Abstract
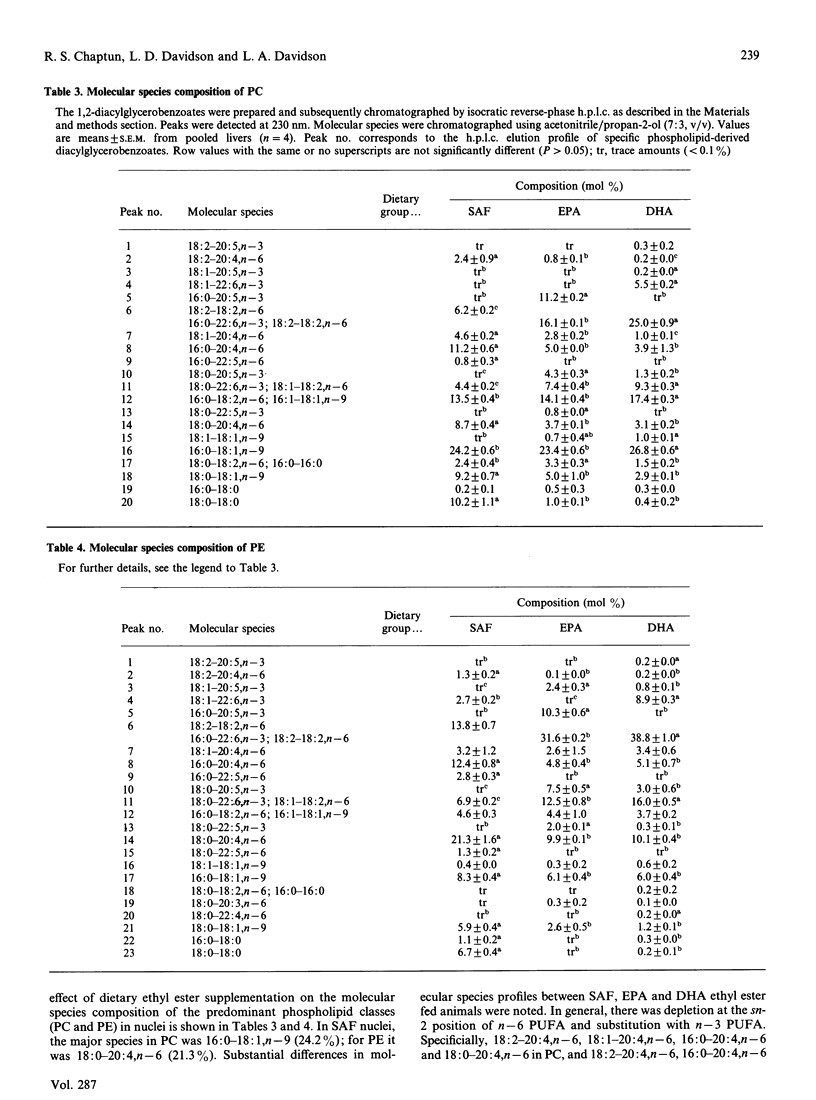
The effect of dietary eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) ethyl esters on the individual molecular species composition of phosphatidylcholine (PC) and phosphatidylethanolamine (PE) was determined in mouse liver nuclei. After a 10 day feeding period, there was a depletion of the sn-2 position of n-6 polyunsaturated fatty acids (PUFA) and substitution with n-3 PUFA. EPA feeding significantly increased (P less than 0.05) diacyl PC and PE 16:0-20:5, n-3, 16:0-22:6,n-3, 18:0-20:5,n-3 and 18:0-22:6,n-3 relative to control (safflower oil ethyl ester fed) animals. In comparison, DHA feeding significantly increased (P less than 0.05) 22:6 n-3-containing species, specifically 18:1-22:6,n-3, 16:0-22:6,n-3 and 18:0-22:6,n-3 in PC, and 18:1-22:6,n-3, 16:0-22:6,n-3 and 18:0-22:6,n-3 in PE. In addition, the presence of 18:0-20:5,n-3 PC in the nuclei of DHA-fed rats and of 18:2-20:5,n-3, 18:1-20:5,n-3 and 18:0-20:5,n-3 in nuclear PE indicate that incorporation of DHA retroconversion (22:6,n-3-->20:5,n-3) products. These results indicate both EPA and DHA are extensively incorporated into nuclear phospholipids, and therefore could potentially influence gene function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akoh C. C., Chapkin R. S. Composition of mouse peritoneal macrophage phospholipid molecular species. Lipids. 1990 Oct;25(10):613–617. doi: 10.1007/BF02536011. [DOI] [PubMed] [Google Scholar]
- Cave W. T., Jr Dietary n-3 (omega-3) polyunsaturated fatty acid effects on animal tumorigenesis. FASEB J. 1991 May;5(8):2160–2166. doi: 10.1096/fasebj.5.8.1673664. [DOI] [PubMed] [Google Scholar]
- Chapkin R. S., Akoh C. C., Miller C. C. Influence of dietary n-3 fatty acids on macrophage glycerophospholipid molecular species and peptidoleukotriene synthesis. J Lipid Res. 1991 Jul;32(7):1205–1213. [PubMed] [Google Scholar]
- Chapkin R. S., Carmichael S. L. Effect of dietary blackcurrant seed oil on mouse macrophage subclasses of choline and ethanolamine glycerophospholipids. J Nutr. 1990 Aug;120(8):825–830. doi: 10.1093/jn/120.8.825. [DOI] [PubMed] [Google Scholar]
- Cocco L., Gilmour R. S., Ognibene A., Letcher A. J., Manzoli F. A., Irvine R. F. Synthesis of polyphosphoinositides in nuclei of Friend cells. Evidence for polyphosphoinositide metabolism inside the nucleus which changes with cell differentiation. Biochem J. 1987 Dec 15;248(3):765–770. doi: 10.1042/bj2480765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes G., Venkatraman J., Khare A., Horbach G. J., Friedrichs W. Modulation of gene expression in autoimmune disease and aging by food restriction and dietary lipids. Proc Soc Exp Biol Med. 1990 Jan;193(1):16–22. doi: 10.3181/00379727-193-42983. [DOI] [PubMed] [Google Scholar]
- Fischer S., Vischer A., Preac-Mursic V., Weber P. C. Dietary docosahexaenoic acid is retroconverted in man to eicosapentaenoic acid, which can be quickly transformed to prostaglandin I3. Prostaglandins. 1987 Sep;34(3):367–375. doi: 10.1016/0090-6980(87)90082-7. [DOI] [PubMed] [Google Scholar]
- Grønn M., Christensen E., Hagve T. A., Christophersen B. O. Peroxisomal retroconversion of docosahexaenoic acid (22:6(n-3)) to eicosapentaenoic acid (20:5(n-3)) studied in isolated rat liver cells. Biochim Biophys Acta. 1991 Jan 4;1081(1):85–91. doi: 10.1016/0005-2760(91)90254-f. [DOI] [PubMed] [Google Scholar]
- Herlan G., Giese G., Wunderlich F. Influence of nuclear membrane lipid fluidity on nuclear RNA release. Exp Cell Res. 1979 Feb;118(2):305–309. doi: 10.1016/0014-4827(79)90155-1. [DOI] [PubMed] [Google Scholar]
- Holub B. J., Kuksis A. Metabolism of molecular species of diacylglycerophospholipids. Adv Lipid Res. 1978;16:1–125. doi: 10.1016/b978-0-12-024916-9.50007-x. [DOI] [PubMed] [Google Scholar]
- Iritani N., Fukuda H., Matsumura Y. Effects of corn oil- and fish oil-supplemented diets on phospholipid fatty acid composition of rat liver nuclei. Biochim Biophys Acta. 1988 Nov 25;963(2):224–230. doi: 10.1016/0005-2760(88)90284-6. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Tamiya-Koizumi K., Kuriki H., Yoshida S., Kojima K. Growth-associated changes in fatty acid compositions of nuclear phospholipids of liver cells. Biochim Biophys Acta. 1991 Jun 19;1084(1):53–59. doi: 10.1016/0005-2760(91)90055-m. [DOI] [PubMed] [Google Scholar]
- Kaizer L., Boyd N. F., Kriukov V., Tritchler D. Fish consumption and breast cancer risk: an ecological study. Nutr Cancer. 1989;12(1):61–68. doi: 10.1080/01635588909514002. [DOI] [PubMed] [Google Scholar]
- Manzoli F. A., Martelli A. M., Capitani S., Maraldi N. M., Rizzoli R., Barnabei O., Cocco L. Nuclear polyphosphoinositides during cell growth and differentiation. Adv Enzyme Regul. 1989;28:25–34. doi: 10.1016/0065-2571(89)90061-7. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Hawthorne J. N. The site of diphosphoinositide synthesis in rat liver. Biochem Biophys Res Commun. 1965 Nov 22;21(4):333–338. doi: 10.1016/0006-291x(65)90198-1. [DOI] [PubMed] [Google Scholar]
- Nicotera P., McConkey D. J., Jones D. P., Orrenius S. ATP stimulates Ca2+ uptake and increases the free Ca2+ concentration in isolated rat liver nuclei. Proc Natl Acad Sci U S A. 1989 Jan;86(2):453–457. doi: 10.1073/pnas.86.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- SMITH L. Spectrophotometric assay of cytochrome c oxidase. Methods Biochem Anal. 1955;2:427–434. doi: 10.1002/9780470110188.ch13. [DOI] [PubMed] [Google Scholar]
- Sylvia V. L., Joe C. O., Norman J. O., Curtin G. M., Busbee D. L. Phosphatidylinositol-dependent activation of DNA polymerase alpha. Biochem Biophys Res Commun. 1986 Mar 28;135(3):880–885. doi: 10.1016/0006-291x(86)91010-7. [DOI] [PubMed] [Google Scholar]
- Upreti G. C., deAntueno R. J., Wood R. Membrane lipids of hepatic tissue. I. Neutral lipids from subcellular fractions of liver and hepatoma 7288CTC. J Natl Cancer Inst. 1983 Mar;70(3):559–566. [PubMed] [Google Scholar]
- Upreti G. C., deAntueno R. J., Wood R. Membrane lipids of hepatic tissue. II. Phospholipids from subcellular fractions of liver and hepatoma 7288CTC. J Natl Cancer Inst. 1983 Mar;70(3):567–573. [PubMed] [Google Scholar]
- Venkatraman J. T., Clandinin M. T. Ribonucleic acid efflux from isolated mouse liver nuclei is altered by diet and genotypically determined change in nuclear envelope composition. Biochim Biophys Acta. 1988 May 9;940(1):33–42. doi: 10.1016/0005-2736(88)90005-3. [DOI] [PubMed] [Google Scholar]
- Venkatraman J. T., Lefebvre Y. A., Clandinin M. T. Diet fat alters the structure and function of the nuclear envelope: modulation of membrane fatty acid composition, NTPase activity and binding of triiodothyronine. Biochem Biophys Res Commun. 1986 Mar 13;135(2):655–661. doi: 10.1016/0006-291x(86)90043-4. [DOI] [PubMed] [Google Scholar]
- Voss A., Reinhart M., Sankarappa S., Sprecher H. The metabolism of 7,10,13,16,19-docosapentaenoic acid to 4,7,10,13,16,19-docosahexaenoic acid in rat liver is independent of a 4-desaturase. J Biol Chem. 1991 Oct 25;266(30):19995–20000. [PubMed] [Google Scholar]



