Abstract
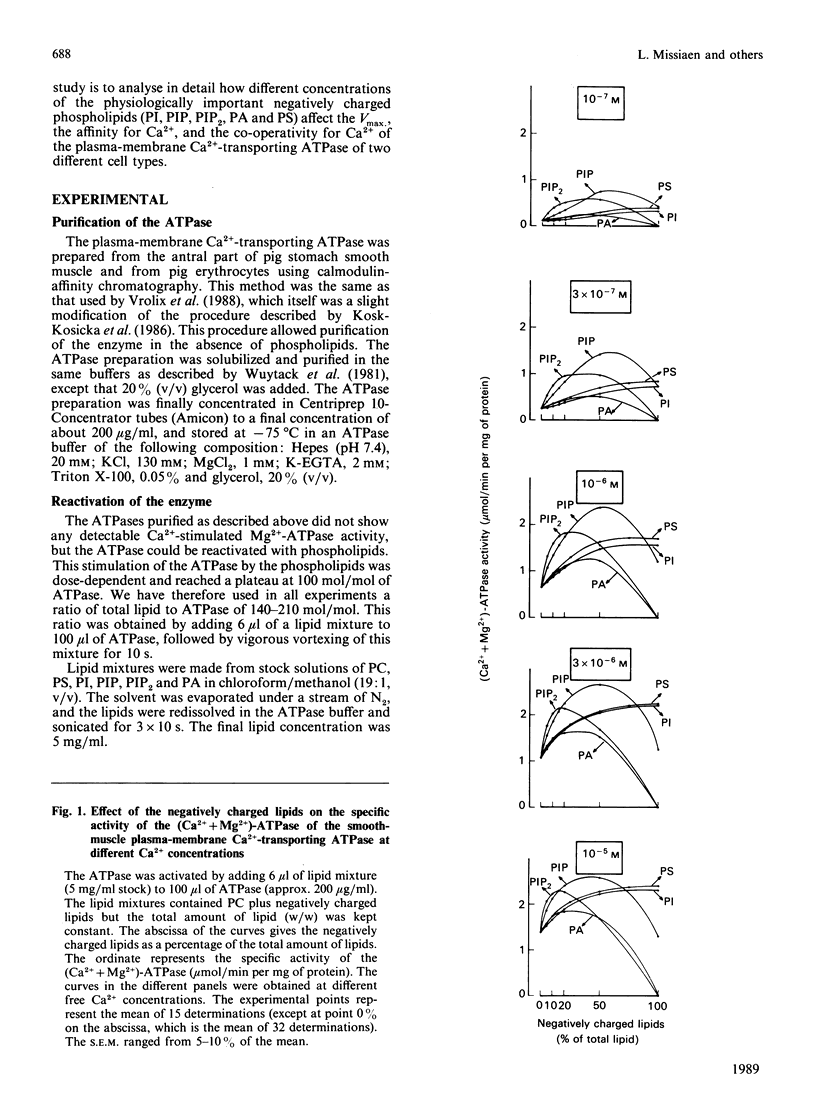
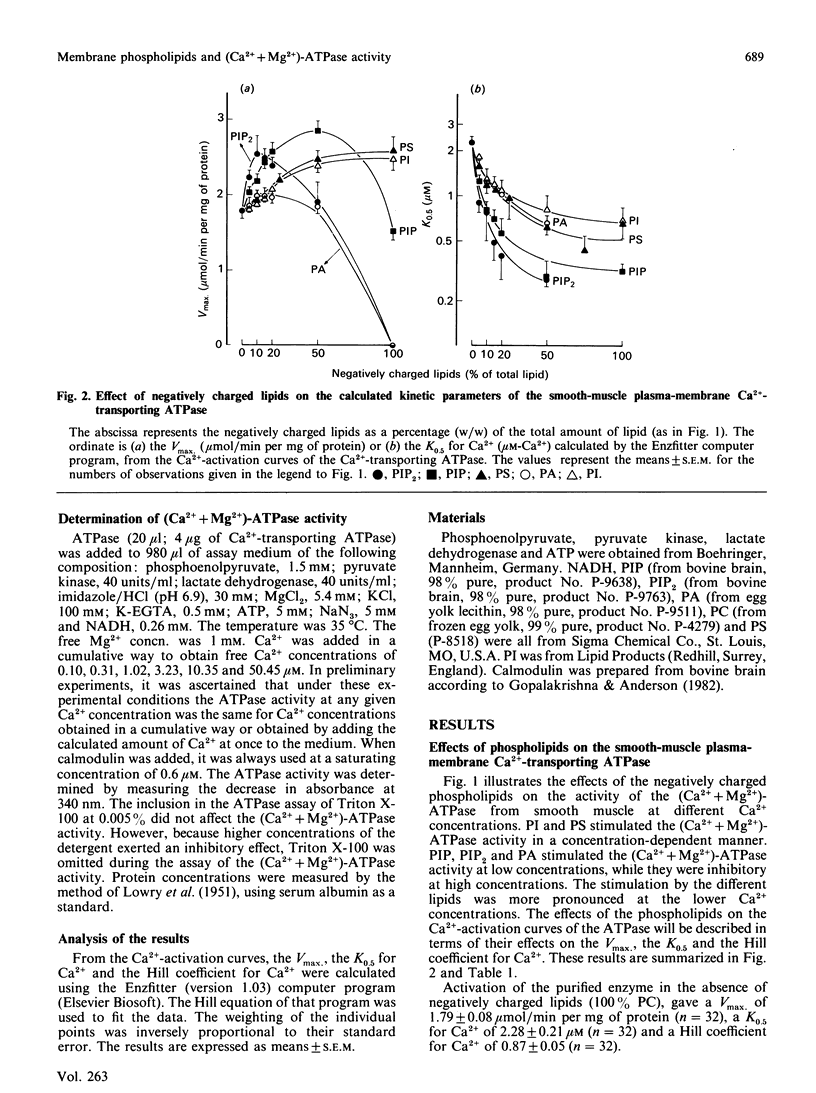
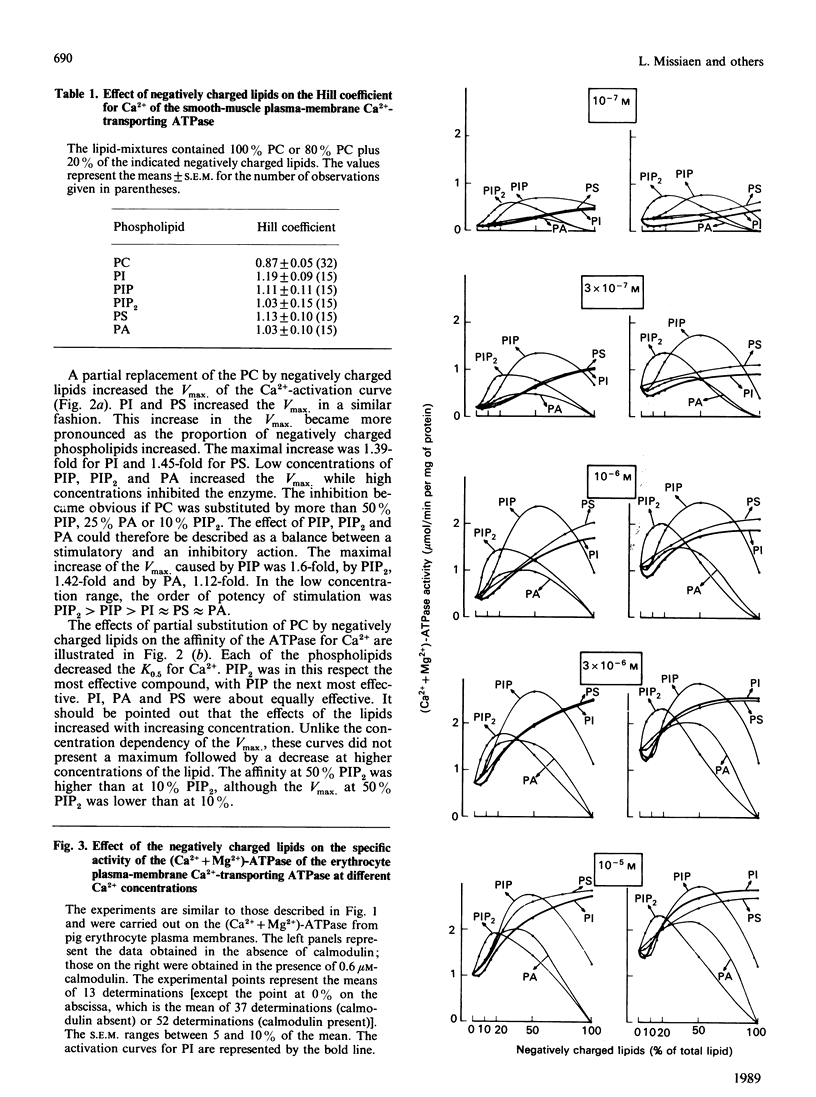
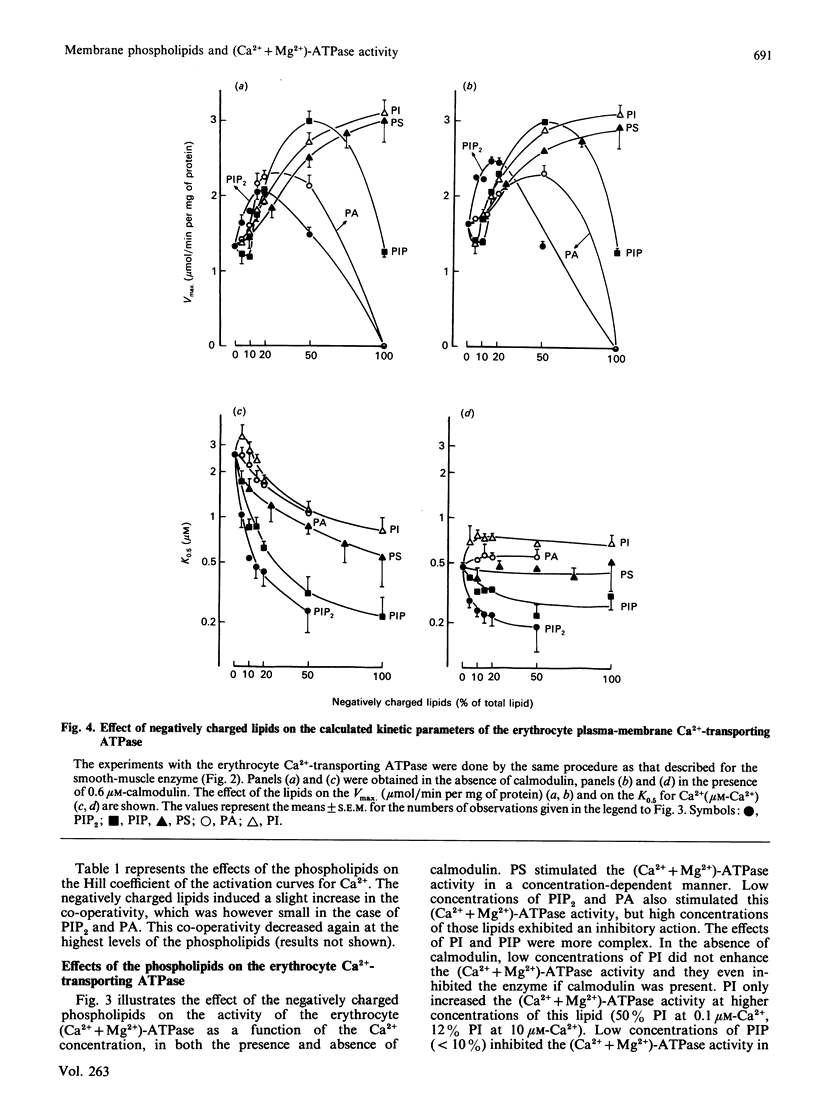
The aim of the present work was to investigate the stimulation of the plasma-membrane Ca2+-transporting ATPase by negatively charged phospholipids. The Ca2+-transporting ATPase was purified from pig stomach smooth muscle and from pig erythrocytes, and was reactivated with phosphatidylcholine (PC) in the presence and absence of negatively charged phospholipids. The substitution of phosphatidylinositol (PI), phosphatidylinositol 4-phosphate (PIP), phosphatidylinositol 4,5-bisphosphate (PIP2), phosphatidic acid (PA) or phosphatidylserine (PS) for PC induced profound changes in the Vmax, the K0.5 and the Hill coefficient of the Ca2+-activation curves for both ATPases. Low concentrations of each of the negatively charged phospholipids increased the Vmax., but high ratios of PIP, PIP2 or PA to PC decreased this parameter. PI, PA and PS increased the Vmax. of the erythrocyte enzyme to a larger extent than that of the smooth-muscle enzyme. This difference was less pronounced for PIP and absent for PIP2. PI (greater than 20% PC substituted), PIP, PIP2, PA and PS all increased the affinity of the two Ca2+-transporting ATPases for Ca2+ in the following order of potency: PIP2 greater than PIP greater than PI approximately PS approximately PA. PI, PA and PS increased the Ca2+ affinity of the smooth-muscle enzyme more than that of the erythrocyte enzyme; this difference was less pronounced for PIP and absent for PIP2. Even in the presence of calmodulin, all of the negatively charged phospholipids were still able to increase the Vmax. of the erythrocyte enzyme, whereas only PIP and PIP2 increased the affinity for Ca2+. The effect of PI at low concentrations (less than 20%) on the erythrocyte enzyme was peculiar in that it caused a decrease in the Ca2+ affinity instead of an increase. This effect was not observed for the smooth-muscle enzyme. All of the negatively charged phospholipids slightly increased the Hill coefficient for Ca2+ of both ATPases, and this effect was additive to that of calmodulin. The stimulation of the erythrocyte enzyme exhibited positive co-operativity towards PI and PIP, whereas that of the smooth-muscle enzyme did not. It is concluded (1) that there is a correlation between the number of negative charges on the phospholipids (PIP2 greater than PIP greater than PA approximately PI approximately PS) and the magnitude of their effect on the Vmax. and the K0.5 for Ca2+, and (2) that the action of the lipids on the smooth-muscle enzyme differs from that on the erythrocyte enzyme, indicating that these two Ca2+-transporting ATPases are not the same.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. The Croonian lecture, 1988. Inositol lipids and calcium signalling. Proc R Soc Lond B Biol Sci. 1988 Sep 22;234(1277):359–378. doi: 10.1098/rspb.1988.0054. [DOI] [PubMed] [Google Scholar]
- Choquette D., Hakim G., Filoteo A. G., Plishker G. A., Bostwick J. R., Penniston J. T. Regulation of plasma membrane Ca2+ ATPases by lipids of the phosphatidylinositol cycle. Biochem Biophys Res Commun. 1984 Dec 28;125(3):908–915. doi: 10.1016/0006-291x(84)91369-x. [DOI] [PubMed] [Google Scholar]
- Enyedi A., Flura M., Sarkadi B., Gardos G., Carafoli E. The maximal velocity and the calcium affinity of the red cell calcium pump may be regulated independently. J Biol Chem. 1987 May 5;262(13):6425–6430. [PubMed] [Google Scholar]
- Filoteo A. G., Gorski J. P., Penniston J. T. The ATP-binding site of the erythrocyte membrane Ca2+ pump. Amino acid sequence of the fluorescein isothiocyanate-reactive region. J Biol Chem. 1987 May 15;262(14):6526–6530. [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Gopinath R. M., Vincenzi F. F. Phosphodiesterase protein activator mimics red blood cell cytoplasmic activator of (Ca2+-Mg2+)ATPase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1203–1209. doi: 10.1016/s0006-291x(77)80107-1. [DOI] [PubMed] [Google Scholar]
- James P., Maeda M., Fischer R., Verma A. K., Krebs J., Penniston J. T., Carafoli E. Identification and primary structure of a calmodulin binding domain of the Ca2+ pump of human erythrocytes. J Biol Chem. 1988 Feb 25;263(6):2905–2910. [PubMed] [Google Scholar]
- James P., Zvaritch E. I., Shakhparonov M. I., Penniston J. T., Carafoli E. The amino acid sequence of the phosphorylation domain of the erythrocyte Ca2+ ATPase. Biochem Biophys Res Commun. 1987 Nov 30;149(1):7–12. doi: 10.1016/0006-291x(87)91597-x. [DOI] [PubMed] [Google Scholar]
- Jarrett H. W., Penniston J. T. Partial purification of the Ca2+-Mg2+ ATPase activator from human erythrocytes: its similarity to the activator of 3':5' - cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1210–1216. doi: 10.1016/s0006-291x(77)80108-3. [DOI] [PubMed] [Google Scholar]
- Kosk-Kosicka D., Scaillet S., Inesi G. The partial reactions in the catalytic cycle of the calcium-dependent adenosine triphosphatase purified from erythrocyte membranes. J Biol Chem. 1986 Mar 5;261(7):3333–3338. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Missiaen L., Kanmura Y., Wuytack F., Casteels R. Carbachol partially inhibits the plasma-membrane Ca2+-pump in microsomes from pig stomach smooth muscle. Biochem Biophys Res Commun. 1988 Jan 29;150(2):681–686. doi: 10.1016/0006-291x(88)90445-7. [DOI] [PubMed] [Google Scholar]
- Missiaen L., Wuytack F., De Smedt H., Amant F., Casteels R. AIF4-induced inhibition of the ATPase activity, the Ca2+-transport activity and the phosphoprotein-intermediate formation of plasma-membrane and endo(sarco)plasmic-reticulum Ca2+-transport ATPases in different tissues. Evidence for a tissue-dependent functional difference. Biochem J. 1989 Jul 15;261(2):655–660. doi: 10.1042/bj2610655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Hanahan D. J. Phospholipid and detergent effects on (Ca2+ + Mg2+)ATPase purified from human erythrocytes. Arch Biochem Biophys. 1985 Feb 1;236(2):720–730. doi: 10.1016/0003-9861(85)90678-2. [DOI] [PubMed] [Google Scholar]
- Niggli V., Adunyah E. S., Carafoli E. Acidic phospholipids, unsaturated fatty acids, and limited proteolysis mimic the effect of calmodulin on the purified erythrocyte Ca2+ - ATPase. J Biol Chem. 1981 Aug 25;256(16):8588–8592. [PubMed] [Google Scholar]
- Shull G. E., Greeb J. Molecular cloning of two isoforms of the plasma membrane Ca2+-transporting ATPase from rat brain. Structural and functional domains exhibit similarity to Na+,K+- and other cation transport ATPases. J Biol Chem. 1988 Jun 25;263(18):8646–8657. [PubMed] [Google Scholar]
- Verma A. K., Filoteo A. G., Stanford D. R., Wieben E. D., Penniston J. T., Strehler E. E., Fischer R., Heim R., Vogel G., Mathews S. Complete primary structure of a human plasma membrane Ca2+ pump. J Biol Chem. 1988 Oct 5;263(28):14152–14159. [PubMed] [Google Scholar]
- Vrolix M., Raeymaekers L., Wuytack F., Hofmann F., Casteels R. Cyclic GMP-dependent protein kinase stimulates the plasmalemmal Ca2+ pump of smooth muscle via phosphorylation of phosphatidylinositol. Biochem J. 1988 Nov 1;255(3):855–863. doi: 10.1042/bj2550855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuytack F., De Schutter G., Casteels R. Purification of (Ca2+ + Mg2+)-ATPase from smooth muscle by calmodulin affinity chromatography. FEBS Lett. 1981 Jul 6;129(2):297–300. doi: 10.1016/0014-5793(81)80187-1. [DOI] [PubMed] [Google Scholar]


