Abstract
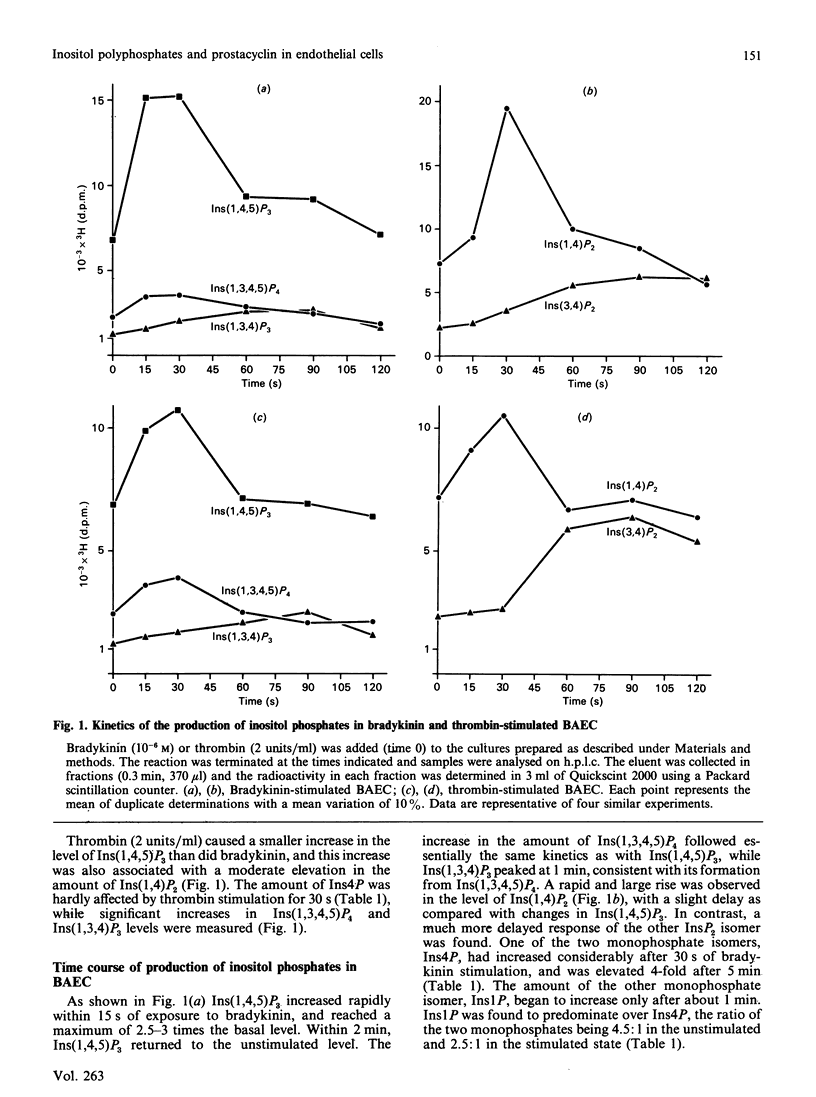
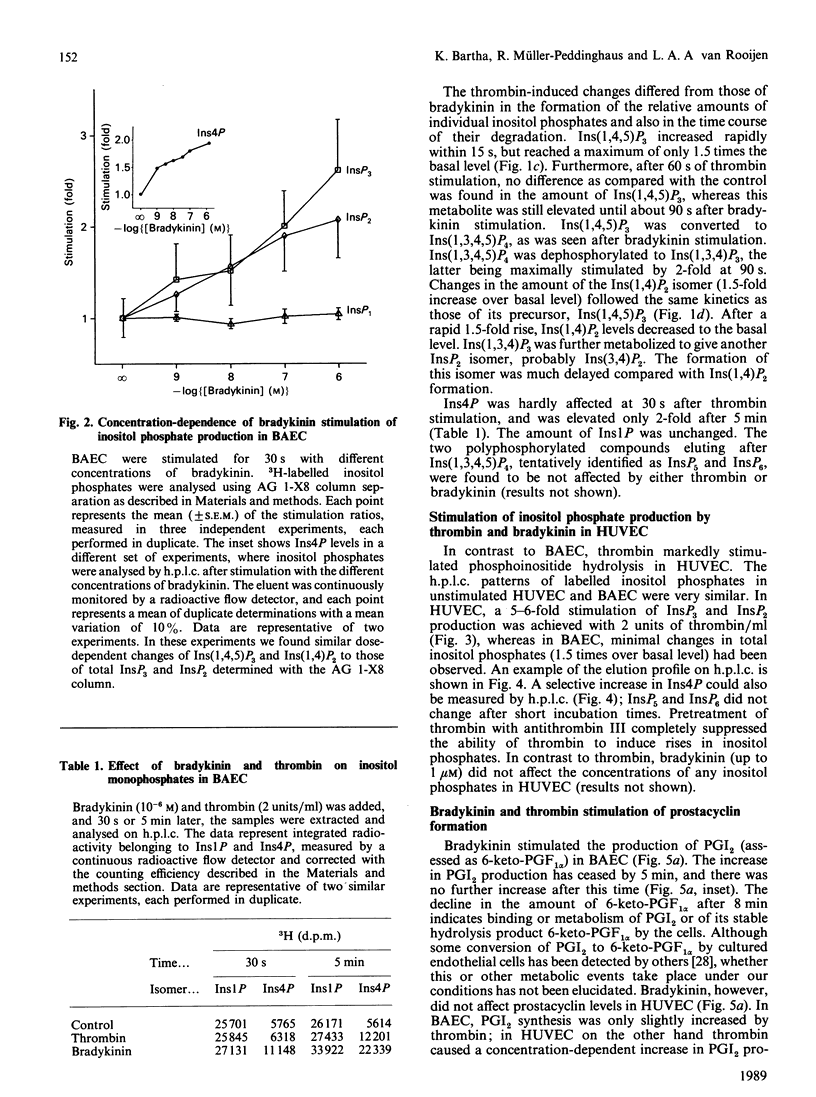
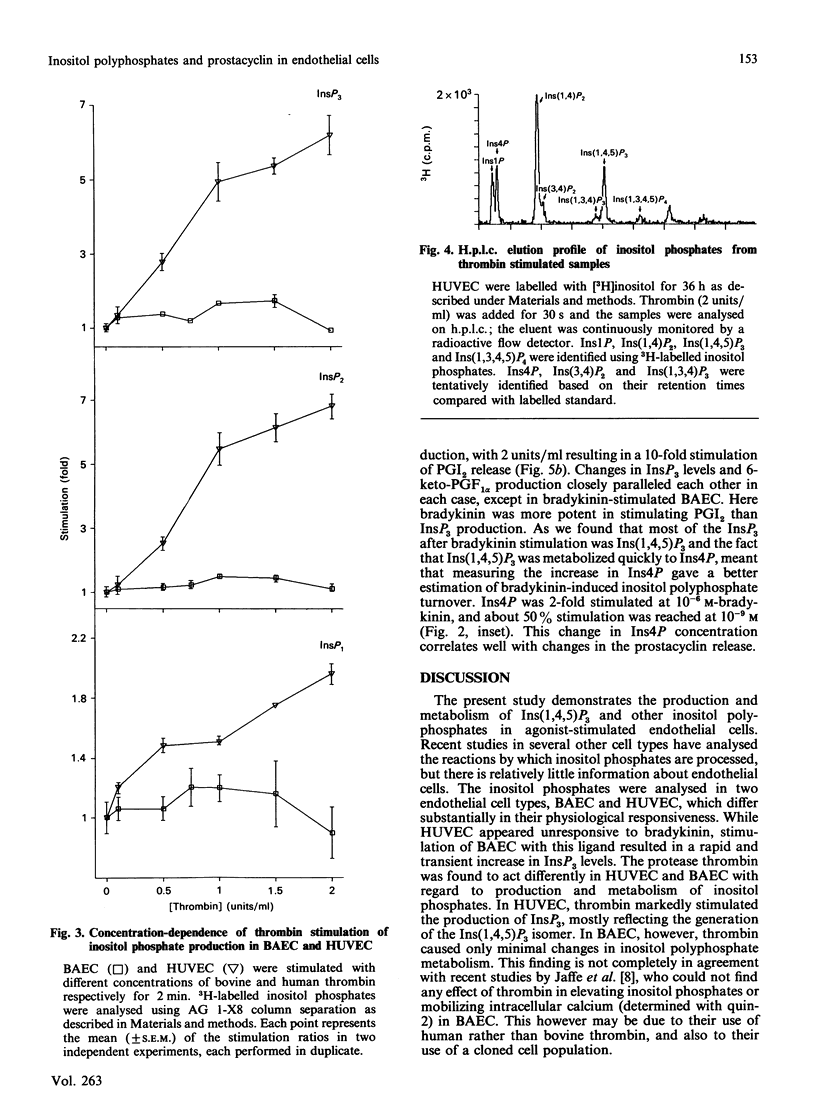
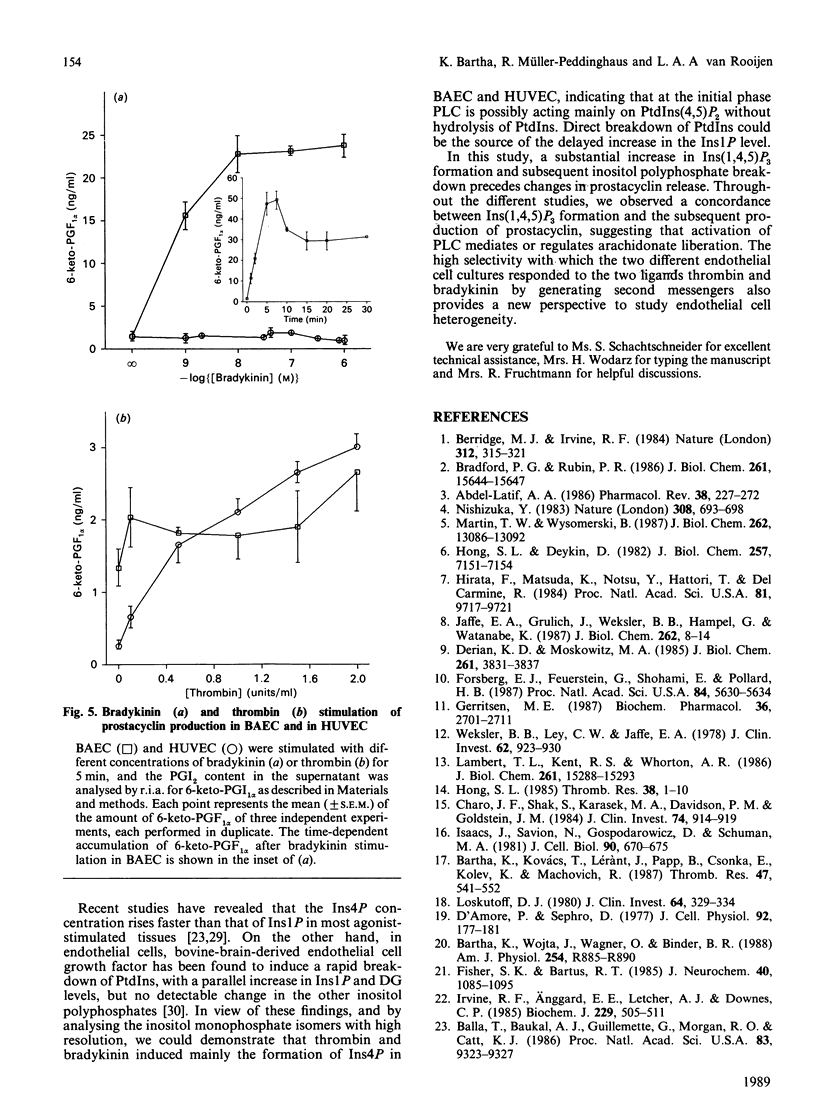
Prostacyclin (PGI2) production by thrombin- and bradykinin-stimulated bovine aortic endothelial cells (BAEC) and human umbilical vein endothelial cells (HUVEC) was related to the receptor-linked activation of inositide hydrolysis. Bradykinin caused a rapid and transient 3-fold increase in the formation of inositol polyphosphates in BAEC. The increase in InsP3 reflected changes mainly in the Ins(1,4,5)P3 isomer. Thrombin was less effective than bradykinin in increasing InsP3 levels and appeared to only minimally stimulate the production of PGI2 in BAEC. In HUVEC, thrombin caused a 5-fold elevation of Ins(1,4,5)P3, closely related to a rise in PGI2 production. However, bradykinin did not affect inositol phosphates and PGI2 production in HUVEC. Other inositol phosphates were also assessed to obtain information on putative metabolism of Ins(1,4,5)P3. The present study supports the notion that formation of Ins(1,4,5)P3 is linked to an increase in PGI2 production in endothelial cells and furthermore provides evidence for a large degree of heterogeneity in the responses of BAEC and HUVEC to thrombin and bradykinin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Balla T., Baukal A. J., Guillemette G., Morgan R. O., Catt K. J. Angiotensin-stimulated production of inositol trisphosphate isomers and rapid metabolism through inositol 4-monophosphate in adrenal glomerulosa cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9323–9327. doi: 10.1073/pnas.83.24.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartha K., Kovács T., Léránt I., Papp B., Csonka E., Kolev K., Machovich R. Interaction of antithrombin III and thrombin-antithrombin III complex with cultured aortic endothelial cells. Thromb Res. 1987 Sep 1;47(5):541–552. doi: 10.1016/0049-3848(87)90359-8. [DOI] [PubMed] [Google Scholar]
- Bartha K., Wojta J., Wagner O. F., Binder B. R. Comparison of fibrinolytic activities of human and bovine endothelial cells. Am J Physiol. 1988 Jun;254(6 Pt 2):R885–R890. doi: 10.1152/ajpregu.1988.254.6.R885. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. Quantitative changes in inositol 1,4,5-trisphosphate in chemoattractant-stimulated neutrophils. J Biol Chem. 1986 Nov 25;261(33):15644–15647. [PubMed] [Google Scholar]
- Charo I. F., Shak S., Karasek M. A., Davison P. M., Goldstein I. M. Prostaglandin I2 is not a major metabolite of arachidonic acid in cultured endothelial cells from human foreskin microvessels. J Clin Invest. 1984 Sep;74(3):914–919. doi: 10.1172/JCI111509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amore P., Shepro D. Stimulation of growth and calcium influx in cultured, bovine, aortic endothelial cells by platelets and vasoactive substances. J Cell Physiol. 1977 Aug;92(2):177–183. doi: 10.1002/jcp.1040920206. [DOI] [PubMed] [Google Scholar]
- Derian C. K., Moskowitz M. A. Polyphosphoinositide hydrolysis in endothelial cells and carotid artery segments. Bradykinin-2 receptor stimulation is calcium-independent. J Biol Chem. 1986 Mar 15;261(8):3831–3837. [PubMed] [Google Scholar]
- Fisher S. K., Bartus R. T. Regional differences in the coupling of muscarinic receptors to inositol phospholipid hydrolysis in guinea pig brain. J Neurochem. 1985 Oct;45(4):1085–1095. doi: 10.1111/j.1471-4159.1985.tb05527.x. [DOI] [PubMed] [Google Scholar]
- Forsberg E. J., Feuerstein G., Shohami E., Pollard H. B. Adenosine triphosphate stimulates inositol phospholipid metabolism and prostacyclin formation in adrenal medullary endothelial cells by means of P2-purinergic receptors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5630–5634. doi: 10.1073/pnas.84.16.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen M. E., Cheli C. D. Arachidonic acid and prostaglandin endoperoxide metabolism in isolated rabbit and coronary microvessels and isolated and cultivated coronary microvessel endothelial cells. J Clin Invest. 1983 Nov;72(5):1658–1671. doi: 10.1172/JCI111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen M. E. Functional heterogeneity of vascular endothelial cells. Biochem Pharmacol. 1987 Sep 1;36(17):2701–2711. doi: 10.1016/0006-2952(87)90252-8. [DOI] [PubMed] [Google Scholar]
- Heslop J. P., Irvine R. F., Tashjian A. H., Jr, Berridge M. J. Inositol tetrakis- and pentakisphosphates in GH4 cells. J Exp Biol. 1985 Nov;119:395–401. doi: 10.1242/jeb.119.1.395. [DOI] [PubMed] [Google Scholar]
- Hong S. L., Deykin D. Activation of phospholipases A2 and C in pig aortic endothelial cells synthesizing prostacyclin. J Biol Chem. 1982 Jun 25;257(12):7151–7154. [PubMed] [Google Scholar]
- Hong S. L., McLaughlin N. J., Tzeng C. Y., Patton G. Prostacyclin synthesis and deacylation of phospholipids in human endothelial cells: comparison of thrombin, histamine and ionophore A23187. Thromb Res. 1985 Apr 1;38(1):1–10. doi: 10.1016/0049-3848(85)90002-7. [DOI] [PubMed] [Google Scholar]
- Inhorn R. C., Bansal V. S., Majerus P. W. Pathway for inositol 1,3,4-trisphosphate and 1,4-bisphosphate metabolism. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2170–2174. doi: 10.1073/pnas.84.8.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Downes C. P. Inositol trisphosphates in carbachol-stimulated rat parotid glands. Biochem J. 1984 Oct 1;223(1):237–243. doi: 10.1042/bj2230237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs J., Savion N., Gospodarowicz D., Shuman M. A. Effect of cell density on thrombin binding to a specific site on bovine vascular endothelial cells. J Cell Biol. 1981 Sep;90(3):670–674. doi: 10.1083/jcb.90.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Loskutoff D. J. Effect of thrombin on the fibrinolytic activity of cultured bovine endothelial cells. J Clin Invest. 1979 Jul;64(1):329–332. doi: 10.1172/JCI109457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Bansal V. S., Inhorn R. C., Ross T. S., Lips D. L. Inositol phosphates: synthesis and degradation. J Biol Chem. 1988 Mar 5;263(7):3051–3054. [PubMed] [Google Scholar]
- Martin T. W., Wysolmerski R. B. Ca2+-dependent and Ca2+-independent pathways for release of arachidonic acid from phosphatidylinositol in endothelial cells. J Biol Chem. 1987 Sep 25;262(27):13086–13092. [PubMed] [Google Scholar]
- Moscat J., Moreno F., Herrero C., López C., García-Barreno P. Endothelial cell growth factor and ionophore A23187 stimulation of production of inositol phosphates in porcine aorta endothelial cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):659–663. doi: 10.1073/pnas.85.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Stephens L. R., Hawkins P. T., Barker C. J., Downes C. P. Synthesis of myo-inositol 1,3,4,5,6-pentakisphosphate from inositol phosphates generated by receptor activation. Biochem J. 1988 Aug 1;253(3):721–733. doi: 10.1042/bj2530721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]


