Abstract
Alzheimer's disease (AD) is a severe progressive neurodegenerative condition associated with neuronal damage and reduced cognitive function that primarily affects the aged worldwide. While there is increasing evidence suggesting that mitochondrial dysfunction is one of the most significant factors contributing to AD, its accurate pathobiology remains unclear. Mitochondrial bioenergetics and homeostasis are impaired and defected during AD pathogenesis. However, the potential of mutations in nuclear or mitochondrial DNA encoding mitochondrial constituents to cause mitochondrial dysfunction has been considered since it is one of the intracellular processes commonly compromised in early AD stages. Additionally, electron transport chain dysfunction and mitochondrial pathological protein interactions are related to mitochondrial dysfunction in AD. Many mitochondrial parameters decline during aging, causing an imbalance in reactive oxygen species (ROS) production, leading to oxidative stress in age-related AD. Moreover, neuroinflammation is another potential causative factor in AD-associated mitochondrial dysfunction. While several treatments targeting mitochondrial dysfunction have undergone preclinical studies, few have been successful in clinical trials. Therefore, this review discusses the molecular mechanisms and different therapeutic approaches for correcting mitochondrial dysfunction in AD, which have the potential to advance the future development of novel drug-based AD interventions.
Keywords: Alzheimer’s disease, mitochondria, mitochondrial dysfunction, drug target, therapeutic approaches, ROS
1. INTRODUCTION
Alzheimer’s disease (AD) is a progressive degenerative disease that impedes the cognitive and memory functions of the elderly worldwide [1]. Numerous studies have found that mitochondrial dysfunction may contribute to AD pathogenesis [2-4]. Important pathological traits in AD include amyloid β (Aβ) plaque accumulation, neurofibrillary tangle (NFT) formation by hyperphosphorylated tau protein (pTau), neuronal dystrophy, astrogliosis, biometal dyshomeostasis, increased oxidative stress, and decreased acetylcholine synthesis in the brain [5]. These phenomena have been associated with pre- and post-synaptic neuronal casualty. However, AD pathogenesis remains unknown, and no curative AD treatment has been found to date. Additionally, mitochondria are involved in key cellular processes, including calcium homeostasis, reactive oxidation species (ROS) production, apoptosis initiation, and metabolite secretion, controlling cell fate determination and function [6]. It has been reported that impaired mitochondrial function may significantly alter cellular and tissue homeostasis in AD. Furthermore, several studies have shown that genetic, biological, and environmental factors are associated with mitochondrial-mediated AD pathogenesis [7]. During AD development, mitochondrial function decreases, leading to changed mitochondrion morphology and synaptic space, reducing mitochondrial axonal transport levels [8]. Therefore, these deficits suggest that mitochondria dysfunction is associated with AD pathogenesis.
Neurodegenerative diseases are chronic debilitating conditions defined by the progressive and selective degeneration of neurons in the central or peripheral nervous system. Primary age-related neurodegenerative illnesses, such as AD and Parkinson's disease (PD), result in dementia, a leading cause of impairment globally and a significant public health burden with escalating healthcare costs. Mitochondrial dysfunction is associated with decreased energy production, poor calcium buffering, protease and phospholipase activation, and increased oxidative stress in AD and PD. Increased oxidative stress and accumulation of mitochondrial DNA (mtDNA) mutations result in mitochondrial malfunction and play a crucial role in the aging process and AD and PD development. AD is the most common cause of dementia. It is marked by the extracellular buildup of Aβ peptides in senile plaques and the intracellular buildup of tau protein in NFTs in the brain’s gray matter. When Aβ and pTau aggregates interact, metabolic decline occurs in limbic structures, such as the cingulate, orbitofrontal, medial, and basal temporal cortices, leading to AD.
Changes in the size and number of mitochondria in neurons are indicative of potential changes in mitochondrial dynamics in AD. However, the presence of proteinaceous deposits within neuronal perikarya (Lewy bodies) and processes (Lewy neurites) is one of the pathological hallmarks of sporadic PD. These deposits primarily comprise alpha-synuclein, ubiquitin (UBB), neurofilaments, and molecular chaperones. What function Lewy bodies play in PD progression and whether they are a pathognomonic sign of PD remains unclear. Rotenone is a well-known inhibitor of complex I of the mitochondrial electron transport chain (ETC) that has been used to induce mitochondrial dysfunction and as a preclinical PD model. It inhibits mitochondrial complex I causing loss of nigrostriatal neurons, rigidity, hypokinesia, and the buildup of alpha-synuclein- and UBB-containing fibrillar inclusions. Moreover, it causes neuronal death via oxidative stress, adenosine triphosphate (ATP) depletion, endoplasmic reticulum stress, and the expression of activating transcription factor 4 (ATF4), phosphorylated pancreatic endoplasmic reticulum kinase/PKR-like endoplasmic reticulum kinase (PERK), immunoglobulin heavy-chain binding protein (HSPA5), and DNA damage-inducible protein/C/EBP homologous protein (CHOP).
There is increasing evidence indicating that mitochondrial dysfunction occurs in healthy aging and diseases, particularly AD [5]. Mitochondrial dysfunction in AD induces phosphorylation of tau and Aβ accumulation. In addition, mitochondria contribute to various biochemical pathways in cells, including steroid hormone synthesis, calcium homeostasis, ATP production, apoptosis, and energy efficiency control [9]. Currently, it is well-known that central cellular pathways are compromised in AD with intraneuronal NFTs made of pTau and extraneuronal senile plaques (SP) made of Aβ, vascular damage, synaptic failure, neuronal and axonal injury, oxidative stress, and microglia-mediated neuroinflammation [10]. Therefore, current efforts are directed toward exploring the potential targets and underlying mechanisms of mitochondrial dysfunction in AD pathogenesis, focusing on describing the function of mitochondrial dysfunction and the status of mitochondrial therapy in AD.
2. MOLECULAR MECHANISM OF AD
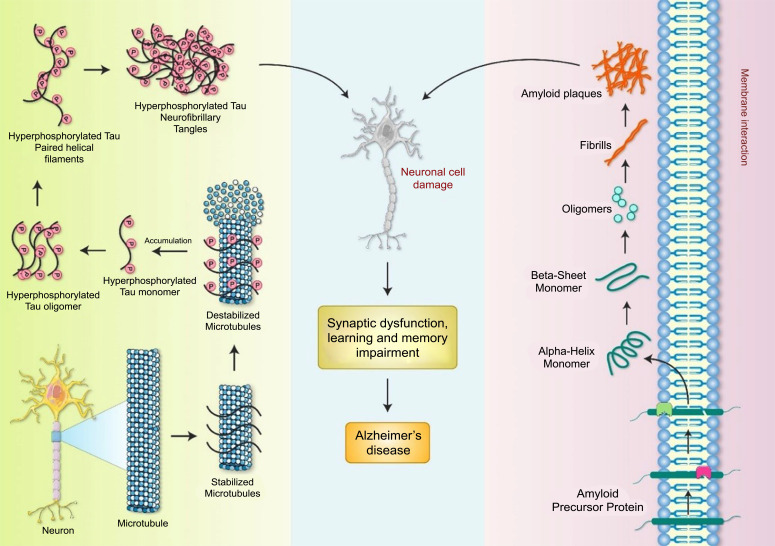
AD is a common neurodegenerative disorder characterized by memory loss and the development of pTau and Aβ aggregates in numerous brain areas, including the hippocampus and cortex [11]. The clinical and pathological AD manifestations include memory impairment and sluggishness when performing typical daily tasks [12]. The principal pathogenic hallmarks are intracellular aggregates formed by extracellular Aβ and pTau protein deposition, creating NFTs and contributing to SP development [13-16] (Fig. 1). Tau protein aggregates have been found in the proximal axon, and large, insoluble tau aggregates containing irreversibly pTau species do not migrate through axons [13]. Toxic tau species may harm the transport mechanism in the axon, reducing amyloid precursor protein (APP) transport to the synapse, thus causing APP buildup in the soma and Aβ aggregation [17]. The hyperphosphorylation of the microtubule-associated protein tau causes it to aggregate in an insoluble state, creating NFTs [18]. The NFT development process and whether NFTs are a major cause of illness or play a more peripheral function remains unknown. The existence of NFTs has been found to be strongly associated with the degree of cognitive impairment in disorders, such as AD [19]. Investigating potential associations between tau and NFTs is crucial for gaining a mechanistic understanding of events contributing to the onset of AD [19]. Mitochondrial dynamics are disrupted in AD, leading to the identification of fission protein inhibitors, such as dynamin-related protein 1 (DRP1) and drugs that induce fusion [20]. Modifications to the adenosine monophosphate (AMP)-activated protein kinase (AMPK), sirtuin 1 (SIRT1), and protein kinase B (AKT) pathways may potentially be superior therapeutic options because these pathways influence mitochondrial activities [21]. Oxidative phosphorylation is a primary source of ROS, which causes mitochondrial damage in AD [22].
Fig. (1).
The molecular pathophysiology of AD. APP is cleaved by α-secretases and β-secretases, resulting in the accumulation of neurotoxic Aβ in plaques. NFTs are aggregates of pTau protein in the nervous system. AD is characterized by synaptic disruption and memory impairment caused by Aβ plaques and NFTs.
3. FUNCTIONAL ROLE OF MITOCHONDRIA
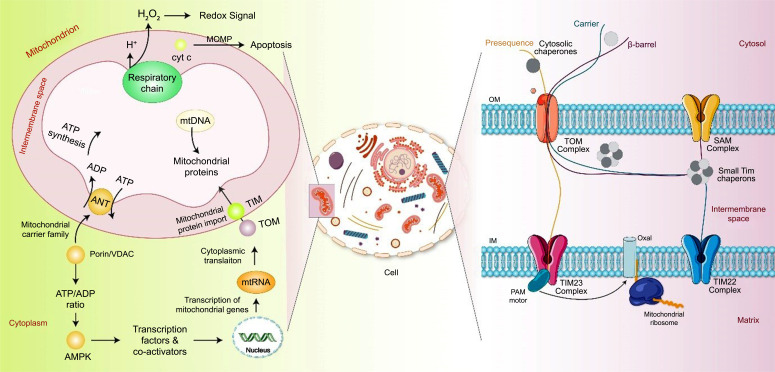
Mitochondria are the cell's energy producers, supplying the majority of ATP via oxidative phosphorylation [23]. Mitochondria are comprised of compact sac-like structures made up of two layers, the outer (OMM) and inner (IMM) mitochondrial membranes, which are chemically and structurally different [6]. The OMM is composed of equal parts lipids and proteins and can be transited by molecules with a molecular weight of 6 kDa [24]. IMM is a highly specialized unit membrane that folds inward to produce a ridge, increasing its surface area [25]. IMM has poor permeability, and proteins make up ~76% of its total weight. Many chemicals, such as hydrogen ions (H+), ATP, and pyruvate, require carriers to transport them across the IMM [26]. The intermembranous gap is located between the OMM and the IMM. The mitochondrial matrix encompassed by the IMM contains different enzymes, ribosomes, and mtDNA and RNA [27]. These traits might be exploited to develop mitochondrial-targeted strategies in AD pathology. A detailed mitochondrial schematic representation is shown in Fig. (2).
Fig. (2).
Mitochondrial biogenesis and function. Mitochondria play an important role in ATP synthesis through oxidative phosphorylation. Glycolysis occurs in the cytosol and is responsible for the initial breakdown of glucose into pyruvate. The mtDNA encodes 37 genes involved in synthesizing the respiratory chain and ATP production. Additional proteins are imported into mitochondria by the translocases of the inner (TIM) and outer (TOM) membranes, which are present in the IMM and OMM and are responsible for transporting nuclear-encoded proteins into mitochondria. Additionally, mitochondrial calcium signaling is facilitated by calcium uptake into the mitochondrial matrix by the calcium uniporter (CaU) in response to intracellular calcium fluctuation. Furthermore, mitochondria play an important role in the apoptosis process. When apoptotic signals are received, the OMM is compromised, resulting in OMM permeabilization (MOMP) and the release of cytochrome c (Cyt-c) and other pro-apoptotic proteins from the intermembrane space into the cytosol, causing apoptotic cell death.
Neurons consume the most ATP of any cell type to sustain the ionic gradients required for continuous neurotransmission, electrophysiological activity, and short-term synaptic plasticity [28]. The primary purpose of mitochondria is to provide ATP and oxygen to the cell, which requires electron transport via the ETC [29]. Located on the IMM, the ETC comprises five proteins, complexes I-V. Complexes I, III, and IV function as proton pumps that transfer H+ from the intermembranous region to the matrix [30]. Consequently, a proton gradient is created, resulting in a significant negative internal membrane potential (ΔΨm) on the IMM of roughly 160 to 180 mV, influencing the entrance of drugs into the mitochondria [31]. Surprisingly, around 0.4-4% of the electrons traveling through the ETC are not fully restored, resulting in the production of primary ROS-superoxide anions (O2-). Excessive superoxide anion production interacts with various different substances to produce secondary ROS and cause oxidative damage [32]. Many ROS by-products are produced in the brain due to high energy demand and rapid ATP production/consumption. ROS overproduction or dysregulation of the antioxidant system disrupts normal mitochondrial activity, resulting in inflammation, apoptosis, memory loss, and brain/neuronal damage [33]. Mitochondrial dysfunction is key in the etiology of AD [34]. Neuronal mitochondrial dysfunction caused by chronic ROS production accelerates the degenerative process underlying AD, such as increased Aβ aggregation and NFT formation [35].
4. MITOCHONDRIAL DYSFUNCTION IN AD PATHOGENESIS
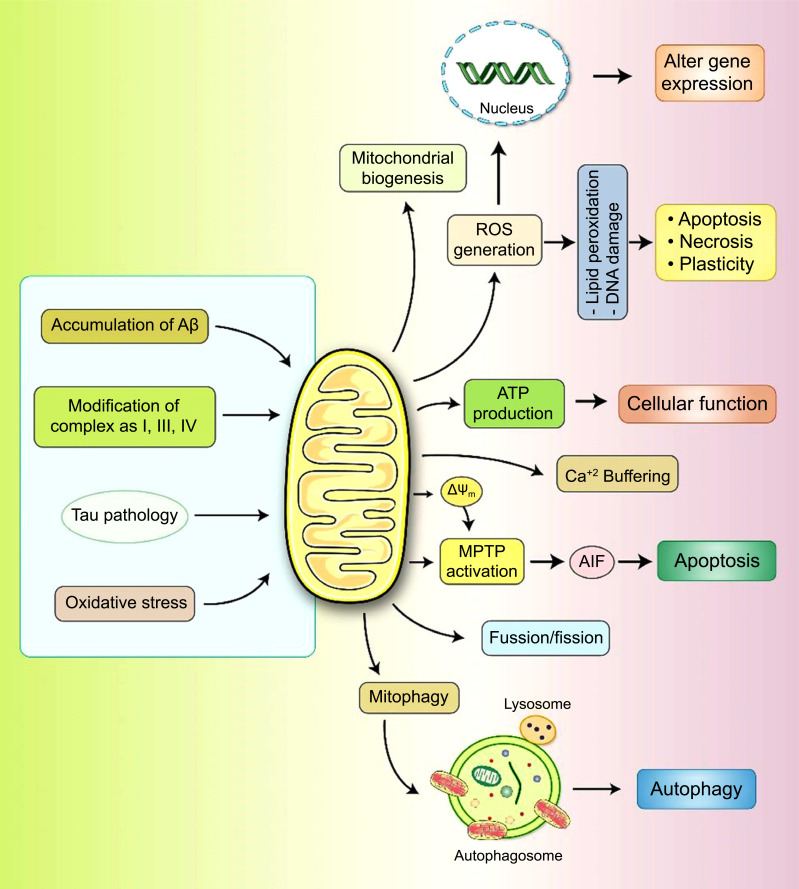
It has been found that mitochondrial functions and cellular homeostasis are sustained by the dedicated mitochondrial quality control system (mtQCS) [1] that contains diverse biochemical mechanisms that act at different levels, from individual polypeptides to the whole organelle [1]. However, the morphology and role of mitochondria are strictly associated with continuous fusion-fission dynamics in the cellular system [36]. The cause and effects of mitochondrial dysfunction in AD pathogenesis are illustrated in Fig. (3). While evidence of mitochondrial dysfunction in AD has been discussed, the actual cause remains unknown, and the defective metabolism characteristic of AD may be simply reflecting mitochondrial dysfunction.
Fig. (3).
The role of mitochondrial dysfunction in AD etiology. Aβ and tau are known to cause mitochondrial dysfunction, resulting in the modulation of many other variables. ROS production results in the formation of lipid peroxidation and DNA damage, triggering apoptosis. The activation of mitochondrial permeability transition pores (mPTPs) results in damaged mitochondria with decreased ΔΨm, causing the release of Cyt-c and apoptosis-inducing factor (AIF) and the induction of the apoptosis pathway. The antioxidants Aβ and pTau enhance mitochondrial fission and mitophagy.
4.1. Dysfunction of Amyloid Cascade in AD
Over the last 30 years, the amyloid cascade hypothesis has dominated AD research and is supported by evidence. Firstly, extraneuronal SPs are comprised of Aβ peptides, and mutations in its precursor APP cause an early-onset form of AD [37,38]. Secondly, Aβ-induced mitochondrial fission is increased in the presence of S-nitrosylated DRP1 [39]. Furthermore, Aβ plaque accumulation causes cellular mitochondrial dysfunction toxicity and abnormal mitochondrial structure [17]. Conversely, dysfunctional mitochondrial morphology in AD neurons inhibits mitochondrial function and reduces mitochondrial content in addition to neuronal function [40]. However, this hypothesis has lost importance following the failure of Phase III clinical AD trials and must be revised or incorporated into other hypotheses [41].
4.2. Dysfunctional Mitochondrial Dynamics in AD
Recently, it has been shown that mitochondrial morphology and function are intimately associated with the continuous fusion-fission dynamics in cells [42]. The five essential proteins involved in controlling dynamics are mitochondrial fission protein 1 (FIS1), DRP1, optic atrophy 1 (OPA1), and mitofusin 1 (MFN1) and 2 (MFN2) [43]. Mitochondria fusion and fission proteins are variably present in the AD hippocampus, with increased FIS1 and significantly decreased DRP1 expression alone or with the fusion proteins OPA1, MFN1, and MFN2 [44]. During cell division, the fusion-fission cycle controls genetic complementation, mitochondrial functionalization, and the correct distribution of newly generated mitochondria [45]. Conversely, in AD, more frequent fission than fusion may increase the overall quantity of palingenetic mitochondria. Similarly, APP overexpression in neural cells was found to cause mitochondrial fragmentation by modifying levels of mitochondrial fusion and fission proteins [46].
4.3. Dysfunctional Mitochondrial Biogenesis in AD
Mitochondrial biogenesis generally occurs regularly in normal cells and in response to oxidative stress and increased energy consumption in some neurodegenerative disorders [47]. Mitochondrial biogenesis compensates for damaged mitochondria and is critical in sustaining an appropriate functional mass of neuronal mitochondria in AD [48]. Coordination and interaction between the nuclear (nDNA) and mtDNA play a significant role in this process during AD. Additionally, recent studies have shown that proliferator-activated receptor coactivator 1 (PGC-1) can control mitochondrial biogenesis by activating transcription factors, including nuclear respiratory factors 1 (NRF1) and 2 (NRF2) and mitochondrial transcription factors A (TFAM) [49]. Similarly, PGC-1 activity is influenced by mitochondrial damage, nutrient availability, and energy balance in cells [50]. Damaged mitochondria were associated with lower NRF1, NRF2, TFAM, and PGC-1 levels in the HEK293-APPswe AD cell model [51]. Furthermore, other studies have found decreased PGC-1-mediated mitochondrial biogenesis signaling in mice with early-stage AD [52]. Therefore, reduced mitochondrial biogenesis is a significant feature of AD.
4.4. Dysfunction of Mitochondrial Membrane Potential in AD
A mitochondrial membrane potential (MMP) is generated when protons are pumped from the mitochondrial matrix into the intermembrane space via the ETC [53]. MMP is the most important component of the mitochondrial electrochemical potential gradient, the depletion of which leads the ETC to uncouple ATP phosphorylation [54]. Therefore, lowering local oxygen tensions by restricting the half-life of ETC intermediates results in a modest drop in MMP and reduced ROS production [55]. However, studies have shown that APP/PS1 transgenic mouse brains have lower ATP levels and complex IV activity and greater oxidative stress than controls [56]. Furthermore, MMP levels are lower in AD animal models and human cortical neurons.
4.5. Dysfunctional Ca2+ Homeostasis in AD
The high-capacity Ca2+ pool decreases with normal neural activity, and mitochondria play an important role in preserving cellular Ca2+ homeostasis by keeping mitochondrial Ca2+ levels aligned with variations in cytosolic Ca2+ load [57]. Mitochondria use an ATP-powered or MMP-driven Ca2+/H+ pump to take up Ca2+ in association with H+ [58]. Furthermore, the opening of Ca2+-mediated permeability holes is functionally connected to another Ca2+ absorption pathway [59]. Excess Ca2+ absorption via mitochondria promotes ROS production, slows ATP synthesis, activates the mitochondrial permeability transition pore (mPTP), and sometimes even causes cell death [60]. Accordingly, Aβ buildup can cause cellular damage in neurons by promoting ROS production and altering Ca2+ homeostasis in mitochondria [61].
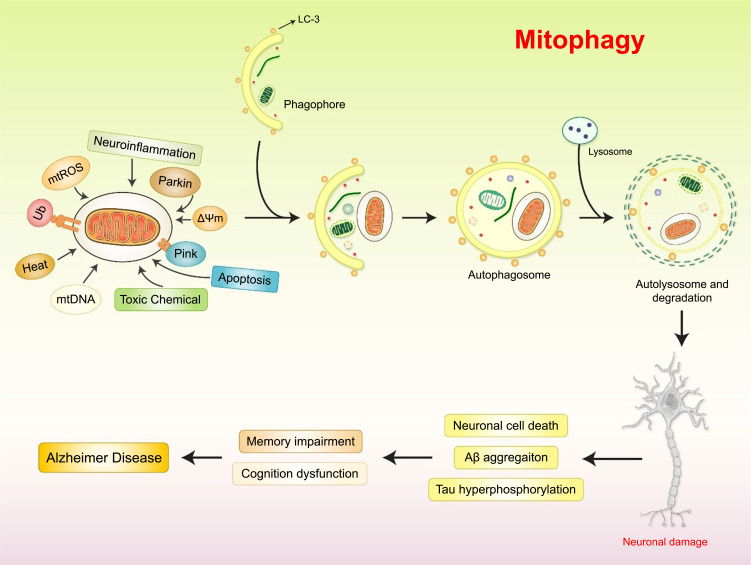
4.6. Dysfunctional Mitophagy in AD
Mitophagy is the process by which injured mitochondria are enveloped by autophagosomes and transported to lysosomes for degradation and recycling [62]. Impaired autophagy has been found to cause a buildup of aberrant mitochondria in AD neuronal cells, exacerbating mitochondrial dysfunction [63]. While mitophagy is induced by ROS produced by injured mitochondria, excessive ROS production suppresses this process [64]. Interestingly, the mammalian target of rapamycin (mTOR) complex, which is highly active in the AD hippocampus brains, directly regulates mitophagy [64]. Recently, mitophagy was found to reduce Aβ and tau pathology and cognitive impairment in AD pathogenesis [65]. Nevertheless, impediments to the clearance of damaged mitochondria and cellular oxidative stress might cause the aggregation of defective AD brain neurons [66]. Consequently, developing drugs that target mitochondrial malfunction to restore MMP and Ca2+ equilibrium might provide a new paradigm for AD treatment. Therefore, if mitophagy is also substantially impaired in AD, it could lead to the buildup of damaged mitochondria and malfunctioning neurons, potentially disrupting the fusion of autophagosomes and lysosomes in AD.
5. EMERGING TREATMENT APPROACHES TO TARGET MITOCHONDRIAL DYSFUNCTION IN AD
A viable and practical method for slowing or reducing brain damage is the design of pharmaceutical molecules that target mitochondrial dysfunction to modify mitochondrial bioenergetics for homeostasis by restoring mitochondrial function. Currently, the standard treatments for AD are cholinesterase inhibitors (donepezil, galantamine, and rivastigmine) and memantine, which inhibit the N-methyl-D-aspartate (NMDA) receptor and excess glutamate activities [67]. NMDA receptors and acetylcholine (ACh) are essential in memory and learning functions, and their concentration and function are impaired in AD [68]. However, these drugs improve cognitive and memory function without slowing disease development [69]. Moreover, since the concept of AD as a multifaceted illness has gained traction recently, a reassessment of mitochondrial-targeted therapy in combination with other drugs is strongly advised. Currently, the US Food and Drug Administration (FDA) has approved four drugs to treat AD. Acetylcholine esterase (AChE) inhibitors are a group of three drugs: donepezil, rivastigmine, and galantamine. Memantine, which blocks NMDA receptors, is the fourth treatment option, and combined donepezil and memantine is the fifth [70]. Recent AD studies have focused on the gut and its inhabitants, the microbiome, which represents another important therapeutic consideration [70].
5.1. Phytochemicals Targeting Mitochondrial Dysfunction in AD
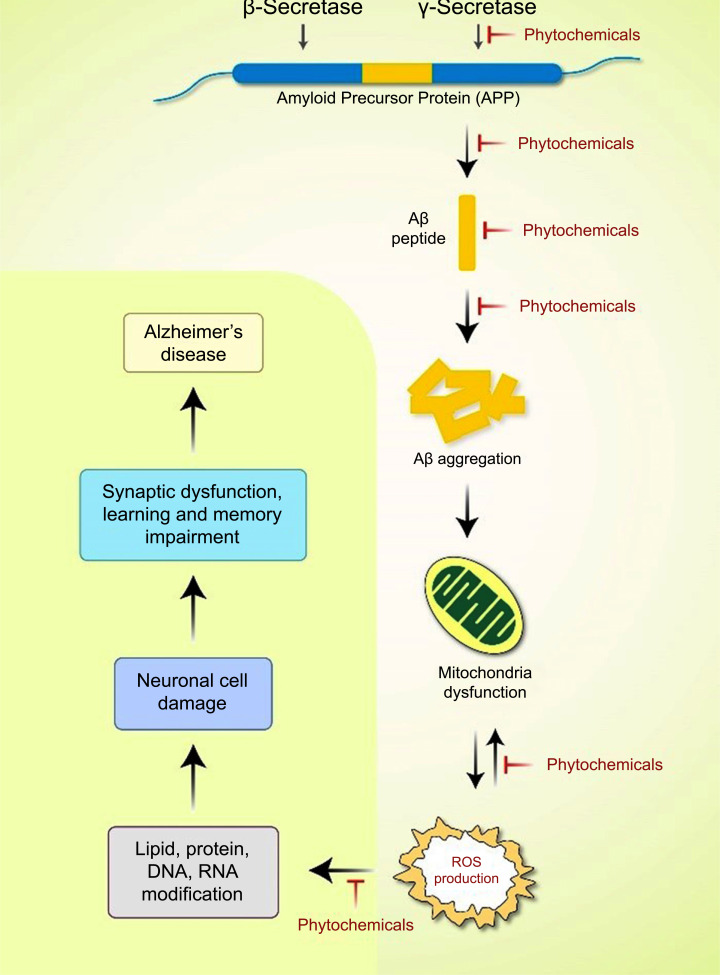
Several studies have shown the beneficial treatment potential of antioxidants and mitochondria-targeting drugs, such as vitamin C, vitamin E, carnitine, and alpha-lipoic acid in AD [71]. Coenzyme Q10, curcumin, piracetam, simvastatin, piracetam, ginkgo biloba, and omega-3 polyunsaturated fatty acids have also been effective therapeutic agents [72]. A viable treatment method for AD that targets mitochondrial proteins can be developed, and various mitochondria-targeted antioxidants have been created with this approach. Changes in mitochondrial mobility have a deleterious effect on mitochondrial function, contributing significantly to AD development [73]. Consequently, efforts to correct faulty mitochondrial mobility and transport may constitute a viable therapeutic strategy for AD treatment. Therapeutics that inhibit the activation of mitochondrial fission proteins, such as DRP1, pTau, and Aβ, can protect neurons against the harmful effects of these drugs and their interaction. A wide range of phytochemicals found in various plant sources show various pharmacological effects, including apoptosis induction [74-81], neuroprotection [82, 83], autophagy activation [84-88], antioxidant effects [89], DNA repair functions [90], and anti-inflammatory activity [91].Therefore, phytochemicals are increasingly evaluated as promising therapeutic possibilities for AD treatment because of these properties [92] (Fig. 4).
Fig. (4).
Phytochemicals are emerging as potential treatments for mitochondrial dysfunction in AD development. Abnormal APP is digested by the β- and γ-secretases, resulting in the buildup of extracellular Aβ. When there is insufficient clearance of Aβ or Aβ production, aggregation occurs, resulting in the buildup of diverse Aβ assembly types. Aβ accumulation is directly associated with mitochondria, and ROS production is directly associated with other intracellular pathways. Neurological degeneration and synaptic function dysregulation in brain regions implicated in learning and memory impairment in AD are caused by these oxidative stress reactions, which have a multifactorial mechanism of action, in addition to neurological degeneration and synaptic dysregulation function.
Melatonin is a natural compound derived from plants and animals that increases mitochondrial biogenesis factors, such as PGC-1, NRF1, NRF2, and TFAM, MMPs, such as Na+-K+-ATPase and cytochrome C (Cyt-c), ATP levels, mtDNA/nDNA ratio, and mitochondrial structure, and decrease amyloidogenic APP processing in AD [51]. Melatonin has a strong neuroprotective effect and can stop or slow AD progression, supporting the view that it could be used to treat AD. Melatonin alters the transcription regulatory network and activity of secretases, inhibiting amyloidogenic APP processing and Aβ production [93]. Therefore, further research is required to investigate the modulating effects of melatonin on the structure and function of mitochondria in AD.
Curcumin's neuroprotective impact on AD is widely recognized. Curcumin protects SH-SY5Y human neuroblastoma cells against Aβ-mediated mitochondrial dysfunction and synaptic damage [94]. In preclinical trials, quercetin restored mitochondrial dysfunction by restoring MMP, resulting in decreased ROS production and restoring ATP synthesis [95]. Moreover, this therapy dramatically increased AMPK expression, reduced dispersed senile plaque formation, and suppressed learning and memory impairment [95]. Long-term oral quercetin treatment to triple transgenic AD mice resulted in decreased tauopathy, astrogliosis, microgliosis, and amyloidosis in the amygdala and hippocampus, which enhanced cognitive function, learning performance, and spatial memory function [96, 97].
Different phytochemicals and other chemicals used in mitochondrial-targeted AD treatments in preclinical and clinical studies are listed in Table 1. Human trials are being conducted to determine whether omega-3 fatty acids obtained by eating fish can prevent coronary artery disease, stroke, aging, dementia, and AD [98]. Conversely, flavonoids and polyphenols from Mediterranean diets have antioxidant and anti-inflammatory effects on cardiovascular disease, type 2 diabetes, cancer prevention, and stroke in humans [98]. Polyphenols found in fruits and vegetables have been shown to control tau hyperphosphorylation and Aβ aggregation in animal AD models [98]. The soy isoflavonoid genistein has therapeutic potential in various aging-related mitochondrial dysfunction in pathological conditions, such as neuroinflammation, oxidative stress, and Aβ aggregation in AD [99]. Its therapeutic effect was related to its capacity to ameliorate mitochondrial function deficits caused by Aβ aggregates [100]. A larger dosage of genistein (150 mg/kg/day) was recently shown to activate autophagy in the streptozotocin-induced rat model of sporadic AD [101]. Furthermore, therapy with genistein resulted in the total degradation of tau hyperphosphorylation and Aβ protein in brains with mitochondrial dysfunction. Recently, genistein-loaded nanocomposites have been developed, showing the potential of oral delivery and overcoming the harmful isoflavonoid effects [102].
Table 1.
The effects of several phytochemicals on mitochondrial dysfunctions in AD pathogenesis.
| Phytochemical | Experimental Model | Pathobiology | Molecular Signaling | Research Outcomes | References |
|---|---|---|---|---|---|
| Liquiritigenin | Aβ-mediated SK-N-MC cell AD model |
Mitochondrial fragmentation |
MFN1, MFN2, and OPA1 signaling accumulation | Prevent cytotoxicity and mitochondrial fragmentation |
[103] |
| Genistein | APP/PS1 rat model of sporadic AD |
Increased Aβ- and tau protein | Autophagy induction and decreased protein aggregates | Enhanced memory and learning function | [101, 104] |
| Anthocyanins | APPswe double mutation | Oxidative stress and mitochondrial dysfunction | Improved NADH levels | Increased mitochondrial dysfunction |
[105] |
| Quercetin | Sprague-Dawley rat H2O2-induced neurotoxicity | Oxidative stress | Improved Aβ clearance | Neuroprotection | [106] |
| Sulfuretin | Aβ neurotoxicity in SH-SY5Y cells and primary hippocampal neurons |
Oxidative stress | PI3K/AKT and NRF2/HO activation | Neuroprotection | [107] |
| Epigallocatechin-3-gallate (EGCG) | Primary cortical rat neurons | Pathological tau species | Improved autophagy and tau clearance |
Improved NRF2-dependent tau degradation |
[108] |
| Curcumin | Sprague-Dawley rats | Cerebral ischemia | Improved autophagy by PI3K/AKT/mTOR pathway | Neuroprotection | [109] |
| Resveratrol | Aβ-induced cytotoxicity in PC12 cells | Oxidative stress | Decreased ROS, activated SOD | Reduced memory impairment and neuroprotection | [110] |
| Polyphenols | SH-SY5Y neuroblastoma cells | Oxidative stress | Initiation of KEAP1-NRF2 signaling | Neuroprotection | [111] |
| Kaempferol | Porcine embryos | Oxidative stress | Activated autophagy | Prevented MMP and ROS | [112] |
5.2. Mitophagy-targeted Mitochondrial Dysfunction in AD
The efficient removal of old and malfunctioning mitochondria via mitophagy, a cargo-selective form of autophagy, is important for the preservation of mitochondrial function and neuronal health [113]. Mitophagy has been the subject of numerous mechanistic studies, which have uncovered a complex and interconnected cellular network governing mitochondrial turnover [114]. Impaired mitophagy occurs early in AD brains and plays a causal role in the development of AD-associated neuropathology. The findings of various AD studies suggest that increased mTOR activity may result in deficient mitophagy in the hippocampus and other brain locations [55]. More importantly, mTOR phosphorylation levels are altered in the brains of AD patients and are associated with the presence of pTau [115]. Consequently, modulation of the mTOR signaling pathways may prove to be an effective strategy for AD intervention and treatment. The mTOR inhibitor rapamycin was shown to be effective in AD treatment, restoring mitophagy/autophagy, increasing the levels of microtubule-associated protein 1 light chain 3 (LC3) II/I and other autophagy-related proteins, suppressing the mTOR pathway [16, 116, 117]. Consequently, mTOR inhibition has garnered much attention as a potential AD treatment. Pharmacological reinstallation of mitophagy has beneficial effects on amyloid and tau pathologies in AD animal models with therapeutic effects on memory loss [118]. Further studies using neurons grown from induced pluripotent stem cells (iPSCs) of sporadic AD or other comparable models could be extremely important in determining whether mitophagy failure is a crucial factor in the development of Aβ/tau proteinopathies.
Mitophagy involves identifying malfunctioning or redundant mitochondria, developing and maturing phagophores, fusion with the lysosome, and degrading mitochondria (Fig. 5). Reduced MMP promotes the stability of PTEN-induced kinase 1 (PINK1) in the OMM. PINK1 is activated by autophosphorylation, after which it phosphorylates MFN2 and UBB, resulting in the recruitment of Parkin to the OMM surface [119]. A basic understanding of mitophagy deficiency in AD progression and the fundamental molecular mechanisms that regulate mitophagy are required before understanding the interplay between AD pathology and mitophagy deficiencies.
Fig. (5).
Mitophagy mechanism in AD pathogenesis. Parkin ubiquitylates various OMM elements, which are then identified by the adaptor proteins optineurin (OPTN), p62, nuclear dot protein 52 kDa (NDP52), and NBR1 autophagy cargo receptor (NBR1), recruiting the damaged mitochondria to the autophagy process and triggering autophagosome production via interactions with LC3. Additionally, MMP, toxic chemicals, mitochondrial ROS (mtROS), heat, and neuroinflammation are keys factor in initiating mitochondrial-mediated autophagy, damaging neuronal cells.
Latrepirdine is an antihistamine drug that reduces mitochondrial enlargement under Aβ stress in cell cultures of AD mice models and stabilized MMPs [120]. The collaboration between latrepirdine and glutamate receptors decreased mitochondrial permeability and blocked voltage-dependent calcium channels in HEKsw cells, preventing unwanted mitophagy or apoptosis [120]. In addition, there is accumulating evidence that EGb761 extract suppressed the mPTP production and reduced tau hyperphosphorylation and cognitive impairment in AD rat models [121]. Clinical trials examining the efficiency of EGb761 extract in treating dementia have indicated that it has beneficial effects on cognition and daily activities [122]. Consequently, mPTP inhibition in mitophagy is a promising therapeutic target for AD treatment.
Inducing mitophagy via pharmaceutical treatment reduced neuroinflammation and improved cognitive performance in an AD mouse model. Increased expression and activity of the NLR family pyrin domain containing 3 (NLRP3) inflammasome have been found in the brain tissues of APP/PS1 AD mice [123]. However, restoration of neuronal mitophagy with the mitophagy-inducing chemicals urolithin A and actinonin reduced neuroinflammation, indicated by decreased levels of cleaved caspase 1 (CASP1), proinflammatory interleukin 1β (IL-1β), and IL-1β [124]. Furthermore, it may be beneficial to design drugs or techniques to activate the NAD+‐dependent protein deacetylase SIRT1 to protect against AD [125]. Photobiomodulation therapy (PBMT) activated the cyclic AMP (cAMP)/protein kinase A (PKA) pathway, increasing SIRT1 deacetylase activity in APP/PS1 AD neurons [120]. Therefore, AD could be treated by PBMT-mediated SIRT1 overexpression, thus reducing Aβ production [126], indicating that targeting mitophagy pathways may have potential therapeutic effects on AD.
5.3. Lifestyle Modification and Physical Activity in AD
A Mediterranean diet, calorie restriction, and physical activity can improve human aging and decrease the risk of neurological diseases [127]. The Mediterranean diet is comprised mostly of fruits, vegetables, and omega-3 fatty acids, which are abundant in olive oil and fish. Studies have found that extra-virgin oil rich in polyphenols decreased mitochondrial-associated oxidative stress and insulin resistance in rats fed a high-fat diet [128]. In addition, oleuropein aglycone (OLE), another polyphenol ingredient of olive oil, stimulated autophagy, decreased aggregated protein levels, and decreased cognitive impairment in AD patients’ brains [129]. Another bioactive component in olive oil, hydroxytyrosol (HT), improved mitochondrial dysfunction in an AD animal model [130].
When there is a limited supply of glucose available in the brain, ketones can be used as an alternative energy source. A ketone ester diet had beneficial effects on mitochondrial function in the 3xTg AD model [127]. Therapeutic ketosis has been proposed to prevent AD brain pathology progression, including the buildup of plaques and NFT [131]. Additionally, calorie restriction (CR), reduced calorie consumption without nutrient deficiency, is a promising approach to enhance longevity and insulin sensitivity and prevent age-related diseases [132]. CR increased mitophagy and ATP levels, decreased ROS, and improved mitochondrial quality and cell bioenergetics in AD [133]. Positive effects of CR were observed at the mitochondrial level, where it was found to influence mitochondrial biogenesis via activation of NO synthase (eNOS) [134].
Physical exercise (PE) has a positive effect on physical and mental health, including brain plasticity and cognitive function. PE treatment improved mitochondrial respiratory function by increasing respiratory chain complex (RCC) activity and reducing ROS production capacity in conjunction with Aβ1-42 peptide levels and improved cognitive function in the hippocampus of the APP/PS1 transgenic AD mouse model [135]. Maternal exercise while pregnant had a beneficial effect on mitochondrial function at the onset of AD. Moreover, it also had a preventive role against Aβ oligomer-induced neurotoxicity in the brains of adult offspring rats [136]. Clinical PE trials were conducted in older individuals with healthy or impaired cognitive function, leading to altered Aβ1-42 levels in plasma and cerebrospinal fluid (CSF). Altered cognitive and executive functioning, hippocampus volume, and memory were observed and were associated with reduced brain atrophy [137, 138]. Therefore, all animal-model-based research supports the view that PE may also have beneficial effects on mitochondrial function and glucose metabolism in humans.
5.4. Glucose Metabolism Targeting Mitochondrial Dysfunction in AD
In different neurodegeneration models, such as AD, mitochondrial dysfunction, decreased glucose uptake, and diminished glucose metabolism have been observed. During the early stages of AD, impaired insulin signaling, caused by decreased glucose consumption and inefficient energy metabolism, is a critical AD indicator [139]. A decreased insulin response is associated with defective mitochondrial glucose consumption in the 3xTg AD mouse model [140]. Insulin treatment in the hippocampus can improve Aβ-induced memory dysfunction by activating major hippocampal extracellular signal-regulated (ERK) and mitogen-activated protein (p38) kinases [120, 141]. The effects of that long-acting insulin medication detemir, delivered intranasally for three weeks, have been examined in individuals with AD or amnestic moderate cognitive impairment [142]. Consequently, efforts to alter the regulatory link between insulin signaling and glucose metabolism in mitochondrial dysfunction are promising options for memory restoration and AD treatment.
5.5. ETC Targeting in Mitochondrial Dysfunction in AD
Candidate ETC-targeting compounds have been explored as potential AD treatments. The disruption of ETC function caused by Aβ accelerated tau phosphorylation, and polymerization caused NFT formation in AD [143]. Neurotrophic treatment agents, such as the curcumin derivative J147, have a beneficial effect in preventing or reducing AD progression [144]. Additionally, the J147 treatment improved memory and cognition in APP/PS1 mice while still maintaining synaptic protein levels [145]. AMPK/mTOR pathway modulation by J147 protected against age-related brain toxicities by directing ATP synthase and modifying the mTOR pathway [145]. Consequently, using agents and drugs that target the ETC in dysfunctional mitochondria is a potential treatment approach for AD.
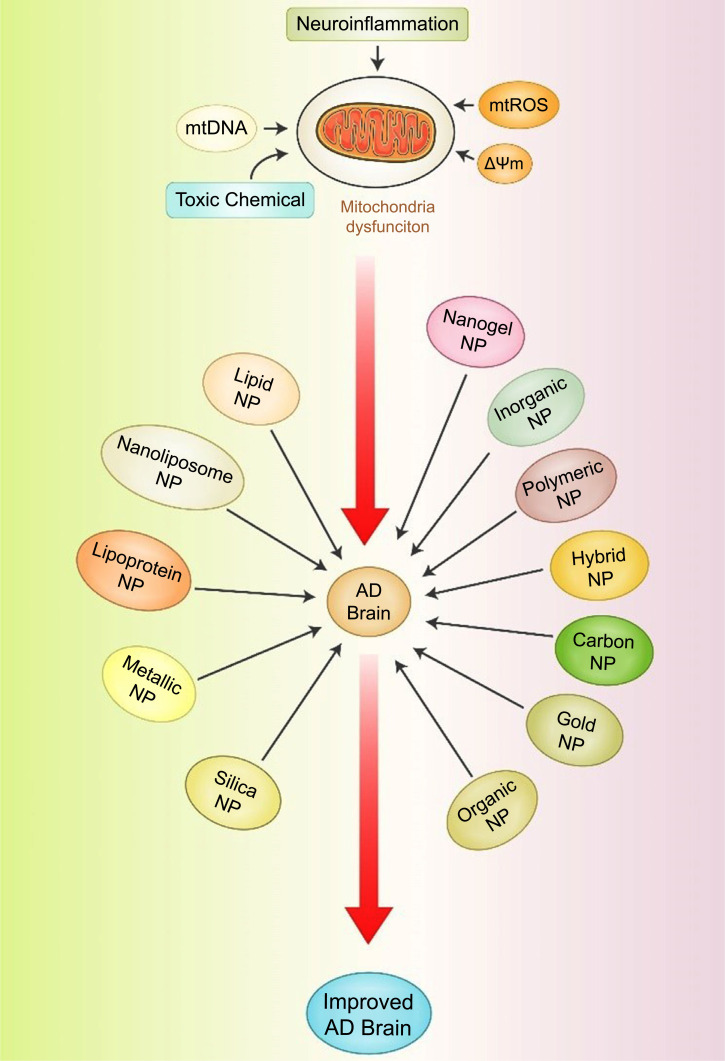
5.6. Nanoparticle-based Treatment of Mitochondrial Dysfunction in AD
Currently, targeted AD drug delivery to the central nervous system (CNS) is hindered by the difficulties posed by the blood-brain barrier (BBB) surrounding the CNS, limiting the bioavailability of therapeutic agents [146]. Nanoparticles (NPs) are a promising new strategy being developed to overcome these limitations and successfully deliver drugs to the CNS, providing new therapeutic options for efficiently transporting drugs across the BBB and into the brain [146]. The most common NP forms used in developing innovative AD therapeutics are illustrated in Fig. (6). Additional research is needed to identify the most effective NP-based therapeutics for mitochondrial dysfunction in AD.
Fig. (6).
NP-based drug delivery systems for treating AD. NPs can bypass biological barriers, and their use in precision medicine applications may benefit AD treatment. NP designs that increase delivery can potentially improve the performance of precision medicine and the speed at which clinical trials are conducted for AD drugs.
At present, nanotechnology is a potential technique for delivering therapeutic AD drugs into mitochondria. However, NPs created using inorganic materials have several limitations. Nanostructured lipid carriers (NLC) carrying rabies virus glycoprotein RVG29 and triphenylphosphine (TPP) molecules attached to the red blood cell (RBC) membrane surface (RVG/TPP NPs@RBCm) and were effective in targeting neurons and localizing to their mitochondria [147]. In addition, RVG/TPP-respiratory syncytial virus (RSV) NPs@RBCm were effective in alleviating AD symptoms by reducing Aβ-related mitochondrial oxidative stress in cell and animal models [148].
Several antioxidants, including lipoic acid, glutathione, vitamin C, and vitamin E, have proven effective in clinical studies on moderate cognitive impairment and AD [149, 150]. In addition, benzoquinone idebenone effectively inhibited Aβ-induced neurotoxicity in vitro and in vivo by targeting mitochondria [133]. Notably, biodegradable polylactic-co-glycolic acid (PLGA) NPs exert neuroprotective effects in AD treatment [87], and coenzyme Q10 (CoQ10)-loaded PLGA NPs protected against Aβ cytotoxicity and restored memory in an AD mouse model [151].
Inorganic monomer, gold carrier, magnetic core, and carbon-based NPs with graphene oxide (GO) sheets have been used to remove Aβ aggregates and inhibit Aβ fibrillation [152]. The most effective NPs for disrupting Aβ aggregation with low cytotoxicity in vitro were GO/gold nanoparticles. In addition, nano-metallo-supramolecular complexes effectively inhibited Aβ-induced biosynthesis of heme and iron uptake by PC12 cells [153]. Liposomes (LIP) biofunctionalized with murine apolipoprotein E (mApoE) and phosphatidic acid (PA), a high-affinity ligand for Aβ, to facilitate the bridging of the BBB decreased Aβ plaque load, which might be beneficial in AD treatment [154]. Recently, AD treatment has been developed based on solid lipid nanoparticles (SLNs) functionalized with anti-transferrin receptor monoclonal antibody OX26 acting as the carrier and successfully transporting a bioactive extract across an in-vitro human BBB model [155]. Some nanotechnology-based approaches are summarized in Table 2.
Table 2.
Nanotechnology-based approaches for the mitochondrial-targeted treatment of AD.
| Drug Candidate | Nanoparticle | Carrier | Model and Effects | References |
|---|---|---|---|---|
| Anthocyanin | Gold (Au) NPs | Gold colloids | Aβ1–42 mouse | [156] |
| Tanshinone IIA | Cationic bovine serum albumin (BSA) |
Polyethylene glycol (PEG) | Rat MCAO and reperfusion | [157] |
| Berberine | Carbon nanotubes | Phospholipids and polysorbate | Wistar rats in Aβ-induced AD | [158] |
| Iron oxide | Metallic NPs | Iron crystal functionalized with PEG | In vitro amyloid fibrillation experiments | [159] |
| Curcumin derivative | Liposome | Sphingomyelin | Ex vivo CSF model | [160] |
| Rivastigmine hydrogen tartrate | Mesoporous silica NPs | N-cetyl trimethyl ammonium bromide | Neuroblastoma SH-SY5Y cells | [161] |
| Galantamine hydrobromide | Solid lipid NPs | Glyceryl behenate lipids | Cognitive deficiency in rats induced with isoproterenol | [162] |
| Tunable zero-dimension | Carbon dots | Single-walled carbon nanotube | Coarse-grained nanoparticle model | [163] |
| Angiopep-2-conjugated NPs | Polylactic acid | B6 peptide (transferrin substitute PEG) | Mice injected with aggregated Aβ1–40 | [164] |
| Z-DEVD-FMK | Chitosan | Ethylene glycol | Mouse model of middle cerebral artery occlusion (MCAO) and reperfusion | [165] |
5.7. Additional Mitochondria-based AD Therapies
In vitro and in vivo studies on AD models have shown that nicotinamide adenine dinucleotide (NAD) therapies have a direct and favorable effect on mitochondrial function. Surprisingly, after 6 months of treatment, the participants with likely AD showed no cognitive decline, indicating that NAD may represent a promising strategy for preventing AD progression [166]. The antihistamine Dimebon used to treat allergies was examined by an AD clinical study due to the improved cognition and memory of neurotoxic rats treated with it [167].
Pioglitazone is a common global AD preventive treatment that slows AD development from mild cognitive impairment (MCI) depending on their APOE and translocase of OMM 40 (TOMM40) genotypes and Aβ status [168]. The Krebs cycle and gluconeogenesis intermediate oxaloacetate have already been proposed as a novel AD treatment and studied in AD patients [169]. Mouse-based oxaloacetate studies found beneficial effects on glycolysis, respiratory fluxes, mtDNA, mtDNA-encoded proteins, mitochondrial biogenesis, neuroinflammation, hippocampus neurogenesis activity, and altered brain insulin signaling [170].
6. LIMITATIONS AND FUTURE DIRECTIONS OF MITOCHONDRIAL DYSFUNCTION IN AD
Currently, no approved AD-modifying treatments can remove these proteins from AD patients' brains. Mitochondrial function is very important in AD, and correcting mitochondrial deficiency has emerged as an attractive potential approach for preventing or halting AD. Current preventative therapies for mitochondrial dysfunction are mostly focused on anti-apoptotic medicines, antioxidants, and natural agents that improve glucose metabolism and mitochondrial bioenergetics. Numerous therapies have shown clear cognitive benefits in preclinical studies on AD mouse models. Several ongoing studies are seeking new and potentially effective AD drugs. However, most therapies have not been completely successful in preventing, postponing, or retreating cognitive decline in clinical studies. Moreover, there is a general lack of effective cell and animal models that simulate the general pathophysiological circumstances and intricate etiology of AD. In addition, various limitations have been found for these candidates, impeding their use in AD treatment, including poor BBB diffusion, low bioavailability, and a restricted capacity to sustain the half-life of dose-response actions. Consequently, great efforts have recently been made to overcome these limitations by attaching candidates and their derivatives to liposomes, lipids, micelles, metal complexes, and NPs.
Further broad studies on mitochondrial activity are required to discover abnormalities shared by most AD patients since few previous studies have examined mitochondrial function in AD with large patient cohorts. Future methods should target early antecedent impairments in substrate supply apoptotic pathways and mitochondrial functions in bioenergetic systems to avoid the development of permanent AD pathology. Mitochondria play important roles in neurons in maintaining appropriate neural synapses, signal transmission, and other critical neuronal activities, indicating that addressing mitochondria dysfunction may be a promising therapeutic strategy for AD treatment. The failures of previous pharmaceuticals in clinical trials often occurred because the underlying scientific basis was not always robust, or models and instruments used to confirm the base premise were not always clearly defined or confirmed. Consequently, a more logical approach to a complex human disease like AD is required, and improved collaboration between scientific disciplines is needed to better understand AD pathogenesis and create new and more effective AD treatments. Therefore, large numbers of individuals need to be examined to improve high throughput screening tests for functional metabolic abnormalities in peripheral cells from AD patients.
CONCLUSION
Despite the continuing dramatic rise in AD frequency, there are no approved pharmaceutical treatments for curing, delaying, or preventing AD. Several studies showed that mitochondrial activity decreases with age and may worsen in the early stages of AD, contributing to its onset. The potential of mitochondria as a target in AD therapy is still being debated, given that certain pharmacological studies were unsuccessful, and while others were promising, none resulted in a marketable AD medication. Nevertheless, current AD knowledge suggests that a comprehensive treatment may remain out of reach for the foreseeable future. However, the enigmatic processes of AD pathobiology also hinder treatment efforts. From this perspective, continued research should be devoted to elucidating the specific AD pathomechanism and investigating prospective AD treatment approaches. Furthermore, clinical evidence is insufficient compared to preclinical data. Consequently, further human studies are required to convert current research findings into clinical use. Mitochondria have a significant role in neurons, maintaining normal neural synapses, signal transduction, and other critical neuronal activities, indicating that targeting mitochondria may be a promising therapeutic option for AD. Understanding AD pathobiology and the pharmacological mechanisms for creating effective treatments may lead to novel neuroprotective AD therapies in the future.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- AD
Alzheimer’s Disease
- APP
Amyloid Precursor Protein
- ATP
Adenosine Triphosphate
- BBB
Blood-brain Barrier
- CNS
Central Nervous System
- ETC
Electron Transport Chain
- MMP
Mitochondrial Membrane Potential
- mtDNA
Mitochondrial DNA
- mtQCS
Mitochondrial Quality Control System
- NAD
Nicotinamide Adenine Dinucleotide
- NFT
Neurofibrillary Tangle
- NPs
Nanoparticles
- NRF1
Nuclear Respiratory Factors 1
- PD
Parkinson's Disease
- PE
Physical Exercise
- ROS
Reactive Oxidation Species
- SP
Senile Plaques
AUTHORS’ CONTRIBUTIONS
M.A.R contributed to the conceptualization and writing original draft. Figures were drawn by M.H.R. H.R and B.K contributed to the review, visualization, and supervision. All authors have read and agreed to the published version of the manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (Grant no. NRF-2020R1I1A2066868) and the National Research Foundation of Korea (NRF) funded by the Korea government (MSIT) (Grant no. 2020R1A5A2019413). This work was additionally funded by intramural funding from the Korea Institute of Science and Technology (Grant no. 2E31511).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Cenini G., Voos W. Mitochondria as potential targets in alzheimer disease therapy: An update. Front Pharmacol. 2019;10:ARTN 902. doi: 10.3389/fphar.2019.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carvalho C., Correia S.C., Cardoso S., Plácido A.I., Candeias E., Duarte A.I., Moreira P.I. The role of mitochondrial disturbances in Alzheimer, Parkinson and Huntington diseases. Expert Rev. Neurother. 2015;15(8):867–884. doi: 10.1586/14737175.2015.1058160. [DOI] [PubMed] [Google Scholar]
- 3.Correia S.C., Santos R.X., Cardoso S., Carvalho C., Candeias E., Duarte A.I., Plácido A.I., Santos M.S., Moreira P.I. Alzheimer disease as a vascular disorder: Where do mitochondria fit? Exp. Gerontol. 2012;47(11):878–886. doi: 10.1016/j.exger.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia S., Rawal R., Sharma P., Singh T., Singh M., Singh V. Mitochondrial dysfunction in Alzheimer’s disease: Opportunities for drug development. Curr. Neuropharmacol. 2022;20(4):675–692. doi: 10.2174/1570159X19666210517114016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ke J., Tian Q., Xu Q., Fu Z., Fu Q. Mitochondrial dysfunction: A potential target for Alzheimer’s disease intervention and treatment. Drug Discov. Today. 2021;26(8):1991–2002. doi: 10.1016/j.drudis.2021.04.025. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y., Yang H., Wei D., Zhang X., Wang J., Wu X., Chang J. Exploration. Wiley Online Library; 2021. Mitochondria‐targeted nanoparticles in treatment of neurodegenerative diseases. p. 20210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai R., Guo J., Ye X.Y., Xie Y., Xie T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022;77:101619. doi: 10.1016/j.arr.2022.101619. [DOI] [PubMed] [Google Scholar]
- 8.Gowda P., Reddy P.H., Kumar S. Deregulated mitochondrial microRNAs in Alzheimer’s disease: Focus on synapse and mitochondria. Ageing Res. Rev. 2022;73:101529. doi: 10.1016/j.arr.2021.101529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Q., Li Y., Shi L., Hussain R., Mehmood K., Tang Z., Zhang H. Heavy metals induced mitochondrial dysfunction in animals: Molecular mechanism of toxicity. Toxicology. 2022;469:153136. doi: 10.1016/j.tox.2022.153136. [DOI] [PubMed] [Google Scholar]
- 10.Pelucchi S., Gardoni F., Di Luca M., Marcello E. Synaptic dysfunction in early phases of Alzheimer’s disease. Handb. Clin. Neurol. 2022;184:417–438. doi: 10.1016/B978-0-12-819410-2.00022-9. [DOI] [PubMed] [Google Scholar]
- 11.Sorgdrager F.J.H., Vermeiren Y., Faassen M., Ley C., Nollen E.A.A., Kema I.P., De Deyn P.P. Age‐ and disease‐specific changes of the kynurenine pathway in Parkinson’s and Alzheimer’s disease. J. Neurochem. 2019;151(5):656–668. doi: 10.1111/jnc.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castro-Chavira S.A., Fernandez T., Nicolini H., Diaz-Cintra S., Prado-Alcala R.A. Genetic markers in biological fluids for aging-related major neurocognitive disorder. Curr. Alzheimer Res. 2015;12(3):200–209. doi: 10.2174/1567205012666150302155138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahman M.A., Rhim H. Therapeutic implication of autophagy in neurodegenerative diseases. BMB Rep. 2017;50(7):345–354. doi: 10.5483/BMBRep.2017.50.7.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moya-Alvarado G., Gershoni-Emek N., Perlson E., Bronfman F.C. Neurodegeneration and Alzheimer’s disease (AD). What can proteomics tell us about the Alzheimer’s brain? Mol. Cell. Proteomics. 2016;15(2):409–425. doi: 10.1074/mcp.R115.053330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman M.A., Rahman M.S., Uddin M.J., Mamum-Or-Rashid A.N.M., Pang M.G., Rhim H. Emerging risk of environmental factors: Insight mechanisms of Alzheimer’s diseases. Environ. Sci. Pollut. Res. Int. 2020;27(36):44659–44672. doi: 10.1007/s11356-020-08243-z. [DOI] [PubMed] [Google Scholar]
- 16.Rahman M.A., Rahman M.S., Rahman M.H., Rasheduzzaman M., Mamun-Or-Rashid A.N.M., Uddin M.J., Rahman M.R., Hwang H., Pang M.G., Rhim H. Modulatory effects of autophagy on APP processing as a potential treatment target for Alzheimer's disease. Biomedicines. 2020;9:5. doi: 10.3390/biomedicines9010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang S.Y., Wang Z.T., Tan L., Yu J.T. Tau toxicity in neurodegeneration. Mol. Neurobiol. 2022;59(6):3617–3634. doi: 10.1007/s12035-022-02809-3. [DOI] [PubMed] [Google Scholar]
- 18.González A., Singh S.K., Churruca M., Maccioni R.B. Alzheimer’s disease and tau self-assembly: In the search of the missing link. Int. J. Mol. Sci. 2022;23(8):4192. doi: 10.3390/ijms23084192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye H., Han Y., Li P., Su Z., Huang Y. The role of post-translational modifications on the structure and function of tau protein. J. Mol. Neurosci. 2022;72(8):1557–1571. doi: 10.1007/s12031-022-02002-0. [DOI] [PubMed] [Google Scholar]
- 20.Dhapola R., Sarma P., Medhi B., Prakash A., Reddy D.H. Recent advances in molecular pathways and therapeutic implications targeting mitochondrial dysfunction for Alzheimer’s disease. Mol. Neurobiol. 2022;59(2022):535–555. doi: 10.1007/s12035-021-02612-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y., Jia M., Chen W., Liu Z. The neuroprotective effects of intermittent fasting on brain aging and neurodegenerative diseases via regulating mitochondrial function. Free Radic. Biol. Med. 2022;182:206–218. doi: 10.1016/j.freeradbiomed.2022.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Du F., Yu Q., Kanaan N.M., Yan S.S. Mitochondrial oxidative stress contributes to the pathological aggregation and accumulation of tau oligomers in Alzheimer’s disease. Hum. Mol. Genet. 2022;31(15):2498–2507. doi: 10.1093/hmg/ddab363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong W., Xu J., Wang Y., Min Q., Chen X., Zhang W., Chen J., Zhan Q. Nuclear genome-derived circular RNA circPUM1 localizes in mitochondria and regulates oxidative phosphorylation in esophageal squamous cell carcinoma. Signal. Transduct. Target. Ther. 2022;7(1):40. doi: 10.1038/s41392-021-00865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zinovkin R.A., Zamyatnin A.A., Jr Mitochondria-targeted drugs. Curr. Mol. Pharmacol. 2019;12(3):202–214. doi: 10.2174/1874467212666181127151059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almendro-Vedia V., Natale P., Valdivieso González D., Lillo M.P., Aragones J.L., López-Montero I. How rotating ATP synthases can modulate membrane structure. Arch. Biochem. Biophys. 2021;708:108939. doi: 10.1016/j.abb.2021.108939. [DOI] [PubMed] [Google Scholar]
- 26.Garbincius J.F., Elrod J.W. Mitochondrial calcium exchange in physiology and disease. Physiol. Rev. 2022;102(2):893–992. doi: 10.1152/physrev.00041.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schapira A.H.V. Mitochondrial disease. Lancet. 2006;368(9529):70–82. doi: 10.1016/S0140-6736(06)68970-8. [DOI] [PubMed] [Google Scholar]
- 28.Cheung G., Bataveljic D., Visser J., Kumar N., Moulard J., Dallérac G., Mozheiko D., Rollenhagen A., Ezan P., Mongin C., Chever O., Bemelmans A.P., Lübke J., Leray I., Rouach N. Physiological synaptic activity and recognition memory require astroglial glutamine. Nat. Commun. 2022;13(1):753. doi: 10.1038/s41467-022-28331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birsoy K., Wang T., Chen W.W., Freinkman E., Abu-Remaileh M., Sabatini D.M. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell. 2015;162(3):540–551. doi: 10.1016/j.cell.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabassum N., Kheya I.S., Asaduzzaman S., Maniha S., Fayz A.H., Zakaria A., Noor R. A review on the possible leakage of electrons through the electron transport chain within mitochondria. Life Sci. 2020;6:105–113. [Google Scholar]
- 31.Mani S., Swargiary G., Tyagi S., Singh M., Jha N.K., Singh K.K. Nanotherapeutic approaches to target mitochondria in cancer. Life Sci. 2021;281:119773. doi: 10.1016/j.lfs.2021.119773. [DOI] [PubMed] [Google Scholar]
- 32.Horie M., Tabei Y. Role of oxidative stress in nanoparticles toxicity. Free Radic. Res. 2021;55(4):331–342. doi: 10.1080/10715762.2020.1859108. [DOI] [PubMed] [Google Scholar]
- 33.Aruoma O. Alzheimer’s disease and Parkinson’s disease: A nutritional toxicology perspective of the impact of oxidative Str. doi: 10.1080/07315724.2019.1683379. [DOI] [PubMed] [Google Scholar]
- 34.Rahman M.A., Rahman M.D.H., Biswas P., Hossain M.S., Islam R., Hannan M.A., Uddin M.J., Rhim H. Potential therapeutic role of phytochemicals to mitigate mitochondrial dysfunctions in Alzheimer’s disease. Antioxidants. 2020;10(1):23. doi: 10.3390/antiox10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma C., Kim S., Nam Y., Jung U.J., Kim S.R. Mitochondrial dysfunction as a driver of cognitive impairment in Alzheimer’s disease. Int. J. Mol. Sci. 2021;22(9):4850. doi: 10.3390/ijms22094850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brillo V., Chieregato L., Leanza L., Muccioli S., Costa R. Mitochondrial dynamics, ROS, and cell signaling: A blended overview. Life (Basel) 2021;11(4):332. doi: 10.3390/life11040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahman M., Hannan M., Uddin M., Rahman M., Rashid M., Kim B. Exposure to environmental arsenic and emerging risk of Alzheimer’s disease: Perspective mechanisms, management strategy, and future directions. Toxics. 2021;9(8):188. doi: 10.3390/toxics9080188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman M.A., Rahman M.H., Mamun-Or-Rashid A.N.M., Hwang H., Chung S., Kim B., Rhim H. Autophagy modulation in aggresome formation: Emerging implications and treatments of Alzheimer’s disease. Biomedicines. 2022;10(5):1027. doi: 10.3390/biomedicines10051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bera A., Lavanya G., Reshmi R., Dev K., Kumar R. Mechanistic and therapeutic role of Drp1 in the pathogenesis of Alzheimer’s disease. Eur. J. Neurosci. 2022;56:5516–5531. doi: 10.1111/ejn.15611. [DOI] [PubMed] [Google Scholar]
- 40.Mondala T., Samantaa S., Kumara A., Govindarajua T. Alzheimer’s Disease: Recent Findings in Pathophysiology, Diagnostic and Therapeutic Modalities. Royal Society of Chemistry; 2022. Multifunctional inhibitors of multifaceted Aβ toxicity of Alzheimer's disease. [Google Scholar]
- 41.Taliyan R., Kakoty V., Sarathlal K.C., Kharavtekar S.S., Karennanavar C.R., Choudhary Y.K., Singhvi G., Riadi Y., Dubey S.K., Kesharwani P. Nanocarrier mediated drug delivery as an impeccable therapeutic approach against Alzheimer’s disease. J. Control. Release. 2022;343:528–550. doi: 10.1016/j.jconrel.2022.01.044. [DOI] [PubMed] [Google Scholar]
- 42.Bomba-Warczak E., Savas J.N. Long-lived mitochondrial proteins and why they exist. Trends Cell Biol. 2022;32(8):646–654. doi: 10.1016/j.tcb.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie L., Shi F., Tan Z., Li Y., Bode A.M., Cao Y. Mitochondrial network structure homeostasis and cell death. Cancer Sci. 2018;109(12):3686–3694. doi: 10.1111/cas.13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X., Su B., Lee H., Li X., Perry G., Smith M.A., Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J. Neurosci. 2009;29(28):9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boguszewska K., Szewczuk M., Kaźmierczak-Barańska J., Karwowski B.T. The similarities between human mitochondria and bacteria in the context of structure, genome, and base excision repair system. Molecules. 2020;25(12):2857. doi: 10.3390/molecules25122857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim D.K., Mook-Jung I. The role of cell type-specific mitochondrial dysfunction in the pathogenesis of Alzheimer’s disease. BMB Rep. 2019;52(12):679–688. doi: 10.5483/BMBRep.2019.52.12.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X., Zhang Y., Ni M., Cao H., Signer R.A.J., Li D., Li M., Gu Z., Hu Z., Dickerson K.E., Weinberg S.E., Chandel N.S., DeBerardinis R.J., Zhou F., Shao Z., Xu J. Regulation of mitochondrial biogenesis in erythropoiesis by mTORC1-mediated protein translation. Nat. Cell Biol. 2017;19(6):626–638. doi: 10.1038/ncb3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding X.W., Robinson M., Li R., Aldhowayan H., Geetha T., Babu J.R. Mitochondrial dysfunction and beneficial effects of mitochondria-targeted small peptide SS-31 in diabetes mellitus and Alzheimer’s disease. Pharmacol. Res. 2021;171:105783. doi: 10.1016/j.phrs.2021.105783. [DOI] [PubMed] [Google Scholar]
- 49.Bilbao-Malavé V., González-Zamora J., de la Puente M., Recalde S., Fernandez-Robredo P., Hernandez M., Layana A.G., Saenz de Viteri M. Mitochondrial dysfunction and endoplasmic reticulum stress in age related macular degeneration, role in pathophysiology, and possible new therapeutic strategies. Antioxidants. 2021;10(8):1170. doi: 10.3390/antiox10081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Machrina Y., Lindarto D., Pane Y.S., Harahap N.S. The pattern of peroxisome proliferator-activated receptor gamma coactivator 1-alpha gene expression in type-2 diabetes mellitus rat model liver: Focus on exercise. Open Access Maced. J. Med. Sci. 2021;9(T3):124–128. doi: 10.3889/oamjms.2021.6362. [DOI] [Google Scholar]
- 51.Wang C.F., Song C.Y., Wang X., Huang L.Y., Ding M., Yang H., Wang P., Xu L.L., Xie Z.H., Bi J.Z. Protective effects of melatonin on mitochondrial biogenesis and mitochondrial structure and function in the HEK293-APPswe cell model of Alzheimer’s disease. Eur. Rev. Med. Pharmacol. Sci. 2019;23(8):3542–3550. doi: 10.26355/eurrev_201904_17723. [DOI] [PubMed] [Google Scholar]
- 52.Singulani M.P., Pereira C.P.M., Ferreira A.F.F., Garcia P.C., Ferrari G.D., Alberici L.C., Britto L.R. Impairment of PGC-1α-mediated mitochondrial biogenesis precedes mitochondrial dysfunction and Alzheimer’s pathology in the 3xTg mouse model of Alzheimer’s disease. Exp. Gerontol. 2020;133:110882. doi: 10.1016/j.exger.2020.110882. [DOI] [PubMed] [Google Scholar]
- 53.Tiwari S., Dewry R.K., Srivastava R., Nath S., Mohanty T.K. Targeted antioxidant delivery modulates mitochondrial functions, ameliorates oxidative stress and preserve sperm quality during cryopreservation. Theriogenology. 2022;179:22–31. doi: 10.1016/j.theriogenology.2021.11.013. [DOI] [PubMed] [Google Scholar]
- 54.Durairajanayagam D., Singh D., Agarwal A., Henkel R. Causes and consequences of sperm mitochondrial dysfunction. Andrologia. 2021;53(1):e13666. doi: 10.1111/and.13666. [DOI] [PubMed] [Google Scholar]
- 55.Wang W., Zhao F., Ma X., Perry G., Zhu X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020;15(1):30. doi: 10.1186/s13024-020-00376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stojakovic A., Trushin S., Sheu A., Khalili L., Chang S.Y., Li X., Christensen T., Salisbury J.L., Geroux R.E., Gateno B., Flannery P.J., Dehankar M., Funk C.C., Wilkins J., Stepanova A., O’Hagan T., Galkin A., Nesbitt J., Zhu X., Tripathi U., Macura S., Tchkonia T., Pirtskhalava T., Kirkland J.L., Kudgus R.A., Schoon R.A., Reid J.M., Yamazaki Y., Kanekiyo T., Zhang S., Nemutlu E., Dzeja P., Jaspersen A., Kwon Y.I.C., Lee M.K., Trushina E. Partial inhibition of mitochondrial complex I ameliorates Alzheimer’s disease pathology and cognition in APP/PS1 female mice. Commun. Biol. 2021;4(1):61. doi: 10.1038/s42003-020-01584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belosludtsev K.N., Sharipov R.R., Boyarkin D.P., Belosludtseva N.V., Dubinin M.V., Krasilnikova I.A., Bakaeva Z.V., Zgodova A.E., Pinelis V.G., Surin A.M. The effect of DS16570511, a new inhibitor of mitochondrial calcium uniporter, on calcium homeostasis, metabolism, and functional state of cultured cortical neurons and isolated brain mitochondria. Biochim. Biophys. Acta, Gen. Subj. 2021;1865(5):129847. doi: 10.1016/j.bbagen.2021.129847. [DOI] [PubMed] [Google Scholar]
- 58.Carafoli E. Historical review: Mitochondria and calcium: Ups and downs of an unusual relationship. Trends Biochem. Sci. 2003;28(4):175–181. doi: 10.1016/S0968-0004(03)00053-7. [DOI] [PubMed] [Google Scholar]
- 59.Zeb A., Kim D., Alam S., Son M., Kumar R., Rampogu S., Parameswaran S., Shelake R., Rana R., Parate S., Kim J.Y., Lee K. Computational simulations identify pyrrolidine-2, 3-dione derivatives as novel inhibitors of Cdk5/p25 complex to attenuate Alzheimer’s pathology. J. Clin. Med. 2019;8(5):746. doi: 10.3390/jcm8050746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonora M., Giorgi C., Pinton P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat. Rev. Mol. Cell Biol. 2022;23:266–285. doi: 10.1038/s41580-021-00433-y. [DOI] [PubMed] [Google Scholar]
- 61.Quintana D.D., Garcia J.A., Anantula Y., Rellick S.L., Engler-Chiurazzi E.B., Sarkar S.N., Brown C.M., Simpkins J.W. Amyloid-β causes mitochondrial dysfunction via a Ca 2+-driven upregulation of oxidative phosphorylation and superoxide production in cerebrovascular endothelial cells. J. Alzheimers Dis. 2020;75(1):119–138. doi: 10.3233/JAD-190964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Filippone A., Esposito E., Mannino D., Lyssenko N., Praticò D. The contribution of altered neuronal autophagy to neurodegeneration. Pharmacol. Ther. 2022;238:108178. doi: 10.1016/j.pharmthera.2022.108178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sorrentino V., Romani M., Mouchiroud L., Beck J.S., Zhang H., D’Amico D., Moullan N., Potenza F., Schmid A.W., Rietsch S., Counts S.E., Auwerx J. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature. 2017;552(7684):187–193. doi: 10.1038/nature25143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Skike C.E., Lin A.L., Roberts Burbank R., Halloran J.J., Hernandez S.F., Cuvillier J., Soto V.Y., Hussong S.A., Jahrling J.B., Javors M.A., Hart M.J., Fischer K.E., Austad S.N., Galvan V. mTOR drives cerebrovascular, synaptic, and cognitive dysfunction in normative aging. Aging Cell. 2020;19(1):e13057. doi: 10.1111/acel.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang W., Xu C., Sun J., Shen H.M., Wang J., Yang C. Impairment of the autophagy-lysosomal pathway in Alzheimer’s diseases: Pathogenic mechanisms and therapeutic potential. Acta Pharm. Sin. B. 2022;12(3):1019–1040. doi: 10.1016/j.apsb.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pradeepkiran J.A., Hindle A., Kshirsagar S., Reddy P.H. Are mitophagy enhancers therapeutic targets for Alzheimer’s disease? Biomed. Pharmacother. 2022;149:112918. doi: 10.1016/j.biopha.2022.112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nazam N., Farhana A., Shaikh S. Recent advances in Alzheimer’s disease in relation to cholinesterase inhibitors and NMDA receptor antagonists, autism spectrum disorder and Alzheimer's disease. 2021:135–151. [Google Scholar]
- 68.Chiang T.I., Yu Y.H., Lin C.H., Lane H.Y. Novel biomarkers of Alzheimer’s disease: Based upon N-methyl-d-aspartate receptor hypoactivation and oxidative stress. Clin. Psychopharmacol. Neurosci. 2021;19(3):423–433. doi: 10.9758/cpn.2021.19.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng Y.J., Lin C.H., Lane H.Y. Involvement of cholinergic, adrenergic, and glutamatergic network modulation with cognitive dysfunction in Alzheimer’s disease. Int. J. Mol. Sci. 2021;22(5):2283. doi: 10.3390/ijms22052283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen V.T.T., Sallbach J., dos Santos Guilherme M., Endres K. Influence of acetylcholine esterase inhibitors and memantine, clinically approved for Alzheimer’s dementia treatment, on intestinal properties of the mouse. Int. J. Mol. Sci. 2021;22(3):1015. doi: 10.3390/ijms22031015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grundman M., Delaney P., Delaney P. Antioxidant strategies for Alzheimer’s disease. Proc. Nutr. Soc. 2002;61(2):191–202. doi: 10.1079/PNS2002146. [DOI] [PubMed] [Google Scholar]
- 72.Malty R.H., Jessulat M., Jin K., Musso G., Vlasblom J., Phanse S., Zhang Z., Babu M. Mitochondrial targets for pharmacological intervention in human disease. J. Proteome Res. 2015;14(1):5–21. doi: 10.1021/pr500813f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang X., Su B., Zheng L., Perry G., Smith M.A., Zhu X. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2009;109(Suppl. 1):153–159. doi: 10.1111/j.1471-4159.2009.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rahman M.A., Bishayee K., Huh S.O. Angelica polymorpha maxim induces apoptosis of human SH-SY5Y neuroblastoma cells by regulating an intrinsic caspase pathway. Mol. Cells. 2016;39(2):119–128. doi: 10.14348/molcells.2016.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kwon Y.H., Bishayee K., Rahman A., Hong J.S., Lim S.S., Huh S.O. Morus alba accumulates reactive oxygen species to initiate apoptosis via FOXO-caspase 3-dependent pathway in neuroblastoma cells. Mol. Cells. 2015;38(7):630–637. doi: 10.14348/molcells.2015.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rahman M.A., Hong J.S., Huh S.O. Antiproliferative properties of Saussurea lappa Clarke root extract in SH-SY5Y neuroblastoma cells via intrinsic apoptotic pathway. Anim. Cells Syst. 2015;19(2):119–126. doi: 10.1080/19768354.2015.1008041. [DOI] [Google Scholar]
- 77.Rahman M.A., Yang H., Kim N.H., Huh S.O. Induction of apoptosis by Dioscorea nipponica Makino extracts in human SH-SY5Y neuroblastoma cells via mitochondria-mediated pathway. Anim. Cells Syst. 2014;18(1):41–51. doi: 10.1080/19768354.2014.880372. [DOI] [Google Scholar]
- 78.Rahman M.A., Yang H., Lim S.S., Huh S.O. Apoptotic effects of melandryum firmum root extracts in human SH-SY5Y neuroblastoma cells. Exp. Neurobiol. 2013;22(3):208–213. doi: 10.5607/en.2013.22.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rahman M.A., Kim N.H., Huh S.O. Cytotoxic effect of gambogic acid on SH-SY5Y neuroblastoma cells is mediated by intrinsic caspase-dependent signaling pathway. Mol. Cell. Biochem. 2013;377(1-2):187–196. doi: 10.1007/s11010-013-1584-z. [DOI] [PubMed] [Google Scholar]
- 80.Rahman M.A., Kim N.H., Kim S.H., Oh S.M., Huh S.O. Antiproliferative and cytotoxic effects of resveratrol in mitochondria-mediated apoptosis in rat b103 neuroblastoma cells. Korean J. Physiol. Pharmacol. 2012;16(5):321–326. doi: 10.4196/kjpp.2012.16.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ataur Rahman M., Kim N.H., Yang H., Huh S.O. Angelicin induces apoptosis through intrinsic caspase-dependent pathway in human SH-SY5Y neuroblastoma cells. Mol. Cell. Biochem. 2012;369(1-2):95–104. doi: 10.1007/s11010-012-1372-1. [DOI] [PubMed] [Google Scholar]
- 82.Hannan M.A., Dash R., Haque M.N., Mohibbullah M., Sohag A.A., Rahman M.A., Uddin M.J., Alam M., Moon I. Neuroprotective potentials of marine algae and their bioactive metabolites: Pharmacological insights and therapeutic advances. Mar. Drugs. 2020;18:347. doi: 10.3390/md18070347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rahman M.A., Rahman M.R., Zaman T., Uddin M.S., Islam R., Abdel-Daim M.M., Rhim H. Emerging potential of naturally occurring autophagy modulators against neurodegeneration. Curr. Pharm. Des. 2020;26(7):772–779. doi: 10.2174/1381612826666200107142541. [DOI] [PubMed] [Google Scholar]
- 84.Rahman M.A., Saha S.K., Rahman M.S., Uddin M.J., Uddin M.S., Pang M.G., Rhim H., Cho S.G. Molecular insights into therapeutic potential of autophagy modulation by natural products for cancer stem cells. Front. Cell Dev. Biol. 2020;8:283. doi: 10.3389/fcell.2020.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rahman M.A., Hwang H., Nah S.Y., Rhim H. Gintonin stimulates autophagic flux in primary cortical astrocytes. J. Ginseng Res. 2020;44(1):67–78. doi: 10.1016/j.jgr.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rahman M.A., Bishayee K., Sadra A., Huh S.O. Oxyresveratrol activates parallel apoptotic and autophagic cell death pathways in neuroblastoma cells. Biochim. Biophys. Acta, Gen. Subj. 2017;1861(2):23–36. doi: 10.1016/j.bbagen.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 87.Rahman M.A., Bishayee K., Habib K., Sadra A., Huh S.O. 18α-Glycyrrhetinic acid lethality for neuroblastoma cells via de-regulating the Beclin-1/Bcl-2 complex and inducing apoptosis. Biochem. Pharmacol. 2016;117:97–112. doi: 10.1016/j.bcp.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 88.Jangra A., Arora M.K., Kisku A., Sharma S. The multifaceted role of mangiferin in health and diseases: A review. Advn Tradi Med. 2021;21(4):619–643. doi: 10.1007/s13596-020-00471-5. [DOI] [Google Scholar]
- 89.Sarikurkcu C., Sahinler S.S., Ceylan O., Tepe B. Onosma pulchra: Phytochemical composition, antioxidant, skin-whitening and anti-diabetic activity. Ind. Crop. Prod. 2020:154. [Google Scholar]
- 90.Franco R., Navarro G., Martinez-Pinilla E. Hormetic and mitochondria-related mechanisms of antioxidant action of phytochemicals. Antioxidants-Basel. 2019;8:373. doi: 10.3390/antiox8090373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu F., Du B., Xu B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018;58(8):1260–1270. doi: 10.1080/10408398.2016.1251390. [DOI] [PubMed] [Google Scholar]
- 92.Vaiserman A., Koliada A., Lushchak O. Neuroinflammation in pathogenesis of Alzheimer’s disease: Phytochemicals as potential therapeutics. Mech. Ageing Dev. 2020;189:111259. doi: 10.1016/j.mad.2020.111259. [DOI] [PubMed] [Google Scholar]
- 93.Li Y., Zhang J., Wan J., Liu A., Sun J. Melatonin regulates Aβ production/clearance balance and Aβ neurotoxicity: A potential therapeutic molecule for Alzheimer’s disease. Biomed. Pharmacother. 2020;132:110887. doi: 10.1016/j.biopha.2020.110887. [DOI] [PubMed] [Google Scholar]
- 94.Reddy P.H., Manczak M., Yin X., Grady M.C., Mitchell A., Kandimalla R., Kuruva C.S. Protective effects of a natural product, curcumin, against amyloid β induced mitochondrial and synaptic toxicities in Alzheimer’s disease. J. Investig. Med. 2016;64(8):1220–1234. doi: 10.1136/jim-2016-000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang D.M., Li S.Q., Wu W.L., Zhu X.Y., Wang Y., Yuan H.Y. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer’s disease. Neurochem. Res. 2014;39(8):1533–1543. doi: 10.1007/s11064-014-1343-x. [DOI] [PubMed] [Google Scholar]
- 96.Paula P.C., Angelica M.S.G., Luis C.H., Gloria P.C.G. Preventive effect of quercetin in a triple transgenic Alzheimer's disease mice model. Molecules. 2019:24. doi: 10.3390/molecules24122287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sabogal-Guáqueta A.M., Muñoz-Manco J.I., Ramírez-Pineda J.R., Lamprea-Rodriguez M., Osorio E., Cardona-Gómez G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology. 2015;93:134–145. doi: 10.1016/j.neuropharm.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Román G.C., Jackson R.E., Gadhia R., Román A.N., Reis J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. (Paris) 2019;175(10):724–741. doi: 10.1016/j.neurol.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 99.Sohel M., Biswas P., Al Amin M., Hossain M.A., Sultana H., Dey D., Aktar S., Setu A., Khan M.S., Paul P., Islam M.N., Rahman M.A., Kim B., Al Mamun A. Genistein, a potential phytochemical against breast cancer treatment-Insight into the molecular mechanisms. Processes (Basel) 2022;10(2):415. doi: 10.3390/pr10020415. [DOI] [Google Scholar]
- 100.Uddin M.S., Kabir M.T. Emerging signal regulating potential of genistein against Alzheimer’s disease: A promising molecule of interest. Front. Cell Dev. Biol. 2019;7:197. doi: 10.3389/fcell.2019.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pierzynowska K., Podlacha M., Gaffke L., Majkutewicz I., Mantej J., Węgrzyn A., Osiadły M., Myślińska D., Węgrzyn G. Autophagy-dependent mechanism of genistein-mediated elimination of behavioral and biochemical defects in the rat model of sporadic Alzheimer’s disease. Neuropharmacology. 2019;148:332–346. doi: 10.1016/j.neuropharm.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 102.Rassu G., Porcu E., Fancello S., Obinu A., Senes N., Galleri G., Migheli R., Gavini E., Giunchedi P. Intranasal delivery of genistein-loaded nanoparticles as a potential preventive system against neurodegenerative disorders. Pharmaceutics. 2018;11(1):8. doi: 10.3390/pharmaceutics11010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jo D.S., Shin D.W., Park S.J., Bae J.E., Kim J.B., Park N.Y., Kim J.S., Oh J.S., Shin J.W., Cho D.H. Attenuation of Aβ toxicity by promotion of mitochondrial fusion in neuroblastoma cells by liquiritigenin. Arch. Pharm. Res. 2016;39(8):1137–1143. doi: 10.1007/s12272-016-0780-2. [DOI] [PubMed] [Google Scholar]
- 104.Valles S.L., Dolz-Gaiton P., Gambini J., Borras C., LLoret A., Pallardo F.V., Viña J. Estradiol or genistein prevent Alzheimer’s disease-associated inflammation correlating with an increase PPARγ expression in cultured astrocytes. Brain Res. 2010;1312:138–144. doi: 10.1016/j.brainres.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 105.Parrado-Fernández C., Sandebring-Matton A., Rodriguez-Rodriguez P., Aarsland D., Cedazo-Mínguez A. Anthocyanins protect from complex I inhibition and APPswe mutation through modulation of the mitochondrial fission/fusion pathways. Biochim. Biophys. Acta Mol. Basis Dis. 2016;1862(11):2110–2118. doi: 10.1016/j.bbadis.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 106.Godoy J.A., Lindsay C.B., Quintanilla R.A., Carvajal F.J., Cerpa W., Inestrosa N.C. Quercetin exerts differential neuroprotective effects against H2O2 and Aβ aggregates in hippocampal neurons: The role of mitochondria. Mol. Neurobiol. 2017;54(9):7116–7128. doi: 10.1007/s12035-016-0203-x. [DOI] [PubMed] [Google Scholar]
- 107.Kwon S.H., Ma S.X., Hwang J.Y., Lee S.Y., Jang C.G. Involvement of the Nrf2/HO-1 signaling pathway in sulfuretin-induced protection against amyloid beta25–35 neurotoxicity. Neuroscience. 2015;304:14–28. doi: 10.1016/j.neuroscience.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 108.Chesser A.S., Ganeshan V., Yang J., Johnson G.V.W. Epigallocatechin-3-gallate enhances clearance of phosphorylated tau in primary neurons. Nutr. Neurosci. 2016;19(1):21–31. doi: 10.1179/1476830515Y.0000000038. [DOI] [PubMed] [Google Scholar]
- 109.Huang L., Chen C., Zhang X., Li X., Chen Z., Yang C., Liang X., Zhu G., Xu Z. Neuroprotective effect of curcumin against cerebral ischemia-reperfusion via mediating autophagy and inflammation. J. Mol. Neurosci. 2018;64(1):129–139. doi: 10.1007/s12031-017-1006-x. [DOI] [PubMed] [Google Scholar]
- 110.Sousa J.C., Santana A.C.F., Magalhães G.J.P. Resveratrol in Alzheimer’s disease: A review of pathophysiology and therapeutic potential. Arq. Neuropsiquiatr. 2020;78(8):501–511. doi: 10.1590/0004-282x20200010. [DOI] [PubMed] [Google Scholar]
- 111.Qi G., Mi Y., Wang Y., Li R., Huang S., Li X., Liu X. Neuroprotective action of tea polyphenols on oxidative stress-induced apoptosis through the activation of the TrkB/CREB/BDNF pathway and Keap1/Nrf2 signaling pathway in SH-SY5Y cells and mice brain. Food Funct. 2017;8(12):4421–4432. doi: 10.1039/C7FO00991G. [DOI] [PubMed] [Google Scholar]
- 112.Yao X., Jiang H., NanXu Y., Piao X., Gao Q., Kim N.H. Kaempferol attenuates mitochondrial dysfunction and oxidative stress induced by H2O2 during porcine embryonic development. Theriogenology. 2019;135:174–180. doi: 10.1016/j.theriogenology.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 113.Korolchuk V.I., Miwa S., Carroll B., von Zglinicki T. Mitochondria in cell senescence: Is mitophagy the weakest link? EBioMedicine. 2017;21:7–13. doi: 10.1016/j.ebiom.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ashrafi G., Schwarz T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20(1):31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li X., Alafuzoff I., Soininen H., Winblad B., Pei J.J. Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer’s disease brain. FEBS J. 2005;272(16):4211–4220. doi: 10.1111/j.1742-4658.2005.04833.x. [DOI] [PubMed] [Google Scholar]
- 116.Perluigi M., Di Domenico F., Butterfield D.A. mTOR signaling in aging and neurodegeneration: At the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol. Dis. 2015;84:39–49. doi: 10.1016/j.nbd.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 117.Pan T., Rawal P., Wu Y., Xie W., Jankovic J., Le W. Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience. 2009;164(2):541–551. doi: 10.1016/j.neuroscience.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 118.Morton H., Kshirsagar S., Orlov E., Bunquin L.E., Sawant N., Boleng L., George M., Basu T., Ramasubramanian B., Pradeepkiran J.A., Kumar S., Vijayan M., Reddy A.P., Reddy P.H. Defective mitophagy and synaptic degeneration in Alzheimer’s disease: Focus on aging, mitochondria and synapse. Free Radic. Biol. Med. 2021;172:652–667. doi: 10.1016/j.freeradbiomed.2021.07.013. [DOI] [PubMed] [Google Scholar]
- 119.Wang W.W., Han R., He H.J., Wang Z., Luan X.Q., Li J., Feng L., Chen S.Y., Aman Y., Xie C.L. Delineating the role of mitophagy inducers for Alzheimer disease patients. Aging Dis. 2021;12(3):852–867. doi: 10.14336/AD.2020.0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jurcau A. Insights into the pathogenesis of neurodegenerative diseases: Focus on mitochondrial dysfunction and oxidative stress. Int. J. Mol. Sci. 2021;22(21):11847. doi: 10.3390/ijms222111847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Friedland-Leuner K., Stockburger C., Denzer I., Eckert G.P., Müller W.E. Mitochondrial dysfunction. Prog. Mol. Biol. Transl. Sci. 2014;127:183–210. doi: 10.1016/B978-0-12-394625-6.00007-6. [DOI] [PubMed] [Google Scholar]
- 122.von Gunten A., Schlaefke S., Überla K. Efficacy of Ginkgo biloba extract EGb 761 ® in dementia with behavioural and psychological symptoms: A systematic review. World J. Biol. Psychiatry. 2016;17(8):622–633. doi: 10.3109/15622975.2015.1066513. [DOI] [PubMed] [Google Scholar]
- 123.Heckmann B.L., Teubner B.J., Tummers B., Boada-Romero E., Harris L., Yang M., Guy C.S., Zakharenko S.S., Green D.R. LC3-associated endocytosis facilitates β-amyloid clearance and mitigates neurodegeneration in murine Alzheimer’s disease. Cell. 2019;178:536–551. doi: 10.1016/j.cell.2019.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fang E.F., Hou Y., Palikaras K., Adriaanse B.A., Kerr J.S., Yang B., Lautrup S., Hasan-Olive M.M., Caponio D., Dan X., Rocktäschel P., Croteau D.L., Akbari M., Greig N.H., Fladby T., Nilsen H., Cader M.Z., Mattson M.P., Tavernarakis N., Bohr V.A. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019;22(3):401–412. doi: 10.1038/s41593-018-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yeong K.Y., Berdigaliyev N., Chang Y. Sirtuins and their implications in neurodegenerative diseases from a drug discovery perspective. ACS Chem. Neurosci. 2020;11(24):4073–4091. doi: 10.1021/acschemneuro.0c00696. [DOI] [PubMed] [Google Scholar]
- 126.Zhang Z., Shen Q., Wu X., Zhang D., Xing D. Activation of PKA/SIRT1 signaling pathway by photobiomodulation therapy reduces Aβ levels in Alzheimer’s disease models. Aging Cell. 2020;19(1):e13054. doi: 10.1111/acel.13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chu C.Q., Yu L., Qi G., Mi Y.S., Wu W.Q., Lee Y., Zhai Q.X., Tian F.W., Chen W. Can dietary patterns prevent cognitive impairment and reduce Alzheimer’s disease risk: Exploring the underlying mechanisms of effects. Neurosci. Biobehav. Rev. 2022;135:104556. doi: 10.1016/j.neubiorev.2022.104556. [DOI] [PubMed] [Google Scholar]
- 128.Alemany-Cosme E., Sáez-González E., Moret I., Mateos B., Iborra M., Nos P., Sandoval J., Beltrán B. Oxidative stress in the pathogenesis of crohn’s disease and the interconnection with immunological response, microbiota, external environmental factors, and epigenetics. Antioxidants. 2021;10(1):64. doi: 10.3390/antiox10010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hadrich F., Chamkha M., Sayadi S. Protective effect of olive leaves phenolic compounds against neurodegenerative disorders: Promising alternative for Alzheimer and Parkinson diseases modulation. Food Chem. Toxicol. 2022;159:112752. doi: 10.1016/j.fct.2021.112752. [DOI] [PubMed] [Google Scholar]
- 130.Abdallah I.M., Al-Shami K.M., Yang E., Wang J., Guillaume C., Kaddoumi A. Oleuropein-rich olive leaf extract attenuates neuroinflammation in the Alzheimer’s disease mouse model. ACS Chem. Neurosci. 2022;13:1002–1013. doi: 10.1021/acschemneuro.2c00005. [DOI] [PubMed] [Google Scholar]
- 131.Sridharan B., Lee M.J. Ketogenic diet: A promising neuroprotective composition for managing Alzheimer’s diseases and its pathological mechanisms. Curr. Mol. Med. 2022;22(7):640–656. doi: 10.2174/1566524021666211004104703. [DOI] [PubMed] [Google Scholar]
- 132.Napoleão A., Fernandes L., Miranda C., Marum A.P. Effects of calorie restriction on health span and insulin resistance: Classic calorie restriction diet vs. ketosis-inducing diet. Nutrients. 2021;13(4):1302. doi: 10.3390/nu13041302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Misrani A., Tabassum S., Yang L. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease. Front. Aging Neurosci. 2021;13:617588. doi: 10.3389/fnagi.2021.617588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nisoli E., Tonello C., Cardile A., Cozzi V., Bracale R., Tedesco L., Falcone S., Valerio A., Cantoni O., Clementi E., Moncada S., Carruba M.O. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310(5746):314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 135.Bo H., Kang W., Jiang N., Wang X., Zhang Y., Ji L.L. Exercise-induced neuroprotection of hippocampus in APP/PS1 transgenic mice via upregulation of mitochondrial 8-oxoguanine DNA glycosylase. Oxid. Med. Cell. Longev. 2014;2014:1–14. doi: 10.1155/2014/834502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klein C.P., Hoppe J.B., Saccomori A.B., dos Santos B.G., Sagini J.P., Crestani M.S., August P.M., Hözer R.M., Grings M., Parmeggiani B., Leipnitz G., Navas P., Salbego C.G., Matté C. Physical exercise during pregnancy prevents cognitive impairment induced by amyloid-β in adult offspring rats. Mol. Neurobiol. 2019;56(3):2022–2038. doi: 10.1007/s12035-018-1210-x. [DOI] [PubMed] [Google Scholar]
- 137.Longobardi A., Nicsanu R., Bellini S., Squitti R., Catania M., Tiraboschi P., Saraceno C., Ferrari C., Zanardini R., Binetti G., Di Fede G., Benussi L., Ghidoni R. Cerebrospinal fluid EV concentration and size are altered in Alzheimer’s disease and dementia with lewy bodies. Cells. 2022;11(3):462. doi: 10.3390/cells11030462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yokoyama H., Okazaki K., Imai D., Yamashina Y., Takeda R., Naghavi N., Ota A., Hirasawa Y., Miyagawa T. The effect of cognitive-motor dual-task training on cognitive function and plasma amyloid β peptide 42/40 ratio in healthy elderly persons: A randomized controlled trial. BMC Geriatr. 2015;15(1):60. doi: 10.1186/s12877-015-0058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang S., Lachance B.B., Mattson M.P., Jia X. Glucose metabolic crosstalk and regulation in brain function and diseases. Prog. Neurobiol. 2021;204:102089. doi: 10.1016/j.pneurobio.2021.102089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Terzo S., Amato A., Mulè F. From obesity to Alzheimer’s disease through insulin resistance. J. Diabetes Complications. 2021;35(11):108026. doi: 10.1016/j.jdiacomp.2021.108026. [DOI] [PubMed] [Google Scholar]
- 141.De Felice F.G., Gonçalves R.A., Ferreira S.T. Impaired insulin signalling and allostatic load in Alzheimer disease. Nat. Rev. Neurosci. 2022;23(4):215–230. doi: 10.1038/s41583-022-00558-9. [DOI] [PubMed] [Google Scholar]
- 142.Hallschmid M. Intranasal insulin for Alzheimer’s disease. CNS Drugs. 2021;35(1):21–37. doi: 10.1007/s40263-020-00781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chadha S., Behl T., Sehgal A., Kumar A., Bungau S. Exploring the role of mitochondrial proteins as molecular target in Alzheimer’s disease. Mitochondrion. 2021;56:62–72. doi: 10.1016/j.mito.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 144.Athar T., Al Balushi K., Khan S.A. Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Mol. Biol. Rep. 2021;48(7):5629–5645. doi: 10.1007/s11033-021-06512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Austad S.N., Ballinger S., Buford T.W., Carter C.S., Smith D.L., Jr, Darley-Usmar V., Zhang J. Targeting whole body metabolism and mitochondrial bioenergetics in the drug development for Alzheimer’s disease. Acta Pharm. Sin. B. 2022;12(2):511–310. doi: 10.1016/j.apsb.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nguyen T.T., Nguyen T.T.D., Nguyen T.K.O., Vo T.K., Vo V.G. Advances in developing therapeutic strategies for Alzheimer’s disease. Biomed. Pharmacother. 2021;139:111623. doi: 10.1016/j.biopha.2021.111623. [DOI] [PubMed] [Google Scholar]
- 147.Han Y., Chu X., Cui L., Fu S., Gao C., Li Y., Sun B. Neuronal mitochondria-targeted therapy for Alzheimer’s disease by systemic delivery of resveratrol using dual-modified novel biomimetic nanosystems. Drug Deliv. 2020;27(1):502–518. doi: 10.1080/10717544.2020.1745328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ordóñez-Gutiérrez L., Re F., Bereczki E., Ioja E., Gregori M., Andersen A.J., Antón M., Moghimi S.M., Pei J.J., Masserini M., Wandosell F. Repeated intraperitoneal injections of liposomes containing phosphatidic acid and cardiolipin reduce amyloid-β levels in APP/PS1 transgenic mice. Nanomedicine. 2015;11(2):421–430. doi: 10.1016/j.nano.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 149.Kryscio R.J., Abner E.L., Caban-Holt A., Lovell M., Goodman P., Darke A.K., Yee M., Crowley J., Schmitt F.A. Association of antioxidant supplement use and dementia in the prevention of Alzheimer’s disease by vitamin E and selenium trial (PREADViSE). JAMA Neurol. 2017;74(5):567–573. doi: 10.1001/jamaneurol.2016.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rahman M.A., Cho Y., Nam G., Rhim H. Antioxidant compound, oxyresveratrol, inhibits APP production through the AMPK/ULK1/mTOR-mediated autophagy pathway in mouse cortical astrocytes. Antioxidants. 2021;10(3):408. doi: 10.3390/antiox10030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Luo Q., Lin Y.X., Yang P.P., Wang Y., Qi G.B., Qiao Z.Y., Li B.N., Zhang K., Zhang J.P., Wang L., Wang H. A self-destructive nanosweeper that captures and clears amyloid β-peptides. Nat. Commun. 2018;9(1):1802. doi: 10.1038/s41467-018-04255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang S., Zheng J., Ma L., Petersen R.B., Xu L., Huang K. Inhibiting protein aggregation with nanomaterials: The underlying mechanisms and impact factors. Biochim. Biophys. Acta, Gen. Subj. 2022;1866(2):130061. doi: 10.1016/j.bbagen.2021.130061. [DOI] [PubMed] [Google Scholar]
- 153.Nguyen T.T., Vo T.K., Vo G.V. Therapeutic strategies and nano-drug delivery applications in management of aging Alzheimer’s disease. Rev. New Drug Targets in Age-Related Disorders. 2021:183–198. doi: 10.1007/978-3-030-55035-6_13. [DOI] [PubMed] [Google Scholar]
- 154.Gobbi M., Re F., Canovi M., Beeg M., Gregori M., Sesana S., Sonnino S., Brogioli D., Musicanti C., Gasco P., Salmona M., Masserini M.E. Lipid-based nanoparticles with high binding affinity for amyloid-β1-42 peptide. Biomaterials. 2010;31(25):6519–6529. doi: 10.1016/j.biomaterials.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 155.Poudel P., Park S. Recent advances in the treatment of Alzheimer’s disease using nanoparticle-based drug delivery systems. Pharmaceutics. 2022;14:835. doi: 10.3390/pharmaceutics14040835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ali T., Kim M.J., Rehman S.U., Ahmad A., Kim M.O. Anthocyanin-loaded PEG-gold nanoparticles enhanced the neuroprotection of anthocyanins in an Aβ1-42 mouse model of Alzheimer’s disease. Mol. Neurobiol. 2017;54(8):6490–6506. doi: 10.1007/s12035-016-0136-4. [DOI] [PubMed] [Google Scholar]
- 157.Liu X., An C., Jin P., Liu X., Wang L. Protective effects of cationic bovine serum albumin-conjugated PEGylated tanshinone IIA nanoparticles on cerebral ischemia. Biomaterials. 2013;34(3):817–830. doi: 10.1016/j.biomaterials.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 158.Lohan S., Raza K., Mehta S.K., Bhatti G.K., Saini S., Singh B. Anti-Alzheimer’s potential of berberine using surface decorated multi-walled carbon nanotubes: A preclinical evidence. Int. J. Pharm. 2017;530(1-2):263–278. doi: 10.1016/j.ijpharm.2017.07.080. [DOI] [PubMed] [Google Scholar]
- 159.Mirsadeghi S., Shanehsazzadeh S., Atyabi F., Dinarvand R. Effect of PEGylated superparamagnetic iron oxide nanoparticles (SPIONs) under magnetic field on amyloid beta fibrillation process. Mater. Sci. Eng. C. 2016;59:390–397. doi: 10.1016/j.msec.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 160.Conti E., Gregori M., Radice I., Da Re F., Grana D., Re F., Salvati E., Masserini M., Ferrarese C., Zoia C.P., Tremolizzo L. Multifunctional liposomes interact with Abeta in human biological fluids: Therapeutic implications for Alzheimer’s disease. Neurochem. Int. 2017;108:60–65. doi: 10.1016/j.neuint.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 161.Karimzadeh M., Rashidi L., Ganji F. Mesoporous silica nanoparticles for efficient rivastigmine hydrogen tartrate delivery into SY5Y cells. Drug Dev. Ind. Pharm. 2017;43(4):628–636. doi: 10.1080/03639045.2016.1275668. [DOI] [PubMed] [Google Scholar]
- 162.Misra S., Chopra K., Sinha V.R., Medhi B. Galantamine-loaded solid-lipid nanoparticles for enhanced brain delivery: Preparation, characterization, in vitro and in vivo evaluations. Drug Deliv. 2016;23(4):1434–1443. doi: 10.3109/10717544.2015.1089956. [DOI] [PubMed] [Google Scholar]
- 163.Li H., Luo Y., Derreumaux P., Wei G. Carbon nanotube inhibits the formation of β-sheet-rich oligomers of the Alzheimer’s amyloid-β(16-22) peptide. Biophys. J. 2011;101(9):2267–2276. doi: 10.1016/j.bpj.2011.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu Z., Gao X., Kang T., Jiang M., Miao D., Gu G., Hu Q., Song Q., Yao L., Tu Y., Chen H., Jiang X., Chen J. B6 peptide-modified PEG-PLA nanoparticles for enhanced brain delivery of neuroprotective peptide. Bioconjug. Chem. 2013;24(6):997–1007. doi: 10.1021/bc400055h. [DOI] [PubMed] [Google Scholar]
- 165.Karatas H., Aktas Y., Gursoy-Ozdemir Y., Bodur E., Yemisci M., Caban S., Vural A., Pinarbasli O., Capan Y., Fernandez-Megia E., Novoa-Carballal R., Riguera R., Andrieux K., Couvreur P., Dalkara T. A nanomedicine transports a peptide caspase-3 inhibitor across the blood-brain barrier and provides neuroprotection. J. Neurosci. 2009;29(44):13761–13769. doi: 10.1523/JNEUROSCI.4246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O., Szoeke C., Macaulay S.L., Martins R., Maruff P., Ames D., Rowe C.C., Masters C.L. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013;12(4):357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 167.Schaffhauser H., Mathiasen J.R., DiCamillo A., Huffman M.J., Lu L.D., McKenna B.A., Qian J., Marino M.J. Dimebolin is a 5-HT6 antagonist with acute cognition enhancing activities. Biochem. Pharmacol. 2009;78(8):1035–1042. doi: 10.1016/j.bcp.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 168.Burns D.K., Chiang C., Welsh-Bohmer K.A., Brannan S.K., Culp M., O’Neil J., Runyan G., Harrigan P., Plassman B.L., Lutz M., Lai E., Haneline S., Yarnall D., Yarbrough D., Metz C., Ponduru S., Sundseth S., Saunders A.M. The TOMMORROW study: Design of an Alzheimer’s disease delay‐of‐onset clinical trial. Alzheimers Dement. (N. Y.) 2019;5(1):661–670. doi: 10.1016/j.trci.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Swerdlow R.H., Bothwell R., Hutfles L., Burns J.M., Reed G.A. Tolerability and pharmacokinetics of oxaloacetate 100mg capsules in Alzheimer’s subjects. BBA Clin. 2016;5:120–123. doi: 10.1016/j.bbacli.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mani S., Swargiary G., Singh M., Agarwal S., Dey A., Ojha S., Jha N.K. Mitochondrial defects: An emerging theranostic avenue towards Alzheimer’s associated dysregulations. Life Sci. 2021;285:119985. doi: 10.1016/j.lfs.2021.119985. [DOI] [PubMed] [Google Scholar]