Abstract
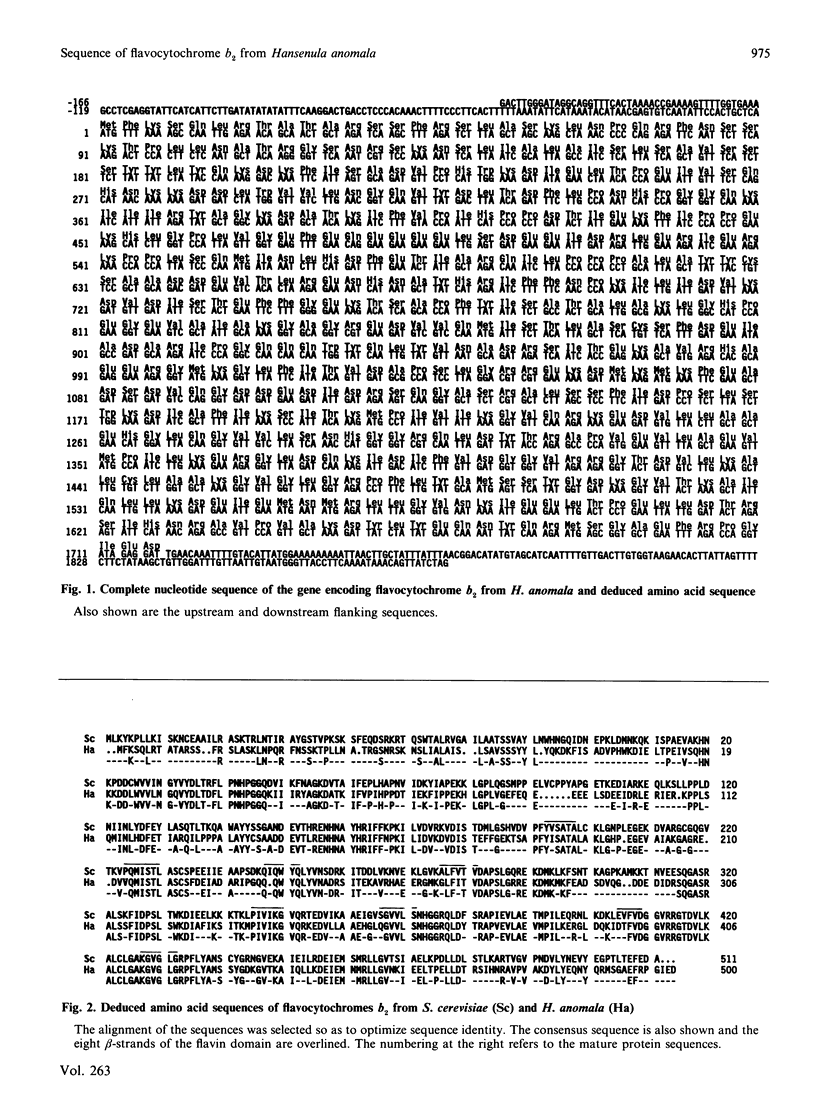
To understand the structural basis for the different catalytic behaviour of the flavocytochromes b2 from Saccharomyces cerevisiae and Hansenula anomala we have cloned and sequenced the gene encoding the latter. We have compared the amino acid sequences of the mature proteins in the context of the known crystal structure of S. cerevisiae flavocytochrome b2. Overall there is 60% sequence identity, but two surface loops in particular are strikingly different in primary structure and net charge.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEBY C. A., MORTON R. K. Crystalline cytochrome b2 and lactic dehydrogenase of yeast. Nature. 1954 Apr 24;173(4408):749–752. doi: 10.1038/173749a0. [DOI] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Capeillère-Blandin C., Albani J. Cytochrome b2, an electron carrier between flavocytochrome b2 and cytochrome c. Rapid kinetic characterization of the electron-transfer parameters with ionic-strength-dependence. Biochem J. 1987 Jul 1;245(1):159–165. doi: 10.1042/bj2450159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capeillère-Blandin C., Barber M. J., Bray R. C. Comparison of the processes involved in reduction by the substrate for two homologous flavocytochromes b2 from different species of yeast. Biochem J. 1986 Sep 15;238(3):745–756. doi: 10.1042/bj2380745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gervais M., Corazzin S., Risler Y. How the loss of several residues, at the level of one interglobule junction, modulates the lactate dehydrogenase activity of yeast flavocytochrome b2: a study of the nicked enzymes resulting from clostripain and trypsin action. Biochimie. 1982 Jul;64(7):509–522. doi: 10.1016/s0300-9084(82)80167-3. [DOI] [PubMed] [Google Scholar]
- Guiard B. Structure, expression and regulation of a nuclear gene encoding a mitochondrial protein: the yeast L(+)-lactate cytochrome c oxidoreductase (cytochrome b2). EMBO J. 1985 Dec 1;4(12):3265–3272. doi: 10.1002/j.1460-2075.1985.tb04076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haumont P. Y., Thomas M. A., Labeyrie F., Lederer F. Amino-acid sequence of the cytochrome-b5-like heme-binding domain from Hansenula anomala flavocytochrome b2. Eur J Biochem. 1987 Dec 15;169(3):539–546. doi: 10.1111/j.1432-1033.1987.tb13642.x. [DOI] [PubMed] [Google Scholar]
- Kaput J., Goltz S., Blobel G. Nucleotide sequence of the yeast nuclear gene for cytochrome c peroxidase precursor. Functional implications of the pre sequence for protein transport into mitochondria. J Biol Chem. 1982 Dec 25;257(24):15054–15058. [PubMed] [Google Scholar]
- Labeyrie F., Baudras A., Lederer F. Flavocytochrome b 2 or L-lactate cytochrome c reductase from yeast. Methods Enzymol. 1978;53:238–256. doi: 10.1016/s0076-6879(78)53030-9. [DOI] [PubMed] [Google Scholar]
- Lathe R., Vilotte J. L., Clark A. J. Plasmid and bacteriophage vectors for excision of intact inserts. Gene. 1987;57(2-3):193–201. doi: 10.1016/0378-1119(87)90122-3. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Pompon D., Iwatsubo M., Lederer F. Flavocytochrome b2 (Baker's yeast). Deuterium isotope effect studied by rapid-kinetic methods as a probe for the mechanism of electron transfer. Eur J Biochem. 1980 Mar;104(2):479–488. doi: 10.1111/j.1432-1033.1980.tb04450.x. [DOI] [PubMed] [Google Scholar]
- Pratje E., Guiard B. One nuclear gene controls the removal of transient pre-sequences from two yeast proteins: one encoded by the nuclear the other by the mitochondrial genome. EMBO J. 1986 Jun;5(6):1313–1317. doi: 10.1002/j.1460-2075.1986.tb04361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G. A., White S., Black M. T., Lederer F., Mathews F. S., Chapman S. K. Probing the active site of flavocytochrome b2 by site-directed mutagenesis. Eur J Biochem. 1988 Dec 15;178(2):329–333. doi: 10.1111/j.1432-1033.1988.tb14454.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegoni M., Silvestrini M. C., Labeyrie F., Brunori M. A temperature-jump study of the electron transfer reactions in Hansenula anomala flavocytochrome b2. Eur J Biochem. 1984 Apr 2;140(1):39–45. doi: 10.1111/j.1432-1033.1984.tb08064.x. [DOI] [PubMed] [Google Scholar]
- Xia Z. X., Shamala N., Bethge P. H., Lim L. W., Bellamy H. D., Xuong N. H., Lederer F., Mathews F. S. Three-dimensional structure of flavocytochrome b2 from baker's yeast at 3.0-A resolution. Proc Natl Acad Sci U S A. 1987 May;84(9):2629–2633. doi: 10.1073/pnas.84.9.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]


