ABSTRACT
The postprandial glycemic response is an important metabolic health factor, which, from laboratory studies, is known to change from low to high over the course of the day, and from which negative health outcomes have been linked to nightly eating. We applied interstitial continuous glucose monitoring to examine the glycemic response to a standardized carbohydrate-rich snack (198 kcal) across the day in a real-life setting. Twenty-four healthy participants (12 men, 12 women, 27–61 y old) consumed the snack nine times during 6 d in a crossover design, altering the time of consumption between morning, afternoon and evening. The snack was consumed in the participant’s own environment with a preceding fast of at least 2.5 h between their customary main meals and practices. Linear mixed models were used with fixed effect of timing, and participant as random effect, to assess incremental area under the curve, peak value and time-to-peak of the glycemic response. Overall, the highest glycemic excursions were observed in the morning, while a more dampened but prolonged response was observed in the evening. These findings do not concur with previously published laboratory studies. This implies that results obtained under controlled experimental conditions in laboratories cannot be generalized directly to predict chrononutritional effects on the glycemic response in healthy individuals and their daily routines.
KEYWORDS: Postprandial glycemic response, real-life setting, continuous glucose monitoring, chrononutrition
Introduction
Proper control of blood glucose is essential to one’s health. For example, an abnormal postprandial glycemic response is a major risk factor for developing type 2 diabetes mellitus (DM2) (Augustin et al. 2015; Gallwitz 2009; Torquati et al. 2018). Postprandial glycemic response is defined as the change in blood glucose concentration in response to consumption of a carbohydrate-containing meal (Augustin et al. 2015). Higher and/or prolonged glycemic excursions are considered to have a negative effect on health due to resulting increase in inflammation, endothelial dysfunction and oxidative stress (Gallwitz 2009).
The postprandial glycemic response is largely dependent on the amount and type of food ingested (Wheeler and Pi-Sunyer 2008) with large interpersonal variability in identical meals (Zeevi et al. 2015). However, life on earth evolved in an environment of a 24-h rhythm of night and day. As a result, many species developed a circadian molecular clock enabling them to anticipate the changes in conditions and opportunities coinciding with the continuous alternations of light and dark. Present in every cell, these circadian clocks are entrained by external cues and internal hormones, preparing the body for its daily (in)activities through modulation of the physiological functions fitting the sleep/wake cycle of the organism. These include metabolic processes regarding food consumption and digestion, typically occurring during the wake period, and the predominant fasting state during the sleep period of the cycle (Stenvers et al. 2019). Decades of research indicate that the timing of consumption affects the glycemic response as well. Several intervention studies in healthy adults have shown that eating at night or late in the evening was associated with lower glucose tolerance and reduced insulin sensitivity, resulting in higher postprandial glycemic responses in the late hours (Al-Naimi et al. 2004; Biston et al. 1996; Bo et al. 2017; Gibbs et al. 2014; Gil-Lozano et al. 2016; Grant et al. 2017; Leung et al. 2019; Morris et al. 2015; Owens et al. 1996). These findings support the concept of chrononutrition, which builds on the hypothesis that our endogenous circadian system tightly interacts with metabolic functions (Kessler and Pivovarova-Ramich 2019). The functions involved include digestion, insulin production, insulin sensitivity and glucose tolerance which are all related to glucose metabolism and thus may affect the glycemic response. While this knowledge may be of some benefit to persons eating during normal habitual circadian schedules, the impact may be even larger for those who are active and eat during the night (Morris et al. 2015). Research shows that night and shift workers have an increased risk of developing DM2 and cardiovascular disease (CVD) (Proper et al. 2016; Torquati et al. 2018; Wang et al. 2011; Wu et al. 2022).
However, intervention studies on chrononutrition were carried out in strictly controlled laboratory settings, such as conditioning through standard meals, fasting periods and supine positions during the recorded glycemic response of the assigned oral intake. Whilst this may have been to achieve comparable conditions between different mealtimes and may have been required because of experimental restrictions, such as obtaining regular sequential blood samples, these environments do not reflect natural situations. Interstitial continuous glucose monitoring (CGM) devices enable measurements in real-life settings, and the practical restrictions associated with intravenous blood sampling no longer exist. This provides research opportunities towards truly applicable advice on dietary control of postprandial glycemic excursions.
As a first step, and prior to monitoring glycemic responses in shift workers during various shifts, this study aimed to investigate the effect of snack timing on the glycemic response in healthy individuals in a real-life setting for the first time. Therefore, the following research question was formulated: “What are the differences between glycemic responses in the morning, afternoon and evening in healthy adults after consuming a standardized carbohydrate-rich snack between their customary main meals and practices?” A straightforward interpretation of the results collected under strictly controlled laboratory settings might lead to the hypothesis that the postprandial glycemic response to a standard snack in a real-life setting will be more extensive in the evening than at the start of the day.
Materials and methods
This study was a randomized, three-way, crossover trial conducted in The Netherlands between 15 February and 24 February 2021, or 8 March and 17 March 2021. The study took place during Central European Time (CET), i.e. prior to daylight savings time. Several governmental Covid-19 pandemic measures were in place, such as a curfew from 21:00h to 04:30h, and strong recommendations to work from home and to limit social visits to one person per day.
This study was approved by the Medical Ethical Committee Utrecht, and all participants provided written, informed consent prior to the eligibility interview. The study was registered as GLUCOZOND at the Dutch Trial Registry (www.trialregister.nl; NL9113) on 8 December 2020.
Study population
Internal advertisements were placed at the National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands to recruit employees and/or their relatives. Interested volunteers were sent a document with all relevant information regarding study procedures. A selection of respondents were invited for an online eligibility interview. Respondents were considered eligible if they were aged between 18 and 65 y, had a BMI between 18.5 and 30 kg/m2 (based on self-reported height/weight during the interview) and did not report any health problems. Exclusion criteria comprised analphabetism; being unable to provide informed consent; not having a general practitioner; using medication that might affect glucose metabolism (e.g. beta-blockers); suffering from any metabolic, hormonal (e.g. diabetes mellitus) or medical condition that may obstruct adherence to the study protocol; being pregnant/lactating or having the intention to become pregnant during the study period; smoking; having difficulties with swallowing; suffering from a delayed gastric emptying; suffering from food allergies related to the test product (e.g. gluten intolerance) or contact allergies related to plasters and/or performing a physically demanding occupation.
Eligible volunteers were invited to perform an oral glucose tolerance test at any of the available diagnostic centers of Saltro, Unilabs, The Netherlands. Blood samples were drawn in a fasted state and 2 h after ingestion of a 75 g glucose load. When a state of (pre)diabetes was observed (fasted glucose ≥5.6 mmol/L and/or 2 h glucose ≥ 7.8 mmol/L) (American Diabetes Association 2020), volunteers were excluded.
Study procedures
Participants were monitored during a 10-d period starting on a Monday (Figure 1). The first day was considered a lead-in day at which participants followed their usual life-style pattern. Subsequently, six test days followed during which the participants ate at scheduled times (see Study protocol), limited exercise and refrained from alcohol and high-fat foods (days 2, 3, 4, 5, 8 and 9). Test days were by design scheduled on workdays to facilitate adherence to the study protocol (with the assumption that the study protocol is easier to adhere to on a customary workday, than during the weekend) and to facilitate a more uniform daily schedule between test days.
Figure 1.
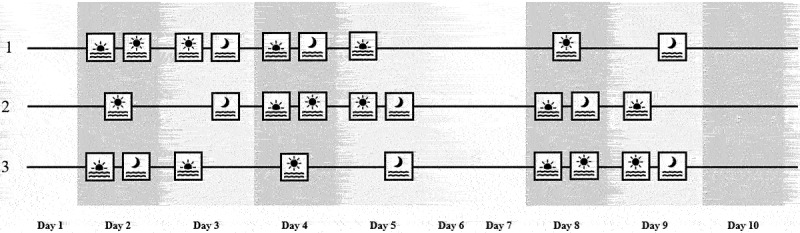
Schematic snack consumption timing across the three test sequences. Twenty four healthy adults (12 men, 12 women) were assigned to one of the test sequences (1, 2 or 3). The study lasted 10 days, of which on days 1, 6 and 7 participants followed their usual lifestyle pattern, and where day 1 was used as a lead-in day for the CGM. Days 2, 3, 4, 5, 8 and 9 were test days at which participants ate breakfast, lunch and dinner of their own choice at preset times and between these main meals either consumed a standard snack or remained fasted. Day 10 was an optional test day and was used in case a previous test day did not run according to protocol, otherwise participants could follow their usual lifestyle pattern on day 10. Test days were deliberately set on week days to facilitate adherence to the study protocol, reserving days 6 and 7 for the weekend. Consumption of a standard snack is indicated with an icon representing the preset time of consumption, either being morning ( ), afternoon (
), afternoon ( ), and/or evening (
), and/or evening ( ). In this study, “morning” implied that the snack was consumed between breakfast and lunch, “afternoon” implied consumption between lunch and dinner and “evening” implied consumption after dinner.
). In this study, “morning” implied that the snack was consumed between breakfast and lunch, “afternoon” implied consumption between lunch and dinner and “evening” implied consumption after dinner.
On days 6 and 7, in the weekend, participants followed their usual lifestyle pattern and were still monitored. If one of the test days was not conducted according to the protocol (determined in consultation with one of the researchers), that test day was repeated correctly at day 10. In case all six test days were conducted properly, participants were free to follow their usual lifestyle pattern on day 10.
Participants visited the research center on day 1 for a 1-h study intake during which height and weight were measured, instructions were given, and the required testing supplies, products and devices were provided. Additionally, a continuous glucose monitor (CGM) was fitted to the participant’s upper arm of choice and worn during the 10-d study period. After the intake session, participants left the research center and continued the study protocol themselves, alongside their usual lifestyle pattern, simulating a real-life setting. During the study, researchers were always available for participants by phone in case issues arose. On day 11, participants removed the CGM and all materials were returned to the research center by mail. Afterwards, participants filled out an evaluation survey in which the acceptability of the study protocol and the wearables used were assessed.
Study protocol
On test days, participants had breakfast, lunch and dinner of their own choice at preset times. Apart from the main meals, participants remained fasted, except for the consumption of the test product (consumption of non-caloric beverages, such as water, coffee or tea, was always allowed). The test product was a single-packed gingerbread bar (Snelle Jelle Kruidkoek®, 65 g) containing 198 kcal, 0.7 g fat, of which 0.1 g saturated fat, 45.3 g carbohydrates, of which 26.6 g sugars, 2.3 g dietary fibers and 1.5 g protein per unit. The test product will hereon in be referred to as “snack”.
The snack was consumed nine times by every participant in a crossover design. The timing of the snack alternated between morning, afternoon and evening, with a maximum of two snacks per test day. In this manner, every participant consumed the snack three times in the morning, three times in the afternoon and three times in the evening. Participants were assigned to one of the test sequences (1, 2 or 3) (see Figure 1 for a schematic overview). In this study, ”morning” implied that the snack was consumed between breakfast and lunch, ”afternoon” implied consumption between lunch and dinner and ”evening” implied consumption after dinner.
To align the physical nutritional state during snack consumptions over the day, snack consumption was scheduled 2.5 h after breakfast and lunch and 3 h after dinner. The following meal was planned at least 2 h after snack consumption. For convenience, participants were provided a meal schedule summarizing the timing of breakfast, lunch, dinner and snacks on test days. The timing of meals was personalized to the participant’s regular eating times and thus could differ per participant. Consequently, the preset timing of snack consumption could vary between participants (morning snack between 08:45h and 12:00h, afternoon snack between 14:30h and 16:30h and evening snack between 20:00h and 22:15h).
Randomization
Participants were randomly assigned to one of the three test sequences in an approximately 1:1:1 ratio in blocks within age strata (18–35 y, 36–50 y and 51–65 y) and gender, aiming to produce an equal number of participants per test sequence, with similar distribution of age and gender. This randomization process was carried out by one of the researchers.
Measurements
Glycemic response
Glycemic response to snack consumption was measured by the Dexcom G6 CGM System (Dexcom, USA). The CGM sensor measured and reported interstitial glucose (mmol/L) percutaneously every 5 min until its removal on day 11. The CGM transmitter, attached to the CGM sensor, sent the measured values to a compatible smartphone which the participants carried with them. Using the Dexcom Clarity reporting software, glucose level data were uploaded from these devices to an external server and accessed by the researchers.
Activity
Over the entire 10-d period (except for the night and when showering/bathing), participants wore a triaxial accelerometer (ActiGraph wGT3X, ActiGraph, USA) around their waist to record activity (VM3counts/min). Accessory software ActiLife 6.13.4 was used to access the data.
Food consumption
A smartphone application (DitEetIk!®, beta version 1.1.122, developed by RIVM) was used by the participants to record their food consumption in a food diary. For foods with barcodes on the food package, the barcode could be scanned, and alternatively text searching of consumed food could be applied. Participants were asked to log everything they either ate or drank, its quantity, the way of preparing, the eating occasion (breakfast, lunch, dinner or in between meals) and time of consumption, with increments of 5 min. The meal schedule provided was intended as a guideline to reflect the small differentiation of food consumption times, which is inevitable in a real-life setting. Participants were instructed to log the exact starting times of snack and meal consumption in the app. This data was downloaded from the backend database of the application. By use of Statistical Analysis System (SAS 9.3) software, all reported foods were linked to the nutrient composition of the corresponding common food items in the NEVO database (RIVM 2019), the nutrient database for the Netherlands. First, nutritional intakes (kcal, carbohydrate, sugar, fiber, fat, protein) for separate food items were assessed by multiplying nutrient levels × amount consumed. Thereafter, nutrient intakes for each meal were calculated by summing the nutrient intakes of all foods consumed in that meal. Single food entries over 1000 g or 1000 ml were considered not realistic and excluded from summary data calculations (reducing the total of 5480 food entries by 8 (1.5‰)).
Study logbook
Participants kept a study log, either online or on paper, in which they recorded the hours during which they wore the accelerometer, every (type of) activity performed, and whether test days ran according to protocol.
Demographics
Demographic data, such as age and gender, were collected during the study eligibility interview.
Statistical analyses
R statistics software version 4.0.2 (R Core Team 2020) was used for analysis. Independent sample t-tests were used to examine differences between gender for age, BMI, fasted glucose and 2-h glucose during the oral glucose tolerance test, for potential subgroup analyses. The values are reported as means with standard deviations (SD).
The main study outcome was the difference between glycemic responses after consumption of a snack in the morning, afternoon and evening as determined by three parameters: incremental area under the curve (iAUC), glucose peak values and time-to-peak after consumption of a snack. The glycemic responses to the snack were quantified during the 120 min after snack consumption for each daypart, and additionally during the 180 min after the snack consumptions in the evening. The glucose iAUC (mmol/L.min) was calculated by first subtracting the baseline value, which was the average over the glucose levels during the 30 min prior to every individual snack consumption. This removes participant variation and day-to-day variation within participants in the baseline glucose values. The actual recorded meal and snack times from the smartphone DitEetIk!-app were used for analysis, rather than the preset times on the provided meal schedules.
The glucose peak value was defined as the highest glucose reading (mmol/L) above baseline during the analyzed time interval after snack consumption. The time-to-peak after consumption of a snack between different dayparts was calculated as the time (minutes) from snack consumption to the glucose peak value.
The values reported for the iAUC, peak value and time-to-peak are daypart means (95% confidence intervals). When missing glucose readings appeared within the mentioned timeframes, they were imputed by linear interpolation. Glycemic responses to snack consumptions were excluded from analysis when the following main meal overlapped the analyzed time interval after snack consumption (recorded meal and snack times obtained from food diary), or when activity of moderate or higher intensity took place for more than 10 min within the 30 min before, or within the analyzed time interval after snack consumption (>2600 VM3counts/min, based on accelerometer measurements, cut-point based on previous research (Sasaki et al. 2011)). When day 10 was used to replace an incorrect test day, the original day was omitted from analysis and the glycemic responses to the snack consumption(s) of day 10 were included instead.
Linear mixed model analyses were used to analyze iAUC, peak value and time-to-peak with “timing” (morning, afternoon and evening) as fixed effect and a random effect of “participant” on the intercept. Post-hoc pairwise t-tests were conducted when the effect of timing appeared significant (morning versus afternoon, morning versus evening, afternoon versus evening) and p values were adjusted according to the Holm-Bonferroni correction for multiple comparisons. In all analyses, (corrected) p values < 0.05 were considered significant.
Results
Participant enrolment
A total of 35 volunteers were invited for an eligibility interview. All completed the interview and 29 were considered eligible. The other six volunteers met one or more of the exclusion criteria. The 29 volunteers who passed the eligibility interview underwent an oral glucose tolerance test (OGTT). Of these individuals, two had elevated fasting blood glucose levels and one had elevated glucose levels at the 2 h time point, which both led to exclusion. A total of 26 participants were eligible to enroll in the Glucozond study. However, two participants had to withdraw before commencement because of personal circumstances. For a schematic overview of the participant selection flow, see Figure 2.
Figure 2.
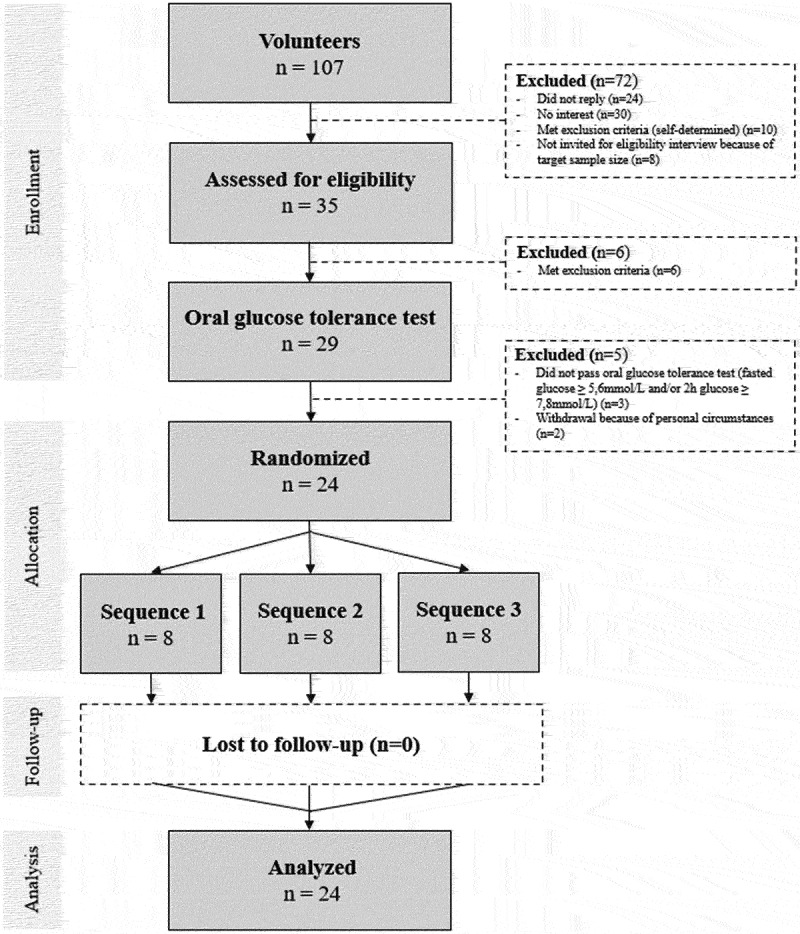
Participant flow diagram of the Glucozond study.
Participant characteristics
Baseline characteristics of the 24 enrolled participants are shown in Table 1. In all, the mean age was 43.7 y, the mean BMI was 24.0 kg/m2 and the mean fasted and 2-h blood glucose of the OGTT were 4.9 and 4.7 mmol/L, respectively. We found no significant difference between men and women for any of these characteristics.
Table 1.
Baseline characteristics for the total study population, and per gender. The Glucozond study population consisted of healthy adults of which 12 men and 12 women.
| Characteristic | Total (n = 24) | Men (n = 12) | Women (n = 12) | p (men vs women) |
|---|---|---|---|---|
| Age (y) | 43.7±11.2 | 42.8±11.1 | 44.6±11.6 | 0.912 |
| BMI (kg/m2) | 24.0±2.3 | 23.2±2.1 | 24.9±2.3 | 0.831 |
| Glucose fasted (mmol/L) | 4.9±0.4 | 4.9±0.3 | 4.8±0.4 | 0.155 |
| Glucose 2 h (mmol/L) | 4.7±1.0 | 4.4±0.8 | 4.9±1.3 | 0.064 |
Values are means ± SD. Glucose values were obtained from blood samples drawn during an oral glucose tolerance test (in fasted state and at timepoint 2 h). p values between men and women are based on an unpaired two-sided t-test. All characteristics of the study population comply with the inclusion and exclusion criteria specified in the Materials and Methods section.
Main meal composition and timing
Table 2 shows a summary of the mean meal compositions for breakfast, lunch and dinner on test days across all participants prior to the eligible snack consumptions (see next paragraph) and the mean time of day these meals were eaten. According to the food diaries, main meal skipping did not take place. As personalized meal schedules were provided, variation in meal time occurred. However, this did not lead to excessive variation in the time interval to the subsequent snack (Table 2).
Table 2.
Mean composition and timing of the main meals prior to all eligible snack consumptions analyzed for the glycemic response in the Glucozond study.
| Composition or time | Breakfast (n = 58) |
Lunch (n = 59) |
Dinner (n = 71) |
|---|---|---|---|
| Energy (kcal) | 398±197 | 631±242 | 764±349 |
| Total fat (g) | 13.2±9.8 | 26.0±12.6 | 32.2±24.3 |
| Saturated fat (g) | 4.3±3.9 | 9.4±4.5 | 9.8±8.8 |
| Total carbohydrates (g) | 51.0±25.9 | 65.8±29.1 | 80.1±46.0 |
| Total sugars (g) | 23.2±13.9 | 19.6±14.3 | 20.3±15.8 |
| Dietary fiber (g) | 5.6±3.6 | 8.4±4.3 | 9.9±5.5 |
| Protein (g) | 15.2±8.4 | 28.8±11.1 | 34.2±14.9 |
| Time range (hh:mm) | 06:05–09:45 | 11:45–14:15 | 17:10–19:20 |
| Time interval to snack (h:mm) | 2:30 ± 0:07 | 2:32 ± 0:13 | 3:00 ± 0:14 |
Values are means ± SD for dietary components. The time range indicates the earliest and the latest (start) time of the respective mean meals. Time interval indicates the mean ± SD time until subsequent snack consumption.
Glycemic response
Glycemic excursions
One of the participants experienced failure of the CGM, which led to premature removal of the sensor; this participant could only complete four instead of six test days. This reduced the total number of snack consumptions to be analyzed by three. Of 212 consumptions, a total of 188 snack consumptions were eligible for analyses, of which 58 were morning, 59 were afternoon and 71 were evening snack consumptions. Data on 24 snack consumptions were excluded because of interference of activity or main meals. Figure 3 presents the glycemic excursions by the snack at different dayparts from baseline. Mean (±SD) baseline values per time of consumption were 5.4 ± 1.0 mmol/L, 6.4 ± 1.4 mmol/L and 6.0 ± 1.0 mmol/L, for breakfast, lunch and dinner, respectively. The baseline values in the morning differed significantly from the baseline values in both the afternoon and the evening (p < .05) (results not shown). Glycemic excursions in the morning and afternoon can be analyzed for 120 min past snack consumption, after which the snack consumption was followed by the subsequent regular meal in most cases, being lunch or dinner, respectively. The 120-min follow-up matched the approximate return to baseline of the mean glucose excursion for the morning and afternoon snacks (Figure 3). However, the mean glucose excursion for the snack consumptions in the evening required approximately 180 min to return to baseline in a time frame not hampered by the subsequent regular meal, breakfast the next morning. Therefore, a two-pronged approach was used for the subsequent comparisons between different parameters of the glycemic response: a 120-min postprandial interval for all snack consumptions (Table 3), and with an extension of the postprandial interval to 180 min for the evening snack (Table 4).
Figure 3.
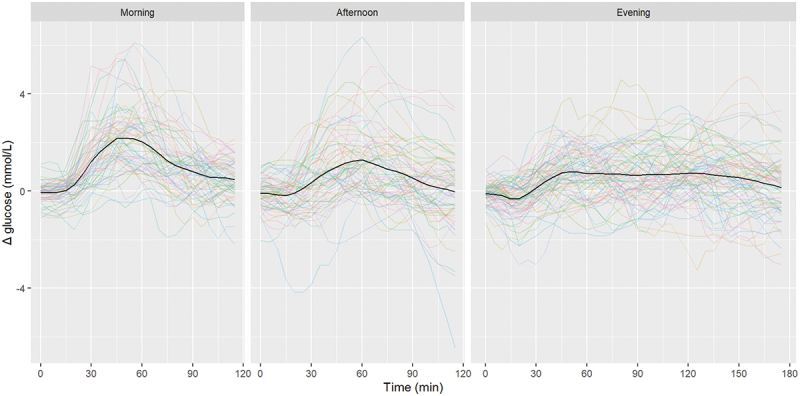
Glycemic excursions from baseline after consumption of a standard snack in the morning (59 consumptions), afternoon (59 consumptions) and evening (71 consumptions) by 24 healthy participants in the Glucozond study. Snack consumption took place at t = 0. Glucose levels were measured interstitially by continuous glucose monitoring. Glucose readings during the 30 min prior to every individual snack consumption were averaged to be taken as baseline value. The black line provides the mean of the individual glucose excursions depicted in color.
Table 3.
Glycemic response (iAUC, peak value and time-to-peak) after consumption of a standard snack in the morning (58 consumptions), afternoon (59 consumptions) and evening (71 consumptions) by 24 healthy participants in the Glucozond study for a postprandial time interval of 120 min for all snack consumptions.
| Time of day | iAUC (mmol/L.120 min) |
Peak value (mmol/L) |
Time-to-peak (min) |
|---|---|---|---|
| Morning | 24.6(19.5–29.8) | 2.7 (2.4–3.1) | 57 (50–65) |
| Afternoon | 12.1(7.0–17.2) | 1.9 (1.6–2.3) | 57 (50–64) |
| Evening | 10.5(5.7–15.3) | 1.8 (1.4–2.1) | 76 (69–83) |
| p (morning versus afternoon) | 2.2 × 10−4 | 8.4 × 10−5 | 8.9 × 10−1 |
| p (morning versus evening) | 1.8 × 10−5 | 5.6 × 10−7 | 1.7 × 10−4 |
| p (afternoon versus evening) | 6.0 × 10−1 | 3.0 × 10−1 | 1.4 × 10−4 |
Upper half: values are means (95% confidence interval). Glucose levels were measured interstitially by continuous glucose monitoring. Glucose readings during the 30 min prior to every individual snack consumption were averaged to be taken as baseline value for the calculation of glucose iAUC and glucose peak value. iAUC, incremental area under the curve. Lower half: Holm-Bonferroni corrected p values of pairwise comparisons for the three parameters of the glycemic response analyzed; values ≤ 0.05 are printed in bold.
Table 4.
Glycemic response (iAUC, peak value and time-to-peak) after consumption of a standard snack in the morning (58 consumptions), afternoon (59 consumptions) and evening (71 consumptions) by 24 healthy participants in the Glucozond study with an extended postprandial time interval of 180 min for evening snacks versus 120 min for morning and afternoon snack consumptions.
| Time of day | iAUC (mmol/L.min) |
Peak value (mmol/L) |
Time-to-peak (min) |
|---|---|---|---|
| Morning (120 min) | 24.6(18.4–30.7) | 2.7 (2.4–3.1) | 57 (48–66) |
| Afternoon (120 min) | 12.1(6.0–18.2) | 1.9 (1.6–2.3) | 57 (47–66) |
| Evening (180 min) | 16.7(11.0–22.4) | 2.0 (1.6–2.3) | 96 (87–104) |
| p (morning versus afternoon) | 4.7 × 10−3 | 2.0 × 10−4 | 9.2 × 10−1 |
| p (morning versus evening) | 7.1 × 10−2 | 2.0 × 10−4 | 1.7 × 10−10 |
| p (afternoon versus evening) | 2.1 × 10−1 | 8.1 × 10−1 | 1.3 × 10−10 |
Upper half: values are means (95% confidence interval, lower bound – upper bound). Glucose levels were measured interstitially by continuous glucose monitoring. Glucose readings during the 30 min prior to every individual snack consumption were averaged to be taken as baseline value for the calculation of glucose iAUC and glucose peak value. iAUC, incremental area under the curve. Lower half: Holm-Bonferroni corrected p values of pairwise comparisons for the three parameters of the glycemic response analyzed; values ≤ 0.05 are printed in bold.
iAUC
Within the 120-min postprandial interval, average glucose iAUC after snack consumption was the highest in the morning (24.6 mmol/L.120 min), compared to afternoon (12.1 mmol/L.120 min) and evening (10.5 mmol/L.120 min). No significant difference in iAUC was observed between the afternoon and the evening (Table 3). When the 180-min postprandial interval for the evening snack was considered, iAUC in the morning (24.6 mmol/L.120 min) tended to remain larger than in the evening (16.7 mmol/L.180 min), albeit no longer significant (Table 4).
Peak value
The average peak value above baseline after snack consumption was higher in the morning (2.7 mmol/L), compared to that in the afternoon (1.9 mmol/L), and in the evening (1.8 mmol/L) within the 120-min postprandial interval (Table 3). No significant difference was found between the glucose peak value in the afternoon and evening. For the 180-min postprandial period of the evening snack, a small shift in peak value was observed (2.0 mmol/L), but the difference with the peak value for the morning snack remained significant (Table 4).
Time-to-peak
Within the 120-min postprandial intervals, the average time-to-peak was significantly longer in the evening (76 min) than during the other two dayparts (57 min; Table 3). Compared to morning and afternoon, the time-to-peak was approximately 33% longer in the evening. No significant difference was found between the time-to-peak in the morning and in the afternoon. Taking the 180-min postprandial interval into consideration for the evening snack, the time-to-peak shifted even further out (96 min), providing even smaller p values when compared to the time-to-peak for the morning or afternoon snack (Table 4) and extending the time-to-peak delay to 68% in de evening.
Discussion
This study aimed to investigate whether timing of standard snack consumption affects the postprandial glycemic response of healthy individuals in a real-life setting. Twenty-four healthy participants enrolled in this study. All participants ate a carbohydrate-rich standard snack at preset times over 6 d, between their usual main meals and practices. The results show that the glycemic response to consumption of the snack differed over the course of the day, as determined by three characteristics. Overall, mean glucose iAUC and mean glucose peak values above baseline were highest for snacks consumed between breakfast and lunch. Though mean iAUC and mean peak values were reduced in the afternoon compared to the morning, mean time-to-peak was similar for these two dayparts. Whereas peak values of the snack after dinner were comparable to the afternoon snack, the evening snack showed extended time-to-peak values, up to 68%.
Earlier studies have consistently demonstrated that glycemic responses in the evening or night were more pronounced than in the day after identical food intake (Al-Naimi et al. 2004; Biston et al. 1996; Bo et al. 2017; Gibbs et al. 2014; Gil-Lozano et al. 2016; Grant et al. 2017; Leung et al. 2019; Morris et al. 2015; Owens et al. 1996). The results of this study contrast with these previous findings, showing the most pronounced glycemic response, i.e. high glucose iAUC and peak values, in the morning.
However, there were substantial differences in protocol between the previous studies and ours. First and foremost, previous studies were conducted under strongly controlled laboratory settings, while the current study was designed to resemble a real-life situation and was conducted by the participants themselves in their regular surroundings. In daily life, most people vary their meals, and quantities and macronutrient compositions differ between the three regular meals, breakfast, lunch and dinner (van Rossum et al. 2020) (see also Table 2). As different meal quantities and compositions directly relate to their postprandial glycemic response (Wheeler and Pi-Sunyer 2008), the postprandial response of a standard snack between main meals was studied in the current study. Previous studies mainly focused on the glycemic response of the same main meal provided at different times of the day. Consequently, multiple factors that can influence the glycemic response were different.
Specifically, most laboratory studies incorporated an extended fast (4–10 h) prior to the consumption of the test meal to exclude any effect of the preceding meal on the outcome measure, and some even specified conditions of the preceding meal. Since our test meal comprised a snack, it was planned between meals and therefore a fast longer than 2.5 h before snack consumption while allowing for a fasting 2-h postprandial snack time frame as well, was not feasible. Although we found that mean baseline glucose values were close (5.4–6.4 mmol/L, see Results) prior to the snack consumptions in the morning, afternoon and evening, these levels were higher with 2 to 3 times larger standard deviations than the fasted glucose values prior to the OGTT (Table 1). Therefore, the preceding meals could well have affected the observed glycemic responses to the snacks, as the meal composition varied between breakfast, lunch and dinner (Table 2). For example, it is shown that protein consumption attenuates the glycemic response of a subsequent food (Meng et al. 2017). Similar to the dietary intake of the Dutch population (van Rossum et al. 2020), protein intake was highest at dinner, compared to breakfast and lunch in our study participants (Table 2). The higher protein intake might have had a suppressing effect on the glycemic response to the consumption of the snack after dinner and potentially explains the delayed and flattened glycemic response in the evening compared to the morning and afternoon (Figure 2). On the other hand, prolonged fasting periods prior to the study meal might affect the endogenous circadian rhythm (Kessler and Pivovarova-Ramich 2019) and consequently influence the glycemic responses differently at different dayparts in studies using such dietary preconditioning.
A second difference in the study protocol is that the test product, the snack that was used in the current study, was relatively low in energy (198 kcal). Most studies used, instead of a snack, a complete meal as a test product, with an energy content ranging from 300 to more than 1000 kcal (Bo et al. 2017; Leung et al. 2019). A higher energy content, and presumably also higher carbohydrate intake, obviously requires a higher load of insulin, which might enlarge the effect of a lower insulin sensitivity in the evening. This could explain why others found a higher glycemic response in the evening, while we did not. Though the test product was a very convenient product to eat between meals, it might have been too small to serve as a comparable test product to complete meals, because it requires a relatively small insulin response. However, eating a snack at different times of the day between meals is more in line with the subjects’ usual diet than an additional complete meal.
The third difference in study protocols was that our participants were instructed to refrain from heavy exercise during the test days and to minimize activity in the hours around the snack consumption but were allowed to move about, such as by walking or cycling at casual commuting speeds, climbing an ordinary staircase or performing light household chores. Participants in the laboratory studies were restricted to sedentary activities or were assigned to bed. Although we aimed to exclude every snack consumption that was interfered by exercise classified higher than moderately intense, contrary to the laboratory studies, participants were thus allowed to engage in their daily activities, to which the metabolic system will adapt, and hence lead to a better reflection of real-life glycemic responses for non-exercise conditions.
Finally, our observation that the glucose peak emerges significantly later upon snack consumption in the evening than during the day is in line with results of the laboratory studies. A later peak glucose value might imply that digestion and/or absorption of nutrients was delayed. Previous research suggests insulin levels in response to the test product might have been higher in the evening, compared to the morning and the afternoon (Bo et al. 2017; Gibbs et al. 2014; Gil-Lozano et al. 2016; Grant et al. 2017; Leung et al. 2019). Together with the slower rate of nutrients entering the circulation, this could result in a lower glycemic response in the evening hours.
Strengths and limitations
The use of CGM to measure glycemic responses is a major strength since it produces glucose readings almost continuously. When blood samples are used to estimate the glycemic response, they are mostly drawn with intervals of 15–30 min, inducing the risk of missing the actual peak value. Therefore, the CGM readings might produce a better estimate of glycemic response, than blood samples. In addition, using a CGM device is considered less invasive than repeated blood sample drawing.
A strength was the crossover design, which allowed the glycemic responses to be compared in the absence of interpersonal variation, which is known to be high in postprandial responses (Zeevi et al. 2015). Moreover, our sample size was relatively large compared to other studies, and included participants in a wide age range, which increases generalization of the results. However, as a real-life situation inevitably leads to more variation in outcomes, an even larger sample size would be ideal to further examine potential covariates such as gender, age or main meal compositions.
A number of limitations should be named. Kroger, et al. (Kroger et al. 2021) mentioned that there should be a period of fasting at least 3 h before consumption of a test meal when assessing postprandial responses. It was not feasible to incorporate this into our study prior to the morning and afternoon snack consumption, and the preceding meals might have affected the observed glycemic response to the snack. However, snacking between meals is a common habit and, therefore, our study provides new insights into the concept of chrononutrition for real-life settings.
We had no sight nor control on the speed of intake of the test product, and the consumption rate might have differed between participants and consumption events. The speed of intake is known to affect the postprandial glycemic response (Kroger et al. 2021). A higher ingestion rate may induce a faster entrance of glucose in the blood, leading to a higher glucose peak value, and thereafter a steeper decrease in glucose levels because of high insulin responses (Argyrakopoulou et al. 2020). Since the snack was small in comparison to a meal, and does not require preparation or an extensive amount of chewing, we assume variations in consumption rates to be minimal.
Consumption speed of the main meals was also not monitored. However, as dinner was typically the largest meal (Table 2) and was not followed by another main meal the same day, we incorporated a 3-h time window after the start of dinner and before the subsequent evening snack, allowing an additional 30 min for meal consumption and digestion compared to the morning and the afternoon 2.5-h time window. The mean baseline glucose value prior to the evening snack was in the middle of the mean baseline values prior to the morning and afternoon snacks (see Results).
While the use of CGM is considered to produce reliable glucose readings within 20% deviation of blood glucose measurements (Dexcom 2020) and is generally accepted as a management tool in diabetics (Carlson et al. 2017), miscalibration, noise spikes or pressure applied to the site of sensor application, may cause inaccurate glucose readings (Baysal et al. 2014). Kulcu, et al. (Kulcu et al. 2003) showed that interstitial glucose levels significantly differ from blood glucose levels. These differences included a lag time in interstitial glucose levels compared to blood glucose levels, and other responses to increase and decrease in blood glucose concentrations. In a state of increasing glucose, the observed increase in interstitial glucose levels was smaller than the increase in blood glucose, while in a state of decreasing glucose, the change in interstitial glucose was larger than in blood glucose. These limitations, relating to the CGM, may have led to over- or underestimating of the actual glycemic response. Lastly, it should be realized that the current results pertain to individuals with a healthy OGTT only, and that for (pre)diabetics, postprandial responses over the day may be quite different.
Conclusions
The aim of the present research was to examine the differences in postprandial glycemic response to a standard snack over the course of a day in healthy individuals. To the best of our knowledge, this is the first study assessing this in a real-life setting. In contrast to earlier work, performed in tightly controlled settings, the most pronounced glycemic responses occurred during the morning rather than the evening. Our results indicate that, for healthy individuals, insights into chrononutrition based on well-controlled experimental conditions in laboratories cannot be applied plainly when giving diet and lifestyle advice aimed at controlled and moderate postprandial glucose excursions in real life. On the other hand, real-life studies coincide with uncontrolled variables that could affect the outcome. The postprandial response in daily life is influenced by many factors, intertwined with the circadian rhythm. Extended real-life studies in combination with controlled specific variables, applied CGM and good documentation of the diets consumed and activities undertaken, should provide more insights into these complex interactions.
Acknowledgements
The authors are grateful to all participants of the “Glucozond” study and thank Ido Toxopeus for his assistance in the use of the DitEetIk!-app and its data extraction, Marja Beukers for her help in the calculations of energy and nutrient intakes by meal, Roel Schreurs for his assistance with constructing the participant database, and Rose Maase for proofreading. Koninklijke Peijnenburg BV kindly provided the gingerbread bars as a gift.
Funding Statement
This research was funded by the Ministry of Health, Welfare and Sport of the Netherlands, The National Institute for Public Health and the Environment, grant number S/010007 and through Bioclock, a project within the NWA-ORC programme of the Dutch Research Council (project number 1292.19.077).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Medical research Ethics Committee United, Nieuwegein, The Netherlands, under protocol code NL71117.041.20 on June 9, 2020.
Informed consent was obtained from all subjects involved in the study.
References
- Al-Naimi S, Hampton SM, Richard P, Tzung C, Morgan LM.. 2004. Postprandial metabolic profiles following meals and snacks eaten during simulated night and day shift work. Chronobiol Int. 21:937–947. doi: 10.1081/CBI-200037171 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association . 2020. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 43:S14–S31. doi: 10.2337/dc20-S002 [DOI] [PubMed] [Google Scholar]
- Argyrakopoulou G, Simati S, Dimitriadis G, Kokkinos A.. 2020. How important is eating rate in the physiological response to food intake, control of body weight, and glycemia? Nutrients. 12:1734. doi: 10.3390/nu12061734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin LS, Kendall CW, Jenkins DJ, Willett WC, Astrup A, Barclay AW, Bjorck I, Brand-Miller JC, Brighenti F, Buyken AE, et al. 2015. Glycemic index, glycemic load and glycemic response: An international scientific consensus summit from the international carbohydrate quality consortium (ICQC). Nutr Metab Cardiovasc Dis. 25:795–815. doi: 10.1016/j.numecd.2015.05.005 [DOI] [PubMed] [Google Scholar]
- Baysal N, Cameron F, Buckingham BA, Wilson DM, Chase HP, Maahs DM, Bequette BW, In Home Closed-Loop Study G . 2014. A novel method to detect pressure-induced sensor attenuations (PISA) in an artificial pancreas. J Diabetes Sci Technol. 8:1091–1096. doi: 10.1177/1932296814553267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biston P, Van Cauter E, Ofek G, Linkowski P, Polonsky KS, Degaute JP.. 1996. Diurnal variations in cardiovascular function and glucose regulation in normotensive humans. Hypertension. 28:863–871. doi: 10.1161/01.HYP.28.5.863 [DOI] [PubMed] [Google Scholar]
- Bo S, Broglio F, Settanni F, Parasiliti Caprino M, Ianniello A, Mengozzi G, De Francesco A, Fadda M, Fedele D, Guggino A, et al. 2017. Effects of meal timing on changes in circulating epinephrine, norepinephrine, and acylated ghrelin concentrations: A pilot study. Nutr Diabetes. 7:303. doi: 10.1038/s41387-017-0010-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson AL, Mullen DM, Bergenstal RM. 2017. Clinical use of continuous glucose monitoring in adults with type 2 diabetes. Diabetes Technol Ther. 19:S-4-S–11. doi: 10.1089/dia.2017.0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexcom . 2020. Is my Dexcom sensor accurate?; [accessed 2020 September 10]. https://www.dexcom.com/faqs/is-my-dexcom-sensor-accurate.
- Gallwitz B. 2009. Implications of postprandial glucose and weight control in people with type 2 diabetes: Understanding and implementing the international diabetes federation guidelines. Diabetes Care. 32:S322–325. doi: 10.2337/dc09-S331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs M, Harrington D, Starkey S, Williams P, Hampton S. 2014. Diurnal postprandial responses to low and high glycaemic index mixed meals. Clin Nutr. 33:889–894. doi: 10.1016/j.clnu.2013.09.018 [DOI] [PubMed] [Google Scholar]
- Gil-Lozano M, Hunter PM, Behan LA, Gladanac B, Casper RF, Brubaker PL. 2016. Short-term sleep deprivation with nocturnal light exposure alters time-dependent glucagon-like peptide-1 and insulin secretion in male volunteers. Am J Physiol Endocrinol Metab. 310:E41–50. doi: 10.1152/ajpendo.00298.2015 [DOI] [PubMed] [Google Scholar]
- Grant CL, Coates AM, Dorrian J, Kennaway DJ, Wittert GA, Heilbronn LK, Pajcin M, Della Vedova C, Gupta CC, Banks S. 2017. Timing of food intake during simulated night shift impacts glucose metabolism: A controlled study. Chronobiol Int. 34:1003–1013. doi: 10.1080/07420528.2017.1335318 [DOI] [PubMed] [Google Scholar]
- Kessler K, Pivovarova-Ramich O. 2019. Meal timing, aging, and metabolic health. Int J Mol Sci. 20:1911. doi: 10.3390/ijms20081911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroger J, Siegmund T, Schubert-Olesen O, Keuthage W, Lettmann M, Richert K, Pfeiffer AFH. 2021. AGP and nutrition - analysing postprandial glucose courses with CGM. Diabetes Res Clin Pract. 174:108738. doi: 10.1016/j.diabres.2021.108738 [DOI] [PubMed] [Google Scholar]
- Kulcu E, Tamada JA, Reach G, Potts RO, Lesho MJ. 2003. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care. 26:2405–2409. doi: 10.2337/diacare.26.8.2405 [DOI] [PubMed] [Google Scholar]
- Leung GKW, Huggins CE, Bonham MP. 2019. Effect of meal timing on postprandial glucose responses to a low glycemic index meal: A crossover trial in healthy volunteers. Clin Nutr. 38:465–471. doi: 10.1016/j.clnu.2017.11.010 [DOI] [PubMed] [Google Scholar]
- Meng H, Matthan NR, Ausman LM, Lichtenstein AH. 2017. Effect of prior meal macronutrient composition on postprandial glycemic responses and glycemic index and glycemic load value determinations. Am J Clin Nutr. 106:1246–1256. doi: 10.3945/ajcn.117.162727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, Scheer FA. 2015. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A. 112:E2225–2234. doi: 10.1073/pnas.1418955112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DS, Macdonald I, Benton D, Sytnik N, Tucker P, Folkard S. 1996. A preliminary investigation into individual differences in the circadian variation of meal tolerance: Effects on mood and hunger. Chronobiol Int. 13:435–447. doi: 10.3109/07420529609020914 [DOI] [PubMed] [Google Scholar]
- Proper KI, van de Langenberg D, Rodenburg W, Vermeulen RCH, van der Beek AJ, van Steeg H, van Kerkhof LWM. 2016. The relationship between shift work and metabolic risk factors: A systematic review of longitudinal studies. Am J Prev Med. 50:e147–e157. doi: 10.1016/j.amepre.2015.11.013 [DOI] [PubMed] [Google Scholar]
- R Core Team R Foundation for Statistical Computing, Vienna, Austria . 2020. A language and environment for statistical computing. https://www.R-project.org/.
- RIVM . 2019. Nederlands voedingsstoffenbestand (NEVO). [accessed 2019/6.0]. https://nevo-online.rivm.nl/.
- Sasaki JE, John D, Freedson PS. 2011. Validation and comparison of ActiGraph activity monitors. J Sci Med Sport. 14:411–416. doi: 10.1016/j.jsams.2011.04.003 [DOI] [PubMed] [Google Scholar]
- Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, Kalsbeek A. 2019. Circadian clocks and insulin resistance. Nat Rev Endocrinol. 15:75–89. doi: 10.1038/s41574-018-0122-1 [DOI] [PubMed] [Google Scholar]
- Torquati L, Mielke GI, Brown WJ, Kolbe-Alexander T. 2018. Shift work and the risk of cardiovascular disease. A systematic review and meta-analysis including dose-response relationship. Scand J Work Environ Health. 44:229–238. doi: 10.5271/sjweh.3700 [DOI] [PubMed] [Google Scholar]
- van Rossum C, Buurma-Rethans E, Dinnissen C, Beukers M, Brants H, Ocké M. 2020. The diet of the Dutch: Results of the Dutch national food consumption survey 2012-2016. Bilthoven: RIVM. doi: 10.21945/RIVM-2020-0083 [DOI] [Google Scholar]
- Wang XS, Armstrong ME, Cairns BJ, Key TJ, Travis RC. 2011. Shift work and chronic disease: The epidemiological evidence. Occup Med (Lond). 61:78–89. doi: 10.1093/occmed/kqr001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ML, Pi-Sunyer FX. 2008. Carbohydrate issues: Type and amount. J Am Diet Assoc. 108:S34–39. doi: 10.1016/j.jada.2008.01.024 [DOI] [PubMed] [Google Scholar]
- Wu QJ, Sun H, Wen ZY, Zhang M, Wang HY, He XH, Jiang YT, Zhao YH. 2022. Shift work and health outcomes: An umbrella review of systematic reviews and meta-analyses of epidemiological studies. J Clin Sleep Med. 18:653–662. doi: 10.5664/jcsm.9642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, et al. 2015. Personalized nutrition by prediction of glycemic responses. Cell. 163:1079–1094. doi: 10.1016/j.cell.2015.11.001 [DOI] [PubMed] [Google Scholar]


