Abstract
1. A new procedure for purifying pig heart NAD+-isocitrate dehydrogenase from mitochondrial extracts has been developed. This relies on the use of f.p.l.c. techniques and exploits the hydrophobic properties of the gel-filtration medium Superose 6 at high ionic strength. A 300-fold purification to apparent homogeneity is achieved within 5 h and with a yield of greater than 20%. 2. The enzyme had an apparent native molecular mass on gel filtration of 320 kDa. In agreement with previous studies [Ramachandran & Colman (1980) J. Biol. Chem. 255, 8859-8864], three subunits (all close to 38 kDa) were separable by isoelectric focusing 3. This preparation was used to investigate the effects of adenine nucleotides, KCl and the required bivalent metal ions, Mg2+ and Mn2+, on the regulation of the enzyme by Ca2+. 4. In the presence of 1.5 mM-ADP, increasing the concentration of Mg2+ from 20 microM to 6.0 mM raised the concentration of Ca2+ required for half-maximal effect (K0.5 value) from 1.2 microM to 232 microM. Similarly, in the presence of 2.5 microM-Mn2+, a K0.5 value for Ca2+ of 3.3 microM was obtained, and this value was increased to 8.9 microM in the presence of 100 microM-Mn2+. In the presence of 1 mM-Mg2+ and 1.5 mM-ADP, the K0.5 value for Ca2+ was raised from 4.7 microM to 10 microM by 75 mM-KCl.
Full text
PDF

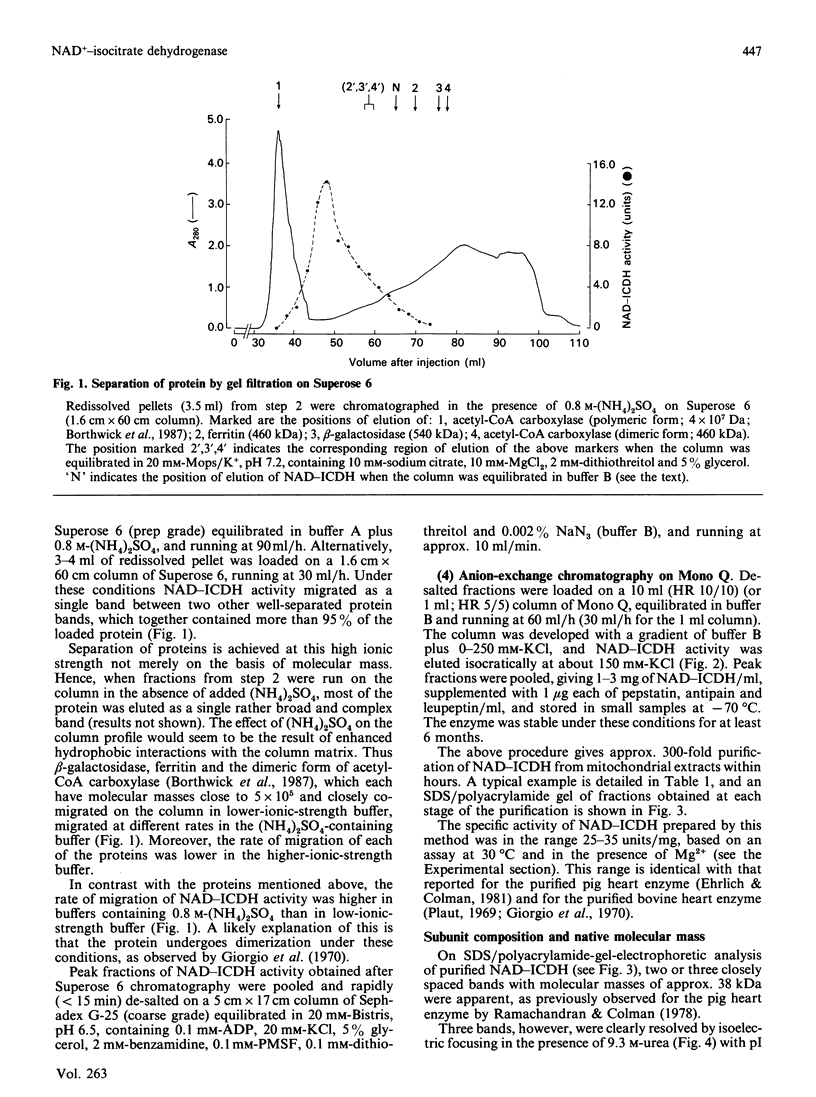
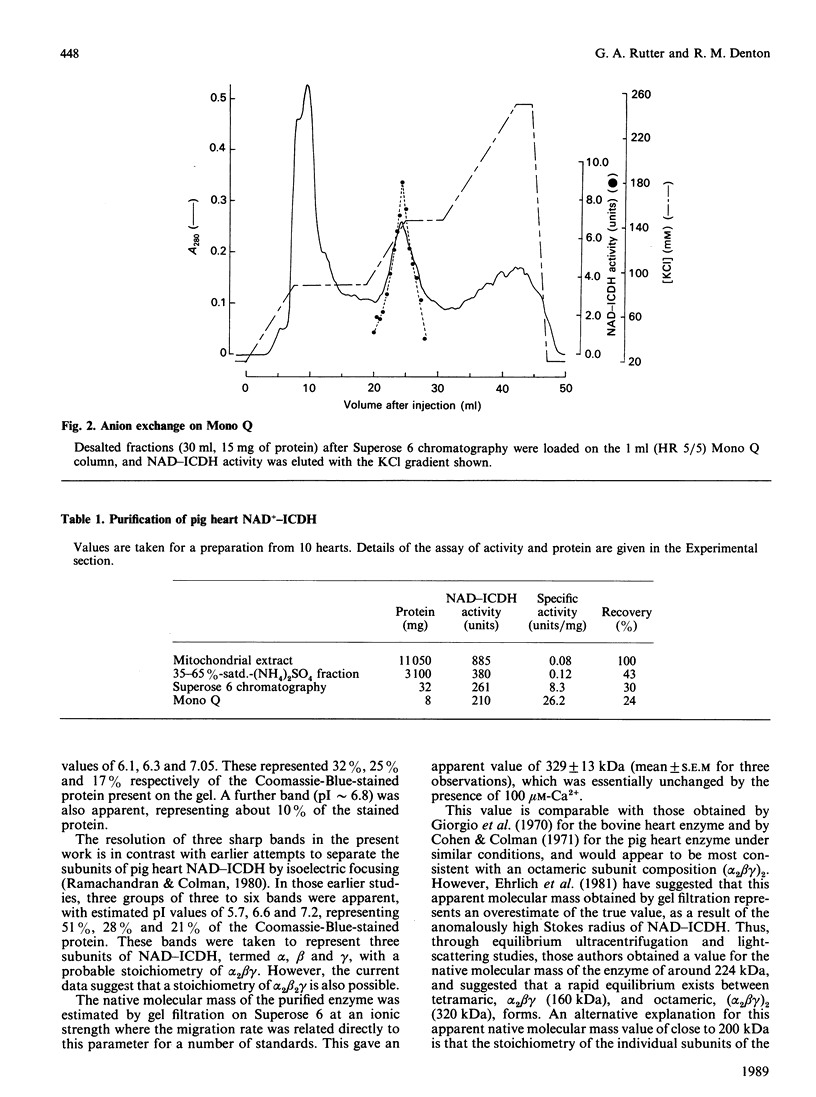
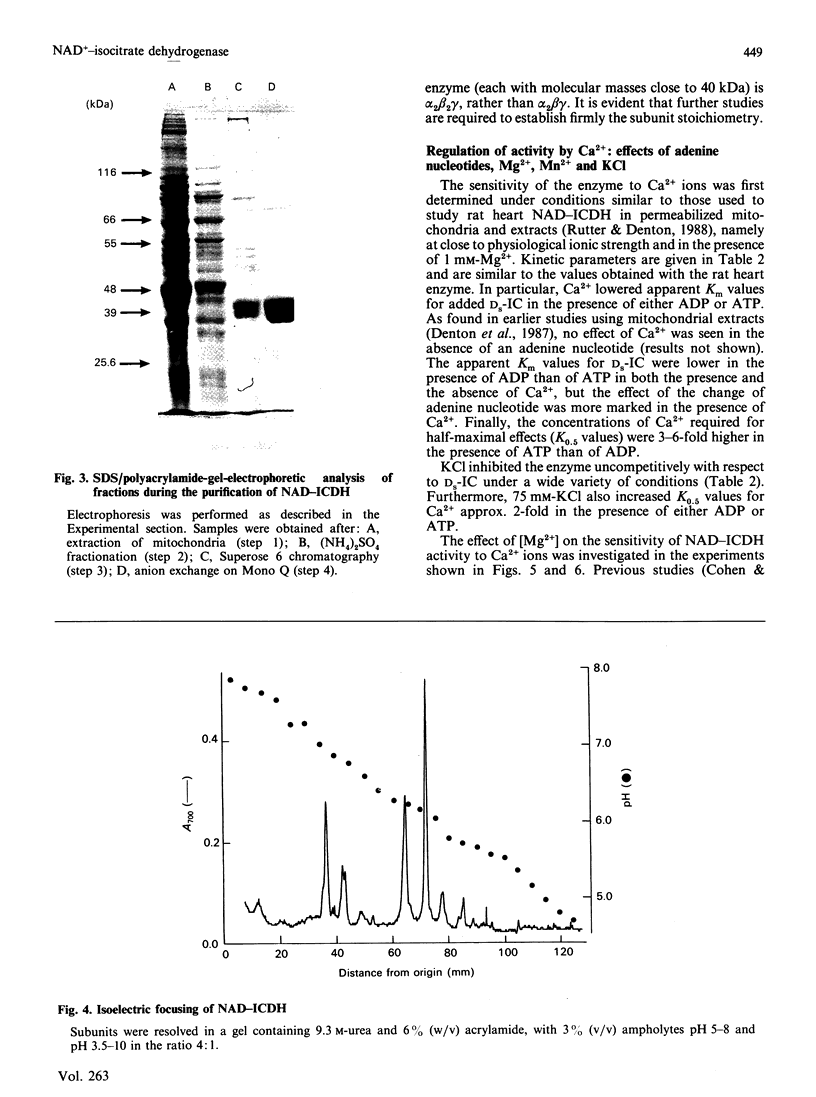
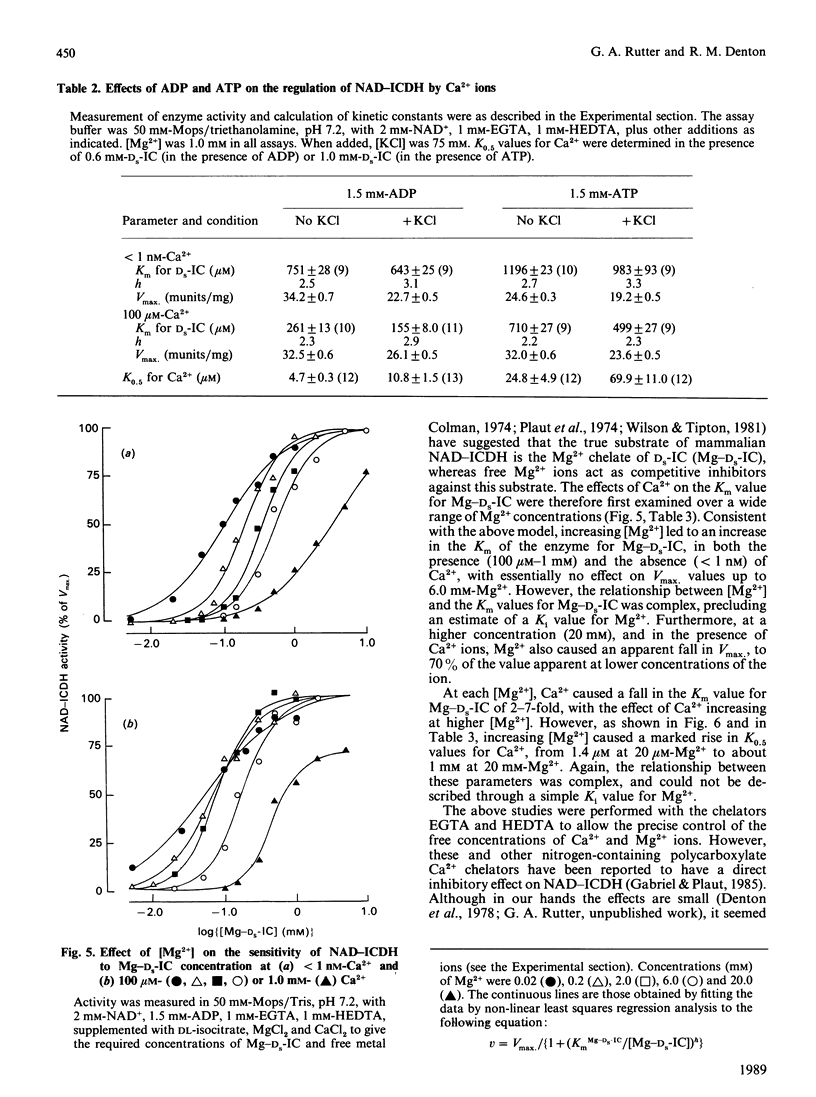
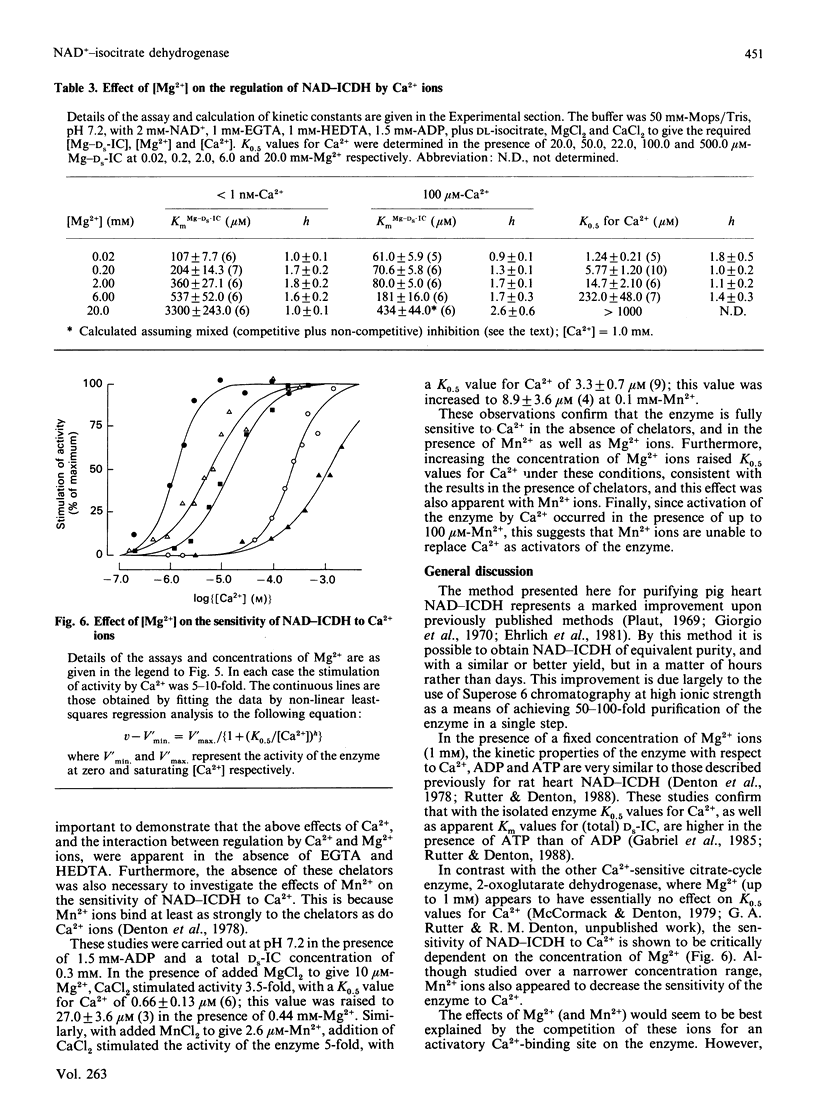

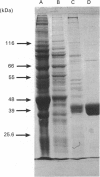
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aogaichi T., Evans J., Gabriel J., Plaut G. W. The effects of calcium and lanthanide ions on the activity of bovine heart nicotinamide adenine dinucleotide-specific isocitrate dehydrogenase. Arch Biochem Biophys. 1980 Oct 1;204(1):350–356. doi: 10.1016/0003-9861(80)90043-0. [DOI] [PubMed] [Google Scholar]
- Belsham G. J., Denton R. M., Tanner M. J. Use of a novel rapid preparation of fat-cell plasma membranes employing Percoll to investigate the effects of insulin and adrenaline on membrane protein phosphorylation within intact fat-cells. Biochem J. 1980 Nov 15;192(2):457–467. doi: 10.1042/bj1920457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick A. C., Edgell N. J., Denton R. M. Use of rapid gel-permeation chromatography to explore the inter-relationships between polymerization, phosphorylation and activity of acetyl-CoA carboxylase. Effects of insulin and phosphorylation by cyclic AMP-dependent protein kinase. Biochem J. 1987 Feb 1;241(3):773–782. doi: 10.1042/bj2410773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brownsey R. W., Edgell N. J., Hopkirk T. J., Denton R. M. Studies on insulin-stimulated phosphorylation of acetyl-CoA carboxylase, ATP citrate lyase and other proteins in rat epididymal adipose tissue. Evidence for activation of a cyclic AMP-independent protein kinase. Biochem J. 1984 Mar 15;218(3):733–743. doi: 10.1042/bj2180733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN R. F., PLAUT G. W. ACTIVATION AND INHIBITION OF DPN-LINKED ISOCITRATE DEHYDROGENASE OF HEART BY CERTAIN NUCLEOTIDES. Biochemistry. 1963 Sep-Oct;2:1023–1032. doi: 10.1021/bi00905a020. [DOI] [PubMed] [Google Scholar]
- Cohen P. F., Colman R. F. Purification of NAD-specific isocitrate dehydrogenase from porcine heart. Biochim Biophys Acta. 1971 Aug 20;242(2):325–330. doi: 10.1016/0005-2744(71)90224-5. [DOI] [PubMed] [Google Scholar]
- Cohen P. F., Colman R. F. Role of manganous ion in the kinetics of pig-heart NAD-specific isocitrate dehydrogenase. Eur J Biochem. 1974 Aug 15;47(1):35–45. doi: 10.1111/j.1432-1033.1974.tb03665.x. [DOI] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974 Dec;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkey B. E., Duszynski J., Rich T. L., Matschinsky B., Williamson J. R. Regulation of free and bound magnesium in rat hepatocytes and isolated mitochondria. J Biol Chem. 1986 Feb 25;261(6):2567–2574. [PubMed] [Google Scholar]
- Davidson A. M., Halestrap A. P. Inhibition of mitochondrial-matrix inorganic pyrophosphatase by physiological [Ca2+], and its role in the hormonal regulation of mitochondrial matrix volume. Biochem J. 1989 Mar 15;258(3):817–821. doi: 10.1042/bj2580817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985 Dec;249(6 Pt 1):E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G., Edgell N. J. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J. 1980 Jul 15;190(1):107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G., Midgley P. J., Rutter G. A. Hormonal regulation of fluxes through pyruvate dehydrogenase and the citric acid cycle in mammalian tissues. Biochem Soc Symp. 1987;54:127–143. [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Richards D. A., Chin J. G. Calcium ions and the regulation of NAD+-linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem J. 1978 Dec 15;176(3):899–906. doi: 10.1042/bj1760899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich R. S., Colman R. F. Binding of ligands to half of subunits of NAD-dependent isocitrate dehydrogenase from pig heart. Binding of manganous ion, isocitrate, ADP and NAD. J Biol Chem. 1981 Feb 10;256(3):1276–1282. [PubMed] [Google Scholar]
- Ehrlich R. S., Hayman S., Ramachandran N., Colman R. F. Re-evaluation of molecular weight of pig heart NAD-specific isocitrate dehydrogenase. J Biol Chem. 1981 Oct 25;256(20):10560–10564. [PubMed] [Google Scholar]
- GOEBELL H., KLINGENBERG M. DPN-SPEZIFISCHE ISOCITRAT-DEHYDROGENASE DER MITOCHONDRIEN. I. KINETISCHE EIGENSSCHAFTEN, VORKOMMEN UND FUNKTION DER DPN-SPEZIFISCHEN ISOCITRAT-DEHYDROGENASE. Biochem Z. 1964 Sep 28;340:441–464. [PubMed] [Google Scholar]
- Gabriel J. L., Milner R., Plaut G. W. Inhibition and activation of bovine heart NAD-specific isocitrate dehydrogenase by ATP. Arch Biochem Biophys. 1985 Jul;240(1):128–134. doi: 10.1016/0003-9861(85)90015-3. [DOI] [PubMed] [Google Scholar]
- Gabriel J. L., Plaut G. W. NAD-specific isocitrate dehydrogenase from bovine heart. Interaction with Ca2+ chelators. Biochem J. 1985 Aug 1;229(3):817–822. doi: 10.1042/bj2290817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel J. L., Zervos P. R., Plaut G. W. Activity of purified NAD-specific isocitrate dehydrogenase at modulator and substrate concentrations approximating conditions in mitochondria. Metabolism. 1986 Jul;35(7):661–667. doi: 10.1016/0026-0495(86)90175-7. [DOI] [PubMed] [Google Scholar]
- Giorgio N. A., Jr, Yip A. T., Fleming J., Plaut G. W. Diphosphopyridine nucleotide-linked isocitrate dehydrogenase from bovine heart. Polymeric forms and subunits. J Biol Chem. 1970 Oct 25;245(20):5469–5477. [PubMed] [Google Scholar]
- Hansford R. G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- Jung D. W., Brierley G. P. Matrix magnesium and the permeability of heart mitochondria to potassium ion. J Biol Chem. 1986 May 15;261(14):6408–6415. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawlis V. B., Roche T. E. Effect of micromolar Ca2+ on NADH inhibition of bovine kidney alpha-ketoglutarate dehydrogenase complex and possible role of Ca2+ in signal amplification. Mol Cell Biochem. 1980 Nov 20;32(3):147–152. doi: 10.1007/BF00227441. [DOI] [PubMed] [Google Scholar]
- McCarthy A. D., Hardie D. G. Evidence that the acyl-O-esters are intermediates in the catalysis. The mechanism of rabbit mammary fatty acid synthase. FEBS Lett. 1982 Dec 13;150(1):181–184. doi: 10.1016/0014-5793(82)81330-6. [DOI] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J. 1979 Jun 15;180(3):533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley P. J., Rutter G. A., Thomas A. P., Denton R. M. Effects of Ca2+ and Mg2+ on the activity of pyruvate dehydrogenase phosphate phosphatase within toluene-permeabilized mitochondria. Biochem J. 1987 Jan 15;241(2):371–377. doi: 10.1042/bj2410371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLAUT G. W., SUNG S. C. Diphosphopyridine nucleotide isocitric dehydrogenase from animal tissues. J Biol Chem. 1954 Mar;207(1):305–314. [PubMed] [Google Scholar]
- Plaut G. W., Aogaichi T. Purification and properties of diphosphopyridine nuleotide-linked isocitrate dehydrogenase of mammalian liver. J Biol Chem. 1968 Nov 10;243(21):5572–5583. [PubMed] [Google Scholar]
- Plaut G. W., Schramm V. L., Aogaichi T. Action of magnesium ion on diphosphopyridine nucleotide-linked isocitrate dehydrogenase from bovine heart. Characterization of the forms of the substrate and the modifier of the reaction. J Biol Chem. 1974 Mar 25;249(6):1848–1856. [PubMed] [Google Scholar]
- Ramachandran N., Colman R. F. Chemical characterization of distinct subunits of pig heart DPN-specific isocitrate dehydrogenase. J Biol Chem. 1980 Sep 25;255(18):8859–8864. [PubMed] [Google Scholar]
- Ramachandran N., Colman R. F. Evidence for the presence of two nonidentical subunits in NAD-dependent isocitrate dehydrogenase of pig heart. Proc Natl Acad Sci U S A. 1978 Jan;75(1):252–255. doi: 10.1073/pnas.75.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter G. A., Denton R. M. Regulation of NAD+-linked isocitrate dehydrogenase and 2-oxoglutarate dehydrogenase by Ca2+ ions within toluene-permeabilized rat heart mitochondria. Interactions with regulation by adenine nucleotides and NADH/NAD+ ratios. Biochem J. 1988 May 15;252(1):181–189. doi: 10.1042/bj2520181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter G. A., Denton R. M. The binding of Ca2+ ions to pig heart NAD+-isocitrate dehydrogenase and the 2-oxoglutarate dehydrogenase complex. Biochem J. 1989 Oct 15;263(2):453–462. doi: 10.1042/bj2630453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W. C., Mauck L., Colman R. F. Physicochemical properties of the diphosphopyridine nucleotide-specific isocitrate dehydrogenase of pig heart. J Biol Chem. 1974 Dec 25;249(24):7942–7949. [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H. Regulation of the citric acid cycle in mammalian systems. FEBS Lett. 1980 Aug 25;117 (Suppl):K73–K85. doi: 10.1016/0014-5793(80)80572-2. [DOI] [PubMed] [Google Scholar]
- Willson V. J., Tipton K. F. The activation of ox-brain NAD+-dependent isocitrate dehydrogenase by magnesium ions. Eur J Biochem. 1981 Jan;113(3):477–483. doi: 10.1111/j.1432-1033.1981.tb05088.x. [DOI] [PubMed] [Google Scholar]
- Wong S. T., Mastropaolo W., Henshaw E. C. Differential phosphorylation of soluble versus ribosome-bound eukaryotic initiation factor 2 in the Ehrlich ascites tumor cell. J Biol Chem. 1982 May 10;257(9):5231–5238. [PubMed] [Google Scholar]