Abstract
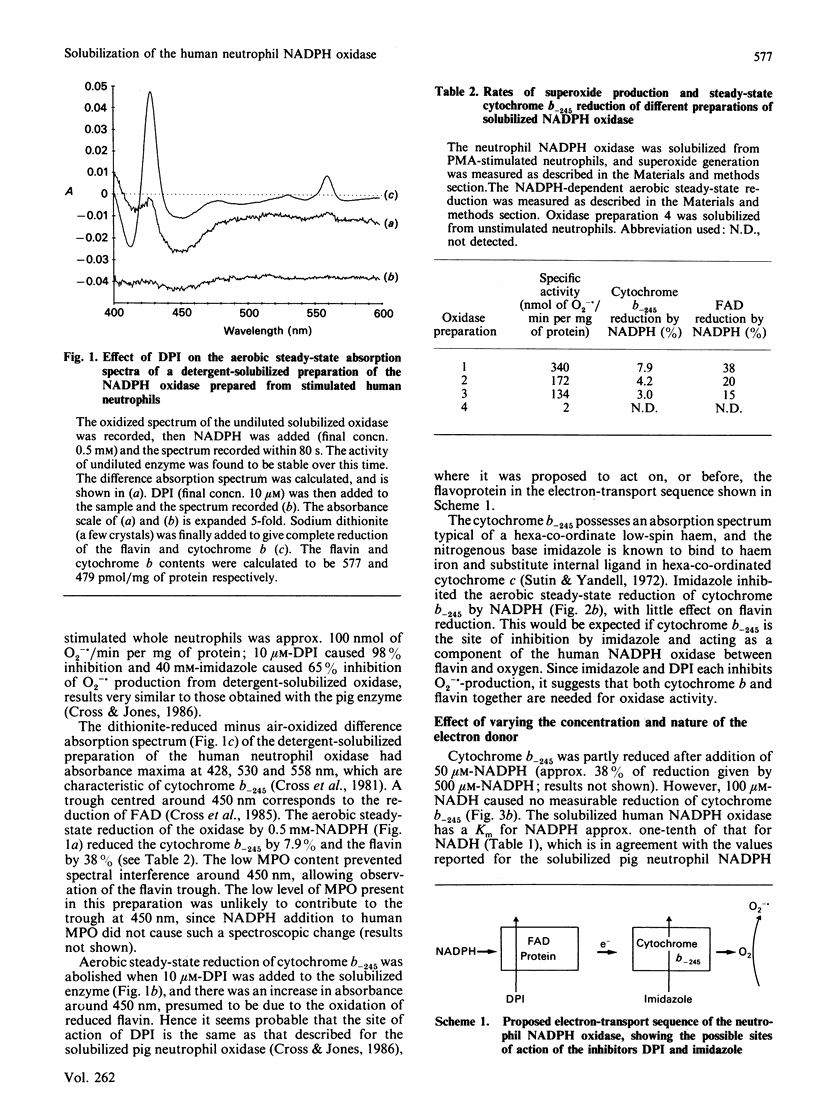
A superoxide-generating NADPH oxidase was solubilized from phorbol 12-myristate 13-acetate-activated human neutrophils with a mixture of sodium deoxycholate (0.125%, w/v) and Lubrol-PX (0.125%, v/v). The solubilized preparation contained FAD (577 pmol/mg of protein) and cytochrome b-245 (479 pmol/mg of protein) and produced 11.61 mol of O2-./s per mol of cytochrome b (340 nmol of O2-./min per mg of protein). On addition of NADPH, the cytochrome b-245 was reduced by 7.9% and the FAD by 38% in the aerobic steady state; NADH addition caused little steady-state reduction of cytochrome b and FAD. In this preparation, and several others, the measured rate of O2-. production correlated with the turnover of cytochrome b calculated from the extent of cytochrome b-245 reduction under aerobic conditions. Addition of diphenyleneiodonium abolished the reduction of both the FAD and cytochrome b-245 components and inhibited O2-. production. The haem ligand imidazole inhibited O2-. generation and cytochrome b reduction while permitting FAD reduction. These results support the suggestion that the human neutrophil NADPH oxidase has the electron-transport sequence: NADPH----FAD----cytochrome b-245----O2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggiolini M. The isolation of granules from neutrophile polymorphonuclear leukocytes (PMN's). Methods Enzymol. 1974;31:345–353. doi: 10.1016/0076-6879(74)31037-3. [DOI] [PubMed] [Google Scholar]
- Bellavite P., Papini E., Zeni L., Della Bianca V., Rossi F. Studies on the nature and activation of O2(-)-forming NADPH oxidase of leukocytes. Identification of a phosphorylated component of the active enzyme. Free Radic Res Commun. 1985;1(1):11–29. doi: 10.3109/10715768509056533. [DOI] [PubMed] [Google Scholar]
- Bellavite P. The superoxide-forming enzymatic system of phagocytes. Free Radic Biol Med. 1988;4(4):225–261. doi: 10.1016/0891-5849(88)90044-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bulkley G. B. The role of oxygen free radicals in human disease processes. Surgery. 1983 Sep;94(3):407–411. [PubMed] [Google Scholar]
- Cochrane C. G., Spragg R., Revak S. D. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983 Mar;71(3):754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Higson F. K., Jones O. T., Harper A. M., Segal A. W. The enzymic reduction and kinetics of oxidation of cytochrome b-245 of neutrophils. Biochem J. 1982 May 15;204(2):479–485. doi: 10.1042/bj2040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T., Garcia R., Segal A. W. The association of FAD with the cytochrome b-245 of human neutrophils. Biochem J. 1982 Dec 15;208(3):759–763. doi: 10.1042/bj2080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T., Harper A. M., Segal A. W. Oxidation-reduction properties of the cytochrome b found in the plasma-membrane fraction of human neutrophils. A possible oxidase in the respiratory burst. Biochem J. 1981 Feb 15;194(2):599–606. doi: 10.1042/bj1940599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J. 1986 Jul 1;237(1):111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Parkinson J. F., Jones O. T. Mechanism of the superoxide-producing oxidase of neutrophils. O2 is necessary for the fast reduction of cytochrome b-245 by NADPH. Biochem J. 1985 Mar 15;226(3):881–884. doi: 10.1042/bj2260881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Parkinson J. F., Jones O. T. The superoxide-generating oxidase of leucocytes. NADPH-dependent reduction of flavin and cytochrome b in solubilized preparations. Biochem J. 1984 Oct 15;223(2):337–344. doi: 10.1042/bj2230337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R. The inhibitory effects of some iodonium compounds on the superoxide generating system of neutrophils and their failure to inhibit diaphorase activity. Biochem Pharmacol. 1987 Feb 15;36(4):489–493. doi: 10.1016/0006-2952(87)90356-x. [DOI] [PubMed] [Google Scholar]
- Dieppe P. A., Doherty M. The role of particles in the pathogenesis of joint disease. Curr Top Pathol. 1982;71:199–233. doi: 10.1007/978-3-642-68382-4_7. [DOI] [PubMed] [Google Scholar]
- Doussiere J., Vignais P. V. Purification and properties of an O2-.-generating oxidase from bovine polymorphonuclear neutrophils. Biochemistry. 1985 Dec 3;24(25):7231–7239. doi: 10.1021/bi00346a032. [DOI] [PubMed] [Google Scholar]
- Ellis J. A., Mayer S. J., Jones O. T. The effect of the NADPH oxidase inhibitor diphenyleneiodonium on aerobic and anaerobic microbicidal activities of human neutrophils. Biochem J. 1988 May 1;251(3):887–891. doi: 10.1042/bj2510887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faeder E. J., Siegel L. M. A rapid micromethod for determination of FMN and FAD in mixtures. Anal Biochem. 1973 May;53(1):332–336. doi: 10.1016/0003-2697(73)90442-9. [DOI] [PubMed] [Google Scholar]
- Gabig T. G., Lefker B. A. NADPH oxidase from polymorphonuclear cells. Methods Enzymol. 1986;132:355–364. doi: 10.1016/s0076-6879(86)32020-2. [DOI] [PubMed] [Google Scholar]
- Glass G. A., DeLisle D. M., DeTogni P., Gabig T. G., Magee B. H., Markert M., Babior B. M. The respiratory burst oxidase of human neutrophils. Further studies of the purified enzyme. J Biol Chem. 1986 Oct 5;261(28):13247–13251. [PubMed] [Google Scholar]
- Hancock J. T., Jones O. T. The inhibition by diphenyleneiodonium and its analogues of superoxide generation by macrophages. Biochem J. 1987 Feb 15;242(1):103–107. doi: 10.1042/bj2420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyneman R. A., Bauwens-Monbaliu D. Kinetics of nicotinamide adenine dinucleotides in oleate-stimulated polymorphonuclear leukocytes. FEBS Lett. 1981 May 5;127(1):87–90. doi: 10.1016/0014-5793(81)80347-x. [DOI] [PubMed] [Google Scholar]
- Iizuka T., Kanegasaki S., Makino R., Tanaka T., Ishimura Y. Pyridine and imidazole reversibly inhibit the respiratory burst in porcine and human neutrophils: evidence for the involvement of cytochrome b558 in the reaction. Biochem Biophys Res Commun. 1985 Jul 31;130(2):621–626. doi: 10.1016/0006-291x(85)90462-0. [DOI] [PubMed] [Google Scholar]
- Ragan C. I., Bloxham D. P. Specific labelling of a constituent polypeptide of bovine heart mitochondrial reduced nicotinamide-adenine dinucleotide-ubiquinone reductase by the inhibitor diphenyleneiodonium. Biochem J. 1977 Jun 1;163(3):605–615. doi: 10.1042/bj1630605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin N., Yandell J. K. Mechanisms of the reactions of cytochrome c. Rate and equilibrium constants for ligand binding to horse heart ferricytochrome c. J Biol Chem. 1972 Nov 10;247(21):6932–6936. [PubMed] [Google Scholar]


