Abstract
TP53, a guardian of the genome, suppresses or enhances tumors through various regulatory pathways. However, the role of p53-related long non-coding RNAs (lncRNAs) in immune regulation of tumor microenvironment and prognosis of gastric cancer (GC) is so far unelucidated. We analyzed the role of TP53-associated lncRNAs (obtained from the TP53LNC-DB database) in immune regulation, immune cell infiltration and RNA modification in gastric cancer. Firstly, using multivariate COX regression analysis, we identified eight lncRNAs related to the prognosis of GC. Furthermore, based on the expression of the lncRNA signature and risk score, the GC patients were divided into high-risk and low-risk groups. We found that M2-macrophages have significantly higher infiltration in the high-risk group. Similarly, significant differences in immune function (APC_co_stimulation, CCR, and checkpoint) and m6A modification (FTO, ZC3H13, YTHDC1, and RBM15), and m5C modification (NOP2 and TET1) between both groups were also observed. These signature lncRNAs were also positively associated with oxidative stress-related genes (MPO, MAPK14, HMOX1, and APP). Additionally, we found that high expression of GAS5 and low expression of MALAT1 in Helicobacter pylori (H-pylori) positive GC patients. Finally, GC patients in the low-risk group showed higher resistance to immunotherapy while patients in the high-risk group were more sensitive to various chemotherapy drugs. Based on these findings, we conclude that p53-associated lncRNAs signature could potentially predict the immune status and overall survival, and may also be used for risk management and planning immunotherapy for gastric cancer patients.
Keywords: P53-related lncRNAs, lncRNA signature, Gastric cancer, Immune cell infiltration, Tumor microenvironment, Immunotherapy, m6A, m5C
1. Introduction
Gastric cancer (GC) is considered one of the most common cancers and ranked as the top deadly cancer worldwide [1]. Although recent therapeutic advancements such as immunotherapy have brought some success in treatment outcomes of advanced-stage gastric cancer patients [2,3], unfortunately, the overall survival and prognosis of GC patients are still poor [4]. Identifying more effective molecular markers for clinical diagnosis and therapeutics is highly valuable for improving disease prognosis, reducing the disease burden [5,6], and planning better clinical management and therapeutics [7,8].
The underlying regulatory mechanisms of cancer pathogenesis and development are highly complex [9]. The regulatory factors, including coding and noncoding genes, play a critical role in the cancer development [10]. Noncoding genes, especially long non-coding RNAs (lncRNAs), perform various regulatory functions in biological systems, including transcriptional, translational, and posttranslational regulation of the DNA, RNAs, and proteins [11]. LncRNAs can be oncopromotors and oncosuppressors or regulators of non-malignant diseases, such as lncRNA LINC-PINT plays a crucial role in various diseases, including cancers, neurological disorders, diabetes, and viral infections [12,13]. LncRNA linc00152 controls the proliferation and transition of the epithelial cell to mesenchymal cells [14], and lncRNA GAS5 causes cell cycle arrest and inhibits tumor growth and cellular proliferation [15]. A lncRNA PVT1 is one of the critical lncRNAs that promotes gastric cancer phenotype through activating angiogenesis and vasculogenic mimicry through STAT3 [[16], [17], [18]]. LncRNAs are also considered key players in cancer immunity, such as lncRNA NKILA regulates tumor-specific cytotoxic T lymphocytes and T helper cells for the cancer evasion [19], and Lnc-MM2P activates macrophages in the osteosarcoma [20]. Recently, several studies have predicted the role of lncRNAs in the prognosis and overall survival of gastric cancer [[21], [22], [23]].
Nevertheless, lncRNA models demand a decent number of functional analyses in an independent cohort. So far, no comprehensive studies have shown the role of p53-associated lncRNAs in immune cell infiltration in gastric cancer. In the current study, we performed a detailed analysis of the p53-associated lncRNAs for their roles in immune function, survival, and prognosis of gastric cancer. We further altered the expression of p53 in gastric cancer cell lines to validate the p53-associated lncRNAs signature. Moreover, lncRNA signatures were validated in normal gastric epithelial and cancer cell lines. The finding of our study may provide a base for planning strategies for risk management and therapeutics for gastric cancer patients.
2. Methods
2.1. Data sources
The TP53-associated lncRNAs were obtained from a previously published database (TP53LNC-DB:) [24]. The database consists of 4851 lncRNA entries in different categories of lncRNA, but we only chose experimentally verified 227 lncRNAs. However, after initial filtering, we selected 128 lncRNA linked with p53. Moreover, we downloaded RNA-sequencing (RNA-seq) data (TCGA-STAD) and complete clinical information of the 375 gastric cancer patients and 32 normal controls from The Cancer Genome Atlas (TCGA) (https://portal.gdc.cancer.gov/) database. The RNA-seq data were processed and normalized using R version 3.6.1 software.
3. Identification of the prognostic gene signature
We extracted the expression levels of all lncRNAs in the samples and normalized all genes using "edgeR". P53-related lncRNAs and patient survival status were combined using "strawberry perl" (version 3.0.0, http://strawberryperl.com/). Multivariate COX regression analysis was performed using the "survival" package and the "glmnet" package to screen out the gene signatures associated with GC prognosis.
3.1. Building a prognostic model
The gastric cancer patients were divided into high-risk and low-risk groups based on the median risk score. Using the ‘survival’ package in the R software [25], a prognostic model was constructed for each high-risk and low-risk group. The model's predictive performance was analyzed by time-dependent receiver operating characteristic (ROC) analysis, and Area Under the Curve (AUC) was calculated for prognostic prediction.
3.2. Relationship between gene signature and clinical characteristics
Age, gender, stage, Tumor Node Metastasis (TNM), and hub lncRNAs were used to construct a heat map to show the relationship between gene expression and clinical features. In addition, multivariate COX regression was used to analyze the risk scores and Hazard Ratio (HR) of different clinical characteristics of the patients.
3.3. ssGSEA analysis
The Gene Set Enrichment Analysis (GSEA) was used to test whether genes in the high-risk score group or low-risk score group were enriched as predefined Hallmark gene sets (v6.2, downloaded from http://software.broadinstitute. org/gsea/downloads.jsp) with the GSEA 3.0 application under the JAVA platform [26]. P-value ≤0.05 was considered a screening convenience standard for false discovery rate (FDR). The top five most meaningful pathways in the high-risk and low-risk groups were selected, and a scatter chart was drawn.
3.4. The relationship between risk score and tumor infiltration of immune cells
The TIMER, CIBERSORT, CIBERSORT-ABS, QUANTISEQ, MCPCOUNTER, XCELL, and EPIC methods were used in the R software to calculate the differential infiltration of the immune cells in both high-risk and low-risk groups. The CIBERSORT package was used to perform a quantitative analysis of immune cells, and the ggplot2 package was used to draw the heat maps of differentially infiltrated immune cells.
3.5. The relationship between risk score and immune marker genes
The immune cells and m6A were obtained from hallmark gene sets in the Molecular Signature Database (MSigDB) (https://www.gsea-msigdb.org/gsea/msigdb/) and imported only related genes through IMPORT, too (https://www.immport.org). The Wilcox test was applied to compare the infiltration level of immune cells in both risk groups. Box plots and scatter plots were drawn using the ‘ggplot2’ package in the R software.
3.6. The correlation between lncRNAs and oxidative stress-related genes (OSRGs)
We extracted Oxidative Stress-related genes (OSRGs) from GeneCards (https://www.genecards.org); genes having correlation scores higher than 40 were selected (Table S1) for determining their correlations with signature lncRNAs. Spearman's test was applied to analyze the correlation of lncRNAs with OSRGs, and a heat map was drawn using the "ggplot2″ package.
3.7. LncRNA expression in gastric cancer cell lines
Gastric cancer cell lines (AGS, SGC7901, MGC803, MKN45, and NCIN87) and normal gastric epithelial cell lines (GES-1) were purchased from American Type Culture Collection (ATCC Manassas, VA, USA). All cells were cultured in RPMI-1640 (Biological Industries, Israel) supplemented with 10 % FBS, and culturing conditions were set in a 37 °C humidified incubator with a constant supply of 5 % CO2. After achieving logarithmic growth, cells were trypsinized and collected in 1 ml pure TRIzol (TaKaRa, Japan), and RNA was extracted using the chloroform method. RT-qPCR was performed using Quantinova Syber-green Real-time PCR Kit (Qiagen, Germany). Relative expression of lncRNAs was calculated using the 2‐ΔΔCt method, and primers used for qPCR are mentioned in Table S2.
3.8. Effect of P53 silencing and overexpression on lncRNAs
The lentiviral particles for P53 knockdown were prepared in HEK293T cells by cotransfecting PLKO.1-EV and PLKO.1-P53 with pREV, pGag/Pol/PRE, and pVSVG (2:2:2:1 ratio) for 48h. Then viral particles in culture media were collected and sh-P53 was transduced to SGC7901 cells using 8 mg/ml polybrene (Sigma); 48h later, cells were selected using puromycin (5 mg/ml) for an additional 24–48h. After achieving a stable cell line, protein and RNA samples were prepared to confirm knockdown efficiency and signature lncRNA expression. Similarly, P53 was cloned into the PSin-3x-flag vector and transiently transfected SGC7901 cells for 48h. Later, the expression of P53 was analyzed by western blotting, and lncRNA expression was evaluated by qualitative real-time PCR.
3.9. Western blot analysis
Total protein samples were prepared using RIPA lysis buffer (Solarbio, Beijing) and quantified using BCA Kit (Solarbio, Beijing) and a microplate reader. An equal amount of protein samples was electrophoresed using SDS-PAGE, transferred to a nitrocellulose membrane, then blocked with 5 % milk, washed with 1 % TBST, and incubated with anti-P53 primary antibody overnight. After removing the primary antibody and washing it with 1 % TBST, the secondary antibody was added for 1h at room temperature. The protein expression was measured using an ECL kit (Epizyme Biomed Tech, Shanghai).
3.10. P53 immunoprecipitation and interacting LncRNAs
The cell lysate was prepared by using IP lysis buffer (0.5 % NP-40, 150 mM NaCl, 20 mM HEPES, pH 7.4, 2 mM EDTA, and 1.5 mM MgCl2, containing RNAse inhibitors and protease inhibitor cocktail) and incubated with protein A/G Sepharose beads tagged with anti-P53, and IgG antibodies for 2–4h at 4 °C. After washing beads, protein and RNA samples were prepared for western blotting and qPCR analysis.
3.11. Statistical analysis
All statistical analyses were performed using R software (version 3.6.1; https://www.R-project.org), and a multivariate Cox proportional hazard regression analysis was performed to assess the association between risk scores and overall survival. The Wilcox test was applied to determine the differences between both groups through R software. P-value P ≤ 0.05 was considered significant for all the analyses.
4. Results
4.1. Screening of prognostic signatures of TCGA gastric cancer
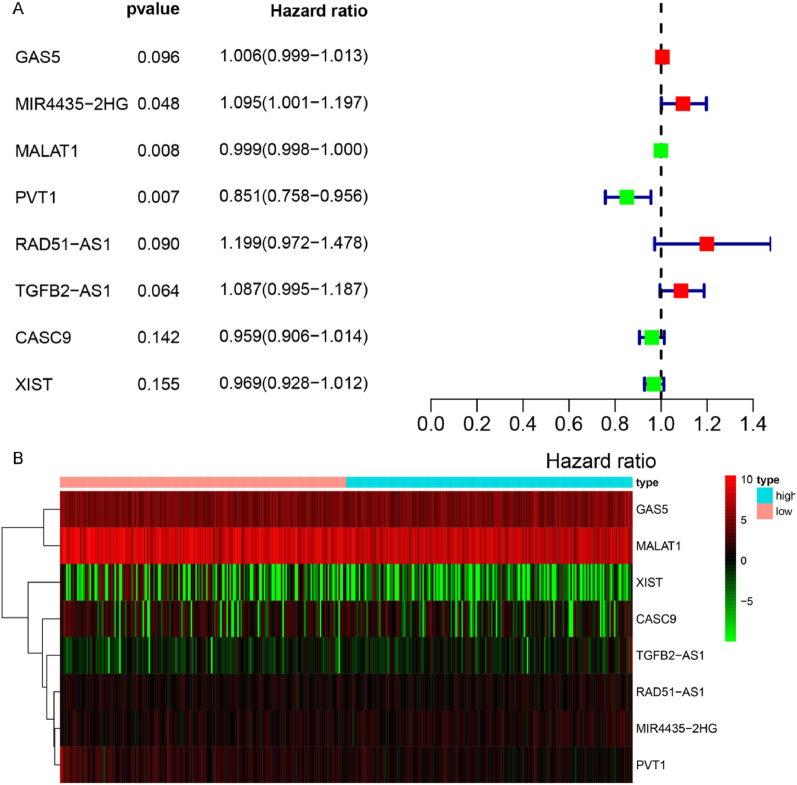
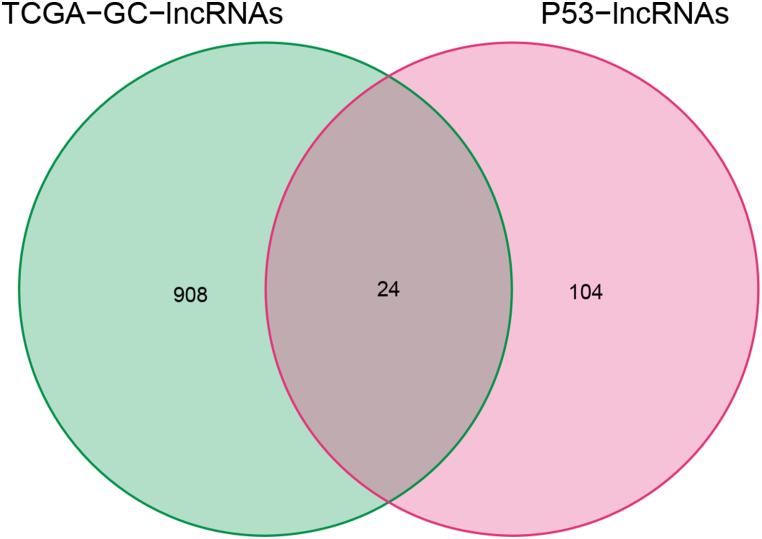
As mentioned, we only considered experimentally verified p53-associated lncRNAs from the TP53LNC-DB database. Additionally, we collected RNA-seq data of gastric cancer patients from TCGA, along with their clinicopathological information. After normalizing, we obtained 932 lncRNAs in TCGA samples, and intersecting 128 p53-related lncRNAs, only 24 lncRNAs were filtered out; we calculated their prognostic characteristics in gastric cancer patients (Fig. S1). Multivariate COX regression analysis was performed for lncRNAs, and the gene signature was selected. The investigation revealed eight lncRNAs (GAS5, MALAT1, XIST, CASC9, TGFB2-AS1, RAD51-AS1, MIR4435-2HG, and PVT1) were related to the survival of gastric cancer patients (Fig. 1A). Based on the expression of eight signature lncRNAs, the risk score for each gastric cancer patient was calculated. GC patients were divided into high-risk and low-risk groups using the median risk scores. The expression levels of the eight signature lncRNAs in the high-risk and low-risk groups are presented as the heat map (Fig. 1B).
Fig. 1.
Establishing prognostic gene characteristics through multivariate COX regression analysis. (A) We used multivariate COX regression analysis to screen out gene sets meaningful for patient survival status. (B) Heat map of the expression levels of 8 hub genes in the high and low expression groups.
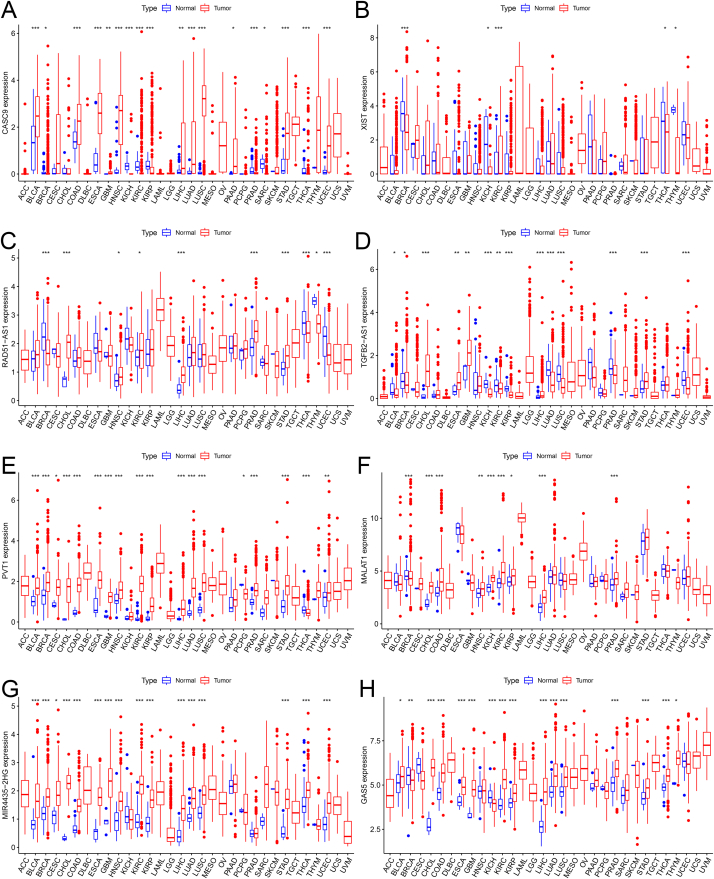
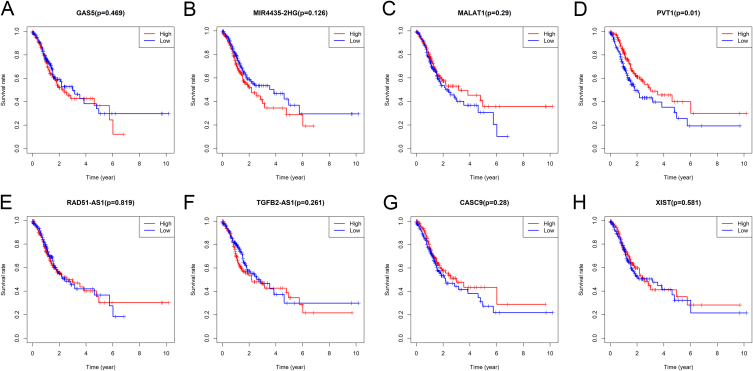
In addition to gastric cancer, we collected RNA sequencing data and clinical clinicopathological information of 32 cancers from the TCGA database. All expression data were normalized via the log2 conversion method. The expression differences of eight lncRNAs in pan-cancer were plotted into a boxplot (Fig. S2). Moreover, the survival curves of eight signature lncRNAs in gastric cancer were plotted using the "survival" package (Fig. S3). Data showed that the expression of lncRNA PVT1 significantly affects the survival of gastric cancer patients.
4.2. Construction of the prognostic model
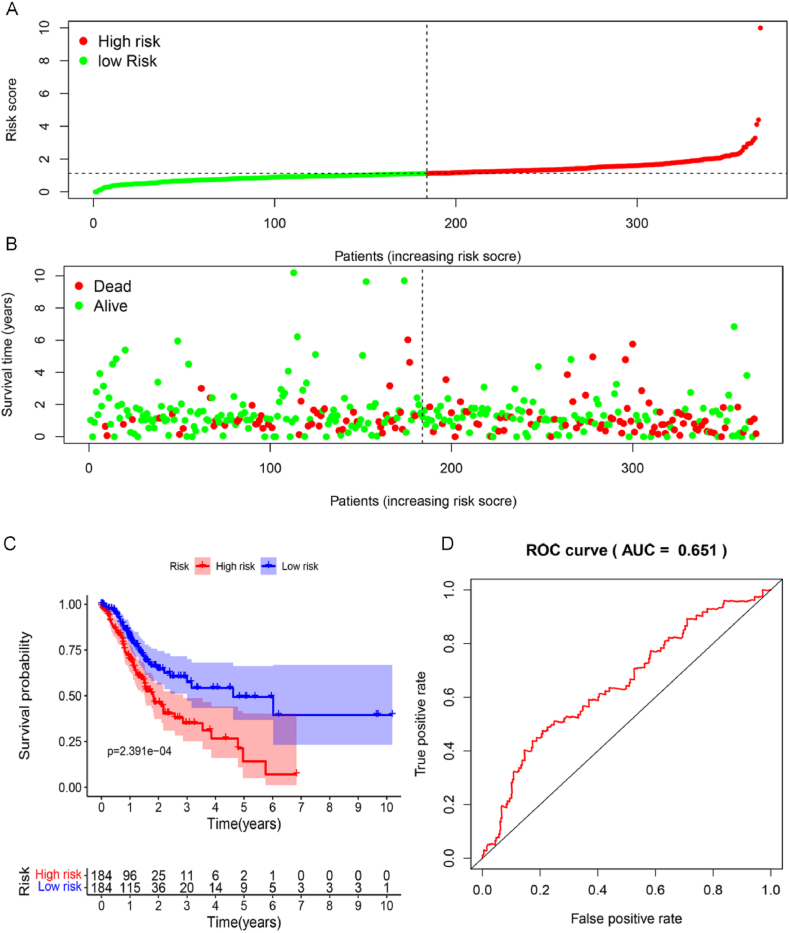
Furthermore, the relationship between risk score distribution and survival time of the STAD patients was calculated, which showed less survival time in the high-risk score group (Fig. 2A and B). Similarly, the survival curve revealed that the patients with low-risk scores survive much longer than those with high-risk scores (Fig. 2C). The highest area under the curve (AUC) for patients' risk scores was detected as 0.651 (Fig. 2D), confirming the prognostic model's reliability.
Fig. 2.
The characteristics of p53-related genes are significantly related to the survival rate of gastric cancer patients. (A&B) The distribution of risk score and patient's survival time. (C) Kaplan-Meier analysis of TCGA gastric cancer patients stratified by the median risk score. (D) Draw the ROC curve and calculate AUC to evaluate the accuracy of the prognostic model.
4.3. Characteristics of the prognostic gene signature
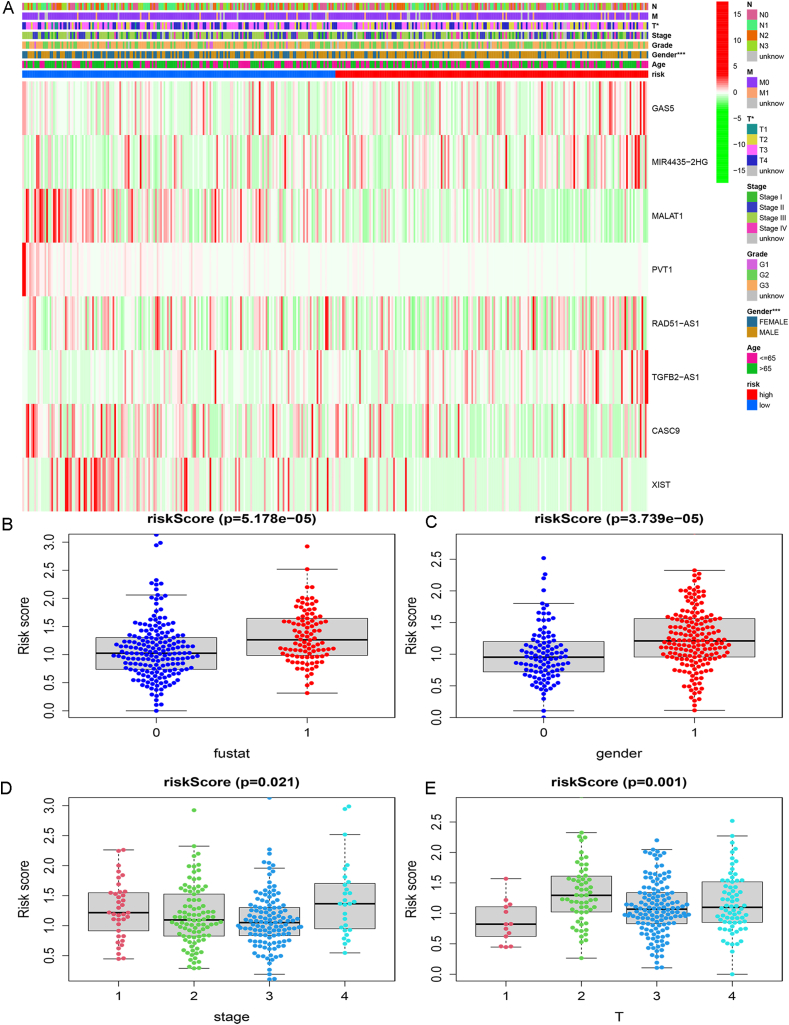
In the TCGA-STAD samples, heat maps were drawn to show the relationship between the expression of signature lncRNAs in both risk groups and the clinical characteristics of the patients. These results showed a significant correlation between risk score with gender and T stage (Fig. S4A). Furthermore, we compared the risk score with survival time, gender, and T stage of the patients in both groups (Figs. S4B–E), which revealed the critical role of P53-associated lncRNA signature in the overall survival of gastric cancer patients (Table 1).
Table 1.
Clinical characteristics of gastric cancer patients according to p53 associated lncRNAs.
| id | futime | fustat | age | gender | grade | stage | T |
|---|---|---|---|---|---|---|---|
| M | 238.411(0.390) | −1.163(0.246) | 54.815(0.150) | 1.467(0.144) | 0.848(0.654) | 160.226 (1.638e-34) | 14.592(0.002) |
| N | 223.52(0.661) | −2.231(0.027) | 35.767(0.836) | 0.54(0.590) | 4.754(0.093) | 158.099 (4.714e-34) | 23.625(2.992e-05) |
| GAS5 | 240.878(0.348) | −0.399(0.690) | 45.535(0.450) | 0.636(0.526) | 1.269(0.530) | 10.033(0.018) | 1.91(0.591) |
| MIR4435-2HG | 241.32(0.340) | −2.289(0.023) | 51.075(0.247) | −1.152(0.250) | 3.526(0.172) | 6.744(0.081) | 5.542(0.136) |
| MALAT1 | 239.743(0.367) | 1.238(0.217) | 35.085(0.856) | −0.375(0.708) | 5.038(0.081) | 15.262(0.002) | 18.328(3.763e-04) |
| PVT1 | 231.538(0.515) | 1.626(0.105) | 68.316(0.014) | 0.968(0.335) | 0.291(0.864) | 1.633(0.652) | 12.253(0.007) |
| RAD51-AS1 | 244.3(0.293) | −0.118(0.906) | 47.671(0.365) | −0.567(0.571) | 3.766(0.152) | 2.011(0.570) | 12.011(0.007) |
| TGFB2-AS1 | 231.1(0.523) | −1.104(0.271) | 43.958(0.516) | −0.808(0.420) | 6.291(0.043) | 0.979(0.806) | 11.358(0.010) |
| CASC9 | 237.237(0.411) | 1.396(0.164) | 46.499(0.410) | −0.051(0.959) | 0.694(0.707) | 0.778(0.855) | 2.738(0.434) |
| XIST | 240.347(0.357) | 1.76(0.080) | 54.626(0.154) | 11.624(5.959e-21) | 0.49(0.783) | 0.687(0.876) | 6.334(0.096) |
| riskScore | 242.676(0.318) | −4.146(5.178e-05) | 58.626(0.084) | −4.198(3.739e-05) | 1.58(0.454) | 9.774(0.021) | 15.515(0.001) |
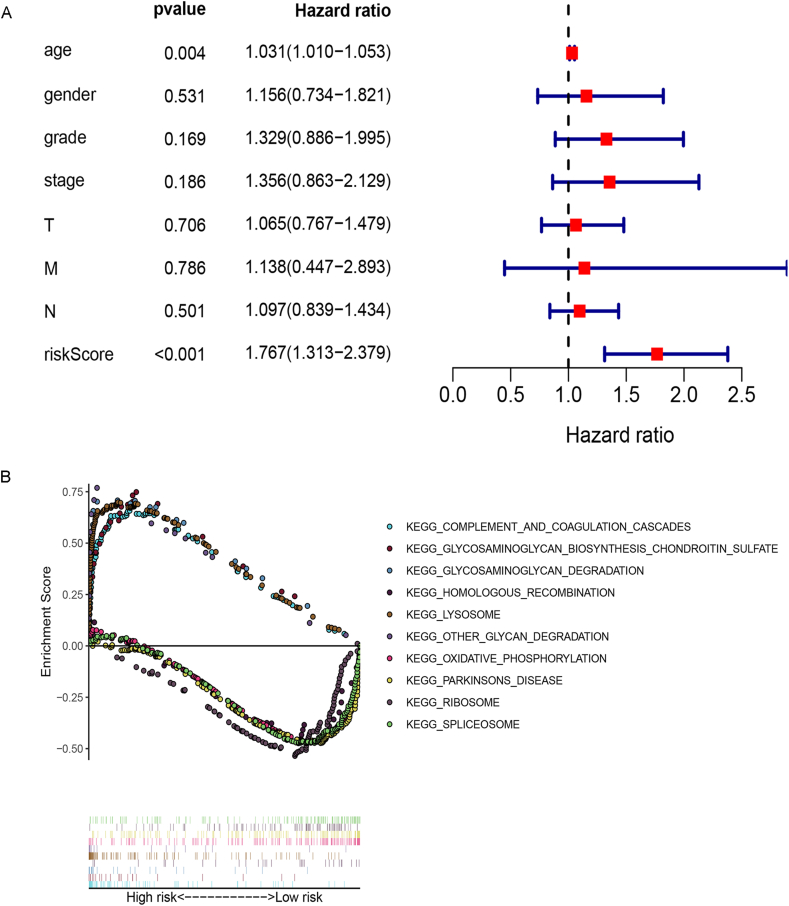
4.4. Identifying signaling pathways involved
Through multivariate COX regression analysis, we found the association of risk score with gender, Hazard Ratio (HR) >1 was considered a risk factor (Fig. S5A). GSEA analysis showed that lncRNA signatures in the high-risk group were significantly enriched in several common pathways, including top-five: COMPLEMENT_AND_COAGULATION_CASCADES, GLYCOSAMINOGLYCAN_BIOSYNTHESIS_CHONDROITIN_SULFATE, GLYCOSAMINOGLYCAN_DEGRADATION, HOMOLOGOUS_RECOMBINATION, and LYSOSOME. While lncRNAs in the low-risk group were associated with the top five pathways; OTHER_GLYCAN_DEGRADATION, OXIDATIVE_PHOSPHORYLATION, PARKINSONS_DISEASE, RIBOSOME, and SPLICEOSOME (Fig. S5B).
The GSVA results suggest that the eight lncRNAs and their constitutive gene sets are associated with most tumor-associated pathways, including the P53 pathway (Fig. S9).
4.5. The relationship between risk score and immune cell tumor infiltration
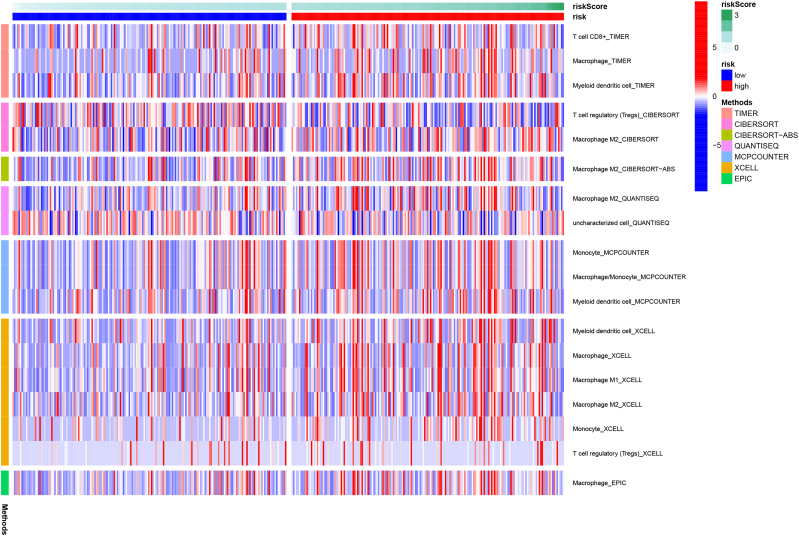
To explore the relationship between p53-associated lncRNA signatures and immune cell infiltration in the gastric cancer tumor microenvironment, we used various methods, including TIMER, CIBERSORT, CIBERSORT-ABS, QUANTISEQ, MCPCOUNTER, XCELL, and EPIC. Using TIMER: T cell CD8+, macrophage, myeloid dendritic cell; CIBERSORT: T cell regulatory (Tregs) and M2-macrophage; CIBERSORT-ABS: M2-macrophage; QUANTISEQ: M2-macrophage, uncharacterized cells; MCPCOUNTER: monocyte, macrophage, myeloid dendritic cell; XCELL: myeloid dendritic cell, macrophage, macrophage M1, M2-macrophage, monocyte, T cell regulatory (Tregs); EPIC: macrophage were detected in the gastric cancer tumor microenvironment (Fig. 3). In general, the higher infiltration of M2-macrophages was detected through several methods, thus showing increased tumor invasion in the high-risk group. This recognizes p53-associated lncRNAs as new factors influencing the infiltration of macrophages in the gastric tumor microenvironment.
Fig. 3.
Differential heat map of immune cell infiltration in the tumor microenvironment patients in the high-risk and low-risk groups.
4.6. The relationship between risk score and different immune functions
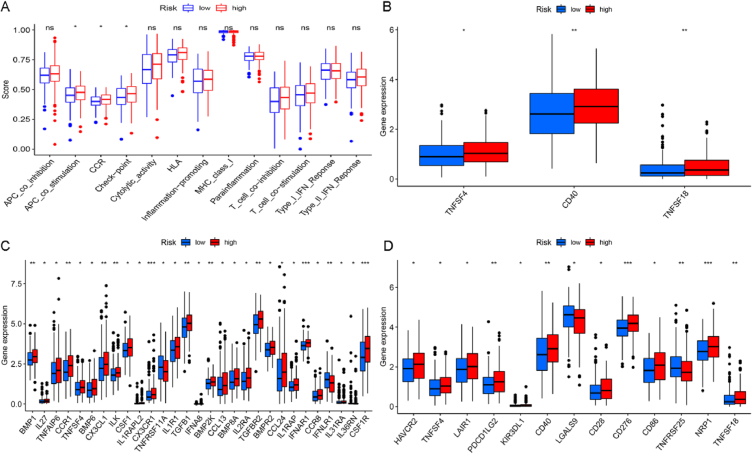
We further analyzed the relationship between risk score and tumor immune function based on differences in immune cell infiltration. Comparing immune functions including APC_co_inhibition, APC_co_stimulation, CCR, Checkpoint, Cytolytic_activity, HLA, Inflammation-promoting, MHC_class_I, Parainflammation, T_cell_co-inhibition, T_cell_co-stimulation, Type_I_IFN_Reponse and Type_II_IFN_Reponse. The data analysis revealed that three functions of APC_co_stimulation, CCR, and Check-point showed a significant difference between high-risk and low-risk groups, showing significant downregulation in the high-risk group (Fig. 4A). Then, a box plot was drawn to show the differentially expressed genes in the three significantly affected immune pathways in both risk groups (Fig. 4B–D). We believe lncRNAs may also regulate these immune-related genes directly or through regulating p53 in gastric cancer.
Fig. 4.
The relationship between risk score and different immune functions. (A) The relationship between risk score and immune function in 13. (B–D) the expression of marker genes differs between the high-risk and low-risk groups in the immune APC_co_stimulation, CCR, and Check-point.
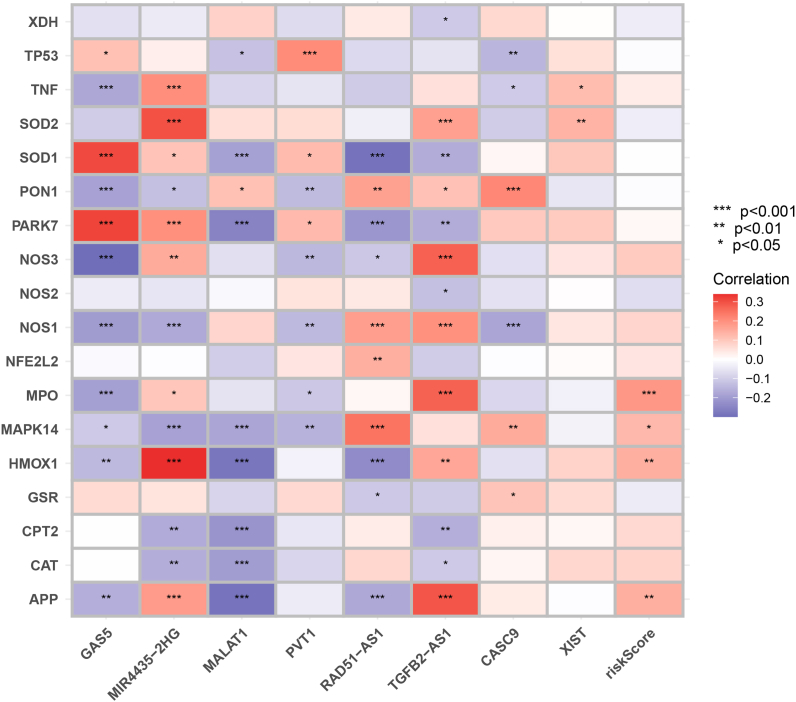
4.7. Correlation of signature LncRNAs with oxidative stress-related genes
A total of 18 genes were found to have correlation scores of more than 40, so we selected them to analyze their association with p53-associated lncRNAs. Overall, gene signature-based risk scores were positively correlated with MPO, MAPK14, HMOX1, and APP in OSRGs (Fig. S6 and Table S3).
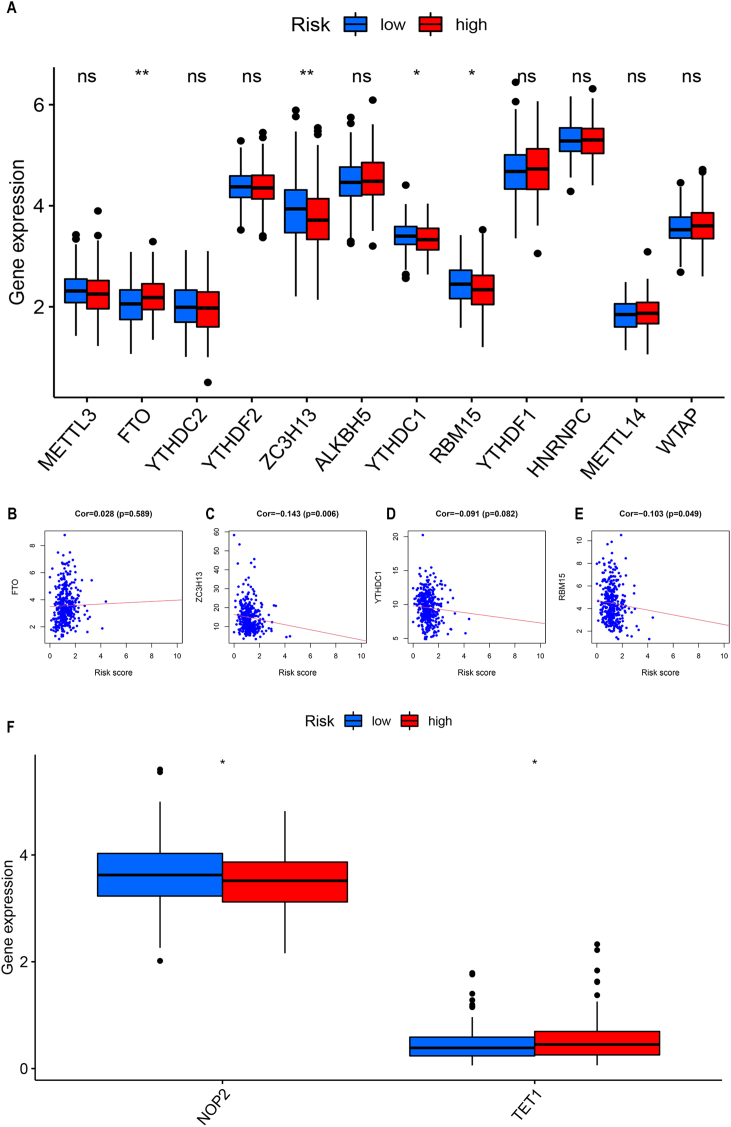
4.8. The relationship between risk score and m6A regulatory function
The relationship between P53 and m6A RNA modifications has been reported in many cancers [[27], [28], [29]]. However, the relationship and role of p53-associated lncRNAs are not yet known in RNA modifications in gastric cancer. Therefore, we specifically studied the role of signature lncRNAs in m6A RNA modifications and compared the expression of m6A-related genes in both risk groups. The gene expression differences in both groups are presented as a box plot, which revealed a significant difference in expression levels of FTO, ZC3H13, YTHDC1, and RBM15 in both risk groups (Fig. S7A). However, ZC3H13 significantly upregulated in the low-risk group, while FTO, YTHDC1, and RBM15 upregulated considerably in the high-risk group. Subsequently, the correlation between the four m6A-related differential genes and the risk scores was plotted as a scatter diagram FTO: Cor = 0.028 (p = 0.589); ZC3H13: Cor = −0.143 (p = 0.006); YTHDC1: Cor = −0.091 (p = 0.082), RBM15: Cor = −0.103 (p = 0.049) (Figs. S7B–E). Except for FTO, all other genes significantly correlated with risk scores in gastric cancer.
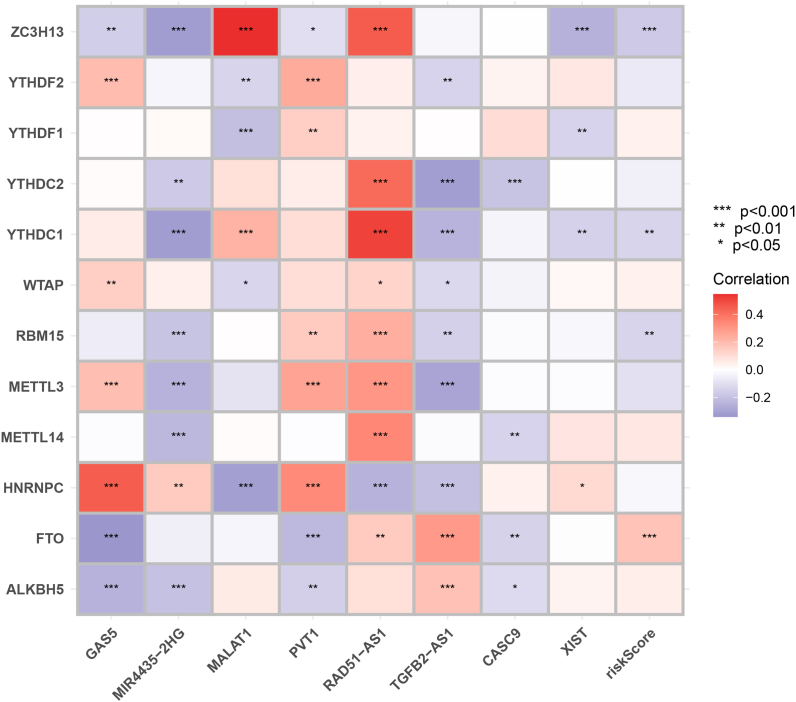
The signature lncRNAs significantly correlated with the m6A factor; for example, RAD51-AS1 was positively correlated with ZC3H13, YTHDC2, YTHDC1, and MALAT1 showed a significant positive correlation with ZC3H13 (Fig. S8 and Table S4).
4.9. The relationship between lncRNAs, risk score, and m5C RNA modification regulators
In total, 18 m5C genes including 11 writers (NOP2, NSUN2, NSUN3, NSUN4, NSUN5, NSUN6, NSUN7, DNMT1, TRDMT1, DNMT3A, and DNMT3B), three readers (YTHDF2, ALYREF, YBX1), and four erasers (TET1, TET2, TET3, ALKBH1) were systematically analyzed using data from previous studies. We screened only m5C genes that have different expression levels in high-risk and low-risk groups. We found that NOP2 had significantly higher expression in the low-risk group, while TET1 showed significantly higher expression in the high-risk group (Figure S7 F).
4.10. Association of lncRNAs with H-pylori infection in gastric cancer patients
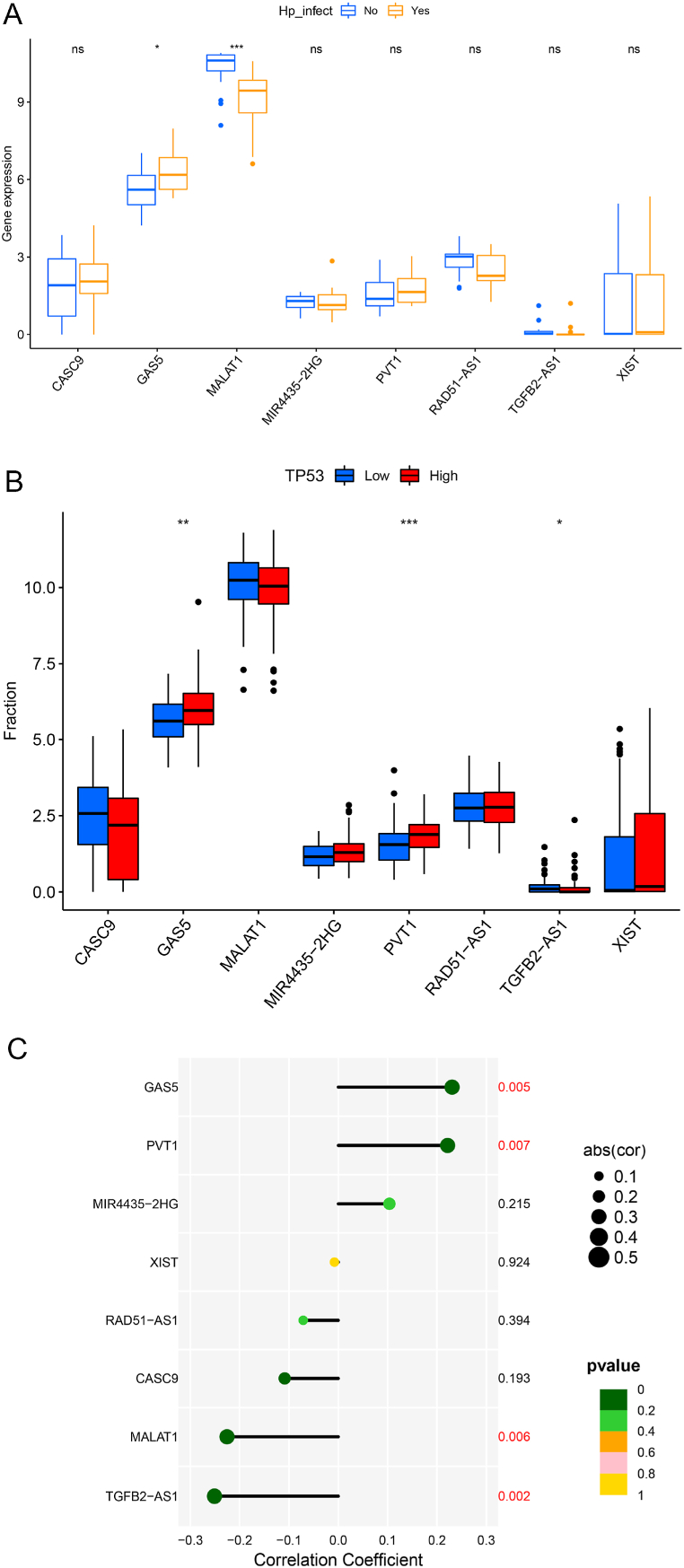
We screened GC patients with clear Helicobacter pylori infection records from TCGA-STAD, and 145 patients were found to have H-pylori infection. To study the relationship between P53-Hp-lncRNA, we sorted 145 patients with H-pylori infected according to the expression level of P53 and screened out the top 18 H-pylori + patients and compared them with 18 GC patients without H-pylori infection. H-pylori + GC patients showed higher expression of GAS5 and low expression of MALAT1 than those without H. pylori infection (Fig. 5A).
Fig. 5.
Relationship between Hp infection and eight lncRNAs. (A) Box plot of the difference in expression of 8 lncRNAs between H. pylori-infected patients with high P53 expression and H. pylori-uninfected patients. (B) Boxplot of differences in the expression of 8 lncRNAs in the P53 high-expression and low-expression sample groups. (C) Lollipop graph of the correlation between P53 and 8 lncRNA expressions. *, P < 0.05. **, P < 0.01. ***, P < 0.001.
Subsequently, we analyzed the relationship between the expression level of P53 and eight signature lncRNAs in H-pylori + samples. We found that GAS5 and PVT1 had higher expression in the P53 high expression group, while TGFB2-AS1 showed an opposite expression pattern (Fig. 5B). Correlation analysis also showed that the expression levels of GAS5 and PVT1 in H-pylori + samples were positively correlated with P53, and MALAT1 and TGFB2-AS1 were negatively correlated with the expression of P53 in GC patients (Fig. 5C).
4.11. P53-associated LncRNA showed better immune response
The Estimation of Stromal and Immune Cells in Malignant Tumors using the Expression Data (ESTIMATE) algorithm has the advantage of the unique properties of the transcriptional profiles to infer the tumor cellularity as the tumor purity.
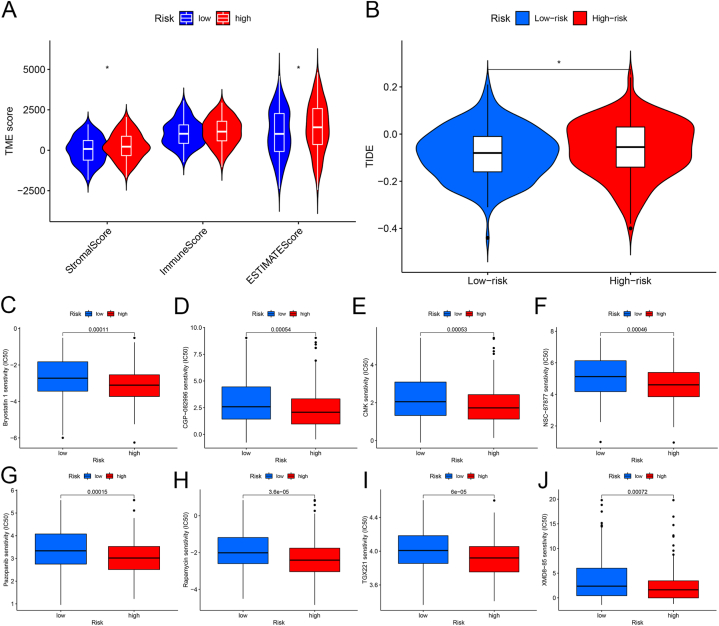
We found that the immune indicators were significantly lower in the high-risk score sample group than in the low-expression group (Fig. 6A), showing that the low-risk score group may have a better immune response.
Fig. 6.
Relationship between risk score and drug efficacy in patients with gastric cancer. (A) Violin plot of differences in immune markers in high and low-risk scores. (B) Relationship between TIDE score and risk score. (C–J) Relationship between risk score and chemotherapeutic drug sensitivity. *, P < 0.05. **, P < 0.01. ***, P < 0.001.
Tumor immune dysfunction and rejection (TIDE; http://tide.dfci.harvard.edu/) were also used to examine immune escape mechanisms and predict the immune response to immunotherapy. Higher TIDE scores indicate a greater likelihood of immune escape, leading to the probability of reduced treatment efficacy. TIDE analysis showed that the low-risk group had higher resistance to immunotherapy (Fig. 6B).
4.12. The response of P53-related LncRNAs to chemotherapy
The Genomics of Drug Sensitivity in Cancer (GDSC) Project (https://www.cancerrxgene.org/) is a database used to analyze the sensitivity of anticancer drugs, which can help to predict the response of molecular targets against anticancer drugs. Based on the GDSC database, we used the “pRRophetic” R package to calculate the response to chemotherapy drugs in GC patients in different risk score groups. We found that the half maximal inhibitory concentration (IC50) values of Bryostatin-1, CGP-082996, CMK, NSC-87877, Pazopanib, Rapamycin, TGX221, and XMD8-85 were lower in the high-risk group (P < 0.05), indicating that the high-risk group patients are more sensitive to chemotherapy drugs (Fig. 6C–J).
4.13. Expression of signature in gastric cancer cell lines
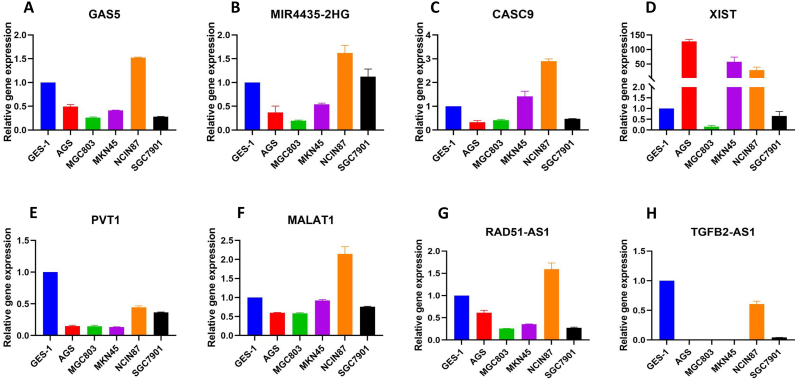
To verify the expression pattern in RNA seq data obtained from TCGA, we analyzed the expression of eight selected lncRNAs in five gastric cancer cell lines (AGS, SGC7901, MGC803, MKN45, and NCIN87) by real-time qPCR and taking normal gastric epithelial cell line GES-1 as control. Data analysis showed that three lncRNAs, Mir4435-2HG, CAS-9, and XIST, had upregulation in two or more GC cell lines (Fig. 7B–D), while PVT1 and TGFB2-ASI were downregulated in all cells (Fig. 7E–H). The remaining lncRNAs GAS5, MALAT1, and RAD51-ASI were upregulated only in NCIN87 cells while down-regulated in other studied cell lines (Fig. 7A–F, G). This analysis shows that all selected P53-associated lncRNAs have a unique role in GC development.
Fig. 7.
The expression of Signature lncRNAs in Gastric cancer cell lines. The expression of 8 lncRNAs (A) GAS5, (B) MIR4435-2HG, (C) CASC9, (D) XIST, (E) PVT1, (F) MALAT1, (G) RAD51-ASI and (H) TGFB2-AS1 was studied in five gastric cancer cell lines (AGS, SGC7901, MGC803, MKN45, and NCIN87) and normal gastric epithelial cell line (GES-1).
4.14. P53 regulates hub genes in gastric cancer cells
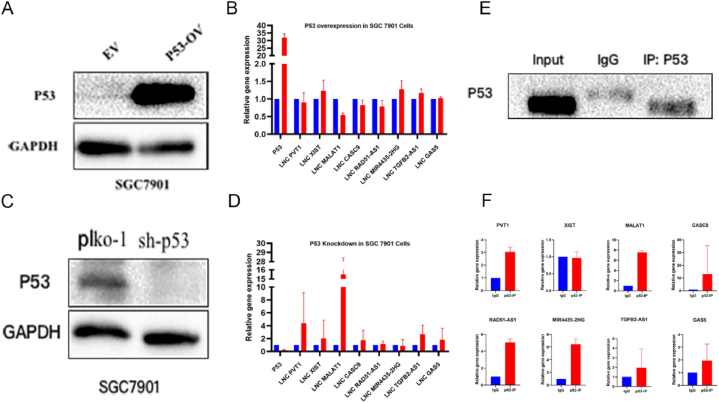
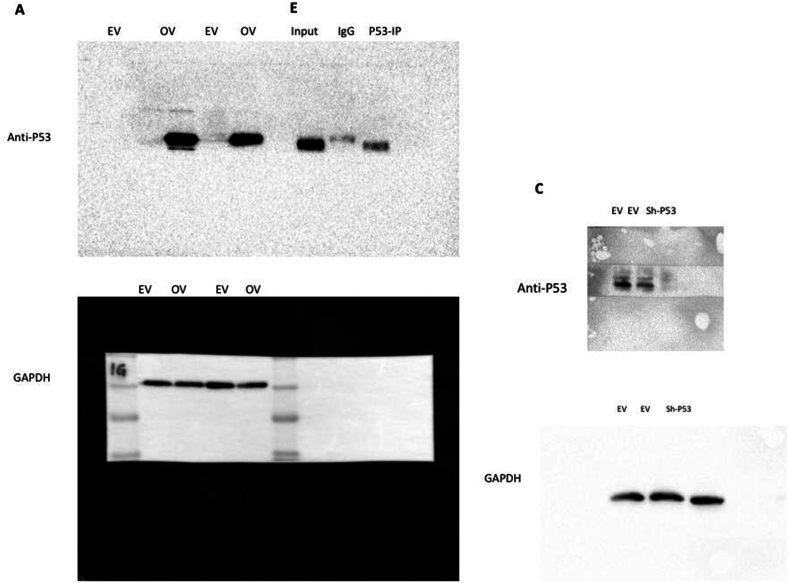
Furthermore, to find whether these lncRNAs are regulated by p53, we transiently overexpressed the p53 in SGC7901 cells and observed no significant change in the expression pattern of the lncRNAs except MALAT1 (Fig. 8A and B, Fig. S10A). Interestingly, sh-RNA-mediated silencing of P53 in SGC7901 showed a substantial increase in the expression of PVT1, XIST, MALAT1, Mir4435-2HG, CAS-9, and GAS5, while no significant change in TGFB2-ASI and RAD51-ASI expression was observed (Fig. 8C and D, Fig. S10C). As shown in Fig. 7, the expression of lncRNAs PVT1, XIST, MALAT1, and TGFB2-ASI in SGC7901 cells was significantly low, while p53 silencing the expression of these lncRNAs is upregulated substantially, showing that P53 is primary regulator these lncRNAs.
Fig. 8.
P53 regulates signature lncRNAs in Gastric cancer cells. The expression of eight lncRNA was analyzed after (A, B) overexpressing and (C, D) silencing P53 in SGC7901 cells, which showed significant alteration of lncRNA expression upon p53 knockdown. Furthermore, (E) RNA-IP by pulling down p53 showed (F) a substantial interaction between signature lncRNAs and P53. For uncropped gel blots of figure A,C and E, please refer to Fig. S10.
4.15. LncRNAs interacting with P53
To further validate the regulation of the lncRNAs by p53, we performed RNA-IP by pulling down p53 in the SGC7901 cells (Fig. 8E, Fig. S10E). The expression profiles of lncRNAs in p53-IP samples revealed that all lncRNAs interacted with P53 except XIST, which showed a weak interaction (Fig. 8F). This analysis revealed that p53 is a primary regulator of selected signature lncRNAs in gastric cancer.
5. Discussion
Gastric cancer is one of the deadliest cancers worldwide due to its late-stage diagnosis [1]. The lack of early-stage diagnostic approaches makes it a heavy health burden. GC is a highly heterogeneous disease in terms of molecular features, and recently, several coding genes have been identified as prognostic markers in gastric cancer patients [11,30]. The immune regulation of the lncRNAs has been studied in many cancers, including the glioblastoma [31], pancreatic cancer [32], and hepatocellular carcinoma [33]. Findings of previous studies have shown that immune-related lncRNA signatures could be used to predict the prognosis and disease outcomes [34,35]. In addition, several non-coding genes (miRNAs and lncRNAs) play a crucial role in the development of gastric cancer and serve as potential diagnostic and therapeutic markers [36]. Noteworthy, p53 is one of the key players in cancer development, so its associated lncRNAs also have equal participation in regulating cancers [12,[37], [38], [39]]. On the other hand, in gastric cancer, H-pylori is considered a vital initiator of the cancer [40,41]; it constantly regulates various biomarkers, including p53 [42] and noncoding RNAs [[43], [44], [45]]. So far, no detailed study has ever reported the role of p53 lncRNAs in immune cell infiltration and the prognosis of gastric cancer.
Therefore, in the current study, we selected experimentally verified p53-associated lncRNAs from a public database TP53LNC-DB [24]. To further validate these lncRNAs, we downloaded gastric cancer RNA-seq data along with the clinical information of the 375 GC patients and 17 normal controls from TCGA. Firstly, we studied the RNA signatures that were only correlated with the immune function and survival of gastric cancer patients. Thus, we filtered out eight lncRNAs, including GAS5, MALAT1, XIST, CASC9, TGFB2-AS1, RAD51-AS1, MIR4435-2HG, and PVT1. These lncRNAs are known to regulate gastric cancer through various pathways. Such as lncRNA MALAT1 act as an oncogene in gastric by inhibiting several miRNAs miR-122, miR1297, miR-202, miR-22-3p, etc., and several pathways or genes like Pi3k/AKT, PCDH10, ZFP91, and SOX2 [[46], [47], [48], [49]]. LncRNA XIST is also a critical lncRNA regulating gastric cancer through binding with miRNAs; it binds with miR-337 and regulates the expression of JAK2, which further governs the proliferation & migration ability of the gastric cancer cells [50]. It also regulates gastric cancer through miR-132 and PXN [51], ceRNA networking like XIST/miR-185/TGF-β1 [52], and via EZH2 modulation by sponging miR-101 [53]. LncRNA CASC9 is also a key player in gastric cancer development. It works through miR-370 and EGFR [54] and suppresses apoptosis by regulating the expression of the BMI1 gene in gastric cancer cells [55]. LncRNA TGFB2-AS1 is known to predict the risk score and immune landscape in the gastric cancer [56]. LncRNA MIR4435-2HG acts as an oncogene in gastric cancer by activating the Wnt/β-catenin signaling [57] and targeting the miR-138-5p/Sox4 axis [58]; it has also been predicted as a prognostic marker for gastric cancer [59,60]. LncRNA PVT1 has also been thoroughly studied in gastric cancer development, showing that it promotes gastric cancer through activating the STAT3/VEGFA axis [16], interacting with FOXM1 [61], or targeting the miR-125 and miR-30a activity [62,63]. Although three lncRNAs, RAD51-AS1 [64], MIR4435-2HG, and PVT1, have been predicted to participate in the prognosis of gastric cancer, their exact role in immune cell infiltration in gastric cancer has not been studied. Therefore, based on the current study's findings, we provide lncRNAs specifically regulated by P53 for survival prediction and risk management. We found that eight signature lncRNAs potentially regulate the immune cell infiltration in gastric cancer. Additionally, signature lncRNAs were associated with immunotherapy and chemotherapy response in gastric cancer patients. We also classified the patients based on the expression of lncRNAs and the risk of disease, showing that high-risk patients have shorter survival and low immune status. These findings were further experimentally validated in the gastric cancer cell lines, which confirms the reliability immune-related lncRNA model. Some of the lncRNAs, such as CAS9, MALAT1, and PVT1 were naturally downregulated in gastric cancer cells, especially in SGC7901; upon silencing the expression of P53, the expression of these lncRNAs significantly upregulated, which confirms their potential involvement in gastric cancer pathogenesis.
Helicobacter Pylori infection is one of the major causes of gastric cancer [65]; it activates various or suppresses pathways, including the P53 pathway [66,67]. H-pylori also affects the expression of multiple lncRNAs, which might be a key factor in H-pylori-induced carcinogenesis [[68], [69], [70], [71]]. Such as lnc-SGK1 induced by H-pylori promotes differentiation of Th2 and Th17 in the gastric cancer [72], and other lncRNAs, including GClnc1, FOXD2-ASI, and NEAT1 have been linked with H-pylori infection in gastric cancer [43,44,73]. So far, no connection has been established between the H-pylori and P53-associated lncRNAs in gastric cancer development, specifically in immune cell infiltration. We believe that H-pylori infection significantly alters the expression of some of the selected lncRNAs. Thus, we collected GC patients having Helicobacter pylori (Hp) infection and analyzed the association of signature lncRNAs. In short, only two lncRNAs (GAS5 and MALAT1) showed a difference in expression in H-pylori-infected patients. Furthermore, we analyzed the association between the p53 expression and signature lncRNAs in H-pylori + GC patients and found that GAS5 and PVT1 positively correlated, while MALAT1 and TGFB2-AS1 showed a negative correlation with P53 in H-pylori + GC patients. Previously, some lncRNAs such as HOTAIR and MEG3 showed a negative association with H-pylori in the gastric cancer [74].
We used different methods to study the infiltrating immune cells related to 8 signature lncRNAs. Overall, patients in the high-risk group were found to have a high proportion of M2-macrophages, which was associated with increased tumor invasion. M2-macrophages have similar properties to tumor-associated macrophages (TAMs), having an oncogenic function, drug resistance, metabolic reprogramming, and immune suppressor function [75]. A study shows that TAMs release certain factors that facilitate the transportation of lncRNA-HISLA to breast cancer cells, stabilizing the HIF-1α level, increasing glycolysis rate, and reducing drug resistance [76]. These findings reveal that P53-associated lncRNAs could be the new factors influencing the infiltration of macrophages in the gastric cancer tumor microenvironment.
Recently, chemical modification of RNAs has been recognized as an epigenetic mechanism playing an essential role in cancer development [77], and N6-methyladenine (m6A) and m5C mRNA modifications are most frequently found in cancer cells and tissues [78,79]. Based on the well-known relationship between the p53 and m6A RNA modification [[27], [28], [29]], we decided to study the relation of p53-related lncRNAs signatures with common genes involved in the RNA modification. The expression level of genes related to m6A RNA modification in high-risk and low-risk patient groups was significantly different. Several lncRNAs have been found to have association with m6A RNA methylation [80]. Similarly, lncRNA GATA6-AS1 was found to have strong binding ability to FTO (m6A demethylase), which blocks expression of GATA6-AS1 and promotes proliferation of gastric cancer cells [81]. Another RNA modifier KIAA1429 enhances expression of LNCO01106 by promoting m6A which ultimately restrict growth lung adenocarcinoma cells [82]. NEAT1 is well known oncogenic LncRNA, and its expression is stabilized by hnRNPA2B1 via m6A modification. Both work together to enhance cellular proliferation and stemness ability of the gastric cancer cells [83]. In the current study, the expression of ZC3H13 was considerably higher in the low-risk group, while FTO, YTHDC1, and RBM15 had higher expression in the high-risk group. Additionally, we analyzed the correlation of the signature lncRNAs with m5C genes and found that NOP2 (m5C writer) and TET1 (m5C eraser) were associated with these lncRNAs. It suggests that p53 plays a key role in activation of RNA modifiers which then regulate the lncRNAs.
However, our study has tremendous clinical implications but still has some limitations; the lncRNA signatures still need to be clinically tested. Thus, comprehensive large-scale clinical studies are required to confidently imply these lncRNA signatures in clinical practice. Moreover, we could only work on a limited number of p53 lncRNAs because not all lncRNAs mentioned in TP53LNC-DB were functionally validated. The interaction between p53 associated lncRNAs and RNA modifiers still need to be experimentally validated. Recent studies on lncRNAs have substantially contributed to the understanding underlying mechanisms of cancer development but using them as clinical markers or therapeutic target confidently still require long time. LncRNAs associated with immune check points are being used as biomarkers for immunotherapy in breast, bladder and lung cancer patients. The target genes of LINC-PINT including CDK1, CCNA2, AURKA, and PCNA are potentially being used as therapeutic targets in route clinical practice [12]. Based on our findings, we believe most immediate clinical use of lncRNAs could be as diagnostic, therapeutic, and prognosis markers.
6. Conclusion
In conclusion, we provide for the first time the p53-associated lncRNAs in gastric cancer. We further experimentally validated our findings in gastric cancer cell lines and cells with altered expression of p53. Our results show that the current lncRNA signatures have great potential in predicting gastric cancer patients' immune status and survival. Hence, these lncRNAs could be used for planning strategies for risk and clinical management of gastric cancer patients.
Data availability statement
The lncRNA was selected from the publicly available database (TP53LNC-DB), and their expression profiles and associated clinical parameters were collected from TCGA. However, additional data, files, figures, or tables produced by the current study could be requested from corresponding authors.
Ethical approval
Not applicable.
Funding
This work was supported by Zhengzhou Major Collaborative Innovation Project (No.18XTZX12003); Key projects of discipline construction in Zhengzhou University (No. XKZDJC202001); National Key Research and development program in China (No.2020YFC2006100); Medical service capacity improvement project of Henan Province in China (grant number Yu Wei Medicine [2017] No.66). Fifth affiliated Hospital of Zhengzhou University research start-up grant (2023kyqdj02).
CRediT authorship contribution statement
Zhao Huanjie: Writing – review & editing, Writing – original draft, Validation, Software, Methodology, Investigation, Formal analysis, Data curation. Ihtisham Bukhari: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Li Fazhan: Validation, Software, Methodology, Investigation, Formal analysis, Data curation. Huijuan Wen: Writing – review & editing, Software, Methodology, Investigation, Formal analysis. Jingyun Wang: Writing – review & editing, Software, Methodology, Investigation, Formal analysis, Data curation. Wu Wanqing: Writing – review & editing, Validation, Methodology, Investigation, Formal analysis, Data curation. Fu Yuming: Writing – review & editing, Visualization, Software, Methodology, Formal analysis, Data curation. Tang Youcai: Writing – review & editing, Visualization, Methodology, Investigation, Formal analysis, Data curation. Reem M. Al Jowaie: Writing – review & editing, Validation, Software, Formal analysis, Data curation. Ibrahim M. Aziz: Writing – review & editing, Validation, Software, Methodology, Formal analysis. Chu Xiufeng: Writing – review & editing, Software, Resources, Methodology, Formal analysis, Data curation. Mi Yang: Writing – review & editing, Visualization, Validation, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Zheng Pengyuan: Writing – review & editing, Validation, Software, Resources, Methodology, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We are highly thankful to our colleagues who are not listed as manuscript authors for their support in completing this work. We also thank the Researchers supporting project (RSP2024R418) King Saud University, Riyadh, Saudi Arabia. We also acknowledge the role of institutes and departments participating in the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e35228.
Contributor Information
Chu Xiufeng, Email: xchu@zzu.edu.cn.
Mi Yang, Email: yangmi198@zzu.edu.cn.
Zheng Pengyuan, Email: pyzheng@zzu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
figs6.
figs7.
figs8.
figs9.
figs10.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Kang Y.K., Boku N., Satoh T., Ryu M.H., Chao Y., Kato K., Chung H.C., Chen J.S., Muro K., Kang W.K., Yeh K.H., Yoshikawa T., Oh S.C., Bai L.Y., Tamura T., Lee K.W., Hamamoto Y., Kim J.G., Chin K., Oh D.Y., Minashi K., Cho J.Y., Tsuda M., Chen L.T. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs C.S., Doi T., Jang R.W., Muro K., Satoh T., Machado M., Sun W., Jalal S.I., Shah M.A., Metges J.P., Garrido M., Golan T., Mandala M., Wainberg Z.A., Catenacci D.V., Ohtsu A., Shitara K., Geva R., Bleeker J., Ko A.H., Ku G., Philip P., Enzinger P.C., Bang Y.J., Levitan D., Wang J., Rosales M., Dalal R.P., Yoon H.H. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferro A., Peleteiro B., Malvezzi M., Bosetti C., Bertuccio P., Levi F., Negri E., La Vecchia C., Lunet N. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur. J. Cancer. 2014;50:1330–1344. doi: 10.1016/j.ejca.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 5.Vedeld H.M., Goel A., Lind G.E. Epigenetic biomarkers in gastrointestinal cancers: the current state and clinical perspectives. Semin. Cancer Biol. 2018;51:36–49. doi: 10.1016/j.semcancer.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalnina Z., Meistere I., Kikuste I., Tolmanis I., Zayakin P., Line A. Emerging blood-based biomarkers for detection of gastric cancer. World J. Gastroenterol. 2015;21:11636–11653. doi: 10.3748/wjg.v21.i41.11636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Ishaq R.K., Overy A.J., Busselberg D. Phytochemicals and gastrointestinal cancer: cellular mechanisms and effects to change cancer progression. Biomolecules. 2020;10 doi: 10.3390/biom10010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashktorab H., Kupfer S.S., Brim H., Carethers J.M. Racial disparity in gastrointestinal cancer risk. Gastroenterology. 2017;153:910–923. doi: 10.1053/j.gastro.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Su T., Wang T., Zhang N., Shen Y., Li W., Xing H., Yang M. Long non-coding RNAs in gastrointestinal cancers: implications for protein phosphorylation. Biochem. Pharmacol. 2022;197 doi: 10.1016/j.bcp.2022.114907. [DOI] [PubMed] [Google Scholar]
- 11.Bhan A., Soleimani M., Mandal S.S. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77:3965–3981. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bukhari I., Khan M.R., Hussain M.A., Thorne R.F., Yu Y., Zhang B., Zheng P., Mi Y. PINTology: a short history of the lncRNA LINC-PINT in different diseases. Wiley Interdiscip Rev RNA. 2022 doi: 10.1002/wrna.1705. [DOI] [PubMed] [Google Scholar]
- 13.Shirani K., Sheikhbahaei E., Torkpour Z., Ghadiri Nejad M., Kamyab Moghadas B., Ghasemi M., Akbari Aghdam H., Ehsani A., Saber-Samandari S., Khandan A. A narrative review of COVID-19: the new pandemic disease. Iran. J. Med. Sci. 2020;45:233–249. doi: 10.30476/ijms.2020.85869.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao J., Liu Y., Zhang W., Zhou Z., Wu J., Cui P., Zhang Y., Huang G. Long non-coding RNA Linc00152 is involved in cell cycle arrest, apoptosis, epithelial to mesenchymal transition, cell migration and invasion in gastric cancer. Cell Cycle. 2015;14:3112–3123. doi: 10.1080/15384101.2015.1078034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y., Zhao J., Zhang W., Gan J., Hu C., Huang G., Zhang Y. lncRNA GAS5 enhances G1 cell cycle arrest via binding to YBX1 to regulate p21 expression in stomach cancer. Sci. Rep. 2015;5 doi: 10.1038/srep10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J., Du P., Cui P., Qin Y., Hu C., Wu J., Zhou Z., Zhang W., Qin L., Huang G. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene. 2018;37:4094–4109. doi: 10.1038/s41388-018-0250-z. [DOI] [PubMed] [Google Scholar]
- 17.Zhao J., Liu Y., Huang G., Cui P., Zhang W., Zhang Y. Long non-coding RNAs in gastric cancer: versatile mechanisms and potential for clinical translation. Am. J. Cancer Res. 2015;5:907–927. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J., Wu J., Qin Y., Zhang W., Huang G., Qin L. LncRNA PVT1 induces aggressive vasculogenic mimicry formation through activating the STAT3/Slug axis and epithelial-to-mesenchymal transition in gastric cancer. Cell. Oncol. 2020;43:863–876. doi: 10.1007/s13402-020-00532-6. [DOI] [PubMed] [Google Scholar]
- 19.Huang D., Chen J., Yang L., Ouyang Q., Li J., Lao L., Zhao J., Liu J., Lu Y., Xing Y., Chen F., Su F., Yao H., Liu Q., Su S., Song E. NKILA lncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death. Nat. Immunol. 2018;19:1112–1125. doi: 10.1038/s41590-018-0207-y. [DOI] [PubMed] [Google Scholar]
- 20.Cao J., Dong R., Jiang L., Gong Y., Yuan M., You J., Meng W., Chen Z., Zhang N., Weng Q., Zhu H., He Q., Ying M., Yang B. LncRNA-MM2P identified as a modulator of macrophage M2 polarization. Cancer Immunol. Res. 2019;7:292–305. doi: 10.1158/2326-6066.CIR-18-0145. [DOI] [PubMed] [Google Scholar]
- 21.Fan Q., Liu B. Discovery of a novel six-long non-coding RNA signature predicting survival of colorectal cancer patients. J. Cell. Biochem. 2018;119:3574–3585. doi: 10.1002/jcb.26548. [DOI] [PubMed] [Google Scholar]
- 22.Song P., Jiang B., Liu Z., Ding J., Liu S., Guan W. A three-lncRNA expression signature associated with the prognosis of gastric cancer patients. Cancer Med. 2017;6:1154–1164. doi: 10.1002/cam4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J., Wang W., Xia P., Wan L., Zhang L., Yu L., Wang L., Chen X., Xiao Y., Xu C. Identification of a five-lncRNA signature for predicting the risk of tumor recurrence in patients with breast cancer. Int. J. Cancer. 2018;143:2150–2160. doi: 10.1002/ijc.31573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan M.R., Bukhari I., Khan R., Hussain H.M.J., Wu M., Thorne R.F., Li J., Liu G. TP53LNC-DB, the database of lncRNAs in the p53 signalling network. Database. 2019;2019 doi: 10.1093/database/bay136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iasonos A., Schrag D., Raj G.V., Panageas K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 2008;26:1364–1370. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo X., Li K., Jiang W., Hu Y., Xiao W., Huang Y., Feng Y., Pan Q., Wan R. RNA demethylase ALKBH5 prevents pancreatic cancer progression by posttranscriptional activation of PER1 in an m6A-YTHDF2-dependent manner. Mol. Cancer. 2020;19:91. doi: 10.1186/s12943-020-01158-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghazi T., Nagiah S., Chuturgoon A.A. Fusaric acid decreases p53 expression by altering promoter methylation and m6A RNA methylation in human hepatocellular carcinoma (HepG2) cells. Epigenetics. 2021;16:79–91. doi: 10.1080/15592294.2020.1788324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie J.W., Huang X.B., Chen Q.Y., Ma Y.B., Zhao Y.J., Liu L.C., Wang J.B., Lin J.X., Lu J., Cao L.L., Lin M., Tu R.H., Zheng C.H., Huang C.M., Li P. m(6)A modification-mediated BATF2 acts as a tumor suppressor in gastric cancer through inhibition of ERK signaling. Mol. Cancer. 2020;19:114. doi: 10.1186/s12943-020-01223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cristescu R., Lee J., Nebozhyn M., Kim K.M., Ting J.C., Wong S.S., Liu J., Yue Y.G., Wang J., Yu K., Ye X.S., Do I.G., Liu S., Gong L., Fu J., Jin J.G., Choi M.G., Sohn T.S., Lee J.H., Bae J.M., Kim S.T., Park S.H., Sohn I., Jung S.H., Tan P., Chen R., Hardwick J., Kang W.K., Ayers M., Hongyue D., Reinhard C., Loboda A., Kim S., Aggarwal A. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 31.Zhou M., Zhang Z., Zhao H., Bao S., Cheng L., Sun J. An immune-related six-lncRNA signature to improve prognosis prediction of glioblastoma multiforme. Mol. Neurobiol. 2018;55:3684–3697. doi: 10.1007/s12035-017-0572-9. [DOI] [PubMed] [Google Scholar]
- 32.Wei C., Liang Q., Li X., Li H., Liu Y., Huang X., Chen X., Guo Y., Li J. Bioinformatics profiling utilized a nine immune-related long noncoding RNA signature as a prognostic target for pancreatic cancer. J. Cell. Biochem. 2019;120:14916–14927. doi: 10.1002/jcb.28754. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Zhang L., Xu Y., Wu X., Zhou Y., Mo J. Immune-related long noncoding RNA signature for predicting survival and immune checkpoint blockade in hepatocellular carcinoma. J. Cell. Physiol. 2020;235:9304–9316. doi: 10.1002/jcp.29730. [DOI] [PubMed] [Google Scholar]
- 34.Eptaminitaki G.C., Stellas D., Bonavida B., Baritaki S. Long non-coding RNAs (lncRNAs) signaling in cancer chemoresistance: from prediction to druggability. Drug Resist. Updates. 2022;65 doi: 10.1016/j.drup.2022.100866. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J., Luo Q., Li X., Guo J., Zhu Q., Lu X., Wei L., Xiang Z., Peng M., Ou C., Zou Y. Novel role of immune-related non-coding RNAs as potential biomarkers regulating tumour immunoresponse via MICA/NKG2D pathway. Biomark. Res. 2023;11:86. doi: 10.1186/s40364-023-00530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Poppel H., Haese A., Graefen M., de la Taille A., Irani J., de Reijke T., Remzi M., Marberger M. The relationship between Prostate CAncer gene 3 (PCA3) and prostate cancer significance. BJU Int. 2012;109:360–366. doi: 10.1111/j.1464-410X.2011.10377.x. [DOI] [PubMed] [Google Scholar]
- 37.Ghafouri-Fard S., Sohrabi B., Hussen B.M., Mehravaran E., Jamali E., Arsang-Jang S., Fathi M., Taheri M., Samsami M. Down-regulation of MEG3, PANDA and CASC2 as p53-related lncRNAs in breast cancer. Breast Dis. 2022;41:137–143. doi: 10.3233/BD-210069. [DOI] [PubMed] [Google Scholar]
- 38.Wang C., Yang Y., Wu X., Li J., Liu K., Fang D., Li B., Shan G., Mei X., Wang F., Mei Y. Reciprocal modulation of long noncoding RNA EMS and p53 regulates tumorigenesis. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2111409119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng Z., Hong J., Tang N., Liu F., Gu S., Feng Z. Long non-coding RNA p53 upregulated regulator of p53 levels (PURPL) promotes the development of gastric cancer. Bioengineered. 2022;13:1359–1376. doi: 10.1080/21655979.2021.2017588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei S., Li X., Wang J., Wang Y., Zhang C., Dai S., Wang X., Deng X., Zhao L., Shan B. Outer membrane vesicles secreted by Helicobacter pylori transmitting gastric pathogenic virulence factors. ACS Omega. 2022;7:240–258. doi: 10.1021/acsomega.1c04549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tilahun M., Gedefie A., Belayhun C., Sahle Z., Abera A. Helicobacter pylori pathogenicity islands and giardia lamblia cysteine proteases in role of coinfection and pathogenesis. Infect. Drug Resist. 2022;15:21–34. doi: 10.2147/IDR.S346705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kayapinar A.K., Solakoglu D., Bas K., Oymaci E., Isbilen B., Calik B., Diniz G., Akbulut G. Relationship of prognostic factors in stomach cancer with helicobacter pylori: a retrospective study. Acta Gastroenterol Belg. 2021;84:607–617. doi: 10.51821/84.4.012. [DOI] [PubMed] [Google Scholar]
- 43.Riahi A., Moqadami A., Sgi A.L., Alizadeh M., Rajabi A., Safaralizadeh R. Overexpression of the GClnc1 as a diagnostic biomarker in gastric cancer patients and its link with H. Pylori infection. Clin. Lab. 2021;67 doi: 10.7754/Clin.Lab.2021.210403. [DOI] [PubMed] [Google Scholar]
- 44.Rajabi A., Bastani S., Maydanchi M., Tayefeh-Gholami S., Abdolahi S., Saber A., Safaralizadeh R. Moderate prognostic value of lncRNA FOXD2-AS1 in gastric cancer with Helicobacter pylori infection. J. Gastrointest. Cancer. 2021;53:687–691. doi: 10.1007/s12029-021-00686-y. [DOI] [PubMed] [Google Scholar]
- 45.Ashraf A.A., Gamal S.M., Ashour H., Aboulhoda B.E., Rashed L.A., Harb I.A., Abdelfattah G.H., El-Seidi E.A., Shawky H.M. Investigating Helicobacter pylori-related pyloric hypomotility: functional, histological, and molecular alterations. Am. J. Physiol. Gastrointest. Liver Physiol. 2021;321:G461–G476. doi: 10.1152/ajpgi.00364.2020. [DOI] [PubMed] [Google Scholar]
- 46.Xu W., Ding M., Wang B., Cai Y., Guo C., Yuan C. Molecular mechanism of the canonical oncogenic lncRNA MALAT1 in gastric cancer. Curr. Med. Chem. 2021;28:8800–8809. doi: 10.2174/0929867328666210521213352. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z., Li M., Zhang Z. lncRNA MALAT1 modulates oxaliplatin resistance of gastric cancer via sponging miR-22-3p. OncoTargets Ther. 2020;13:1343–1354. doi: 10.2147/OTT.S196619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao Y., Pan J., Geng Q., Wang G. LncRNA MALAT1 increases the stemness of gastric cancer cells via enhancing SOX2 mRNA stability. FEBS Open Bio. 2019;9:1212–1222. doi: 10.1002/2211-5463.12649. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Dai Q., Zhang T., Li C. LncRNA MALAT1 regulates the cell proliferation and cisplatin resistance in gastric cancer via PI3K/AKT pathway. Cancer Manag. Res. 2020;12:1929–1939. doi: 10.2147/CMAR.S243796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng W., Li J., Zhou X., Cui L., Wang Y. The lncRNA XIST promotes proliferation, migration and invasion of gastric cancer cells by targeting miR-337. Arab J Gastroenterol. 2020;21:199–206. doi: 10.1016/j.ajg.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 51.Li P., Wang L., Li P., Hu F., Cao Y., Tang D., Ye G., Li H., Wang D. Silencing lncRNA XIST exhibits antiproliferative and proapoptotic effects on gastric cancer cells by up-regulating microRNA-132 and down-regulating PXN. Aging (Albany NY) 2020;13:14469–14481. doi: 10.18632/aging.103635. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Zhang Q., Chen B., Liu P., Yang J. XIST promotes gastric cancer (GC) progression through TGF-beta1 via targeting miR-185. J. Cell. Biochem. 2018;119:2787–2796. doi: 10.1002/jcb.26447. [DOI] [PubMed] [Google Scholar]
- 53.Chen D.L., Ju H.Q., Lu Y.X., Chen L.Z., Zeng Z.L., Zhang D.S., Luo H.Y., Wang F., Qiu M.Z., Wang D.S., Xu D.Z., Zhou Z.W., Pelicano H., Huang P., Xie D., Wang F.H., Li Y.H., Xu R.H. Correction to: long non-coding RNA XIST regulates gastric cancer progression by acting as a molecular sponge of miR-101 to modulate EZH2 expression. J. Exp. Clin. Cancer Res. 2021;40:208. doi: 10.1186/s13046-021-02002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li C., Zhang J., Zhou Y., Li B. Long non-coding RNA CASC9 promotes the progression and development of gastric cancer via regulating miR-370/EGFR axis. Dig. Liver Dis. 2021;53:509–516. doi: 10.1016/j.dld.2020.12.115. [DOI] [PubMed] [Google Scholar]
- 55.Fang J., Chen W., Meng X.L. LncRNA CASC9 suppressed the apoptosis of gastric cancer cells through regulating BMI1. Pathol. Oncol. Res. 2020;26:475–482. doi: 10.1007/s12253-019-00703-3. [DOI] [PubMed] [Google Scholar]
- 56.Sun J., Jiang Q., Chen H., Zhang Q., Zhao J., Li H., Wang X., Fang Y., Ruan Y., Sun Y. Genomic instability-associated lncRNA signature predicts prognosis and distinct immune landscape in gastric cancer. Ann. Transl. Med. 2021;9:1326. doi: 10.21037/atm-21-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H., Wu M., Lu Y., He K., Cai X., Yu X., Lu J., Teng L. LncRNA MIR4435-2HG targets desmoplakin and promotes growth and metastasis of gastric cancer by activating Wnt/beta-catenin signaling. Aging (Albany NY) 2019;11:6657–6673. doi: 10.18632/aging.102164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao L.F., Li W., Liu Y.G., Zhang C., Gao W.N., Wang L. Inhibition of mir4435-2HG on invasion, migration, and EMT of gastric carcinoma cells by mediating MiR-138-5p/sox4 Axis. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.661288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ke D., Li H., Zhang Y., An Y., Fu H., Fang X., Zheng X. The combination of circulating long noncoding RNAs AK001058, INHBA-AS1, MIR4435-2HG, and CEBPA-AS1 fragments in plasma serve as diagnostic markers for gastric cancer. Oncotarget. 2017;8:21516–21525. doi: 10.18632/oncotarget.15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miao Y., Sui J., Xu S.Y., Liang G.Y., Pu Y.P., Yin L.H. Comprehensive analysis of a novel four-lncRNA signature as a prognostic biomarker for human gastric cancer. Oncotarget. 2017;8:75007–75024. doi: 10.18632/oncotarget.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu M.D., Wang Y., Weng W., Wei P., Qi P., Zhang Q., Tan C., Ni S.J., Dong L., Yang Y., Lin W., Xu Q., Huang D., Huang Z., Ma Y., Zhang W., Sheng W., Du X. A positive feedback loop of lncRNA-PVT1 and FOXM1 facilitates gastric cancer growth and invasion. Clin. Cancer Res. 2017;23:2071–2080. doi: 10.1158/1078-0432.CCR-16-0742. [DOI] [PubMed] [Google Scholar]
- 62.Niu J., Song X., Zhang X. Regulation of lncRNA PVT1 on miR-125 in metastasis of gastric cancer cells. Oncol. Lett. 2020;19:1261–1266. doi: 10.3892/ol.2019.11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L., Xiao B., Yu T., Gong L., Wang Y., Zhang X., Zou Q., Zuo Q. lncRNA PVT1 promotes the migration of gastric cancer by functioning as ceRNA of miR-30a and regulating Snail. J. Cell. Physiol. 2021;236:536–548. doi: 10.1002/jcp.29881. [DOI] [PubMed] [Google Scholar]
- 64.Ho K.H., Huang T.W., Shih C.M., Lee Y.T., Liu A.J., Chen P.H., Chen K.C. Glycolysis-associated lncRNAs identify a subgroup of cancer patients with poor prognoses and a high-infiltration immune microenvironment. BMC Med. 2021;19:59. doi: 10.1186/s12916-021-01925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oster P., Vaillant L., McMillan B., Velin D. The efficacy of cancer immunotherapies is compromised by Helicobacter pylori infection. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.899161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharndama H.C., Mba I.E. Helicobacter pylori: an up-to-date overview on the virulence and pathogenesis mechanisms. Braz. J. Microbiol. 2022;53:33–50. doi: 10.1007/s42770-021-00675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abu-Lubad M.A., Helaly G.F., Haddadin W.J., Jarajreh D.A.K., Aqel A.A., Al-Zeer M.A. Loss of p53 expression in gastric epithelial cells of Helicobacter pylori-infected Jordanian patients. Internet J. Microbiol. 2022;2022 doi: 10.1155/2022/7779770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yousefi L., Osquee H.O., Ghotaslou R., Rezaee M.A., Pirzadeh T., Sadeghi J., Hemmati F., Yousefi B., Moaddab S.Y., Yousefi M., Shirmohammadi M., Somi M.H., Ganbarov K., Kafil H.S. Dysregulation of lncRNA in Helicobacter pylori-infected gastric cancer cells. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/6911734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dastmalchi N., Khojasteh S.M.B., Nargesi M.M., Safaralizadeh R. The correlation between lncRNAs and Helicobacter pylori in gastric cancer. Pathog Dis. 2019;77 doi: 10.1093/femspd/ftaa004. [DOI] [PubMed] [Google Scholar]
- 70.Yoshida N., Kimura T. Pathogen-associated regulatory non-coding RNAs and oncogenesis. Front Biosci (Landmark Ed) 2017;22:1599–1621. doi: 10.2741/4560. [DOI] [PubMed] [Google Scholar]
- 71.Zhang J., Wei J., Wang Z., Feng Y., Wei Z., Hou X., Xu J., He Y., Yang D. Transcriptome hallmarks in Helicobacter pylori infection influence gastric cancer and MALT lymphoma. Epigenomics. 2020;12:661–671. doi: 10.2217/epi-2019-0152. [DOI] [PubMed] [Google Scholar]
- 72.Yao Y., Jiang Q., Jiang L., Wu J., Zhang Q., Wang J., Feng H., Zang P. Lnc-SGK1 induced by Helicobacter pylori infection and highsalt diet promote Th2 and Th17 differentiation in human gastric cancer by SGK1/Jun B signaling. Oncotarget. 2016;7:20549–20560. doi: 10.18632/oncotarget.7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rao X., Liu X., Liu N., Zhang Y., Zhang Z., Zhou L., Han G., Cen R., Shi N., Zhu H., Gong H., Huang C., Ji Q., Li Q. Long noncoding RNA NEAT1 promotes tumorigenesis in H. pylori gastric cancer by sponging miR-30a to regulate COX-2/BCL9 pathway. Helicobacter. 2021;26 doi: 10.1111/hel.12847. [DOI] [PubMed] [Google Scholar]
- 74.Amini F., Khalaj-Kondori M., Moqadami A., Rajabi A. Expression of HOTAIR and MEG3 are negatively associated with H. pylori positive status in gastric cancer patients. Genes Cancer. 2022;13:1–8. doi: 10.18632/genesandcancer.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sica A., Larghi P., Mancino A., Rubino L., Porta C., Totaro M.G., Rimoldi M., Biswas S.K., Allavena P., Mantovani A. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 76.Chen F., Chen J., Yang L., Liu J., Zhang X., Zhang Y., Tu Q., Yin D., Lin D., Wong P.P., Huang D., Xing Y., Zhao J., Li M., Liu Q., Su F., Su S., Song E. Extracellular vesicle-packaged HIF-1alpha-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat. Cell Biol. 2019;21:498–510. doi: 10.1038/s41556-019-0299-0. [DOI] [PubMed] [Google Scholar]
- 77.Sun T., Wu R., Ming L. The role of m6A RNA methylation in cancer. Biomed. Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108613. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y., Geng X., Li Q., Xu J., Tan Y., Xiao M., Song J., Liu F., Fang C., Wang H. m6A modification in RNA: biogenesis, functions and roles in gliomas. J. Exp. Clin. Cancer Res. 2020;39:192. doi: 10.1186/s13046-020-01706-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma S., Chen C., Ji X., Liu J., Zhou Q., Wang G., Yuan W., Kan Q., Sun Z. The interplay between m6A RNA methylation and noncoding RNA in cancer. J. Hematol. Oncol. 2019;12:121. doi: 10.1186/s13045-019-0805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li X., Ding Z., Tong Y. Correlations of m(6)A methylation-related lncRNAs with the prognosis of papillary thyroid carcinoma. Int. J. Gen. Med. 2024;17:775–790. doi: 10.2147/IJGM.S449827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shen J.J., Li M.C., Tian S.Q., Chen W.M. Long non-coding RNA GATA6-AS1 is mediated by N6-methyladenosine methylation and inhibits the proliferation and metastasis of gastric cancer. World J. Gastrointest. Oncol. 2024;16:1019–1028. doi: 10.4251/wjgo.v16.i3.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu D., Wang Z., Li F. KIAA1429 induces m6A modification of LINC01106 to enhance the malignancy of lung adenocarcinoma cells via the JAK/STAT3 pathway. Crit. Rev. Immunol. 2024;44:49–61. doi: 10.1615/CritRevImmunol.2024052728. [DOI] [PubMed] [Google Scholar]
- 83.Wang J., Zhang J., Liu H., Meng L., Gao X., Zhao Y., Wang C., Gao X., Fan A., Cao T., Fan D., Zhao X., Lu Y. N6-methyladenosine reader hnRNPA2B1 recognizes and stabilizes NEAT1 to confer chemoresistance in gastric cancer. Cancer Commun. 2024;44:469–490. doi: 10.1002/cac2.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The lncRNA was selected from the publicly available database (TP53LNC-DB), and their expression profiles and associated clinical parameters were collected from TCGA. However, additional data, files, figures, or tables produced by the current study could be requested from corresponding authors.