Abstract
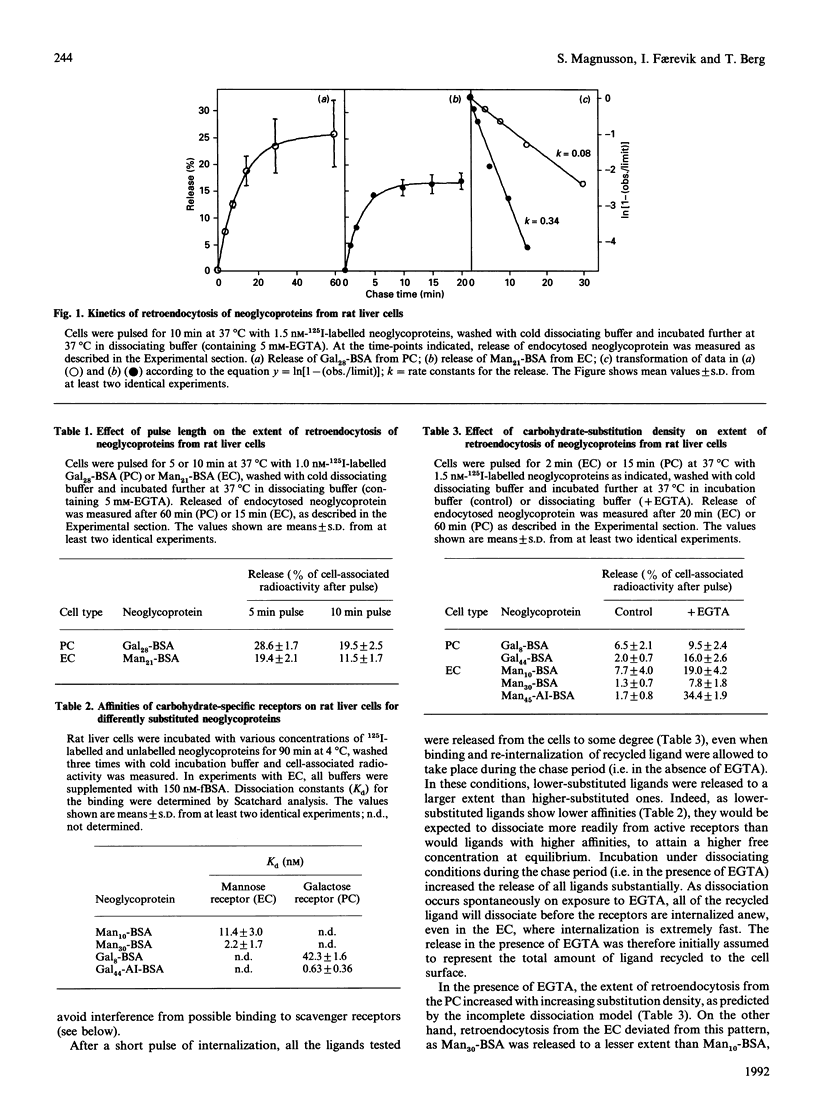
After receptor-mediated endocytosis, internalized ligands may be recycled to the cell surface instead of being routed to lysosomes for degradation, a process termed retroendocytosis. We have investigated the kinetics and extent of retroendocytosis of neoglycoproteins after internalization via two carbohydrate-specific receptors in rat liver cells: galactose receptors in parenchymal cells (PC) and mannose receptors in sinusoidal endothelial cells (EC). Retroendocytosis in both cell types occurred with first-order kinetics, and the rate of recycling of internalized ligands was about 4 times higher in EC than in PC. As the length of the internalization pulse was increased, the extent of subsequent retroendocytosis decreased, indicating that retroendocytosis takes place from a relatively early stage in the endocytic pathway. Furthermore, as the degree of carbohydrate substitution of the neoglycoprotein ligands increased, the affinities of the receptors for the ligands and the extent of ligand retroendocytosis increased. In the EC, the relationship between degree of substitution and extent of retroendocytosis was not immediately apparent, as some of the neoglycoprotein ligands used may also bind to and be internalized by scavenger receptors on the EC, causing a decreased apparent retroendocytosis. However, when this interaction was inhibited, this relationship was restored. We conclude that retroendocytosis mainly occurs because of incomplete dissociation of ligands from receptors before receptor recycling to the cell surface and that the affinities of a receptor for its ligand at the cell surface and in the endosomal environment are major factors in determining the extent of retroendocytosis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aulinskas T. H., Oram J. F., Bierman E. L., Coetzee G. A., Gevers W., van der Westhuyzen D. R. Retro-endocytosis of low density lipoprotein by cultured human skin fibroblasts. Arteriosclerosis. 1985 Jan-Feb;5(1):45–54. doi: 10.1161/01.atv.5.1.45. [DOI] [PubMed] [Google Scholar]
- Aulinskas T. H., van der Westhuyzen D. R., Bierman E. L., Gevers W., Coetzee G. A. Retro-endocytosis of low density lipoprotein by cultured bovine aortic smooth muscle cells. Biochim Biophys Acta. 1981 May 22;664(2):255–265. doi: 10.1016/0005-2760(81)90048-5. [DOI] [PubMed] [Google Scholar]
- Blomhoff R., Green M. H., Green J. B., Berg T., Norum K. R. Vitamin A metabolism: new perspectives on absorption, transport, and storage. Physiol Rev. 1991 Oct;71(4):951–990. doi: 10.1152/physrev.1991.71.4.951. [DOI] [PubMed] [Google Scholar]
- Braciale T. J., Braciale V. L. Antigen presentation: structural themes and functional variations. Immunol Today. 1991 Apr;12(4):124–129. doi: 10.1016/0167-5699(91)90096-C. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Casanova J. E., Breitfeld P. P., Ross S. A., Mostov K. E. Phosphorylation of the polymeric immunoglobulin receptor required for its efficient transcytosis. Science. 1990 May 11;248(4956):742–745. doi: 10.1126/science.2110383. [DOI] [PubMed] [Google Scholar]
- Connolly D. T., Townsend R. R., Kawaguchi K., Bell W. R., Lee Y. C. Binding and endocytosis of cluster glycosides by rabbit hepatocytes. Evidence for a short-circuit pathway that does not lead to degradation. J Biol Chem. 1982 Jan 25;257(2):939–945. [PubMed] [Google Scholar]
- Connolly D. T., Townsend R. R., Kawaguchi K., Hobish M. K., Bell W. R., Lee Y. C. Binding and endocytosis of glycoproteins and neoglycoproteins by isolated rabbit hepatocytes. Biochem J. 1983 Aug 15;214(2):421–431. doi: 10.1042/bj2140421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D. C., Tsao T., Duckworth W. C., Mahoney M. J., Rabkin R. Retroendocytosis of insulin in a cultured kidney epithelial cell line. Am J Physiol. 1989 Aug;257(2 Pt 1):C190–C196. doi: 10.1152/ajpcell.1989.257.2.C190. [DOI] [PubMed] [Google Scholar]
- Diment S., Martin K. J., Stahl P. D. Cleavage of parathyroid hormone in macrophage endosomes illustrates a novel pathway for intracellular processing of proteins. J Biol Chem. 1989 Aug 15;264(23):13403–13406. [PubMed] [Google Scholar]
- Dunn K. W., McGraw T. E., Maxfield F. R. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J Cell Biol. 1989 Dec;109(6 Pt 2):3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskild W., Kindberg G. M., Smedsrod B., Blomhoff R., Norum K. R., Berg T. Intracellular transport of formaldehyde-treated serum albumin in liver endothelial cells after uptake via scavenger receptors. Biochem J. 1989 Mar 1;258(2):511–520. doi: 10.1042/bj2580511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan P., St Clair R. W. Retroendocytosis of low density lipoprotein. Effect of lysosomal inhibitors on the release of undegraded 125I-low density lipoprotein of altered composition from skin fibroblasts in culture. J Biol Chem. 1984 Feb 10;259(3):1703–1713. [PubMed] [Google Scholar]
- Gruenberg J., Howell K. E. Membrane traffic in endocytosis: insights from cell-free assays. Annu Rev Cell Biol. 1989;5:453–481. doi: 10.1146/annurev.cb.05.110189.002321. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., McKanna J. A., Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J Cell Biol. 1979 May;81(2):382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe C. A., Lee Y. C. The binding and processing of mannose-bovine serum albumin derivatives by rabbit alveolar macrophages. Effect of the sugar density. J Biol Chem. 1983 Dec 10;258(23):14193–14199. [PubMed] [Google Scholar]
- Jansen R. W., Molema G., Ching T. L., Oosting R., Harms G., Moolenaar F., Hardonk M. J., Meijer D. K. Hepatic endocytosis of various types of mannose-terminated albumins. What is important, sugar recognition, net charge, or the combination of these features. J Biol Chem. 1991 Feb 15;266(5):3343–3348. [PubMed] [Google Scholar]
- Jialal I., King G. L., Buchwald S., Kahn C. R., Crettaz M. Processing of insulin by bovine endothelial cells in culture. Internalization without degradation. Diabetes. 1984 Aug;33(8):794–800. doi: 10.2337/diab.33.8.794. [DOI] [PubMed] [Google Scholar]
- Kindberg G. M., Magnusson S., Berg T., Smedsrød B. Receptor-mediated endocytosis of ovalbumin by two carbohydrate-specific receptors in rat liver cells. The intracellular transport of ovalbumin to lysosomes is faster in liver endothelial cells than in parenchymal cells. Biochem J. 1990 Aug 15;270(1):197–203. doi: 10.1042/bj2700197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. C., Stowell C. P., Krantz M. J. 2-Imino-2-methoxyethyl 1-thioglycosides: new reagents for attaching sugars to proteins. Biochemistry. 1976 Sep 7;15(18):3956–3963. doi: 10.1021/bi00663a008. [DOI] [PubMed] [Google Scholar]
- Levy J. R., Olefsky J. M. Retroendocytosis of insulin in rat adipocytes. Endocrinology. 1986 Aug;119(2):572–579. doi: 10.1210/endo-119-2-572. [DOI] [PubMed] [Google Scholar]
- MEGO J. L., MCQUEEN J. D. THE UPTAKE AND DEGRADATION OF INJECTED LABELED PROTEINS BY MOUSE-LIVER PARTICLES. Biochim Biophys Acta. 1965 Apr 12;100:136–143. doi: 10.1016/0304-4165(65)90436-8. [DOI] [PubMed] [Google Scholar]
- Magnusson S., Berg T. Extremely rapid endocytosis mediated by the mannose receptor of sinusoidal endothelial rat liver cells. Biochem J. 1989 Feb 1;257(3):651–656. doi: 10.1042/bj2570651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson S., Berg T., Turpin E., Frénoy J. P. Interactions of ricin with sinusoidal endothelial rat liver cells. Different involvement of two distinct carbohydrate-specific mechanisms in surface binding and internalization. Biochem J. 1991 Aug 1;277(Pt 3):855–861. doi: 10.1042/bj2770855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S. Dual pathways for the intracellular processing of insulin. Relationship between retroendocytosis of intact hormone and the recycling of insulin receptors. J Biol Chem. 1985 Nov 5;260(25):13524–13531. [PubMed] [Google Scholar]
- McIntosh D., Timar J., Davies A. J. The intracellular movement and cycling of ricin. Eur J Cell Biol. 1990 Jun;52(1):77–86. [PubMed] [Google Scholar]
- Monsigny M., Roche A. C., Midoux P. Uptake of neoglycoproteins via membrane lectin(s) of L1210 cells evidenced by quantitative flow cytofluorometry and drug targeting. Biol Cell. 1984;51(2):187–196. doi: 10.1111/j.1768-322x.1984.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Redshaw M. R., Lynch S. S. An improved method for the preparation of iodinated antigens for radioimmunoassay. J Endocrinol. 1974 Mar;60(3):527–528. doi: 10.1677/joe.0.0600527. [DOI] [PubMed] [Google Scholar]
- Rodman J. S., Mercer R. W., Stahl P. D. Endocytosis and transcytosis. Curr Opin Cell Biol. 1990 Aug;2(4):664–672. doi: 10.1016/0955-0674(90)90108-q. [DOI] [PubMed] [Google Scholar]
- Roupas P., Herington A. C. Processing of growth hormone by rat adipocytes in primary culture: differentiation between release of intact hormone and degradative processing. Endocrinology. 1987 Oct;121(4):1521–1530. doi: 10.1210/endo-121-4-1521. [DOI] [PubMed] [Google Scholar]
- Schmitz G., Robenek H., Lohmann U., Assmann G. Interaction of high density lipoproteins with cholesteryl ester-laden macrophages: biochemical and morphological characterization of cell surface receptor binding, endocytosis and resecretion of high density lipoproteins by macrophages. EMBO J. 1985 Mar;4(3):613–622. doi: 10.1002/j.1460-2075.1985.tb03674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C. F., Jr, Schwartz A. L. Cellular pathways of galactose-terminal ligand movement in a cloned human hepatoma cell line. Mol Pharmacol. 1984 Nov;26(3):509–519. [PubMed] [Google Scholar]
- Tietze C., Schlesinger P., Stahl P. Mannose-specific endocytosis receptor of alveolar macrophages: demonstration of two functionally distinct intracellular pools of receptor and their roles in receptor recycling. J Cell Biol. 1982 Feb;92(2):417–424. doi: 10.1083/jcb.92.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolleshaug H., Chindemi P. A., Regoeczi E. Diacytosis of human asialotransferrin type 3 by isolated rat hepatocytes. J Biol Chem. 1981 Jul 10;256(13):6526–6528. [PubMed] [Google Scholar]
- Weigel P. H., Clarke B. L., Oka J. A. The hepatic galactosyl receptor system: two different ligand dissociation pathways are mediated by distinct receptor populations. Biochem Biophys Res Commun. 1986 Oct 15;140(1):43–50. doi: 10.1016/0006-291x(86)91055-7. [DOI] [PubMed] [Google Scholar]
- Weigel P. H., Oka J. A. Recycling of the hepatic asialoglycoprotein receptor in isolated rat hepatocytes. Receptor-ligand complexes in an intracellular slowly dissociating pool return to the cell surface prior to dissociation. J Biol Chem. 1984 Jan 25;259(2):1150–1154. [PubMed] [Google Scholar]
- Weigel P. H., Oka J. A. The surface content of asialoglycoprotein receptors on isolated hepatocytes is reversibly modulated by changes in temperature. J Biol Chem. 1983 Apr 25;258(8):5089–5094. [PubMed] [Google Scholar]


