Abstract
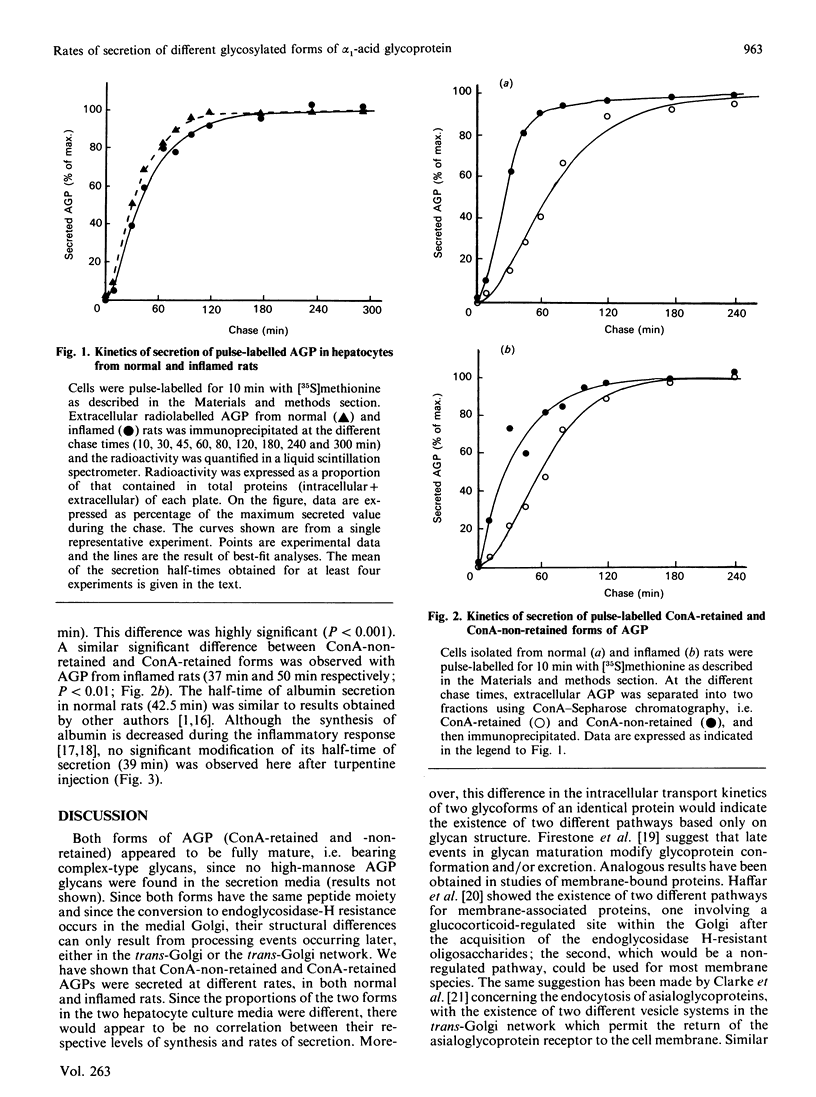
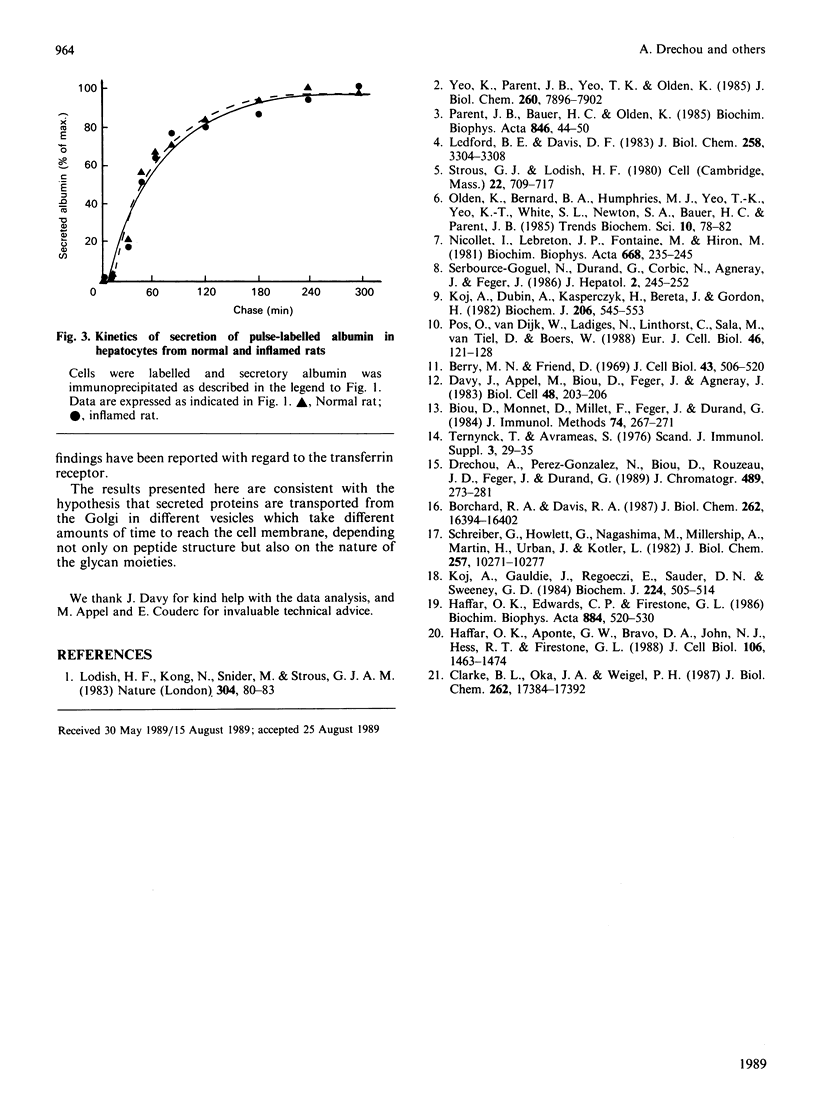
Various studies have shown that oligosaccharides play an important role in the intracellular transport and secretion of glycoproteins. We show here a difference in the rate of secretion of two mature glycoforms of a single protein, alpha 1-acid glycoprotein. This indicates the existence of kinetically different pathways for these two forms for transport from the medial Golgi to the extracellular medium.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biou D., Monnet D., Millet F., Feger J., Durand G. An immunochemical procedure to evaluate the degree of desialylation of alpha 1-acid glycoprotein in rat serum. J Immunol Methods. 1984 Nov 30;74(2):267–271. doi: 10.1016/0022-1759(84)90293-x. [DOI] [PubMed] [Google Scholar]
- Borchardt R. A., Davis R. A. Intrahepatic assembly of very low density lipoproteins. Rate of transport out of the endoplasmic reticulum determines rate of secretion. J Biol Chem. 1987 Dec 5;262(34):16394–16402. [PubMed] [Google Scholar]
- Clarke B. L., Oka J. A., Weigel P. H. Degradation of asialoglycoproteins mediated by the galactosyl receptor system in isolated hepatocytes. Evidence for two parallel pathways. J Biol Chem. 1987 Dec 25;262(36):17384–17392. [PubMed] [Google Scholar]
- Davy J., Appel M., Biou D., Feger J., Agneray J. L'hépatocyte isolé fixé sur collagène, modèle fidèle pour l'étude de la sécrétion des protéines en milieu minimal. Réponse à l'inflammation. Biol Cell. 1983;48(2-3):203–206. doi: 10.1111/j.1768-322x.1984.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Drechou A., Perez-Gonzalez N., Biou D., Rouzeau J. D., Feger J., Durand G. One-step purification of rat plasma alpha 1-acid glycoprotein by antibody affinity chromatography: application to normal and inflamed rat sera. J Chromatogr. 1989 Apr 14;489(2):273–281. doi: 10.1016/s0378-4347(00)82905-9. [DOI] [PubMed] [Google Scholar]
- Haffar O. K., Aponte G. W., Bravo D. A., John N. J., Hess R. T., Firestone G. L. Glucocorticoid-regulated localization of cell surface glycoproteins in rat hepatoma cells is mediated within the Golgi complex. J Cell Biol. 1988 May;106(5):1463–1474. doi: 10.1083/jcb.106.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffar O. K., Edwards C. P., Firestone G. L. Protein glycosylation regulates the externalization of two distinct classes of glucocorticoid-induced glycoproteins in rat hepatoma cells. Biochim Biophys Acta. 1986 Dec 10;884(3):520–530. doi: 10.1016/0304-4165(86)90204-7. [DOI] [PubMed] [Google Scholar]
- Koj A., Dubin A., Kasperczyk H., Bereta J., Gordon A. H. Changes in the blood level and affinity to concanavalin A of rat plasma glycoproteins during acute inflammation and hepatoma growth. Biochem J. 1982 Sep 15;206(3):545–553. doi: 10.1042/bj2060545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koj A., Gauldie J., Regoeczi E., Sauder D. N., Sweeney G. D. The acute-phase response of cultured rat hepatocytes. System characterization and the effect of human cytokines. Biochem J. 1984 Dec 1;224(2):505–514. doi: 10.1042/bj2240505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford B. E., Davis D. F. Kinetics of serum protein secretion by cultured hepatoma cells. Evidence for multiple secretory pathways. J Biol Chem. 1983 Mar 10;258(5):3304–3308. [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Snider M., Strous G. J. Hepatoma secretory proteins migrate from rough endoplasmic reticulum to Golgi at characteristic rates. Nature. 1983 Jul 7;304(5921):80–83. doi: 10.1038/304080a0. [DOI] [PubMed] [Google Scholar]
- Nicollet I., Lebreton J. P., Fontaine M., Hiron M. Evidence for alpha-1-acid glycoprotein populations of different pI values after concanavalin A affinity chromatography. Study of their evolution during inflammation in man. Biochim Biophys Acta. 1981 Apr 28;668(2):235–245. doi: 10.1016/0005-2795(81)90031-3. [DOI] [PubMed] [Google Scholar]
- Parent J. B., Bauer H. C., Olden K. Three secretory rates in human hepatoma cells. Biochim Biophys Acta. 1985 Jul 30;846(1):44–50. doi: 10.1016/0167-4889(85)90108-9. [DOI] [PubMed] [Google Scholar]
- Pos O., van Dijk W., Ladiges N., Linthorst C., Sala M., van Tiel D., Boers W. Glycosylation of four acute-phase glycoproteins secreted by rat liver cells in vivo and in vitro. Effects of inflammation and dexamethasone. Eur J Cell Biol. 1988 Apr;46(1):121–128. [PubMed] [Google Scholar]
- Schreiber G., Howlett G., Nagashima M., Millership A., Martin H., Urban J., Kotler L. The acute phase response of plasma protein synthesis during experimental inflammation. J Biol Chem. 1982 Sep 10;257(17):10271–10277. [PubMed] [Google Scholar]
- Serbource-Goguel Seta N., Durand G., Corbic M., Agneray J., Fegar J. Alterations in relative proportions of microheterogenous forms of human alpha 1-acid glycoprotein in liver disease. J Hepatol. 1986;2(2):245–252. doi: 10.1016/s0168-8278(86)80083-6. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Yeo K. T., Parent J. B., Yeo T. K., Olden K. Variability in transport rates of secretory glycoproteins through the endoplasmic reticulum and Golgi in human hepatoma cells. J Biol Chem. 1985 Jul 5;260(13):7896–7902. [PubMed] [Google Scholar]


