Abstract
Background
The US government considers veterans to have been exposed to Agent Orange if they served in Vietnam while the carcinogen was in use, and these veterans are often deemed at high risk of prostate cancer (PCa). Here, we assess whether presumed Agent Orange exposure is independently associated with increased risk of any metastatic or fatal PCa in a diverse Veteran cohort still alive in the modern era (at least 2011), when accounting for race/ethnicity, family history, and genetic risk.
Patients and Methods
Participants in the Million Veteran Program (MVP; enrollment began in 2011) who were on active duty during the Vietnam War era (August 1964-April 1975) were included (n = 301,470). Agent Orange exposure was determined using the US government definition. Genetic risk was assessed via a validated polygenic hazard score. Associations with age at diagnosis of any PCa, metastatic PCa, and death from PCa were assessed via Cox proportional hazards models.
Results and Interpretation
On univariable analysis, exposure to Agent Orange was not associated with increased PCa (hazard ratio [HR]: 1.02, 95% confidence interval [CI]: 1.00–1.04, p = 0.06), metastatic PCa (HR: 0.98, 95% CI: 0.91–1.05, p = 0.55), or fatal PCa (HR: 0.94, 95% CI: 0.79–1.09, p = 0.41). When accounting for race/ethnicity and family history, Agent Orange exposure was independently associated with slightly increased risk of PCa (HR: 1.06, 95% CI: 1.04–1.09, <10-6) but not with metastatic PCa (HR: 1.07, 95% CI: 0.98–1.15, p = 0.10) or PCa death (HR: 1.02, 95% CI: 0.83–1.23, p = 0.09). Similar results were found when accounting for genetic risk. Agent Orange exposure history may not improve modern PCa risk stratification.
KEYWORDS: Agent Orange, prostate cancer, MVP, race/ethnicity, health disparities
Introduction
Agent Orange, a mixture of herbicides 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-Trichlorophenoxyacetic acid (2,4,5-T), kerosene, and diesel fuel, was used in the Vietnam War to clear dense vegetation and destroy food crops. A potential association between 2,4-D and 2,4,5-T exposure [1–3] increased the risk of non-Hodgkin lymphoma, soft-tissue sarcoma, and bladder and lung cancers and has been debated since the 1980s [4], though no adequate epidemiological evidence has supported that conclusion [5]. Agent Orange and early formulations of 2,4-D and 2,4,5-T, were contaminated with a dioxin compound known as 2,3,7,8-tetrachlorobenzo-p-dioxin (TCDD), which has been classified as a carcinogen since the 1990s. The Agent Orange Act of 1991 defines exposure to include all veterans who served anywhere in Vietnam between January 9, 1962 to May 7,1975; this Federal definition is used to guide current preventive healthcare policies in this population [6–8]. In the 2000s, a potential association was acknowledged between Agent Orange exposure and genitourinary cancers [9]. However, evidence linking Agent Orange exposure to increased PCa risk or associated mortality among Vietnam War Veterans has been limited to small case series [10–16]. These small studies have found Agent Orange to be associated with slightly lower age at PCa diagnosis, higher incidence of Stage IV disease, and lower rates of biochemical control [8, 16].
We investigated the association between Agent Orange exposure and PCa risk in the VA Million Veteran Program (MVP), a population-based cohort that started enrollment in 2011 with genotyping, long-term follow-up, and linked clinical records for over 870,000 participating US veterans. The MVP is one of the largest and most diverse electronic health record-linked biobanks in the world, with a unique structure that allows for detailed investigation into the interactions between inherited risk and Agent Orange exposure in US veterans [17]. We tested the hypothesis that Agent Orange exposure, using the practical government definition, is associated with PCa outcomes and thus might improve modern PCa risk stratification for early detection strategies. Moreover, as MVP data have the potential to inform future clinical care and clinical trials (e.g. NCT05129605), it is important to understand how Agent Orange exposure might influence results in this population.
Methods
Participants
We obtained data from MVP for individuals recruited from 63 VA Medical Centers across the United States (US) beginning in 2011. All veterans were eligible for participation in MVP. Study participation included consenting to access the participant’s electronic health records for research purposes. The MVP received ethical and study protocol approval from the VA Central Institutional Review Board in accordance with the principles outlined in the Declaration of Helsinki. We limited the present study to males on active duty during the Vietnam War era (August 1964–April 1975) (Table 1). We included PCa diagnoses at any point after Vietnam War service, regardless of when the participant enrolled in MVP. At the time of MVP enrollment, 265,146 participants had no known PCa, 22,609 had a non-metastatic PCa diagnosis, and 1,218 had been diagnosed with metastatic PCa.
Table 1.
Participant characteristics for self-reported race/ethnicity groups among MVP participants who served on active duty during the Vietnam War era (August 1964–April 1975).
| All | Self-reported Race/Ethnicity |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Hispanic White | Black or African American | Hispanic White | Asian | Native American | Pacific Islander | Other | Unknown | ||
| Active duty during Vietnam War | 301,470 (84,326) |
230,506 (68,171) |
45,257 (9,216) |
11,009 (3,176) |
1,915 (447) |
3,082 (860) |
1,292 (339) |
4,155 (1,096) |
4,254 (1,021) |
| Fatal prostate cancer | 795 (221) |
525 (155) |
200 (50) |
27 (6) |
<10 (1) |
<10 (1) |
<10 (1) |
13 (2) |
23 (5) |
| Metastatic prostate cancer | 3,828 (1,113) |
2,495 (818) |
1,033 (219) |
125 (35) |
23 (4) |
29 (8) |
12 (4) |
47 (13) |
64 (12) |
| Any prostate cancer | 42,569 (12,822) |
29,482 (9,555) |
10,084 (2,385) |
1,278 (403) |
224 (45) |
366 (130) |
152 (46) |
481 (135) |
501 (123) |
Numbers indicate participants available for analysis. Numbers in parentheses indicate participants with Agent Orange exposure.
Potential Agent Orange exposure
Potential exposure to Agent Orange was determined by the VA Compensation & Pension Committee, as recorded in the MVP data core. As per the legal US government definition, veterans who served physically (on land or inland waterways) in Vietnam during periods of Agent Orange use by the US military were considered exposed to Agent Orange (January 9, 1962–May 7, 1975). Information about the intensity (amount and duration) of Agent Orange exposure for each individual is not known, consistent with routine clinical reality.
Clinical data extraction
PCa diagnosis, age at diagnosis, prostate-specific antigen (PSA) tests, and date of last follow-up were retrieved from the VA Corporate Data Warehouse based on ICD codes and VA Central Cancer Registry data. Age at diagnosis of metastatic PCa indicated the age of the participant when diagnosed with either nodal or distant metastases as determined through a validated natural language processing tool [18]. Fatal PCa information was determined from National Death Index. Participants with ICD10 code ‘C61’ as underlying cause of death were considered to have died from PCa. Family history was recorded as either the presence or absence of one or more first-degree relatives with PCa. Among the participants eligible for analysis, over 99% had received at least one PSA test in the VA system, though the age at testing and frequency of testing were variable, and clinical indications (screening vs. diagnostic workup) are not known.
Genetic risk: Polygenic Hazard Score (PHS290)
Blood sampling, DNA extraction, quality controls, and imputation were conducted by MVP as described previously [9, 14]. The MVP 1.0 genotyping array contains a total of 723,305 variants, enriched for low-frequency variants in African and Hispanic populations and variants associated with diseases common to the VA population [16].
To assess genetic risk, we calculated a previously developed and validated polygenic hazard score using 290 common genetic variants (PHS290) that reliably stratifies men for age-dependent genetic risk of PCa and is associated with PCa, metastatic PCa, and PCa death [18–20]. Details of PHS290 calculation in MVP are described elsewhere [18, 19]. PHS290 performs well in diverse datasets and is independently associated with PCa risk [18, 19].
Cox proportional hazards analysis
We used Cox proportional hazards models to evaluate the association of Agent Orange exposure with three clinical endpoints: age at diagnosis of PCa, age at diagnosis of metastatic PCa, and age at death from PCa. We also analyzed self-reported racial/ethnic subgroups. Participants with both Black race and Hispanic ethnicity were included in a single category for Black or African American race. Where individuals did not meet the endpoint of interest, we censored at age at last follow-up.
To assess for independent association of Agent Orange exposure with PCa endpoints, we used multivariable Cox proportional hazards models with race/ethnicity, family history, and PHS290. For race/ethnicity hazard ratios (HRs), we used Non-Hispanic White as reference. For PHS290, we illustrated the effect size via the HR for the highest 20% versus lowest 20% of genetic risk (HR80/20) and between other strata of PHS290. These percentiles refer to previously defined absolute thresholds of PHS290 [18, 19]. We assessed statistical significance with two-tailed alpha at 0.01.
PSA testing
Screening has been shown in a large, randomized trial to increase PCa incidence and reduce cause-specific mortality [22], raising the possibility that PSA testing may confound any impact of Agent Orange exposure. We ascertained the number of PSA tests each participant underwent and evaluated associations between Agent Orange exposure and number of pre-diagnostic PSA tests (≥2 years prior to PCa diagnosis) via linear regression. Multivariable linear regressions used race/ethnicity, family history, and PHS290 as predictive variables in addition to Agent Orange exposure.
RESULTS
We found 301,470 veterans eligible for this analysis. Median age at PCa diagnosis was 65.3 years [interquartile range (IQR): 61–69]. Median age at last follow-up was 71.3 [68–74].
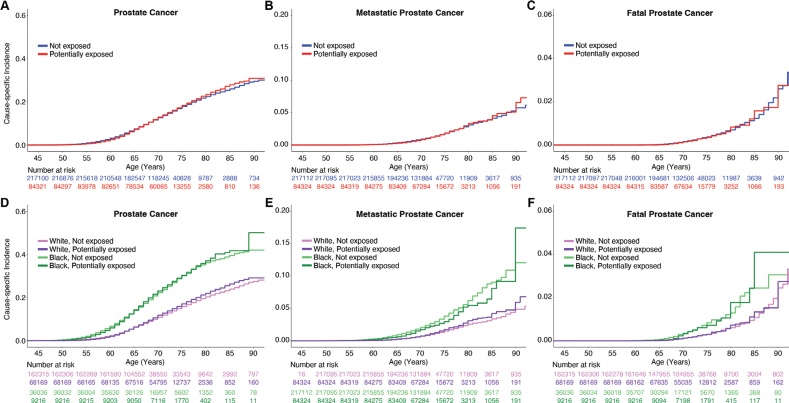
On univariable analysis, Agent Orange exposure was not associated with increased PCa diagnosis (HR: 1.02, 95% confidence interval [CI]: 1.00–1.04, p = 0.06) (Figure 1; Supplemental Table 1). Some statistically significant associations were found in subgroups based on race and ethnicity (Supplemental Table 1). In the Non-Hispanic White group, Agent Orange exposure was associated with increased PCa (HR: 1.08, 95% CI: 1.05–1.10, p < 10-8) and metastatic PCa diagnosis (HR: 1.13, 95% CI: 1.03–1.22, p < 10-2). A statistically significant association in the opposite direction was observed in the Black or African American group: those with Agent Orange exposure were somewhat less likely to develop PCa (HR: 0.82, 95% CI: 0.71–0.95, p < 10-2). No evidence of association with fatal PCa was seen in MVP participants. Cause-specific cumulative incidence curves for PCa were qualitatively similar regardless of Agent Orange exposure status (Figure 1).
Figure 1.
Million Veteran Program (MVP) cause-specific cumulative incidence based on Agent Orange exposure. Cause-specific cumulative incidence among MVP participants on active duty during the Vietnam War, stratified by Agent Orange exposure status (top row) and stratified by self-reported race (bottom row) for (A, D) all prostate cancer, (B, E) metastatic prostate cancer, and (C, F) fatal prostate cancer. ‘White’ indicates Non-Hispanic White participants, and ‘Black’ indicates Black and Hispanic Black participants.
When accounting for race/ethnicity and family history, Agent Orange exposure was an independent risk factor for PCa diagnosis (HR: 1.06, 95% CI: 1.04–1.09, p < 0.05) but not for metastatic PCa or PCa death. (Table 2). Genetic risk (PHS290) was strongly associated with all PCa endpoints, but accounting for this genetic effect had no impact on the association between Agent Orange exposure and PCa diagnosis (Supplemental Table 2). Agent Orange exposure did not differentially modulate PCa risk among men with high genetic risk (PHS290 >80th percentile, as defined previously [21]) or across any PHS290 values (Supplemental Table 3, Supplemental Figure 1).
Table 2.
Multivariable models combining self-reported race/ethnicity, family history, and Agent Orange exposure for three PCa clinical endpoints.
| Clinical Endpoint | Self-Reported Race/Ethnicity |
Family History | Agent Orange Exposure | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Black or African American | Hispanic White | Asian | Native American | Pacific Islander | Unknown | Other | |||
| Fatal Prostate Cancer | 2.34 [1.87–2.88]** | 1.06 [0.53–1.6] | 0.26 [0.0–0.8] | 0.77 [0.0–1.86] | NA | NA | 2.02 [1.04–3.17] | 1.89 [1.45–2.34]* | 1.02 [0.83–1.23] |
| Metastatic Prostate Cancer | 2.49 [2.26–2.72]*** | 1.24 [0.97–1.52] | 1.03 [0.55–1.53] | 0.98 [0.52–1.49] | 0.56 [0.0–1.42] | 1.78 [0.77–3.13] | 1.44 [1.06–1.86] | 1.51 [1.34–1.7]** | 1.07 [0.98–1.17] |
| Prostate Cancer | 2.2 [2.13–2.26]*** | 1.02 [0.95–1.1] | 0.87 [0.75–1.0] | 1.02 [0.9–1.14] | 0.89 [0.62–1.18] | 0.83 [0.62–1.05] | 1.05 [0.95–1.15] | 1.85 [1.79–1.92]*** | 1.06 [1.04–1.09]* |
Cox proportional hazards results for association with age at death from PCa, age at diagnosis of metastatic PCa, and age at diagnosis with PCa. P-values reported are from multivariable models using self-reported race/ethnicity, family history, and Agent Orange exposure (yes or no). Hazard ratios for race/ethnicity were estimated using Non-Hispanic White as the reference. Hazard ratios for family history were for one or more first-degree relatives diagnosed with prostate cancer. This multivariable analysis was limited to the 213,856 participants who were on active duty during the Vietnam War and for whom family history information was available. Numbers in brackets are 95% confidence intervals. Significant predictors in the multivariable model are indicated by
(p < 0.01),
(p < 10-10), and
(p < 10-16).
On univariable and multivariable linear regression analyses in this population, there was no evidence of association between Agent Orange exposure and increased screening. Agent Orange exposure was associated with a statistically significant but small reduction in screening intensity on univariable analysis – 8.3 PSA tests compared to 9 PSA tests for those not exposed. On the other hand, self-reported Black race was associated with increased PSA testing, concordant with guidelines that support stronger consideration of screening for men at higher risk [20] (Supplemental Table 4).
DISCUSSION
In a large, diverse, population-based cohort of US Veterans who served during the Vietnam War and were still alive to enroll in MVP in 2011, Agent Orange exposure was weakly associated with overall PCa, but not metastatic or fatal PCa. Importantly, we present the first multivariable analysis in a population-based cohort to assess whether Agent Orange exposure was an independent risk factor for PCa outcomes when accounting for family history, ancestry, and/or genetic risk. Our findings may have pragmatic implications for early detection strategies and suggest the US definition of Agent Orange exposure does not substantially increase risk of morbidity or mortality from PCa, at least for individuals alive today. Also, this study helps inform inclusion criteria for clinical trial enrollment in the VA and sets the foundation to better understand veteran exposures such as burn pits that need to be monitored
Details confirming actual Agent Orange exposure including duration or intensity are not available in MVP or routine clinical practice. Some veterans who physically served in Vietnam while Agent Orange was in use may have had heavy and/or frequent exposure, whereas others may have escaped with little to no exposure. It is possible that intense Agent Orange exposure is associated with aggressive PCa, though adequate data will likely never be available to answer this question. The definition of Agent Orange exposure used in this study is also used by the VA Compensation & Pension Committee to address the needs of potentially exposed individuals. Use of this definition estimates associations of the average exposure by those veterans serving in Vietnam during use of Agent Orange. Among Veterans surviving to 2011 or later, we can conclude that average Agent Orange exposure among US veterans serving during Vietnam War era has a much smaller effect size than do family history, Black race, or high polygenic risk. On multivariable analysis, potential Agent Orange exposure yielded HRs < 1.10 for all PCa endpoints underscoring the fact that these statistical associations are not likely clinically meaningful, whereas HRs for metastatic PCa were 1.37 for family history, 1.97 for Black race, and 4.42 for individuals with high versus low polygenic risk (PHS290). Notably, effects may be underestimated as our study focused on veterans who were alive for MVP enrollment in 2011 and did not include veterans who may have died prior to 2011 from Agent Orange exposure effects.
Statistically significant associations in subgroup analyses of self-reported race/ethnicity were small and in opposite directions (increased risk after Agent Orange exposure for Non-Hispanic White participants and decreased risk for Black or African American participants). We interpret these subgroup findings cautiously. On the whole, there is not a clear and strong association of Agent Orange exposure and poor PCa outcomes in MVP.
This study was conducted using data from MVP, so the results may not be generalizable beyond the VA population. Potential differences in PCa screening intensity between exposure groups were not completely accounted for, though there was no evidence of increased PSA testing among those exposed to Agent Orange in this study. As sequencing for rare pathogenic mutations was not performed, it was also not possible to assess the impact of Agent Orange exposure on risk arising from, for example, germline BRCA2 mutations, considering Agent Orange mutates genes and induces chromosomal aberrations.
Author contributions
A.J.L., M.S.P., T.M.S. conceived and designed the analysis; A.J.L., M.S.P. and R.K. performed the analysis; J.A.L., K.M.L., S.L.D., J.M.G. contributed data and analysis tools; A.J.L., M.S.P., and T.M.S. wrote the paper with assistance from A.Y.Z., A.P., B.S.R., H.K.C., A.S.K., M.S.P., R.L.H.
Supplementary Material
Acknowledgements
This research was completed as a project in the MVP022 study and used data from the Million Veteran Program, Office of Research and Development, Veterans Health Administration. R.L.H. was funded by VA MVP022 CX001727, the VISN-22 VA Center of Excellence for Stress and Mental Health (CESAMH), and National Institute of Aging RO1 grant AG050595 (The VETSA Longitudinal Twin Study of Cognition and Aging VETSA 4). M.S.P. was supported by the National Institutes of Health (#1F30CA247168, #T32CA067754). A.L. was supported by the Grillo-Marxuach Family Fellowship at the Moores Cancer Institute of UC San Diego. A.P. was supported by the Prostate Cancer Foundation (Young Investigator Award) and the Swedish Cancer Society (Fellowship). T.M.S. and R.K. were supported by the National Institutes of Health (NIH/NIBIB #K08EB026503), the Prostate Cancer Foundation, and the University of California (#C21CR2060).
This paper has been included in the Nordic Precision Cancer Medicine, NPCM, 2023 Symposia Collection
Funding Statement
This work was supported using resources and facilities of the Department of Veterans Affairs (VA) Informatics and Computing Infrastructure (VINCI), funded under the research priority to Put VA Data to Work for Veterans (VA ORD 22-D4V). This publication does not represent the views of the Department of Veterans Affairs or the United States Government.
Disclosure statements
None of the authors have a direct conflict of interest relevant to the subject of this study. More broadly, A.K. reports service on the Data and Safety Monitoring Committee for Bristol Meyers Squib and for Cellvax; he also reports consulting for Janssen, Merck, Bayer, and Blue Earth. A.J.L. reports consulting for MIM Software. T.M.S. reports honoraria from Varian Medical Systems and WebMD; he has an equity interest in CorTechs Labs, Inc. and serves on its Scientific Advisory Board; he has received in-kind research support from GE Healthcare via a research agreement with the University of California San Diego. J.A.L., K.M.L., and S.L.D. report grants from Alnylam Pharmaceuticals, Inc., Astellas Pharma, Inc., AstraZeneca Pharmaceuticals LP, Biodesix, Inc, Celgene Corporation, Cerner Enviza, GSK PLC, IQVIA Inc., Janssen Pharmaceuticals, Inc., Kantar Health, Myriad Genetic Laboratories, Inc., Novartis International AG, Parexel International Corporation through the University of Utah or Western Institute for Veteran Research outside the submitted work. These companies might potentially benefit from the research results. The terms of this arrangement have been reviewed and approved by the University of California San Diego in accordance with its conflict-of-interest policies.
Data availability statement
It is not possible for the authors to directly share the individual-level data that were obtained from the MVP due to constraints stipulated in the informed consent. Anyone wishing to gain access to this data should inquire directly to MVP at MVPLOI@va.gov. The data generated from our analyses are included in the manuscript main text, tables, and figures.
Ethics declarations and trial registry information
The MVP received ethical and study protocol approval from the VA Central Institutional Review Board in accordance with the principles outlined in the Declaration of Helsinki.
References
- [1].Kaur G, Kumar BVS, Singh B, Sethi RS. Exposures to 2,4-Dichlorophenoxyacetic acid with or without endotoxin upregulate small cell lung cancer pathway. J Occup Med Toxicol. 2021. Apr 17;16(1):14. 10.1186/s12995-021-00304-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Coggon D, Ntan G, Harris EC, Jayakody N, Palmer KT. Soft tissue sarcoma, non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia in workers exposed to phenoxy herbicides: extended follow-up of a UK cohort. Occup Environ Med. 2015. Jun;72(6):435–41. 10.1136/oemed-2014-102654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Goodman JE, Loftus CT, Zu K. 2,4-Dichlorophenoxyacetic acid and non-Hodgkin’s lymphoma: results from the Agricultural Health Study and an updated meta-analysis. Ann Epidemiol. 2017. Apr;27(4):290.e5–2.e5. 10.1016/j.annepidem.2017.01.008 [DOI] [PubMed] [Google Scholar]
- [4].Hoar SK, Blair A, Holmes FF, et al. Agricultural herbicide use and risk of lymphoma and soft-tissue sarcoma. JAMA. 1986. Sep 5;256(9):1141–7. 10.1001/jama.256.9.1141 [DOI] [PubMed] [Google Scholar]
- [5].Burns C, Bodner K, Swaen G, Collins J, Beard K, Lee M. Cancer incidence of 2,4-D production workers. Int J Environ Res Public Health. 2011. Sep;8(9):3579–90. 10.3390/ijerph8093579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Carlo GL, Sund KG. Carcinogenicity of dioxin. Lancet. 1991. Nov 30;338(8779):1393. 10.1016/0140-6736(91)92268-7 [DOI] [PubMed] [Google Scholar]
- [7].Manz A, Berger J, Dwyer JH, Flesch-Janys D, Nagel S, Waltsgott H. Cancer mortality among workers in chemical plant contaminated with dioxin. Lancet. 1991. Oct 19;338(8773):959–64. 10.1016/0140-6736(91)91835-I [DOI] [PubMed] [Google Scholar]
- [8].IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, World Health Organization & International Agency for Research on Cancer . Polychlorinated Dibenzo-para-dioxins and Polychlorinated Dibenzofurans. IARC Monographs Evaluat. 1997:69:1–631. [PMC free article] [PubMed] [Google Scholar]
- [9].Chang C, Benson M, Fam MM. A review of Agent Orange and its associated oncologic risk of genitourinary cancers. Urol Oncol. 2017. Nov;35(11):633–9. 10.1016/j.urolonc.2017.08.029 [DOI] [PubMed] [Google Scholar]
- [10].Pavuk M, Michalek JE, Schecter A, Ketchum NS, Akhtar FZ, Fox KA. Did TCDD exposure or service in Southeast Asia increase the risk of cancer in air force Vietnam veterans who did not spray agent orange? J Occup Environ Med. 2005. Apr;47(4):335–42. 10.1097/01.jom.0000158739.56427.24 [DOI] [PubMed] [Google Scholar]
- [11].Pavuk M, Michalek JE, Ketchum NS. Prostate cancer in US Air Force veterans of the Vietnam war. J Expo Sci Environ Epidemiol. 2006. Mar;16(2):184–90. 10.1038/sj.jea.7500448 [DOI] [PubMed] [Google Scholar]
- [12].Akhtar FZ, Garabrant DH, Ketchum NS, Michalek JE. Cancer in US Air Force veterans of the Vietnam War. J Occup Environ Med. 2004. Feb;46(2):123–36. 10.1097/01.jom.0000111603.84316.0f [DOI] [PubMed] [Google Scholar]
- [13].Shah SR, Freedland SJ, Aronson WJ, et al. Exposure to Agent Orange is a significant predictor of prostate-specific antigen (PSA)-based recurrence and a rapid PSA doubling time after radical prostatectomy. BJU Int. 2009. May;103(9):1168–72. 10.1111/j.1464-410X.2009.08405.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chamie K, DeVere White RW, Lee D, Ok J-H, Ellison LM. Agent Orange exposure, Vietnam War veterans, and the risk of prostate cancer. Cancer. 2008. Nov 1;113(9):2464–70. 10.1002/cncr.23695 [DOI] [PubMed] [Google Scholar]
- [15].Ansbaugh N, Shannon J, Mori M, Farris PE, Garzotto M. Agent Orange as a risk factor for high-grade prostate cancer. Cancer. 2013. Jul 1;119(13):2399–404. 10.1002/cncr.27941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wilson EJ, Horsley KW, van der Hoek R. Australian Vietnam Veterans Mortality Study 2005. Canberra: Department of Veterans’ Affairs, 2005. [Google Scholar]
- [17].Gaziano JM, Concato J, Brophy M, et al. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016. Feb:70:214–23. 10.1016/j.jclinepi.2015.09.016 [DOI] [PubMed] [Google Scholar]
- [18].Pagadala MS, Lynch J, Karunamuni R, et al. Polygenic risk of any, metastatic, and fatal prostate cancer in the Million Veteran Program. J Natl Cancer Inst. 2023. Feb 8;115(2):190–9. 10.1093/jnci/djac199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Huynh-Le MP, Karunamuni R, Fan CC, et al. Prostate cancer risk stratification improvement across multiple ancestries with new polygenic hazard score. Prostate Cancer Prostatic Dis. 2022. Apr;25(4):755–61. 10.1038/s41391-022-00497-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moses KA, Sprenkle PC, Bahler C, et al. NCCN Guidelines® Insights: prostate cancer early detection, Version 1.2023. J Natl Compr Canc Netw. 2023. Mar;21(3):236–46. 10.6004/jnccn.2023.0014 [DOI] [PubMed] [Google Scholar]
- [21].Conti DV, Darst BF, Moss LC, et al. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nat Genet. 2021. Jan;53(1):65–75. 10.1038/s41588-020-00748-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hugosson J, Roobol MJ, Månsson M, et al. A 16-yr Follow-up of the European Randomized study of Screening for Prostate Cancer. Eur Urol. 07 2019;76(1):43-51. doi: 10.1016/j.eururo.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
It is not possible for the authors to directly share the individual-level data that were obtained from the MVP due to constraints stipulated in the informed consent. Anyone wishing to gain access to this data should inquire directly to MVP at MVPLOI@va.gov. The data generated from our analyses are included in the manuscript main text, tables, and figures.