Abstract
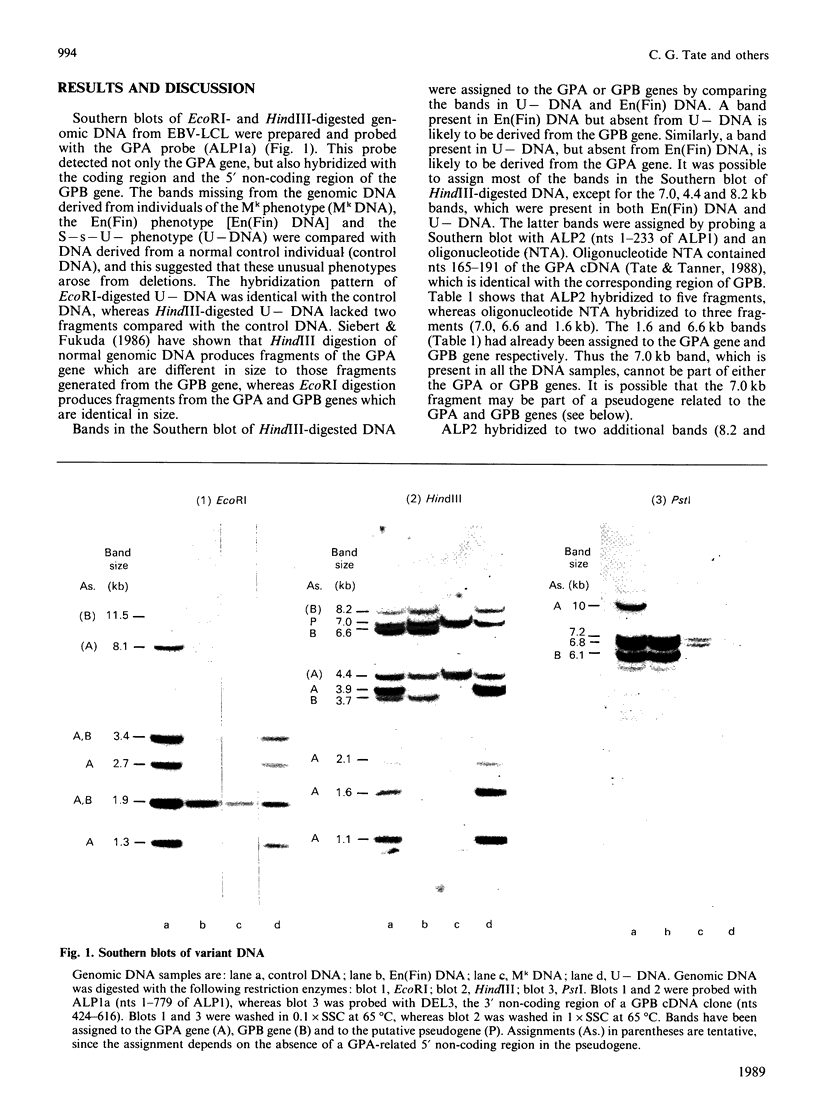
1. Genomic DNA derived from individuals who lack glycophorin A (GPA), glycophorin B (GPB) or both of these proteins was subjected to Southern-blot analysis using GPA and GPB cDNA probes. 2. Bands on the Southern blots were assigned to the GPA gene, GPB gene or to a putative pseudogene. 3. Genomic DNA derived from an individual of the Mk phenotype was shown to have deletions in the GPA and GPB genes. The simplest model for the results obtained is that a single deletion spans the GPA and GPB genes in the individual studied.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anstee D. J., Tanner M. J. Structure and function of the red cell membrane sialoglycoproteins. Br J Haematol. 1986 Oct;64(2):211–215. doi: 10.1111/j.1365-2141.1986.tb04113.x. [DOI] [PubMed] [Google Scholar]
- Anstee D. J. The blood group MNSs-active sialoglycoproteins. Semin Hematol. 1981 Jan;18(1):13–31. [PubMed] [Google Scholar]
- Huang C. H., Johe K., Moulds J. J., Siebert P. D., Fukuda M., Blumenfeld O. O. Delta glycophorin (glycophorin B) gene deletion in two individuals homozygous for the S--s--U-- blood group phenotype. Blood. 1987 Dec;70(6):1830–1835. [PubMed] [Google Scholar]
- Murray J. C., Buetow K. H., Ferrell R. E., Sieberg P. D., Fukuda M. An RFLP for glycoprotein A (MN) is in linkage disequilibrium with MN and Ss. Cytogenet Cell Genet. 1988;47(3):149–151. doi: 10.1159/000132534. [DOI] [PubMed] [Google Scholar]
- Okubo Y., Daniels G. L., Parsons S. F., Anstee D. J., Yamaguchi H., Tomita T., Seno T. A Japanese family with two sisters apparently homozygous for Mk. Vox Sang. 1988;54(2):107–111. doi: 10.1111/j.1423-0410.1988.tb01626.x. [DOI] [PubMed] [Google Scholar]
- Rahuel C., London J., Vignal A., Cherif-Zahar B., Colin Y., Siebert P., Fukuda M., Cartron J. P. Alteration of the genes for glycophorin A and B in glycophorin-A-deficient individuals. Eur J Biochem. 1988 Nov 15;177(3):605–614. doi: 10.1111/j.1432-1033.1988.tb14413.x. [DOI] [PubMed] [Google Scholar]
- Rahuel C., London J., d'Auriol L., Mattei M. G., Tournamille C., Skrzynia C., Lebouc Y., Galibert F., Cartron J. P. Characterization of cDNA clones for human glycophorin A. Use for gene localization and for analysis of normal of glycophorin-A-deficient (Finnish type) genomic DNA. Eur J Biochem. 1988 Feb 15;172(1):147–153. doi: 10.1111/j.1432-1033.1988.tb13866.x. [DOI] [PubMed] [Google Scholar]
- Siebert P. D., Fukuda M. Human glycophorin A and B are encoded by separate, single copy genes coordinately regulated by a tumor-promoting phorbol ester. J Biol Chem. 1986 Sep 25;261(27):12433–12436. [PubMed] [Google Scholar]
- Siebert P. D., Fukuda M. Molecular cloning of a human glycophorin B cDNA: nucleotide sequence and genomic relationship to glycophorin A. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6735–6739. doi: 10.1073/pnas.84.19.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M. J., High S., Martin P. G., Anstee D. J., Judson P. A., Jones T. J. Genetic variants of human red-cell membrane sialoglycoprotein beta. Study of the alterations occurring in the sialoglycoprotein-beta gene. Biochem J. 1988 Mar 1;250(2):407–414. doi: 10.1042/bj2500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate C. G., Tanner M. J. Isolation of cDNA clones for human erythrocyte membrane sialoglycoproteins alpha and delta. Biochem J. 1988 Sep 15;254(3):743–750. doi: 10.1042/bj2540743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. S., Bergren M. O., Busch M. P., Carmody A. M., Perkins H. A. Finnish En(a-) propositus with anti-EnaFS and anti-EnaFR: in vitro and in vivo characteristics. Vox Sang. 1987;52(1-2):103–106. doi: 10.1111/j.1423-0410.1987.tb03001.x. [DOI] [PubMed] [Google Scholar]