracellular fluid due to differences in osmotic pressure. Rapidly after this inward flow, the plasma membrane ruptures, releasing the intracellular contents [75]. RIPK3 activates MLKL, which then promotes membrane disassembly [74].
Like apoptosis, nanoparticle-mediated cell necroptosis promotes tumor immunotherapy. Some studies demonstrated that carbon black NPs (CBNPs) induce lysosome rupture, cell necroptosis, and a series of inflammatory reactions [76]. A self-assembled redox nanoparticle (RNP) has been shown to significantly increase the apoptosis/necroptosis ratio of radiation-induced cell death by RNP pretreatment [77]. As a result of acute cytoskeletal breakdown caused by ZnO NPs, necroptosis and a late reactive ROS-dependent apoptotic pathway were found to occur [78].
Nanomedicine-induced pyroptosis
Pyroptosis is a more effective cancer therapy compared to the former two [60]. Pyroptosis is a gasdermin (GSDM)-mediated pathway of inflammatory PCD [79]. Morphologically, it is characterized by both apoptosis and necroptosis, with early signs of apoptosis-like DNA fragmentation and chromatin condensation, followed by necroptosis-like features, such as transmembrane pore formation, cell swelling like large bubbles, and cell membrane rupture, leading to the release of inflammatory molecules and cellular content, followed by the activation of an intense inflammatory response [16], [60], [80], [81].
In the typical pyroptosis pathway, pattern recognition receptors (PRRs) initiation of cellular pyroptosis is predicated on the prior recognition of DAMP or pathogen-associated molecular pattern (PAMP). In the non-classical pathway, cytoplasmic lipopolysaccharide (LPS) directly triggers caspase-4/5 (human) and caspase-11 (in mice) to trigger the pyroptosis process. These results in the formation of membrane pores and the secretion of trace amounts of IL-1β. When gasdermin-E (GSDME) is expressed at high levels in both healthy and cancerous cells, caspase-3 is activated, which lyses GSDME to produce N-terminal domains, followed by the formation of transmembrane pores, ultimately leading to pyroptosis. Typically, caspases can also trigger pyroptosis and may initiate apoptosis or necroptosis in cells. Under normal conditions, GSDMD is in a self-inhibited state, and caspase-1 is activated to cleave GSDMD. The lytic GSDMD releases the domains of GSDMN and GSDMC, which are then transported to the cell membrane to create membrane pores (GSDMD pores), which promotes cell pyroptosis by causing membrane rupture, cell death, and cell growth [80], [81], [82]. Pores can lead to spillover of cell content, such as inflammatory agents IL-1β and high mobility group protein (HMGB)-1 [83]. The Food and Drug Administration (FDA) has given the drug combination of BRAF and MEK inhibitors permission for the treatment of BRAF V600E/K mutant melanoma because it encourages GSDME cleavage and the release of HMGB-1[84]. CXC chemokine receptor 4 (CXCR4)-targeted toxin NPs can target and kill anti-apoptotic tumors derived from CXCR4+ colon tumor stem cells (CSCs) by triggering pyroptosis [85], [86]. In addition, mixed metal–organic frameworks (MOF) can be constructed from ions released from cells. Degradation of MOF triggered pyroptosis [87], [88]. Regarding immunotherapy relying on the pyroptosis process, nanocomposites, such as Lip-MOFs, can be employed to assault cancer cells in acidic microenvironments. Another study constructed SLR20NPs, a nanoscale delivery system based on SLR20, an agonist designed from a double-stranded, triphosphorylated stem-loop RNA. Due to nanotechnology and pH responsiveness, SLR20NPs facilitated the internalization of tumors and their pro-inflammatory function by SLR20NPs + αPD-L1 via pyroptosis in breast tumors can inhibit tumor growth and metastasis [81]. In addition, cellular components released by pyroptosis, including as lactate dehydrogenase (LDH) and inflammatory cytokines, have an additional anticancer function by initiating an antitumor immune response [89]. Chen et al. designed an ANPS library to induce GSDME-mediated pyroptosis in various GSDME-positive cancer cells by nanosensitizer-mediated oxidative stress in vivo, which has worked well in various GSDME-expressing cancers [90].
Nanomedicine-induced ferroptosis
Because of “iron addiction,” which causes cancer cells to require more iron than healthy cells do, these cells are more vulnerable to ferroptosis. Lipid peroxide (LPO) accumulation brought on by ferroptosis can severely disrupt cellular integrity and structure. Ferroptosis can also bypass apoptosis inhibition and avoid the induction of membrane-specific proteins associated with multidrug resistance (for example, P-glycoprotein and multidrug resistance-associated protein family) [91], could offer a unique therapeutic approach to overcome drug resistance and apoptosis resistance in solid tumors [92], [93].
Ferroptosis is a form of cell death triggered by an overload of intracellular iron ions. Unlike the previous mentioned several types of PCD, ferroptosis is a non-apoptotic form of PCD that requires both iron and reactive oxygen species (ROS) [81], [91], [92], [94], [95]. Reduced ferroptosis is a key mechanism of tumorigenesis [96], [97]. In comparison to normal cells, cancer cells have higher baseline ROS levels, such that increasing ROS levels and inhibiting antioxidant molecular activity induce cancer cell death: an optimal strategy for cancer therapy [91]. Ferroptosis is broadly divided into the typical glutathione peroxidase 4 (GPX4) regulatory pathway, iron metabolic pathway, and lipid metabolic pathway. Different iron collapse-inducing compounds (FINs) can inhibit GPX4 directly or indirectly through glutathione (GSH) depletion [93]. Ferroptosis may result from inhibiting GPX4 or decreasing GSH, which is expressed as a cofactor of GPX4 [98]. A decline in ferrum (Fe)3+ content generates toxic ROS that can undergo an electrochemical Fenton reaction with hydrogen peroxide (H2O2) (Fig. 2). Especially in malignancies, the Fenton reaction is essential for triggering ferroptosis [63], [99]. Due to the high metabolism and proliferation, ROS expression is more pronounced in cancer cells. When ROS-induced intracellular oxidative stress is excessive, the cancer cells undergo the cellular ferroptosis pathway [91]. LPO causes membrane instability and permeability, eventually resulting in cell death [93]. It is proposed that the synthesis of LPO is hypothesized to be a biologically regulated Fe3+-dependent Fenton reaction. However, there is very little free Fe3+ in the human body [99]. Upregulated Fe2+ levels in the cellular iron pool are a viable target for effective ferroptosis treatment [100]. The need for iron to maintain stable development makes cancer cells more vulnerable to ferroptosis [89].
Fig. 2.
Effect of nano-drugs promoting iron apoptosis and FINs in vivo. Schematic of a nanodrug that promotes ferroptosis and a FINs combination that enhances ·OH to improve anticancer activity and therapeutic efficiency.
The combination of ferroptosis and nanotechnology provides effective antitumor responses, and ferroptosis-based nanomedicines offer new strategies for cancer treatment [63]. Various iron-based nanomaterials, such as ferumoxytol (an iron oxide NP for deficiencies in iron therapy that has received FDA approval [101], [102]), amorphous iron NPs, and organic iron frameworks, have been used as ferroptosis inducers [94]. Ferumoxytol included two iron oxidation states that could undergo the Fenton reaction; as a result, oxidative stress and cell death were increased and free Fe2+ generated ROS were produced [102]. Feng et al. developed a metallopolyphenol-ligated nanostructure to deliver and release Fe2+ into tumor cells, which can significantly improve ferroptosis-dependent antitumor therapy [103]. In order to create magnetic vesicles that may be utilized for magnetic resonance imaging (MRI) guidance, Fe modulation, and magnetic targeting to add Fe to the Fenton reaction, Zhang et al. synthesized Fe3O4 magnetic nanoclusters (NCs) with superparamagnetic and magnetic management as the basis [32]. Recently, a number of GPX-4-inhibiting nanotherapies have been created to support ferroptosis-driven cancer therapy. Some specialized nanoparticles or nanocarriers have the ability to block GPX-4; for instance, nanobubble AMSNs and Arg-based ultrathin surface overlays greatly enhance the efficacy of GSH depletion, enabling efficient GPX4 deactivation of tumor cells [94], [104]. Through the targeted distribution of inducers utilizing nanocarriers, “Nanocatalytic Biomedicine (NCB)” has been proposed to kill cancer cells by inducing the Fenton reaction inside tumor cells. In addition, Ag NPs, MnOx-based NPs, and a few organic nanosystems were also present. The development of outstanding performance nanocatalysts and intracellular reactions (such as H2O2 and Fe3+) increased the Fenton reaction based on nano-DDS. For example, mesoporous silica NPs (MS@MnO2) encapsulated by MnO2 can be used for effective Fenton-based cancer therapy [99]. Zinc oxide NPs (ZnONPs) can also cause ferroptosis by regulating ROS and iron metabolism in cells [105]. In order to achieve the goal of eliminating the tumor by a Fenton-like reaction, Wang et al. created nanocatalysts with a biodegradable and catalytic framework (rFeOx-HMSN) that may disproportionate tumor-rich H2O2 into harmful hydroxyl radicals (–OH) [64].
The acidic conditions of TME can trigger an efficient Fenton reaction and induce cancer cell death while leaving normal cells unharmed [99], [106]. Ferromagnetic NPs (γ-Fe2O3 or Fe3O4 NPs) have been shown to effectuate dual enzyme-like activities in vitro and in vivo in a pH-dependent way. These iron oxide NPs (IONPs) can disproportionate H2O2 to the highly toxic ROS-OH and show peroxidase-like activity when exposed to acidic environments [106]. One nanocomposite named FePt@MoS2 can release large amounts of Fe2+ in TME within 72 h, activating ferroptosis by accelerating the Fenton reaction [63]. In most cases, silica NPs have been combined with organic glucose oxidase (GOD, enzyme catalyst) and extremely small Fe3O4 NPs as a nanocatalyst capable of catalyzing Fenton-like reactions in reaction with mildly acidic TME, causing tumor cell death [106]. TME can also be activated by Fe-amycin preloaded with amorphous CaCO3 NPs triggering ferroptosis in target tumor cells [107]. In situ Ph-activated nanoplatform (SR780@Fe-PAE-GP) enhanced the synergistic effect of iron shedding and photodynamic therapy [108]. miRNA-based therapeutic nanomaterials delivered miR-101-3p to tumor cells in vivo and inhibited tumor proliferation by regulating ferroptosis [109]. miR-101-3p can target TBLR1 and form a miR-101-3p/TBLR1 axis to induce ferroptosis in tumor cells [110].
Nanomedicine-induced autophagy
Autophagy is the process by which cells recycle nutrients from destroyed organelles and proteins to maintain cellular homeostasis [111]. The autophagic process can act as a trigger for apoptosis, often before apoptosis or necroptosis but also outside of these processes. Through autophagy, cells destined to die to initiate their catabolism, which exacerbates the cell death process. Second, autophagy also contributes to the maintenance of optimal, high adenosine triphosphate (ATP) levels, which might promote the apoptotic process [113].
Autophagy is a homeostatic regulatory system that affects both cell survival and cell death. Whether it is inflammation, immunity or cell death, are all tightly related to autophagy-mediated DAMP production [114]. This article focuses on the autophagy species microautophagy, chaperone-mediated autophagy, and macroautophagy. Invagination of lysosome or endosomal membranes during microautophagy results in the direct phagocytosis of cytoplasmic payloads. Proteins with motifs similar to Lys-Phe-Glu-Arg-Gln (KFERQ) must be selectively degraded as part of chaperone-mediated autophagy. Autophagosomes with a traditional double membrane make up macroautophagy [115], wherein cargo isolated in autophagosomes is delivered to lysosomes via vesicle fusion [116]. Local tissue macrophages detect DAMP, which sets off an immediate inflammatory reaction. Overactivation of macrophages, however, can promote tumor growth and cell proliferation. Therefore, autophagy also appropriately mediates the end of the inflammatory response. In most cases, DAMP-induced macrophage activation results in mitochondrial stress, which in turn activates mitochondrial signals that affect the release of inflammatory cytokines dependent on inflammatory vesicles to prevent infection and encourage tissue regeneration [115]. Autophagy is also crucial for metabolic communication between tumor and stromal cells and is highly involved in tumor metabolism [117].
Recent studies have shown that nanomaterials are recognized by cells as endosomal pathogens or proteins [118]. In most cases, autophagy triggered by nanoparticles causes cell death; however, many nanomaterials also happen to be capable of triggering pro-survival autophagy. Autophagy can be regulated by nanomaterials for cancer treatment. A large number of nanomaterials, including rare earth, metals and alloys, metal oxides, semiconductors, carbon bases, and NPs, have been demonstrated to trigger autophagy. Potentially an innovative tool in the fight against tumors that are difficult to cure and drug-resistant is nanodrug-induced autophagy [112]. Gold nanoparticles (AuNPs) have been suggested for delivery of medicines, cancer diagnostics, and treatment. Chitosan-coated AuNPs (CH-AuNPs) exert a dose-dependent cytotoxicity in leukemia cells, leading to autophagy [119]. Reportedly, iron oxide NPs made with citric acid to act as surfactant promote pro-death autophagy in A549 lung cancer cells. Endocytosed micellar NPs are said to effectively inhibit breast cancer in vivo by promoting the alkalinization of lysosomes and autolysosomes. In addition, induced autophagy may have pro-death effects [112]. Metal-organic skeleton nanoreactors inhibit autophagy from enhancing the mediated effect of glucose oxidase (GOx) and induce macrophages to polarize into M1 phenotypes and enhance the therapeutic effect [120]. Only over-activated autophagy can increase chemical sensitivity and immune activity, leading to autophagy [89]. Therefore, Wang et al. designed a nanosystem that responds to autophagy and releases drugs, significantly improving immunotherapy's effect [121].
Crosstalk between nanomedicine-mediated PCD pathways
The various forms of cell death work together in a coordinated manner rather than independently of one another. Other regulatory mechanisms make sure the process of cell death occurs when one pathway is abnormally faulty (Fig. 3) [122]. Therefore, using nanomedicines can induce a combination of various PCD treatments.
Fig. 3.
Crosstalk effect between various PCD. Cell death types are not independent but have a close cross-talk, promoting and inhibiting each other. In addition to promoting apoptosis, activated caspase-8 also inhibits lysosome-induced autophagy and GSDm-mediated pyroptosis and promotes the activation of RIPK1/3. In addition to inducing cell necroptosis via MLKL, the activation of RIPK1/3 also promotes the production of inflammasome in the process of pyroptosis. Similarly, autophagosomes activate TNFα and RIPK1/3, which in turn mediates cell death.
The intracellular interaction between gold nanorods (GNRs) and lysosomes mediates the activation and production of caspase-3 and caspase-8 and induces the transition from necroptosis to apoptosis. Switching between necroptosis and apoptosis can also be achieved by tuning the appropriate structural characteristics of this nanomaterial [123]. Interestingly, a powerful nanoplatform, such as the SRF@FeIIITA, can perform multiple integrations of apoptotic and non-apoptotic tools [100]. Nanolongan can also be used as a powerful platform with outstanding EPR effects for combination ferroptosis-apoptosis therapy for cancer, enabling tumor cell uptake and subsequent induction of lysosomal escape [124]. According to a prior study, autophagy and apoptosis have many common inducers. Usually, autophagy occurs before apoptosis, but caspase inhibition in the apoptotic machinery induces autophagic cell death independent of apoptosis. Chromatin condensation, MOMP activation, and cysteine aspartase activation are all indications of apoptosis, which can also be triggered by autophagy [113]. Feng et al. designed supramolecular nanodrugs with Fe (III)-purpurin metal-polyphenol ligands, which decompose into Fe2+ and purpurin upon exposure to increased GSH levels in cancer cells. Purpurin can promote the production of RIP1-RIP3 complex, which is used to treat ferroptosis and necroptosis in tumors [103]. Additionally, active caspase-8 encourages the stimulation of the GSDMD cleavage and pyroptosis as well as the signaling for apoptosis pathway. In contrast, necrosome development and necroptosis are permitted when caspase-8 activation is blocked. RIPK3/MLKL can also stimulate inflammasomes that induce pyroptosis (Fig. 4) [52]. Notably, PD1/PDL-1 inhibitors significantly synergistically affect tumor suppression with pyroptosis/ferroptosis inducers [60].
Fig. 4.
PCD can be mediated by nanomedicine effectively. PCD produces DAMPs that contribute to anti-tumor immunotherapy. It promotes cytophagocytosis and mediates inflammatory responses, recruitment, and activation of immune cells (NK cells, DCs, CD8+ T cells), and proliferation of cytokines. Thus, it plays an enhanced role in tumor immunotherapy.
Strategies to improve immunotherapy: Nanomedicine and PCD
The defense side of immunotherapy involves innate immune sensors that try to employ PRRS to find infections. These sensors set off multiple PCD types, which destroy the damaged host cell ecotone while enhancing a positive immune response [125]. Both in vivo and in vitro, powerful antitumor immunity can be triggered by tumor cells undergoing PCD, and this can synergistically increase the effectiveness of the immune response [60]. The plasma membrane permeability changes and intracellular content release induce immune responses [126]. Among others, PCD that can trigger inflammatory forms alters the influx of TIME and tumor-infiltrating lymphocyte (TIL). By producing DAMPs, inflammatory cytokines, and altering APCs that phagocytose dead cells, the PCD pathway primarily induces inflammation, which in turn can significantly alter the influx of TME and TIL (Fig. 4) [52]. Strikingly, DAMPs possess immunogenicity that can stimulate innate immune pattern recognition receptor (PRR) [75], [127], promote DC maturation, and induce T-cell cross-priming, thus stimulating cross-initiation of cytotoxic T cells and tumor antigen-specific IFN-γ production. In addition, macrophages can be attracted and polarized to promote immune activation [128].
Numerous investigations have demonstrated that PCD can be induced with nanomedicines. Previously, integrating nanomedicines with immunotherapy demonstrated significant promise for enhancing the effectiveness of cancer immunotherapy in patients. Modifiable surfaces, such as those with virus-like dimensions and high surface-to-volume ratios for precision targeting of particular cell types, can be used for cancer vaccine design and delivery of immunomodulators. Designing of cancer vaccines and ACT therapies has been influenced by developing nanomedicine and immunoconjugation therapies in the field of ICB [26]. A growing body of preclinical and clinical research suggested that combining nanomedicines with immunotherapies can transform “cold” non-immunoreactive tumors and metastases into “hot” immunoreactive lesions [13] and can also overcome tumor immune tolerance. The effectiveness of vaccination, immunotherapy, and adjuvant tolerance can all be improved by nanovaccines [13], [54]. Nanomedicines can provide immunogenic stimulation to increase tumor antigen presentation while providing a combination of drugs or artificial molecules to send immune signals of danger or obstruct immune escape, immunosuppression, and T-cell rejection pathways [49]. Cell death with strong immunogenicity can expose CRT to preapoptosis on the cell surface and promote the production of ATP and HMGB-1 in cell death, the vaccination impact of the tumor therapy vaccine [129]. Phenol-based tumor penetration nanoskeleton (EGPt-NF) decreased the expression of PD-L1 in tumor cells, increased the effectiveness of tumor immunotherapy, and increased tumor immunogenicity through immune-stimulating cell death [130]. TAA cross-presentation requires immunogenic tumor cell death [131].
In summary, the PCD pathway mediated by nanomedicine enhances the effect of immunotherapy on tumors. In cancer treatment, the immunogenicity of PCD and dead cells is essential [126]. The combination therapy of PCD and immunotherapy can trigger immunogenicity and stimulate the immune response, thus improving the effect of immunotherapy [89].
Nanomedicine-induced apoptosis enhances the efficacy of immunotherapy
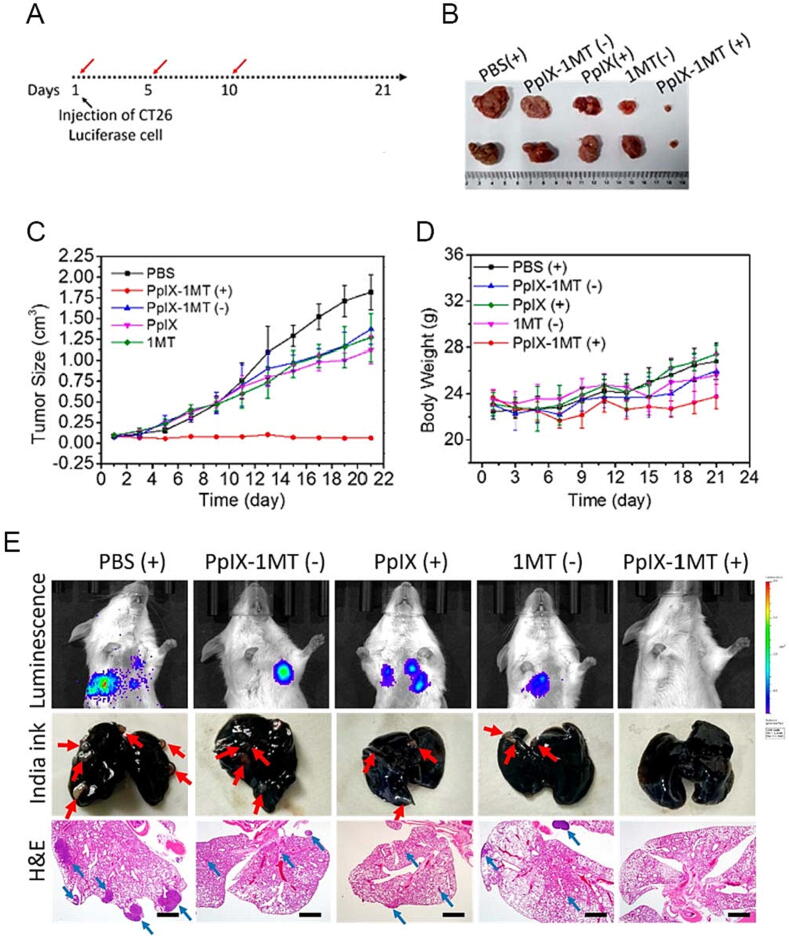
The ineffectiveness of therapeutic tumor treatment is intimately related to the tumor cells' drug-resistant apoptosis. Existing evidence has proved that nanomaterials can overcome apoptotic drug resistance in tumor therapy and can also promote the therapeutic effect in combination with immunotherapy [69]. Moreover, apoptosis initiation in combination with CAR-T cell therapy enhances the antitumor immune response. The intravenous administration of Listeria monocytogenes induced GSDM-dependent apoptosis of tumor cells and stimulated immune responses against the tumor [132]. One study prepared a multifunctional GNR reagent GMPF-siIDO; IDO with photothermal effect kills tumor cells by inducing apoptosis, significantly enhances antitumor immunity and upregulates antitumor cytokines TNF-α and IFN-γ [133]. Polylactide-co-glycolide nanoparticles (PLGA_NPs) labeled with annexin A5 and loaded with neoantigen-treated apoptotic tumor cells express PS as congenital ICI and enhance the efficacy of tumor ICB therapy [134]. Song et al. prepared a chimeric peptide PpIX-1MT that achieves a synergistic cascade through the combination of apoptosis, ICBs, and photodynamic therapy (Fig. 5). It is easy to show from the experimental results that this treatment can effectively kill tumors and inhibit their metastasis [135].
Fig. 5.
The combination of apoptosis, ICBs and photodynamic therapy was used to achieve tumor therapeutic effect. (A) A 21-day treatment period was conducted. The red arrows indicate the time points of intravenous injections of various materials. (B) Representative tumor images of different groups were taken after 21 days of treatment. (C) Absolute tumor size was measured. (D) Relative body weight was recorded. (E) Luminescence imaging was performed on CT26-bearing mice. Images of lung tissue were taken after treatment with India ink. A pulmonary tumorous node is indicated by the red arrow. Lung tissues were also stained with H&E. Lung metastasis tumor cells are indicated by the blue arrow (scale bar: 1 mm). “(+)” and “(−)” represent the sample with and without irradiation (630 nm, 340 mW cm−2, 10 min). Copyright © 2018, American Chemical Society. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Nanomedicine-induced necroptosis enhances the efficacy of immunotherapy
A potential link has been established between nano-induced necroptosis and antitumor immunity, which might enhance the antitumor response to immunotherapy, according to some evidence [74]. In addition to those listed in Fig. 4, necrotic cells secrete inflammatory factors that are linked to the start of adaptive immune responses. In vivo and in vitro experiments demonstrated that necrotic tumor cells stimulate CD8+ T cell proliferation and cross-initiation, which results in excessive IFN- production. In a model of preventive tumor vaccination, necrotic tumor cells serve as effective immunological agents because necrotic tumor cells can induce the maturation of bone marrow-derived DCs even after phagocytosis. Using tailored immunostimulation, a necrotic cancer cell-imimicking nanovaccine has been shown to improve antitumor immunotherapy. Mice vaccinated with the vaccine exhibited efficient lymph node transport and multi-epitope T cell responses. NKG2D+ NK cells and CD8+ T cells that express IFN-γ were both stimulated to grow by the vaccination. Combining ICI improved in vivo tumor regression brought on by immunization [60]. Several studies have introduced nanobubbles (NBs) that triggered the non-dependent necrotizing apoptosis of RIPK3. Under US irradiation, cancer cells treated with NBs generated physiologically active DAMPs, which assisted DC maturation. In addition to PD-L1 blockade, treatment with NBs demonstrated stronger inhibitory impact against both primary and metastatic cancers. This result could be explained by TAA and DAMP's efficient activation of CTL and immunogenic augmentation of DC maturation [74].
Nanomedicine-induced pyroptosis enhances the efficacy of immunotherapy
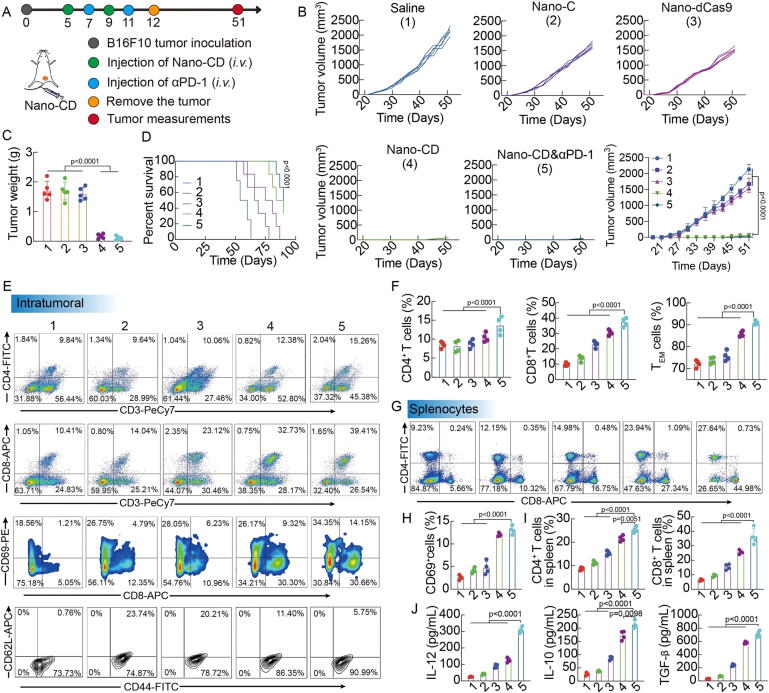
Pyroptosis is an inflammatory and immunogenic form of PCD and is crucial for immunity[136]. The first observation of pyroptosis was also within immune cells [137]. A new approach is needed to induce pyroptosis in cells with targeted effects on the tumor microenvironment, whereby primary tumors are directly affected by nanomaterials that activate the immune system [49]. Tumor cell-derived microparticles can be used as natural biomaterials to reverse tumor resistance to chemotherapeutic drugs and can also be loaded with cell pyroptosis inducers to act as immunomodulators [49], [138]. Table 1 Solid tumor immunotherapy is made possible by the ability of pyroptosis cancer cells to release pro-inflammatory intracellular substances in primary tumors that activate systemic anti-tumor immunity [55], [82]. When cells shed their contents and mature pro-inflammatory cytokines during pyroptosis, this can trigger a powerful immune response by activating T cells that are specific for an antigen [80], [139]. The effectiveness of tumor vaccines is further increased by the pro-inflammatory cytokines' dual roles in innate and adaptive immunity. Cellular pyroptosis therefore plays a vital part in both innate and adaptive immune responses [23]. CAR T cell effectiveness is decreased by cytokine release syndrome (CRS). CRS is caused by pyrogenic release factors in tumor cells. Therefore, GSDME knockout or caspase-1 inhibition can eliminate CRS, and CAR T cells can induce pyrogenic death in target cells [140]. Chimeric costimulatory switching receptors can interfere with the PD-1 pathway to aid CAR-NK therapy. Additionally, the pyrogenic activation-dependent novel complex PD1-NKG2D-41BB receptor increased the antitumor activity of NK92 cells [141]. Herein, a nano-drug scaffold has been proposed to enhance immunotherapy by activating intrinsic pyrodeath in tumors (Fig. 6). This experiment establishes a more efficient nanoplatform that can activate a strong tumor-killing effect and and maintain an efficient immune response [142].
Table 1.
Nanomedicines mediating pyroptosis.
| Pyroptosis-inducing nanomedicines | Type of tumor | Effects in vivo | Effect on immunotherapy | Ref. |
|---|---|---|---|---|
| Lip-MOFs and other similar nanostructures | • Cervical cancer • Skin squamous cell carcinoma • Breast cancer |
• The release of iron induces pyroptosis • Attacks tumor cells in acidic TME via pyroptosis |
• Triggers an immune response | [106] |
| Methotrexate-loaded tumor cell-derived microvesicles (MTX-TMPs) | • Extrahepatic cholangiocarcinoma | •Induces pyroptosis in cholangiocarcinoma (CCA) cells via a GSDME-dependent pathway • Release of intracellular content upon CCA cell death activates macrophages to produce pro-inflammatory cytokines • Attracts a second wave of neutrophils to the tumor site • Promotes DCs to cross-present tumor antigens on TMPs to CD8 + T cells • Induction of DCs upregulation of the IFN-I pathway by cyclic GMP-AMP synthase-stimulator (cGAS-STING) that activates interferon genes • Induces tumor cell pyroptosis, which generates a strong inflammatory signal and confers tumor-infiltrating neutrophils with an antitumor phenotype |
• Improves immune response • Improves tumor vaccine efficacy |
[153] |
| CXCR4-targeted toxin NPs | • Colorectal cancer | • Triggers pyroptosis to target and efficiently kill apoptosis-resistant tumors derived from CXCR4 + colonic CSCs | • Improves issues such as immunotherapy resistance and metastasis | [103] |
| A tumor-targeted nanoliposome loaded with cisplatin (LipoDDP) | • Breast cancer • Adenocarcinoma of the colon • Melanoma |
• For demethylation of DFNA5 gene (a key protein in pyroptosis, GSDME, is translated by the DFNA5 gene) in tumor cells • Triggers cancer cell pyroptosis pathway • Activates the caspase-3 pathway in tumor cells and triggers pyroptosis |
• Enhances antitumor response to immunotherapy | [138] |
| A Phe-BF3 Desilylation-based bioorthogonal system compatible with NP-mediated delivery of controlled-release drugs | • Breast cancer | • Shows tumor selectivity • Releases client proteins, including active GSDM, into tumor cells selectively |
• Triggers robust antitumor immunity and can synergize with checkpoint blockade | [53] |
| A biomimetic NP (BNP) | • Breast cancer | • Design of poly(lactic-co-glycolic acid) (PLGA) polymer core and cancer cell membrane masking to confer homologous tumor-homing properties and low immunogenicity to BNPs • Low-dose photoactivation (60 J) at the tumor site causes local hyperthermia in granules that puncture the cell membrane to induce caspase-3 activation and release drugs to modulate pyroptosis |
• Immunotherapy of solid tumors with minimized systemic toxicity | [55] |
| Smart TME ROS/GSH dual-responsive nanoprodrug (named MCPP) | • Colon cancer | • Induces tumor cell pyroptosis and reduces normal tissue pyroptosis • Causes antigen release, triggers an immune response, and creates an immune memory • Promotes tumor antigen release, initiates APCs, and increases T cell infiltration |
• Enhances the effect of immunotherapy and prevents tumor recurrence • Amplifies response rates to PD-1 blockade therapy |
[154] |
Fig. 6.
Enhancing in vivo experimental results of nano-drugs for immunotherapy by activating intrinsic focal death of tumors. (A) Scheme of mice treated with different formulations. (B-D) Tumor volume, tumor weight and survival curve (Nano-CD vs. Saline) of tumor-bearing mice with different treatments. (E–I) Profiles and percentages of CD4+ T cells, CD8+ T cells, CD69+ activated T cells, CD44+CD62L− TEM cells in tumors and CD4+ T cells/CD8+ T cells in spleens by FCM analysis. (J) Concentration of IL-12, IL-10 and TGF-β cytokines in serum after different treatments. Copyright © 2021, Nature Communications.
Nanomedicine-induced ferroptosis enhances the efficacy of immunotherapy
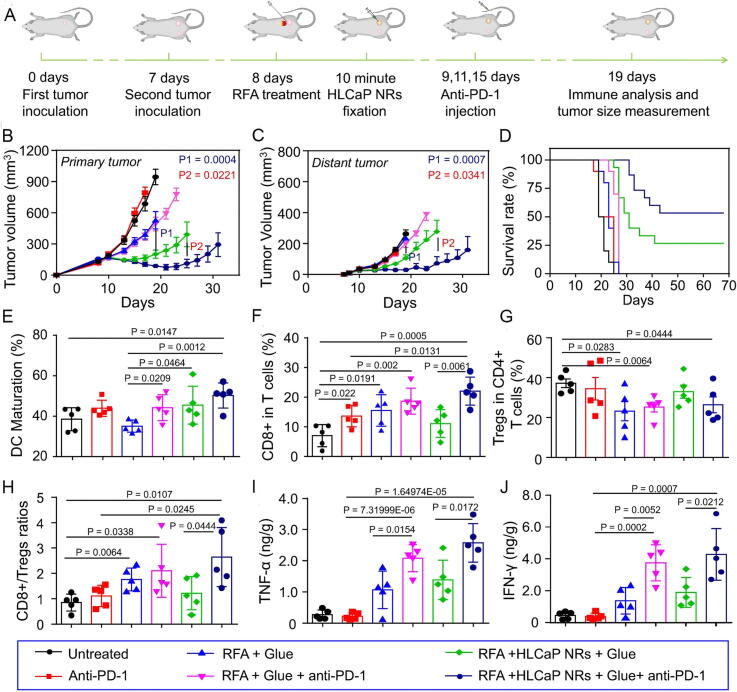
Ferroptosis is highly immunogenic and has synergistic effects with immunomodulation in cancer [32], [143]. An earlier study showed that ferroptosis-specific lipid peroxidation in tumor cells is enhanced by immunotherapy-activated CD8+ T lymphocytes, promoting ferroptosis and increasing the effectiveness of immunotherapy [144]. Several studies have shown that regulation of ferroptosis may overcome resistance to conventional chemotherapy, targeted therapies, and immunotherapy. GPX4 protects Treg from ferroptosis and may reverse immunotherapy resistance by inhibiting GPX4-induced Treg ferroptosis. Regulation of ferroptosis seems promising and challenging as a therapeutic approach to overcome cancer treatment resistance [26], [93]. NP inducers have their unique advantages on ferroptosis (Table 2) [93]. HLCaP NRs enhance anti-tumor immune effects by triggering ferroptosis (Fig. 7). This experiment combined ferroptosis induction as well as anti-PD-l immunotherapy, and the results showed that it exerted good therapeutic and anti-tumor metastatic effects [145].
Table 2.
Nanomedicines mediating ferroptosis.
| Ferroptosis-inducing nanomedicines | Type of tumor | Effects in vivo | Effect on immunotherapy | Ref. |
|---|---|---|---|---|
| A synergistic nanodrug of oxaliplatin (OxPt)/dihydroartemisinin (DHA) NPs | • Colon cancer | • Anticancer and immunostimulatory properties of ROS that favor ferroptosis • Induces CRT exposure and HMGB-1 release • Recruits antigen-presenting DCs and macrophages • Promotes cancer cell phagocytosis and enhances antigen processing and presentation • Increases intratumoral-infiltrating CD8 + T cells |
• OxPt/DHA directly converts treated tumors into in situ vaccines • Enhances ICB treatment significantly |
[149] |
| Ferumoxytol | • Breast cancer • Lung cancer |
• Targets TIME effectively • Converts M2-TAM to M1-TAM • Inhibits the growth of primary and metastatic tumors in the liver and lungs • Enhances tumor endocytosis of macrophages significantly |
• Improves efficacy of ICB therapy | [155] |
| Irradiated tumor cells release microparticles (RT-MPs) | • Malignant Pleural effusion (MPE) | • Induces ferroptosis • Polarization of microenvironmental immunosuppressive M2-TAMs to antitumor M1-TAMs |
• After RT-MPs internalization, TAMs showed increased PD-L1 expression, inducing an immune memory effect | [156] |
| A biomimetic magnetosome with Fe3O4 magnetic nanoclusters as the core and prefabricated leukocyte membrane as the coat | • Breast cancer | • Promotes ferroptosis/immunomodulatory synergy in cancer • Creates an immunogenic TME • Increases H2O2 in M1-TAM to promote Fenton reaction • Produces ·OH induces lethal ferroptosis in tumor cells • Exposed tumor antigens in turn enhance the immunogenicity of the microenvironment |
• Ferroptosis/immune cycle synergy | [32] |
| Biomimetic magnetic NPs Fe3O4-SAS@PLT | • Breast cancer | • Platelet membranes have immune evasion and tumor targeting capabilities to maximize ferroptosis-inducing NPs delivery to tumors • Increases ferroptosis susceptibility and produces mild immunogenicity effectively • Repolarizes macrophages from the M2-TAM phenotype to the M1-TAM effectively |
• Improves the efficacy of ICB significantly | [50] |
| ZnP@DHA/Pyro-Fe particles | • Colon cancer | • Uses nanotechnology can combine DHA and other drugs with ICB to treat tumors • Hydrolysis of stabilized DHA • Prolongs blood circulation of Chol-DHA and Pyro-Fe to enhance their uptake in tumors • Co-delivery of exogenous iron complex and DHA induces more ROS production, leading to ferroptosis in cancer cells • Causes significant tumor suppression |
• ICB sensitizes non-immunogenic colorectal tumors against PD-L1 by increasing tumor immunogenicity | [157] |
| Fe-PDA NPs | • Breast tumor | • Enhance the ability of photoheat conversion • Fe is released in the tumor, promoting TAM polarization into M1 mode • Activate CTLs and T helper cells (Th) |
• Effectively improve the effect of immunotherapy to prevent tumor recurrence and metastasis | [158] |
| Dynamic NPs activated by intracellular acidity | • Melanoma • Breast tumor |
• Tumor-specific delivery of ferroptosis inducer glutathione peroxidase 4 inhibitor RSL-3 • Acid-activated photodynamic therapy by protonation of ionizable cores • Tumor-infiltrating T lymphocytes are recruited, and IFN-g is secreted |
• Ferroptosis is combined with the blocking of PD-L1 | [159] |
| PADO-Fe | • Breast cancer | • Responds to pH and H2O2 • Amplifies the ferroptosis cascade |
• In combination with ICB, it enhances the immune response to PD-L1 ICB immunotherapy | [127] |
| FePd | • Its peak topology can activate autophagy to enhance ferroptosis and synergistic enhancement of cancer immunotherapy |
Fig. 7.
In vivo experimental results of nanomaterials enhancing anti-tumor immunity by triggering ferroptosis. (A) For in vivo anticancer and immune mechanism research, a schematic representation of the inoculation of the bilateral tumor model is shown. (B–D) Primary tumor growth curves (B) and distant tumors (C), together with a similar rate of mobility-free survival (D) of mice with bilateral tumor models following the recommended treatments. (E) DC maturation status following various therapies as indicated in the drain lymph nodes close to the original tumors. (F–H) The frequencies of CD3+CD8+ T cells (F) and CD3+CD4+FoxP3+ Tregs (G), and the ratios (H) after receiving various therapies as specified, inside the distant tumors. (I, J) The amounts of TNF-α and IFN-γ secretion within the distant tumors following different therapies are shown. Copyright © 2023, Nature Communications.
Nanomedicine-induced autophagy enhances the efficacy of immunotherapy
A previous study identified a link between chemotherapy-induced autophagy, ATP release, and anti-cancer immune response in mouse tumors. This has significant implications for developing new strategies that make cancer cell death immunogenic [146]. Recent research has demonstrated that autophagosomes produced from tumor cells are greater antigen carriers than complete tumor cells and trigger a larger anticancer response. Moreover, α-Al2O3 NPs can deliver antigens to autophagosomes in DCs, which present the antigen to T cells via autophagy. α-Al2O3 NPs could be effective adjuvants in the creation of therapeutic cancer vaccines. Recent research revealed the potential relevance of NP-induced autophagy as a cancer vaccination method by enhancing the immunological response of T cells. Autophagosomes of antigen donor cells are related with efficient cross-presentation [57]. Additionally, exosomes have a role in the KRAS G12D protein being released by autophagy from pancreatic ductal adenocarcinoma (PDAC) cells. This secreted protein has the ability to activate STAT3-dependent fatty acid oxidation. Because of this, a pro-tumor phenotype that closely mimics M2 is formed as a result of the activation and polarization of macrophages. PDAC cell tumor development is inhibited by blocking the release of KRAS G12D and the immune system's subsequent uptake of exosomes containing KRAS G12D [114]. Furthermore, NPs can also trigger necrotic tumor cell death through modulation of autophagy [60], thus enhancing the effect of immunotherapy. Autophagy-cascading amplified NPs (ASN) can overactivate autophagy, leading to enhanced following tumor antigen treatment of cells that are dying, exhibiting optimal immunostimulation and antitumor efficiency [121]. An optimal NP can regulate autophagy and combine with anti-PD-L1 antibodies to improve the efficacy of tumor therapy (Fig. 8). This experiment is a very effective combination therapy by combining autophagy disruption with immune checkpoint blockade therapy from the results of the trial [147].
Fig. 8.
Nanomaterials regulate autophagy and bind with anti-PD-L1 antibodies to improve the efficacy of tumor therapy in vivo. (A) Kaplan–Meier survival analysis of C6 glioma-bearing mice treated with different formulations (n = 10). (B) Flow cytometry analysis of mature DCs (CD83+CD11c+) in spleen after treatment, *P < 0.05, **P < 0.01, ***P < 0.001. (C) Flow cytometry analysis of mature DCs (CD86+CD11c+) in spleen after treatment, *P < 0.05, **P < 0.01, ***P < 0.001. (D) IHC staining of mice brains collected after the last administration, CD4, CD8, and Foxp3 were stained brown (Scale bar: 40 μm). (E) Semiquantitative analysis of the percentage of CD4+ T cells, CD8+ T cells, and Foxp3+ Treg of total T cells using ImageJ, *P < 0.05, **P < 0.01, ***P < 0.001 represent statistical significance vs. N.S. group (n = 3). (F) Ratio of CD4+ T cells to Foxp3+ Treg and CD8+ T cells to Foxp3+ Treg, *P < 0.05, **P < 0.01, ***P < 0.001 represent statistical significance vs. N.S. group (n = 3). Copyright © 2019, American Chemical Society.
Conclusions and future perspectives
As a recent hotspot in tumor therapy, the clinical progress in tumor immunotherapy is also very impressive [55]. It has been reported that today's oncology research is no longer limited to the research and application of single therapies, but the direction of development of oncology therapies lies in the fusion of cutting-edge research areas of previous years, such as “nanomedicine”, “tumor immunotherapy”, and the combination of other related therapies. For example, precision medicine and nanomedicine-mediated tumor immunotherapy can be combined. Or, as mentioned in this paper, combining both with PCD [148]. The scope of PCD is more than the classical apoptotic pathway. In recent years, cell necroptosis, pyroptosis, ferroptosis, and autophagy have been elucidated for putative cancer treatment strategies [16]. This review introduces that nanomedicine-mediated PCD is closely related to and can largely enhance the efficacy of tumor immunotherapy. Nanomedicine-induced PCD is mainly summarized as directly inducing cell death, promoting the release of antigen and inflammatory factors, enhancing immune stimulation, inducing immune cell infiltration, thus promoting the killing of tumor and inhibiting tumor metastasis. This combination therapy has shown satisfactory synergistic benefits in preclinical and clinical investigations and is a superior oncology treatment strategy.
The ideal cancer therapy destroys the primary tumor and activates the antitumor immune response to achieve a satisfactory antitumor immune effect [136]. Nano-induced PCD is a possible method to improve the effectiveness of cancer immunotherapy [60]. Among them, NPs have the unique advantage of being a desirable platform for piggybacking drugs, but the existing studies show that nanotechnology has not been perfected. The active targeting ability of nanocarriers improves the drug delivery performance [124]. However, because of the reverse gradient in pressure and transport barrier of tumor physiology, NPs neither accumulate easily nor penetrate through EPR in solid tumors. Despite an equal chance of targeting tumor and non-tumor cells, the net effect of so-called active targeting is not visible in the clinical context. Due to the heterogeneity of tumors and the expression of specific receptors on tumors, targeted delivery of nanomedicines is capable of killing a small portion of tumor cells with particular receptors found in available regions of the overall tumor; the most preclinical experiments with nanomedicines use rodent models. However, in the majority of human malignancies, the expression of certain receptors is not sufficient for successful tumor control by nanomedicines [21]. In fact, for nanoparticles, the clinical environment does not clearly show the net impact of so-called targeted action. Since NP-based drug research is not limited to traditional geometry, size, or chemical structure [149], these issues can be solved using biomimetic methods of immune cell membranes [150] or cancer cell membranes [151]. BNPs have higher targeting properties, biocompatibility, low immunogenicity, and less toxicity than typical NPs [63]. Immune cell surface proteins give NPs a variety of abilities, such as increased blood flow, a strong ability to detect antigens for improved targeting, better cellular contacts, progressive drug release, and decreased in vivo toxicity [150]. Common nanomedicine targets such as lymph nodes and TME can be studied in depth to design better-targeted and more potent drugs [152], [153].
Nevertheless, it is unclear whether the NP-mediated approach to PCD-enhanced immunotherapy triggers autoimmune responses. The sensitivity to nanodrug-mediated PCD can vary greatly among cancer cell lines. Also, several types of cells that may be disrupted by the PCD pathway are involved in TME. It is therefore vital to comprehend the process of sensitivity to PCD in TME in distinct cell types to balance the PCD vulnerability of cancer, anti-tumor immune, and immunosuppressive cells [154]. In addition, light therapy [155] and radiation therapy [156] may be added to the combination of therapies for optimal treatment.
CRediT authorship contribution statement
Jiaye Lu: Conceptualization, Methodology, Writing – original draft. Zongguang Tai: Conceptualization, Writing – review & editing. Junchao Wu: Methodology, Software. Lisha Li: Visualization, Investigation. Tingrui Zhang: Investigation, Software. Jun Liu: Investigation. Quangang Zhu: Resources. Zhongjian Chen: Validation, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [Grant No. 82073385 and 82172706] and the Science and Technology Commission of Shanghai Municipality [Grant No. 21140901900, 21S21900900, and 22S21902700]
Biographies

Jiaye Lu is a joint Master Student in both Department of Pharmacy at Shanghai Skin Disease Hospital, School of Medicine, Tongji University and Shanghai University. Jiaye Lu current research focuses on the therapeutic effect of nanodrug delivery systems for tumors in melanoma.

Zongguang Tai is an associate chief pharmacist of Shanghai Skin Disease Hospital, School of Medicine, Tongji University. He received his PhD from Naval Medical University. His research interest includes innovative drugs for dermatology, emerging immunotherapy, and drug delivery system.

Junchao Wu is a joint Master Student in both Department of Pharmacy at Shanghai Skin Disease Hospital, School of Medicine, Tongji University and Shanghai University. Junchao wu current research focuses on pathogenesis and intervention treatment of atopic dermatitis.

Lisha Li is a joint Master Student in both Department of Pharmacy at Shanghai Skin Disease Hospital, School of Medicine, Tongji University and Shanghai University. Lisha Li current research focuses on ionic liquids and their application in the field of medicine.

Tingrui Zhang is a joint Master Student in both Department of Pharmacy at Shanghai Skin Disease Hospital, School of Medicine, Tongji University and Shanghai University. Tingrui Zhang current research focuses on repairment of skin tissue.

Jun liu is a joint Master Student in both Department of Pharmacy at Shanghai Skin Disease Hospital, School of Medicine, Tongji University. Jun liu current research focuses on the immunotherapy of skin tumors.

Quangang Zhu is the Director of Department of Pharmacy and GCP (Good Clinical Practice) Office of Shanghai Skin Disease Hospital, School of Medicine, Tongji University. He holds a Ph.D. of Pharmacy and is also a Professor of Pharmacy at Tongji University School of Medicine. His research activity includes pharmacology of skin, topical drugs, nanodelivery systems, and novel drug delivery systems.

Zhongjian Chen is the vice president of Shanghai Skin Disease Hospital, School of Medicine, Tongji University. He holds a Ph.D of Medicine and is also a professor of Pharmacy at Tongji University School of Medicine. His research activity includes pharmacology of skin, topical drugs, nanomedicine and novel drug delivery systems.
Contributor Information
Quangang Zhu, Email: qgzhu@126.com.
Zhongjian Chen, Email: aajian818@163.com.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Pützer B, Sabapathy KJFic, d. biology, Editorial: multidisciplinary approaches in exploring cancer heterogeneity, TME and therapy resistance: perspectives for systems medicine, 2022;10: 842596. [DOI] [PMC free article] [PubMed]
- 3.Goliwas K, Deshane J, Elmets C, Athar MJPr. Moving immune therapy forward targeting TME 2021;101(2): 417-425. [DOI] [PMC free article] [PubMed]
- 4.Yadav D, Kwak M, Chauhan P, Puranik N, Lee P, Jin JJSicb. Cancer immunotherapy by immune checkpoint blockade and its advanced application using bio-nanomaterials, 2022; 1-14. [DOI] [PubMed]
- 5.Jiang M., Chen W., Yu W., Xu Z., Liu X., Jia Q., et al. Interfaces, Sequentially pH-responsive drug-delivery nanosystem for tumor immunogenic cell death and cooperating with immune checkpoint blockade for efficient cancer chemoimmunotherapy. 2021;13(37):43963–43974. doi: 10.1021/acsami.1c10643. [DOI] [PubMed] [Google Scholar]
- 6.Cilibrasi C., Papanastasopoulos P., Samuels M., Giamas G. Reconstituting immune surveillance in breast cancer: molecular pathophysiology and current immunotherapy strategies. Int J Mol Sci. 2021;22(21):12015. doi: 10.3390/ijms222112015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emens L.A., Ascierto P.A., Darcy P.K., Demaria S., Eggermont A.M.M., Redmond W.L., et al. Cancer immunotherapy: Opportunities and challenges in the rapidly evolving clinical landscape, European journal of cancer (Oxford. England. 1990;81(2017):116–129. doi: 10.1016/j.ejca.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 8.Zitvogel L, Tesniere A, Kroemer GJNrI. Cancer despite immunosurveillance: immunoselection and immunosubversion 2006;6(10): 715-27. [DOI] [PubMed]
- 9.Shankaran V., Ikeda H., Bruce A., White J., Swanson P., Old L., et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. 2001;410(6832):1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 10.Taieb J, Chaput N, Ménard C, Apetoh L, Ullrich E, Bonmort M, et al. A novel dendritic cell subset involved in tumor immunosurveillance 2006;12(2): 214-9. [DOI] [PubMed]
- 11.Qi J., Jin F., Xu X., Du Y. Combination cancer immunotherapy of nanoparticle-based immunogenic cell death inducers and immune checkpoint inhibitors. Int J Nanomed. 2021;16:1435–1456. doi: 10.2147/IJN.S285999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irvine D.J., Maus M.V., Mooney D.J., Wong W.W. The future of engineered immune cell therapies, Science. 2022;378:853–858. doi: 10.1126/science.abq6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Y, Lammers TJAocr. Combining nanomedicine and immunotherapy 2019;52(6): 1543-1554. [DOI] [PMC free article] [PubMed]
- 14.Filliol A, Saito Y, Nair A, Dapito DH, Yu LX, Ravichandra A, et al. Opposing roles of hepatic stellate cell subpopulations in hepatocarcinogenesis. Nature 2022;610(7931): 356-65. [DOI] [PMC free article] [PubMed]
- 15.Galluzzi L, Vitale I, Aaronson S, Abrams J, Adam D, Agostinis P, et al. Differentiation, Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018; 25(3): 486-541. [DOI] [PMC free article] [PubMed]
- 16.Liao M., Qin R., Huang W., Zhu H., Peng F., Han B., et al. Liu, oncology, Targeting regulated cell death (RCD) with small-molecule compounds in triple-negative breast cancer: a revisited perspective from molecular mechanisms to targeted therapies. 2022;15(1):44. doi: 10.1186/s13045-022-01260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.V. Forest, J.J.A.d.d.r. Pourchez, Nano-delivery to the lung - by inhalation or other routes and why nano when micro is largely sufficient?, 183 (2022) 114173. [DOI] [PubMed]
- 18.Z. Chen, Z. Wang, Z.J.A.o.c.r. Gu, Bioinspired and Biomimetic Nanomedicines, 52(5) (2019) 1255-1264. [DOI] [PMC free article] [PubMed]
- 19.Iqbal J., Abbasi B., Ahmad R., Mahmood T., Ali B., Khalil A., et al. Nanomedicines for developing cancer nanotherapeutics: from benchtop to bedside and beyond. 2018;102(22):9449–9470. doi: 10.1007/s00253-018-9352-3. [DOI] [PubMed] [Google Scholar]
- 20.Peer D, Karp J, Hong S, Farokhzad O, Margalit R, Langer RJNn. Nanocarriers as an emerging platform for cancer therapy, 2007;2(12): 751-60. [DOI] [PubMed]
- 21.Youn Y, Bae YJAddr. Perspectives on the past, present, and future of cancer nanomedicine 2018;130: 3-11. [DOI] [PubMed]
- 22.Maiorino L., Daßler-Plenker J., Sun L., Egeblad M. Innate immunity and cancer pathophysiology. Annu Rev Pathol. 2022;17:425–457. doi: 10.1146/annurev-pathmechdis-032221-115501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.C.J.I.r. Dinarello, Overview of the IL-1 family in innate inflammation and acquired immunity, 281(1) (2018) 8-27. [DOI] [PMC free article] [PubMed]
- 24.S. Nagata, M.J.N.r.I. Tanaka, Programmed cell death and the immune system, 17(5) (2017) 333-340. [DOI] [PubMed]
- 25.S. George, B. Rini, H.J.J.o. Hammers, Emerging Role of Combination Immunotherapy in the First-line Treatment of Advanced Renal Cell Carcinoma: A Review, 5(3) (2019) 411-421. [DOI] [PubMed]
- 26.Y. Mi, C. Hagan, B. Vincent, A.J.A.s. Wang, Emerging Nano-/Microapproaches for Cancer Immunotherapy, 6(6) (2019) 1801847. [DOI] [PMC free article] [PubMed]
- 27.Jia X., Yan B., Tian X., Liu Q., Jin J., Shi J., et al. CD47/SIRPα pathway mediates cancer immune escape and immunotherapy. Int J Biol Sci. 2021;17(13):3281–3287. doi: 10.7150/ijbs.60782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowshanravan B., Halliday N., Sansom D.M. CTLA-4: a moving target in immunotherapy. Blood. 2018;131(1):58–67. doi: 10.1182/blood-2017-06-741033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosseini A., Gharibi T., Marofi F., Babaloo Z., Baradaran B. CTLA-4: From mechanism to autoimmune therapy. Int Immunopharmacol. 2020;80 doi: 10.1016/j.intimp.2020.106221. [DOI] [PubMed] [Google Scholar]
- 30.M.W. Rohaan, T.H. Borch, J.H. van den Berg, Ö. Met, R. Kessels, M.H. Geukes Foppen, J. Stoltenborg Granhøj, B. Nuijen, C. Nijenhuis, I. Jedema, M. van Zon, S. Scheij, J.H. Beijnen, M. Hansen, C. Voermans, I.M. Noringriis, T.J. Monberg, R.B. Holmstroem, L.D.V. Wever, M. van Dijk, L.G. Grijpink-Ongering, L.H.M. Valkenet, A. Torres Acosta, M. Karger, J.S.W. Borgers, R.M.T. Ten Ham, V.P. Retèl, W.H. van Harten, F. Lalezari, H. van Tinteren, A.A.M. van der Veldt, G.A.P. Hospers, M.A.M. Stevense-den Boer, K.P.M. Suijkerbuijk, M.J.B. Aarts, D. Piersma, A.J.M. van den Eertwegh, J.B. de Groot, G. Vreugdenhil, E. Kapiteijn, M.J. Boers-Sonderen, W.E. Fiets, F. van den Berkmortel, E. Ellebaek, L.R. Hölmich, A.C.J. van Akkooi, W.J. van Houdt, M. Wouters, J.V. van Thienen, C.U. Blank, A. Meerveld-Eggink, S. Klobuch, S. Wilgenhof, T.N. Schumacher, M. Donia, I.M. Svane, J. Haanen, Tumor-Infiltrating Lymphocyte Therapy or Ipilimumab in Advanced Melanoma, The New England journal of medicine 387(23) (2022) 2113-2125. [DOI] [PubMed]
- 31.Xu-Monette Z.Y., Zhou J., Young K.H. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131(1):68–83. doi: 10.1182/blood-2017-07-740993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang F., Li F., Lu G., Nie W., Zhang L., Lv Y., et al. Xie, Engineering Magnetosomes for Ferroptosis/Immunomodulation Synergism. Cancer. 2019;13(5):5662–5673. doi: 10.1021/acsnano.9b00892. [DOI] [PubMed] [Google Scholar]
- 33.H. Phuengkham, C. Song, Y.J.A.m. Lim, A Designer Scaffold with Immune Nanoconverters for Reverting Immunosuppression and Enhancing Immune Checkpoint Blockade Therapy, 31(42) (2019) e1903242. [DOI] [PubMed]
- 34.J. Liu, M. Fu, M. Wang, D. Wan, Y. Wei, X.J.J.o.h. Wei, oncology, Cancer vaccines as promising immuno-therapeutics: platforms and current progress, 15(1) (2022) 28. [DOI] [PMC free article] [PubMed]
- 35.M. Saxena, S. van der Burg, C. Melief, N.J.N.r.C. Bhardwaj, Therapeutic cancer vaccines, 21(6) (2021) 360-378. [DOI] [PubMed]
- 36.S. Huang, Y. Zhu, L. Zhang, Z.J.A.m. Zhang, Recent Advances in Delivery Systems for Genetic and Other Novel Vaccines, (2021) e2107946. [DOI] [PubMed]
- 37.Qin L., Zhang H., Zhou Y., Umeshappa C., Gao H.J.S. Nanovaccine-Based Strategies to Overcome Challenges in the Whole Vaccination Cascade for Tumor. Immunotherapy. 2021;17(28):e2006000. doi: 10.1002/smll.202006000. [DOI] [PubMed] [Google Scholar]
- 38.Badrinath S., Dellacherie M., Li A., Zheng S., Zhang X., Sobral M., et al. A vaccine targeting resistant tumours by dual T cell plus NK cell attack. 2022;606(7916):992–998. doi: 10.1038/s41586-022-04772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.J. Huppa, M.J.N.r.I. Davis, T-cell-antigen recognition and the immunological synapse, 3(12) (2003) 973-83. [DOI] [PubMed]
- 40.Liu C., Liu X., Xiang X., Pang X., Chen S., Zhang Y., et al. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy. 2022;17(5):531–540. doi: 10.1038/s41565-022-01098-0. [DOI] [PubMed] [Google Scholar]
- 41.E. Blass, P.J.N.r.C.o. Ott, Advances in the development of personalized neoantigen-based therapeutic cancer vaccines, 18(4) (2021) 215-229. [DOI] [PMC free article] [PubMed]
- 42.Y. Xue, J. Che, X. Ji, Y. Li, J. Xie, X.J.C.S.r. Chen, Recent advances in biomaterial-boosted adoptive cell therapy, 51(5) (2022) 1766-1794. [DOI] [PubMed]
- 43.M. Daher, K.J.C.d. Rezvani, Outlook for New CAR-Based Therapies with a Focus on CAR NK Cells: What Lies Beyond CAR-Engineered T Cells in the Race against Cancer, 11(1) (2021) 45-58. [DOI] [PMC free article] [PubMed]
- 44.C. Brown, C.J.N.r.I. Mackall, CAR T cell therapy: inroads to response and resistance, 19(2) (2019) 73-74. [DOI] [PubMed]
- 45.Zhang D., Zheng Y., Lin Z., Liu X., Li J., Yang H., et al. Equipping Natural Killer Cells with Specific Targeting and Checkpoint Blocking Aptamers for Enhanced Adoptive Immunotherapy in Solid Tumors. 2020;59(29):12022–12028. doi: 10.1002/anie.202002145. [DOI] [PubMed] [Google Scholar]
- 46.A. Finck, T. Blanchard, C. Roselle, G. Golinelli, C.J.N.m. June, Engineered cellular immunotherapies in cancer and beyond, 28(4) (2022) 678-689. [DOI] [PMC free article] [PubMed]
- 47.Yilmaz A., Cui H., Caligiuri M., J. J. J. o.h Yu, oncology, Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. 2020;13(1):168. doi: 10.1186/s13045-020-00998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gong Y., Klein Wolterink R., Wang J., Bos G., W.J.J.o.h Germeraad, oncology, Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. 2021;14(1):73. doi: 10.1186/s13045-021-01083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.A. Nel, K. Mei, Y. Liao, X.J.A.n. Liu, Multifunctional Lipid Bilayer Nanocarriers for Cancer Immunotherapy in Heterogeneous Tumor Microenvironments, Combining Immunogenic Cell Death Stimuli with Immune Modulatory Drugs, 16(4) (2022) 5184-5232. [DOI] [PMC free article] [PubMed]
- 50.Jiang Q., Wang K., Zhang X., Ouyang B., Liu H., Pang Z., et al. Platelet Membrane-Camouflaged Magnetic Nanoparticles for Ferroptosis-Enhanced Cancer. Immunotherapy. 2020;16(22):e2001704. doi: 10.1002/smll.202001704. [DOI] [PubMed] [Google Scholar]
- 51.Genoud V., Espinoza F., Marinari E., Rochemont V., Dietrich P., McSheehy P., et al. Treating ICB-resistant glioma with anti-CD40 and mitotic spindle checkpoint controller BAL101553 (lisavanbulin) 2021;6(18):e142980. doi: 10.1172/jci.insight.142980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenbaum S.R., Wilski N.A., Aplin A.E.J.C.D. Fueling the Fire: Inflammatory Forms of Cell Death and Implications for Cancer. Immunotherapy. 2021;11(2):266–281. doi: 10.1158/2159-8290.CD-20-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Q., Wang Y., Ding J., Wang C., Zhou X., Gao W., et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. 2020;579(7799):421–426. doi: 10.1038/s41586-020-2079-1. [DOI] [PubMed] [Google Scholar]
- 54.Wang D., Wang T., Yu H., Feng B., Zhou L., Zhou F., et al. Engineering nanoparticles to locally activate T cells in the tumor microenvironment. 2019;4(37):eaau6584. doi: 10.1126/sciimmunol.aau6584. [DOI] [PubMed] [Google Scholar]
- 55.Zhao P., Wang M., Chen M., Chen Z., Peng X., Zhou F., et al. Programming cell pyroptosis with biomimetic nanoparticles for solid tumor immunotherapy. 2020;254 doi: 10.1016/j.biomaterials.2020.120142. [DOI] [PubMed] [Google Scholar]
- 56.Cao W., He L., Cao W., Huang X., Jia K., Dai J. Recent progress of graphene oxide as a potential vaccine carrier and adjuvant. Acta Biomater. 2020;112:14–28. doi: 10.1016/j.actbio.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 57.Li H., Li Y., Jiao J., H.J.N.n Hu, Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. 2011;6(10):645–650. doi: 10.1038/nnano.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.L. Wu, W. Xie, Y. Li, Q. Ni, P. Timashev, M. Lyu, L. Xia, Y. Zhang, L. Liu, Y. Yuan, X. Liang, Q.J.A.s. Zhang, Biomimetic Nanocarriers Guide Extracellular ATP Homeostasis to Remodel Energy Metabolism for Activating Innate and Adaptive Immunity System, 9(17) (2022) e2105376. [DOI] [PMC free article] [PubMed]
- 59.J. Lee, D. Kim, Q. Le, Y.J.S.i.c.b. Oh, Nanotherapeutics for immune network modulation in tumor microenvironments, 86 (2022) 1066-1087. [DOI] [PubMed]
- 60.Tang R., Xu J., Zhang B., Liu J., Liang C., Hua J., et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. 2020;13(1):110. doi: 10.1186/s13045-020-00946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.T. Aaes, A. Kaczmarek, T. Delvaeye, B. De Craene, S. De Koker, L. Heyndrickx, I. Delrue, J. Taminau, B. Wiernicki, P. De Groote, A. Garg, L. Leybaert, J. Grooten, M. Bertrand, P. Agostinis, G. Berx, W. Declercq, P. Vandenabeele, D.J.C.r. Krysko, Vaccination with Necroptotic Cancer Cells Induces Efficient Anti-tumor Immunity, 15(2) (2016) 274-287. [DOI] [PubMed]
- 62.Kesavardhana S., Malireddi R.K.S., Kanneganti T.D. Caspases in Cell Death, Inflammation, and Pyroptosis. Annu Rev Immunol. 2020;38:567–595. doi: 10.1146/annurev-immunol-073119-095439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.H. Zafar, F. Raza, S. Ma, Y. Wei, J. Zhang, Q.J.B.s. Shen, Recent progress on nanomedicine-induced ferroptosis for cancer therapy, 9(15) (2021) 5092-5115. [DOI] [PubMed]
- 64.Wang L., Huo M., Chen Y., Shi J.J.B. Iron-engineered mesoporous silica nanocatalyst with biodegradable and catalytic framework for tumor-specific therapy. 2018;163:1–13. doi: 10.1016/j.biomaterials.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 65.Green D.R. Caspase Activation and Inhibition. Cold Spring Harb Perspect Biol. 2022;14(8) doi: 10.1101/cshperspect.a041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Opdenbosch N., Lamkanfi M. Caspases in Cell Death, Inflammation, and Disease. Immunity. 2019;50(6):1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.J. Montero, R.J.C.d. Haq, Adapted to Survive: Targeting Cancer Cells with BH3 Mimetics, 12(5) (2022) 1217-1232. [DOI] [PMC free article] [PubMed]
- 68.He B., Shi Y., Liang Y., Yang A., Fan Z., Yuan L., et al. Single-walled carbon-nanohorns improve biocompatibility over nanotubes by triggering less protein-initiated pyroptosis and apoptosis in macrophages. Nat Commun. 2018;9(1):2393. doi: 10.1038/s41467-018-04700-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng R., Zhao L., Chen X., Liu L., Liu Y., Chen X., et al. Metal-coordinated nanomedicine for combined tumor therapy by inducing paraptosis and apoptosis. J Control Release. 2021;336:159–168. doi: 10.1016/j.jconrel.2021.06.021. [DOI] [PubMed] [Google Scholar]
- 70.Yang X., Zhao J., Duan S., Hou X., Li X., Hu Z., et al. Enhanced cytotoxic T lymphocytes recruitment targeting tumor vasculatures by endoglin aptamer and IP-10 plasmid presenting liposome-based nanocarriers. Theranostics. 2019;9(14):4066–4083. doi: 10.7150/thno.33383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiong Q., Li Y., Zhou K., Chen P., Guo H., Chen L., et al. Optimized fluorodendrimer-incorporated hybrid lipid-polymer nanoparticles for efficient siRNA delivery. 2020;8(3):758–762. doi: 10.1039/c9bm01738k. [DOI] [PubMed] [Google Scholar]
- 72.D. Frank, J.J.C.d. Vince, differentiation, Pyroptosis versus necroptosis: similarities, differences, and crosstalk, 26(1) (2019) 99-114. [DOI] [PMC free article] [PubMed]
- 73.Pasparakis M., Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517(7534):311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 74.Um W., Ko H., You D., Lim S., Kwak G., Shim M., et al. JJAm Park, Necroptosis-Inducible Polymeric Nanobubbles for Enhanced Cancer Sonoimmunotherapy. 2020;32(16):e1907953. doi: 10.1002/adma.201907953. [DOI] [PubMed] [Google Scholar]
- 75.M. Messmer, A. Snyder, A.J.C.d. Oberst, differentiation, Comparing the effects of different cell death programs in tumor progression and immunotherapy, 26(1) (2019) 115-129. [DOI] [PMC free article] [PubMed]
- 76.Yuan X., Nie W., He Z., Yang J., Shao B., Ma X., et al. Carbon black nanoparticles induce cell necrosis through lysosomal membrane permeabilization and cause subsequent inflammatory response. Theranostics. 2020;10(10):4589–4605. doi: 10.7150/thno.34065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim A., Yonemoto C., Feliciano C., Shashni B., Nagasaki Y.J.S. Antioxidant Nanomedicine Significantly Enhances the Survival Benefit of Radiation Cancer Therapy by Mitigating Oxidative Stress-Induced Side Effects. 2021;17(21):e2008210. doi: 10.1002/smll.202008210. [DOI] [PubMed] [Google Scholar]
- 78.García-Hevia L., Valiente R., Martín-Rodríguez R., Renero-Lecuna C., González J., Rodríguez-Fernández L., et al. Nano-ZnO leads to tubulin macrotube assembly and actin bundling, triggering cytoskeletal catastrophe and cell necrosis. 2016;8(21):10963–10973. doi: 10.1039/c6nr00391e. [DOI] [PubMed] [Google Scholar]
- 79.Loveless R., Bloomquist R., Teng Y. Pyroptosis at the forefront of anticancer immunity. Journal of experimental & clinical cancer research : CR. 2021;40(1):264. doi: 10.1186/s13046-021-02065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu M., Liu X., Chen H., Duan Y., Liu J., Pan Y., et al. Activation of Pyroptosis by Membrane-Anchoring AIE Photosensitizer Design: New Prospect for Photodynamic Cancer Cell Ablation. 2021;60(16):9093–9098. doi: 10.1002/anie.202016399. [DOI] [PubMed] [Google Scholar]
- 81.Wu D., Wang S., Yu G., Chen X.J.A.C. Cell Death Mediated by the Pyroptosis Pathway with the Aid of Nanotechnology: Prospects for. Cancer Therapy. 2021;60(15):8018–8034. doi: 10.1002/anie.202010281. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y., Gao W., Shi X., Ding J., Liu W., He H., et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. 2017;547(7661):99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 83.J. Hou, J. Hsu, M.J.M.c. Hung, Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity, 81(22) (2021) 4579-4590. [DOI] [PMC free article] [PubMed]
- 84.Erkes D., Cai W., Sanchez I., Purwin T., Rogers C., Field C., et al. Inhibitors Regulate the Tumor Immune Microenvironment via Pyroptosis. 2020;10(2):254–269. doi: 10.1158/2159-8290.CD-19-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Serna N., Álamo P., Ramesh P., Vinokurova D., Sánchez-García L., Unzueta U., et al. Nanostructured toxins for the selective destruction of drug-resistant human CXCR4 colorectal cancer stem cells. 2020;320:96–104. doi: 10.1016/j.jconrel.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 86.Rioja-Blanco E., Arroyo-Solera I., Álamo P., Casanova I., Gallardo A., Unzueta U., et al. Mangues, ccr CR, CXCR4-targeted nanotoxins induce GSDME-dependent pyroptosis in head and neck squamous cell carcinoma. 2022;41(1):49. doi: 10.1186/s13046-022-02267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.S. Zhou, Q. Shang, J. Ji, Y.J.A.a.m. Luan, interfaces, A Nanoplatform to Amplify Apoptosis-to-Pyroptosis Immunotherapy via Immunomodulation of Myeloid-Derived Suppressor Cells, 13(40) (2021) 47407-47417. [DOI] [PubMed]
- 88.E. Ploetz, A. Zimpel, V. Cauda, D. Bauer, D. Lamb, C. Haisch, S. Zahler, A. Vollmar, S. Wuttke, H.J.A.m. Engelke, Metal-Organic Framework Nanoparticles Induce Pyroptosis in Cells Controlled by the Extracellular pH, 32(19) (2020) e1907267. [DOI] [PubMed]
- 89.Zeng Q., Ma X., Song Y., Chen Q., Jiao Q., Zhou L.J.T. Targeting regulated cell death in tumor nanomedicines. 2022;12(2):817–841. doi: 10.7150/thno.67932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen B., Yan Y., Yang Y., Cao G., Wang X., Wang Y., et al. A pyroptosis nanotuner for cancer therapy. 2022;17(7):788–798. doi: 10.1038/s41565-022-01125-0. [DOI] [PubMed] [Google Scholar]
- 91.Xia Y., Liu S., Li C., Ai Z., Shen W., Ren W., et al. Yang, disease, Discovery of a novel ferroptosis inducer-talaroconvolutin A-killing colorectal cancer cells in vitro and in vivo. 2020;11(11):988. doi: 10.1038/s41419-020-03194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fu J., Li T., Yang Y., Jiang L., Wang W., Fu L., et al. Activatable nanomedicine for overcoming hypoxia-induced resistance to chemotherapy and inhibiting tumor growth by inducing collaborative apoptosis and ferroptosis in solid tumors. 2021;268 doi: 10.1016/j.biomaterials.2020.120537. [DOI] [PubMed] [Google Scholar]
- 93.C. Zhang, X. Liu, S. Jin, Y. Chen, R.J.M.c. Guo, Ferroptosis in cancer therapy: a novel approach to reversing drug resistance, 21(1) (2022) 47. [DOI] [PMC free article] [PubMed]
- 94.Wang S., Li F., Qiao R., Hu X., Liao H., Chen L., et al. Ling, Arginine-Rich Manganese Silicate Nanobubbles as a Ferroptosis-Inducing Agent for Tumor-Targeted Theranostics. 2018;12(12):12380–12392. doi: 10.1021/acsnano.8b06399. [DOI] [PubMed] [Google Scholar]
- 95.Luo M., Wu L., Zhang K., Wang H., Zhang T., Gutierrez L., et al. Yang, differentiation, miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. 2018;25(8):1457–1472. doi: 10.1038/s41418-017-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao L., Zhou X., Xie F., Zhang L., Yan H., Huang J., et al. Ferroptosis in cancer and cancer immunotherapy. 2022;42(2):88–116. doi: 10.1002/cac2.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stockwell B., Friedmann Angeli J., Bayir H., Bush A., Conrad M., Dixon S., et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism. Redox Biology, and Disease. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.An Y., Zhu J., Liu F., Deng J., Meng X., Liu G., et al. Boosting the Ferroptotic Antitumor Efficacy via Site-Specific Amplification of Tailored Lipid Peroxidation. ACS Appl Mater Interfaces. 2019;11(33):29655–29666. doi: 10.1021/acsami.9b10954. [DOI] [PubMed] [Google Scholar]
- 99.Qian X., Zhang J., Gu Z., Chen Y.J.B. Nanocatalysts-augmented Fenton chemical reaction for nanocatalytic tumor therapy. 2019;211:1–13. doi: 10.1016/j.biomaterials.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 100.Liu T., Liu W., Zhang M., Yu W., Gao F., Li C., et al. Ferrous-Supply-Regeneration Nanoengineering for Cancer-Cell-Specific Ferroptosis in Combination with Imaging-Guided Photodynamic. Therapy. 2018;12(12):12181–12192. doi: 10.1021/acsnano.8b05860. [DOI] [PubMed] [Google Scholar]
- 101.Huang Y., Hsu J.C., Koo H., Cormode D.P. Repurposing ferumoxytol: Diagnostic and therapeutic applications of an FDA-approved nanoparticle. Theranostics. 2022;12(2):796–816. doi: 10.7150/thno.67375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trujillo-Alonso V., Pratt E., Zong H., Lara-Martinez A., Kaittanis C., Rabie M., et al. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. 2019;14(6):616–622. doi: 10.1038/s41565-019-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feng W., Shi W., Liu S., Liu H., Liu Y., Ge P., et al. Fe(III)-Shikonin Supramolecular Nanomedicine for Combined Therapy of Tumor via. Ferroptosis and Necroptosis. 2022;11(2):e2101926. doi: 10.1002/adhm.202101926. [DOI] [PubMed] [Google Scholar]
- 104.W. Ou, R. Mulik, A. Anwar, J. McDonald, X. He, I.J.F.r.b. Corbin, medicine, Low-density lipoprotein docosahexaenoic acid nanoparticles induce ferroptotic cell death in hepatocellular carcinoma, 112 (2017) 597-607. [DOI] [PMC free article] [PubMed]
- 105.Y. Liu, W.J.C.d. Gu, differentiation, p53 in ferroptosis regulation: the new weapon for the old guardian, 29(5) (2022) 895-910. [DOI] [PMC free article] [PubMed]
- 106.M. Huo, L. Wang, Y. Chen, J.J.N.c. Shi, Tumor-selective catalytic nanomedicine by nanocatalyst delivery, 8(1) (2017) 357. [DOI] [PMC free article] [PubMed]
- 107.Xue C., Li M., Zhao Y., Zhou J., Hu Y., Cai K., et al. Tumor microenvironment-activatable Fe-doxorubicin preloaded amorphous CaCO nanoformulation triggers ferroptosis in target tumor cells. 2020;6(18):eaax1346. doi: 10.1126/sciadv.aax1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun R., Ma W., Ling M., Tang C., Zhong M., Dai J., et al. Peng, pH-activated nanoplatform for visualized photodynamic and ferroptosis synergistic therapy of tumors. 2022;350:525–537. doi: 10.1016/j.jconrel.2022.08.050. [DOI] [PubMed] [Google Scholar]
- 109.R. Alzeibak, T. Mishchenko, N. Shilyagina, I. Balalaeva, M. Vedunova, D.J.J.f.i.o.c. Krysko, Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future, 9(1) (2021) e001926. [DOI] [PMC free article] [PubMed]
- 110.Luo Y., Niu G., Yi H., Li Q., Wu Z., Wang J., et al. Nanomedicine promotes ferroptosis to inhibit tumour proliferation in vivo. 2021;42 doi: 10.1016/j.redox.2021.101908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Luo M., Ye L., Chang R., Ye Y., Zhang Z., Liu C., et al. Multi-omics characterization of autophagy-related molecular features for therapeutic targeting of autophagy. Nat Commun. 2022;13(1):6345. doi: 10.1038/s41467-022-33946-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Y. Zhang, L. Zhang, J. Gao, L.J.A.o.c.r. Wen, Pro-Death or Pro-Survival: Contrasting Paradigms on Nanomaterial-Induced Autophagy and Exploitations for Cancer Therapy, 52(11) (2019) 3164-3176. [DOI] [PubMed]
- 113.M. Maiuri, E. Zalckvar, A. Kimchi, G.J.N.r.M.c.b. Kroemer, Self-eating and self-killing: crosstalk between autophagy and apoptosis, 8(9) (2007) 741-752. [DOI] [PubMed]
- 114.Dai E., Han L., Liu J., Xie Y., Kroemer G., Klionsky D., et al. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. 2020;16(11):2069–2083. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhong Z., Sanchez-Lopez E., Karin M.J.C. Autophagy. Inflammation, and Immunity: A Troika Governing Cancer and Its Treatment. 2016;166(2):288–298. doi: 10.1016/j.cell.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.S. Kaushik, A.J.N.r.M.c.b. Cuervo, The coming of age of chaperone-mediated autophagy, 19(6) (2018) 365-381. [DOI] [PMC free article] [PubMed]
- 117.A. Kimmelman, E.J.C.m. White, Autophagy and Tumor Metabolism, 25(5) (2017) 1037-1043. [DOI] [PMC free article] [PubMed]
- 118.Zabirnyk O., Yezhelyev M., Seleverstov O.J.A. Nanoparticles as a novel class of autophagy activators. 2007;3(3):278–281. doi: 10.4161/auto.3916. [DOI] [PubMed] [Google Scholar]
- 119.A. Martínez-Torres, H. Lorenzo-Anota, M. García-Juárez, D. Zarate-Triviño, C.J.I.j.o.n. Rodríguez-Padilla, Chitosan gold nanoparticles induce different ROS-dependent cell death modalities in leukemic cells, 14 (2019) 7173-7190. [DOI] [PMC free article] [PubMed]
- 120.Liu X., Gao P., Shi M., Chen Y., Pan W., Li N., et al. An autophagy-inhibitory MOF nanoreactor for tumor-targeted synergistic therapy. 2022;10(12):3088–3091. doi: 10.1039/d2bm00579d. [DOI] [PubMed] [Google Scholar]
- 121.Zhou T., Damsky W., Weizman O., McGeary M., Hartmann K., Rosen C., et al. IL-18BP is a secreted immune checkpoint and barrier to IL-18 immunotherapy. 2020;583(7817):609–614. doi: 10.1038/s41586-020-2422-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu L., Li H., Hu D., Wang Y., Shao W., Zhong J., et al. Insights into N6-methyladenosine and programmed cell death in cancer. 2022;21(1):32. doi: 10.1186/s12943-022-01508-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang F.L., Hou Y., Zhu M.H., Deng B., Zhao M., Zhu X.D., et al. Death Pathways of Cancer Cells Modulated by Surface Molecule Density on Gold Nanorods. Adv Sci. 2021;8(22):e2102666. doi: 10.1002/advs.202102666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bao W., Liu X., Lv Y., Lu G., Li F., Zhang F., et al. Nanolongan with Multiple On-Demand Conversions for Ferroptosis-Apoptosis Combined Anticancer. Therapy. 2019;13(1):260–273. doi: 10.1021/acsnano.8b05602. [DOI] [PubMed] [Google Scholar]
- 125.K. Nozaki, L. Li, E.J.A.r.o.i. Miao, Innate Sensors Trigger Regulated Cell Death to Combat Intracellular Infection, 40 (2022) 469-498. [DOI] [PMC free article] [PubMed]
- 126.Y. Zhang, X. Chen, C. Gueydan, J.J.C.r. Han, Plasma membrane changes during programmed cell deaths, 28(1) (2018) 9-21. [DOI] [PMC free article] [PubMed]
- 127.Y. Ma, J. Pitt, Q. Li, H.J.I.r. Yang, The renaissance of anti-neoplastic immunity from tumor cell demise, 280(1) (2017) 194-206. [DOI] [PubMed]
- 128.Zhang J., Sun X., Zhao X., Yang C., Shi M., Zhang B., et al. Combining immune checkpoint blockade with ATP-based immunogenic cell death amplifier for cancer chemo-immunotherapy. 2022;12(9):3694–3709. doi: 10.1016/j.apsb.2022.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.S. Gara, J. Lack, L. Zhang, E. Harris, M. Cam, E.J.N.c. Kebebew, Metastatic adrenocortical carcinoma displays higher mutation rate and tumor heterogeneity than primary tumors, 9(1) (2018) 4172. [DOI] [PMC free article] [PubMed]
- 130.Sun X., Zhang J., Xiu J., Zhao X., Yang C., Li D., et al. A phenolic based tumor-permeated nano-framework for immunogenic cell death induction combined with PD-L1 immune checkpoint blockade. 2022;10(14):3808–3822. doi: 10.1039/d2bm00455k. [DOI] [PubMed] [Google Scholar]
- 131.Hammerich L., Marron T., Upadhyay R., Svensson-Arvelund J., Dhainaut M., Hussein S., et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. 2019;25(5):814–824. doi: 10.1038/s41591-019-0410-x. [DOI] [PubMed] [Google Scholar]
- 132.Liu Y., Lu Y., Ning B., Su X., Yang B., Dong H., et al. Listeria monocytogenesIntravenous Delivery of Living Elicits Gasdmermin-Dependent Tumor Pyroptosis and Motivates Anti-Tumor Immune Response. 2022;16(3):4102–4115. doi: 10.1021/acsnano.1c09818. [DOI] [PubMed] [Google Scholar]
- 133.Y. Zhang, Y. Feng, Y. Huang, Y. Wang, L. Qiu, Y. Liu, S. Peng, R. Li, N. Kuang, Q. Shi, Y. Shi, Y. Chen, R. Joshi, Z. Wang, K. Yuan, W.J.F.i.i. Min, Tumor-Targeted Gene Silencing IDO Synergizes PTT-Induced Apoptosis and Enhances Anti-tumor Immunity, 11 (2020) 968. [DOI] [PMC free article] [PubMed]
- 134.Lee S., Lee C., Won J., Jang G., Lee J., Park S., et al. Enhancement of anticancer immunity by immunomodulation of apoptotic tumor cells using annexin A5 protein-labeled nanocarrier system. 2022;288 doi: 10.1016/j.biomaterials.2022.121677. [DOI] [PubMed] [Google Scholar]
- 135.Song W., Kuang J., Li C.X., Zhang M., Zheng D., Zeng X., et al. Enhanced immunotherapy based on photodynamic therapy for both primary and lung metastasis tumor eradication. ACS Nano. 2018;12(2):1978–1989. doi: 10.1021/acsnano.7b09112. [DOI] [PubMed] [Google Scholar]
- 136.Su X., Wang W., Cao Q., Zhang H., Liu B., Ling Y., et al. A Carbonic Anhydrase IX (CAIX)-Anchored Rhenium(I) Photosensitizer Evokes Pyroptosis for Enhanced Anti-Tumor. Immunity. 2022;61(8):e202115800. doi: 10.1002/anie.202115800. [DOI] [PubMed] [Google Scholar]
- 137.Zychlinsky A., Prevost M., Sansonetti P.J.N. Shigella flexneri induces apoptosis in infected macrophages. 1992;358(6382):167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 138.Jin X., Ma J., Liang X., Tang K., Liu Y., Yin X., et al. Pre-instillation of tumor microparticles enhances intravesical chemotherapy of nonmuscle-invasive bladder cancer through a lysosomal pathway. 2017;113:93–104. doi: 10.1016/j.biomaterials.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 139.Fan J., Deng R., Wang H., Liu X., Wang X., Qin R., et al. Epigenetics-based tumor cells pyroptosis for enhancing the immunological effect of chemotherapeutic nanocarriers. 2019;19(11):8049–8058. doi: 10.1021/acs.nanolett.9b03245. [DOI] [PubMed] [Google Scholar]
- 140.Liu FYY, Chen X, Wang Z, Liang X, Zhang T, Liu M, et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome J Sci Immunol J, 2020;5(43): eaax7969. [DOI] [PubMed]
- 141.Du T., Gao J., Li P., Wang Y., Qi Q., Liu X., et al. t medicine, Pyroptosis, metabolism, and tumor immune microenvironment. 2021;11(8):e492. doi: 10.1002/ctm2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang N., Liu C., Li Y., Huang D., Wu X., Kou X., et al. A cooperative nano-CRISPR scaffold potentiates immunotherapy via activation of tumour-intrinsic pyroptosis. Nat Commun. 2023;14(1):779. doi: 10.1038/s41467-023-36550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hu R., Dai C., Dai X., Dong C., Huang H., Song X., et al. Topology regulation of nanomedicine for autophagy-augmented ferroptosis and cancer immunotherapy. 2023;68(1):77–94. doi: 10.1016/j.scib.2022.12.030. [DOI] [PubMed] [Google Scholar]
- 144.Wang W., Green M., Choi J., Gijón M., Kennedy P., Johnson J., et al. CD8 T cells regulate tumour ferroptosis during cancer immunotherapy. 2019;569(7755):270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang Z., Zhu Y., Dong Z., Li W., Yang N., Wang X., et al. Tumor-killing nanoreactors fueled by tumor debris can enhance radiofrequency ablation therapy and boost antitumor immune responses. Nat Commun. 2021;12(1):4299. doi: 10.1038/s41467-021-24604-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Michaud M., Martins I., Sukkurwala A., Adjemian S., Ma Y., Pellegatti P., et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. 2011;334(6062):1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 147.Ruan S., Xie R., Qin L., Yu M., Xiao W., Hu C., et al. Aggregable nanoparticles-enabled chemotherapy and autophagy inhibition combined with anti-PD-L1 antibody for improved glioma treatment. Nano Lett. 2019;19(11):8318–8332. doi: 10.1021/acs.nanolett.9b03968. [DOI] [PubMed] [Google Scholar]
- 148.Pei Z., Chen S., Ding L., Liu J., Cui X., Li F., et al. Current perspectives and trend of nanomedicine in cancer: A review and bibliometric analysis. J Control Release. 2022;352:211–241. doi: 10.1016/j.jconrel.2022.10.023. [DOI] [PubMed] [Google Scholar]
- 149.Blanco E, Shen H, Ferrari MJNb. Principles of nanoparticle design for overcoming biological barriers to drug delivery 2015;33(9): 941-51. [DOI] [PMC free article] [PubMed]
- 150.Oroojalian F., Beygi M., Baradaran B., Mokhtarzadeh A., Shahbazi M.J.S. Immune cell membrane-coated biomimetic nanoparticles for targeted. Cancer Therapy. 2021;17(12):e2006484. doi: 10.1002/smll.202006484. [DOI] [PubMed] [Google Scholar]
- 151.Duan X, Chan C, Han W, Guo N, Weichselbaum R, Lin WJNc. Immunostimulatory nanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors 2019;10(1): 1899. [DOI] [PMC free article] [PubMed]
- 152.Gao S., Yang X., Xu J., Qiu N., Zhai G. Nanotechnology for boosting cancer immunotherapy and remodeling tumor microenvironment: the horizons in cancer treatment. ACS Nano. 2021;15(8):12567–12603. doi: 10.1021/acsnano.1c02103. [DOI] [PubMed] [Google Scholar]
- 153.Xia Y., Fu S., Ma Q., Liu Y., Zhang N. Application of nano-delivery systems in lymph nodes for tumor immunotherapy. Nano-micro letters. 2023;15(1):145. doi: 10.1007/s40820-023-01125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lei G, Zhuang L, Gan BJNrC. Targeting ferroptosis as a vulnerability in cancer 2022;22(7): 381-96. [DOI] [PMC free article] [PubMed]
- 155.Li Z., Lai X., Fu S., Ren L., Cai H., Zhang H., et al. Immunogenic cell death activates the tumor immune microenvironment to boost the immunotherapy efficiency. 2022;9(22):e2201734. doi: 10.1002/advs.202201734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Min Y., Roche K., Tian S., Eblan M., McKinnon K., Caster J., et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. 2017;12(9):877–882. doi: 10.1038/nnano.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Han W., Duan X., Ni K., Li Y., Chan C., Lin W. Co-delivery of dihydroartemisinin and pyropheophorbide-iron elicits ferroptosis to potentiate cancer. immunotherapy. 2022;280:121315. doi: 10.1016/j.biomaterials.2021.121315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xu N., Hu A., Pu X., Li J., Wang X., Wang J., et al. Fe(III)-Chelated Polydopamine Nanoparticles for Synergistic Tumor Therapies of Enhanced Photothermal Ablation and Antitumor Immune. Activation. 2022;14(14):15894–15910. doi: 10.1021/acsami.1c24066. [DOI] [PubMed] [Google Scholar]
- 159.Song R., Li T., Ye J., Sun F., Hou B., Saeed M., et al. Acidity-Activatable Dynamic Nanoparticles Boosting Ferroptotic Cell Death for Immunotherapy of. Cancer. 2021;33(31) doi: 10.1002/adma.202101155. [DOI] [PubMed] [Google Scholar]