Abstract
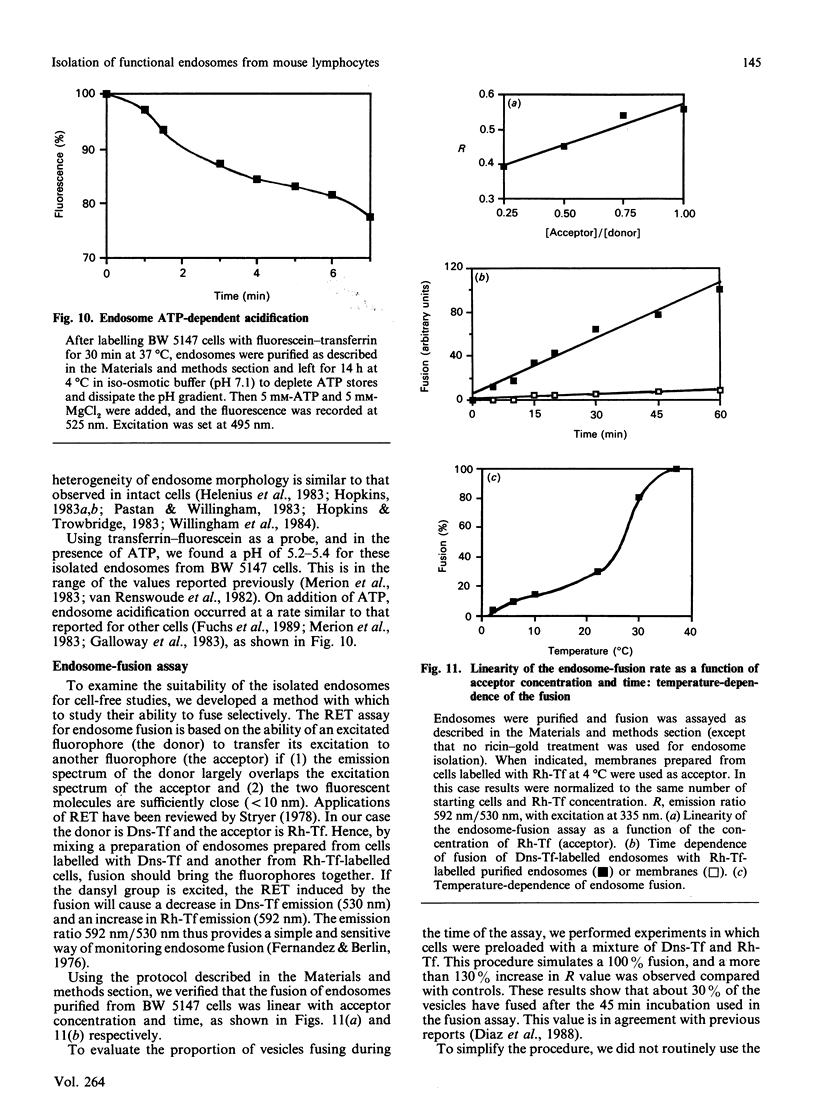
A discontinuous-sucrose-gradient procedure for isolating endosomes from mouse lymphoma cells has been developed. After centrifugation, most organelles (especially mitochondria and lysosomes) are recovered in the denser fractions of the gradient, whereas a mixture of plasma membrane and endosomes is present at lighter densities. The endosome recovery in this fraction can be increased (by 100%) by (a) a mild trypsin treatment of the postnuclear supernatant and (b) loading the cell endosomes with a saturating concentration of low-density lipoproteins. Removal of the plasma-membrane contamination was achieved by preincubating the cells with a gold-ricin complex at 4 degrees C. On centrifugation, the gold-loaded membranes sediment to the bottom of the gradient. The endosome preparation isolated by these procedures is less than 6% contaminated by other organelles and contains 42% of internalized 125I-transferrin. We show that these isolated endosomes are functional, as displayed by their ability to fuse and to acidify in a cell-free system. Endosome fusion was studied by a new assay based on the use of fluorescence resonance energy transfer. This fusion is dependent on ATP and on a cytosolic, thermoresistant but trypsin- and N-ethylmaleimide-sensitive, protein factor. Early endosomes fuse more actively among themselves than with late-endocytic vesicles, and they fuse only slowly with plasma-membrane vesicles.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajioka R. S., Kaplan J. Intracellular pools of transferrin receptors result from constitutive internalization of unoccupied receptors. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6445–6449. doi: 10.1073/pnas.83.17.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan D., Crumpton M. J. Preparation and characterization of the plasma membrane of pig lymphocytes. Biochem J. 1970 Nov;120(1):133–143. doi: 10.1042/bj1200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardmore J., Howell K. E., Miller K., Hopkins C. R. Isolation of an endocytic compartment from A431 cells using a density modification procedure employing a receptor-specific monoclonal antibody complexed with colloidal gold. J Cell Sci. 1987 May;87(Pt 4):495–506. doi: 10.1242/jcs.87.4.495. [DOI] [PubMed] [Google Scholar]
- Belcher J. D., Hamilton R. L., Brady S. E., Hornick C. A., Jaeckle S., Schneider W. J., Havel R. J. Isolation and characterization of three endosomal fractions from the liver of estradiol-treated rats. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6785–6789. doi: 10.1073/pnas.84.19.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Suchard S. J., Nagpal M. L., Glenney J. R., Jr A T-lymphoma transmembrane glycoprotein (gp180) is linked to the cytoskeletal protein, fodrin. J Cell Biol. 1985 Aug;101(2):477–487. doi: 10.1083/jcb.101.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braell W. A. Fusion between endocytic vesicles in a cell-free system. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1137–1141. doi: 10.1073/pnas.84.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch W. J., Mullock B. M., Luzio J. P. Rapid subcellular fractionation of the rat liver endocytic compartments involved in transcytosis of polymeric immunoglobulin A and endocytosis of asialofetuin. Biochem J. 1987 Jun 1;244(2):311–315. doi: 10.1042/bj2440311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S., Thomson J. N., Pearse B. M. Coated pits act as molecular filters. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4156–4159. doi: 10.1073/pnas.77.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M. A., Bale W. F., Spar I. L. Iodine monochloride (IC1) iodination techniques. Methods Enzymol. 1983;92:277–292. [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J., Hurtley S. M., Warren G. Reconstitution of an endocytic fusion event in a cell-free system. Cell. 1985 Dec;43(3 Pt 2):643–652. doi: 10.1016/0092-8674(85)90236-3. [DOI] [PubMed] [Google Scholar]
- DeBanne M. T., Bolyos M., Gauldie J., Regoeczi E. Two populations of prelysosomal structures transporting asialoglycoproteins in rat liver. Proc Natl Acad Sci U S A. 1984 May;81(10):2995–2999. doi: 10.1073/pnas.81.10.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Mayorga L., Stahl P. In vitro fusion of endosomes following receptor-mediated endocytosis. J Biol Chem. 1988 May 5;263(13):6093–6100. [PubMed] [Google Scholar]
- Dickson R. B., Beguinot L., Hanover J. A., Richert N. D., Willingham M. C., Pastan I. Isolation and characterization of a highly enriched preparation of receptosomes (endosomes) from a human cell line. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5335–5339. doi: 10.1073/pnas.80.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diment S., Leech M. S., Stahl P. D. Cathepsin D is membrane-associated in macrophage endosomes. J Biol Chem. 1988 May 15;263(14):6901–6907. [PubMed] [Google Scholar]
- Enrich C., Bachs O., Evans W. H. A 115 kDa calmodulin-binding protein is located in rat liver endosome fractions. Biochem J. 1988 Nov 1;255(3):999–1005. doi: 10.1042/bj2550999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Hardison W. G. Phospholipid, cholesterol, polypeptide and glycoprotein composition of hepatic endosome subfractions. Biochem J. 1985 Nov 15;232(1):33–36. doi: 10.1042/bj2320033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber E., Resch K., Wallach D. F., Imm W. Isolation and characterization of lymphocyte plasma membranes. Biochim Biophys Acta. 1972 May 9;266(2):494–504. doi: 10.1016/0005-2736(72)90105-8. [DOI] [PubMed] [Google Scholar]
- Fernandez S. M., Berlin R. D. Cell surface distribution of lectin receptors determined by resonance energy transfer. Nature. 1976 Dec 2;264(5585):411–415. doi: 10.1038/264411a0. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Schmid S., Mellman I. A possible role for Na+,K+-ATPase in regulating ATP-dependent endosome acidification. Proc Natl Acad Sci U S A. 1989 Jan;86(2):539–543. doi: 10.1073/pnas.86.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway C. J., Dean G. E., Marsh M., Rudnick G., Mellman I. Acidification of macrophage and fibroblast endocytic vesicles in vitro. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3334–3338. doi: 10.1073/pnas.80.11.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. E., Kornfeld S. Evidence for extensive subcellular organization of asparagine-linked oligosaccharide processing and lysosomal enzyme phosphorylation. J Biol Chem. 1983 Mar 10;258(5):3159–3165. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Gruenberg J. E., Howell K. E. Reconstitution of vesicle fusions occurring in endocytosis with a cell-free system. EMBO J. 1986 Dec 1;5(12):3091–3101. doi: 10.1002/j.1460-2075.1986.tb04615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J., Griffiths G., Howell K. E. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol. 1989 Apr;108(4):1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D. K., Tartakoff A. M. A novel lectin-gold density perturbation eliminates plasma membrane contaminants from Golgi-enriched subcellular fractions. Eur J Cell Biol. 1989 Feb;48(1):64–70. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms E., Kartenbeck J., Darai G., Schneider J. Purification and characterization of human lysosomes from EB-virus transformed lymphoblasts. Exp Cell Res. 1981 Feb;131(2):251–266. doi: 10.1016/0014-4827(81)90230-5. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hoessli D. C., Rungger-Brändle E. Isolation of plasma membrane domains from murine T lymphocytes. Proc Natl Acad Sci U S A. 1983 Jan;80(2):439–443. doi: 10.1073/pnas.80.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
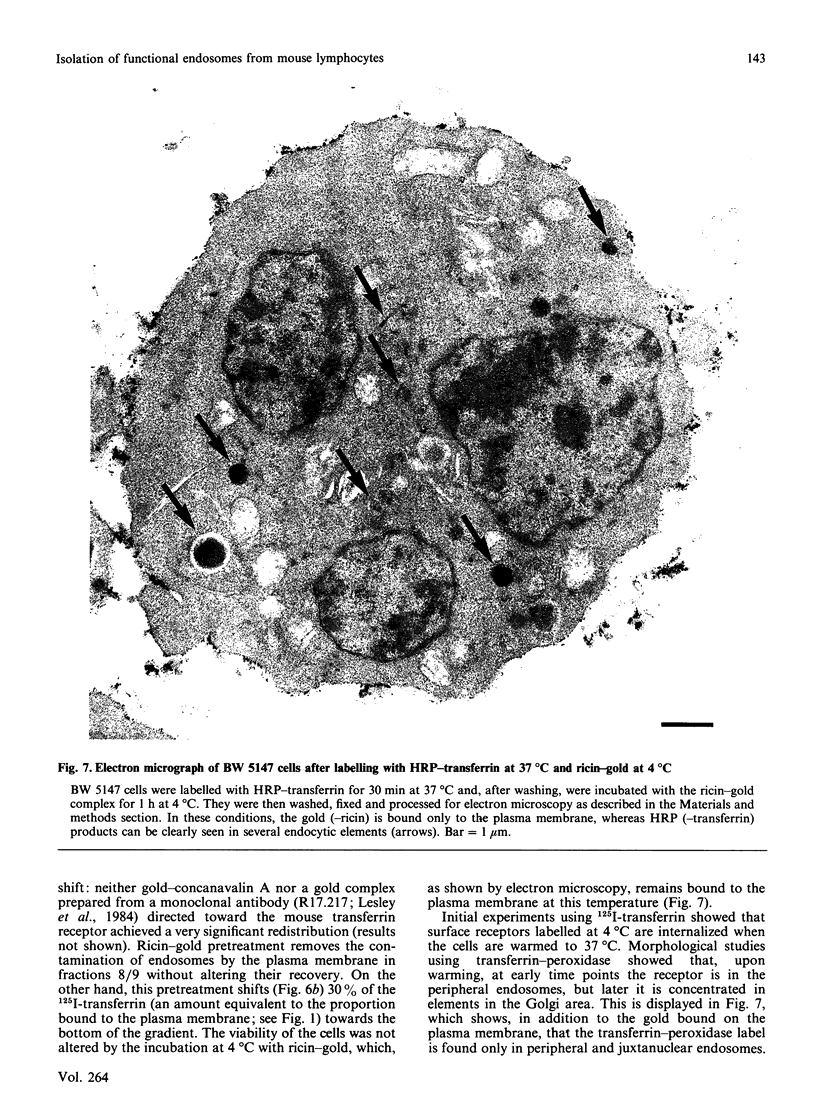
- Hopkins C. R. Intracellular routing of transferrin and transferrin receptors in epidermoid carcinoma A431 cells. Cell. 1983 Nov;35(1):321–330. doi: 10.1016/0092-8674(83)90235-0. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R. The importance of the endosome in intracellular traffic. Nature. 1983 Aug 25;304(5928):684–685. doi: 10.1038/304684a0. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Trowbridge I. S. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983 Aug;97(2):508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
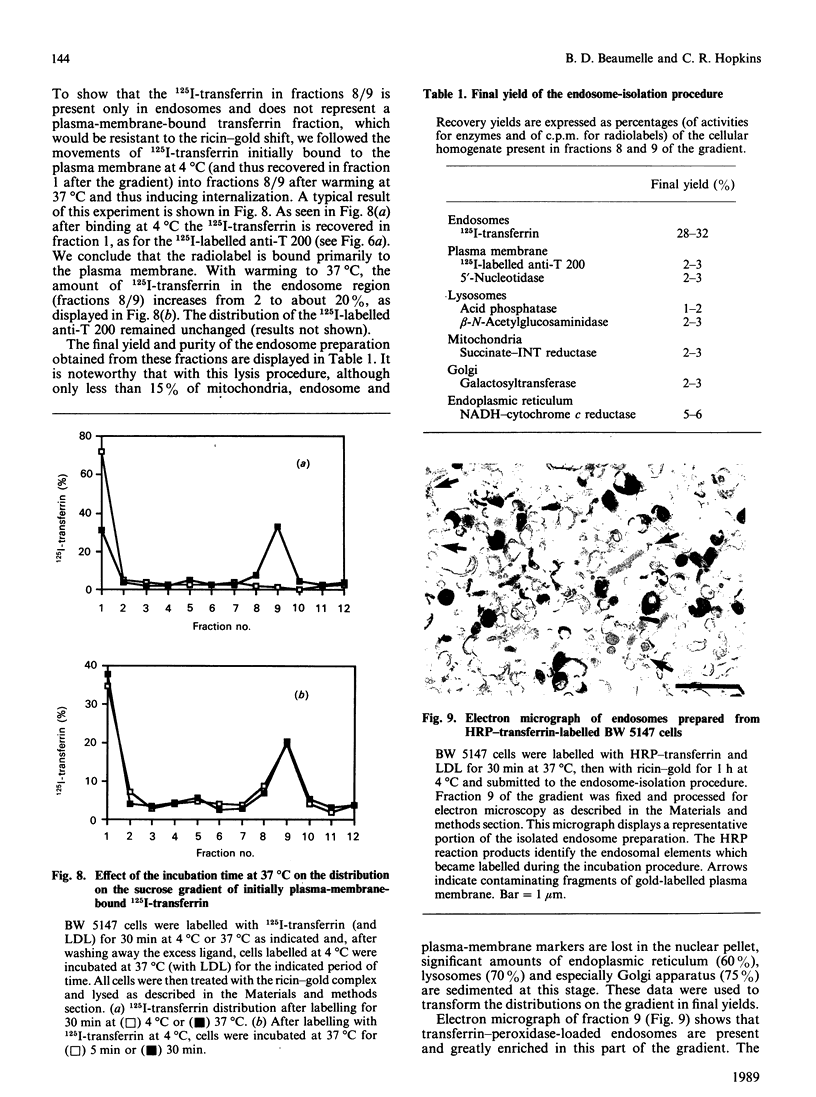
- Howell K. E., Devaney E., Gruenberg J. Subcellular fractionation of tissue culture cells. Trends Biochem Sci. 1989 Feb;14(2):44–47. doi: 10.1016/0968-0004(89)90040-6. [DOI] [PubMed] [Google Scholar]
- Hyman R., Stallings V. Complementation patterns of Thy-1 variants and evidence that antigen loss variants "pre-exist" in the parental population. J Natl Cancer Inst. 1974 Feb;52(2):429–436. doi: 10.1093/jnci/52.2.429. [DOI] [PubMed] [Google Scholar]
- Jacobsen C., Jacobsen J. Dansylation of human serum albumin in the study of the primary binding sites of bilirubin and L-tryptophan. Biochem J. 1979 Jul 1;181(1):251–253. doi: 10.1042/bj1810251. [DOI] [PMC free article] [PubMed] [Google Scholar]
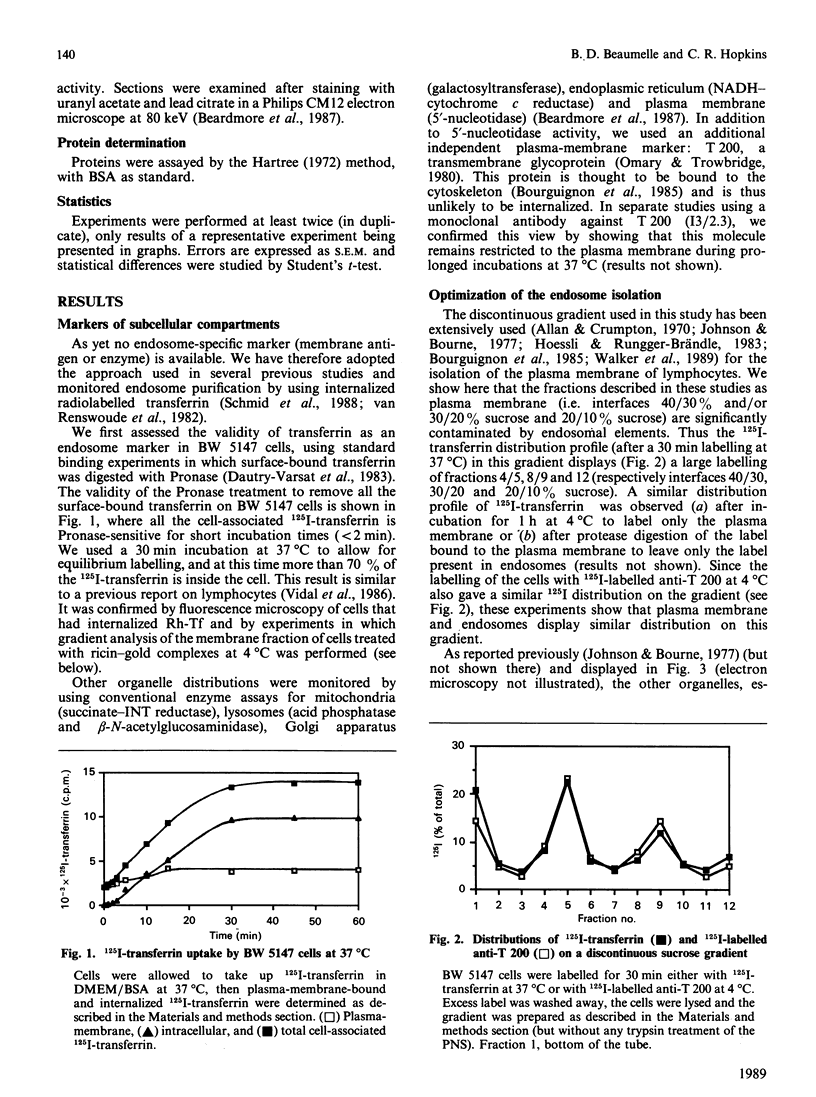
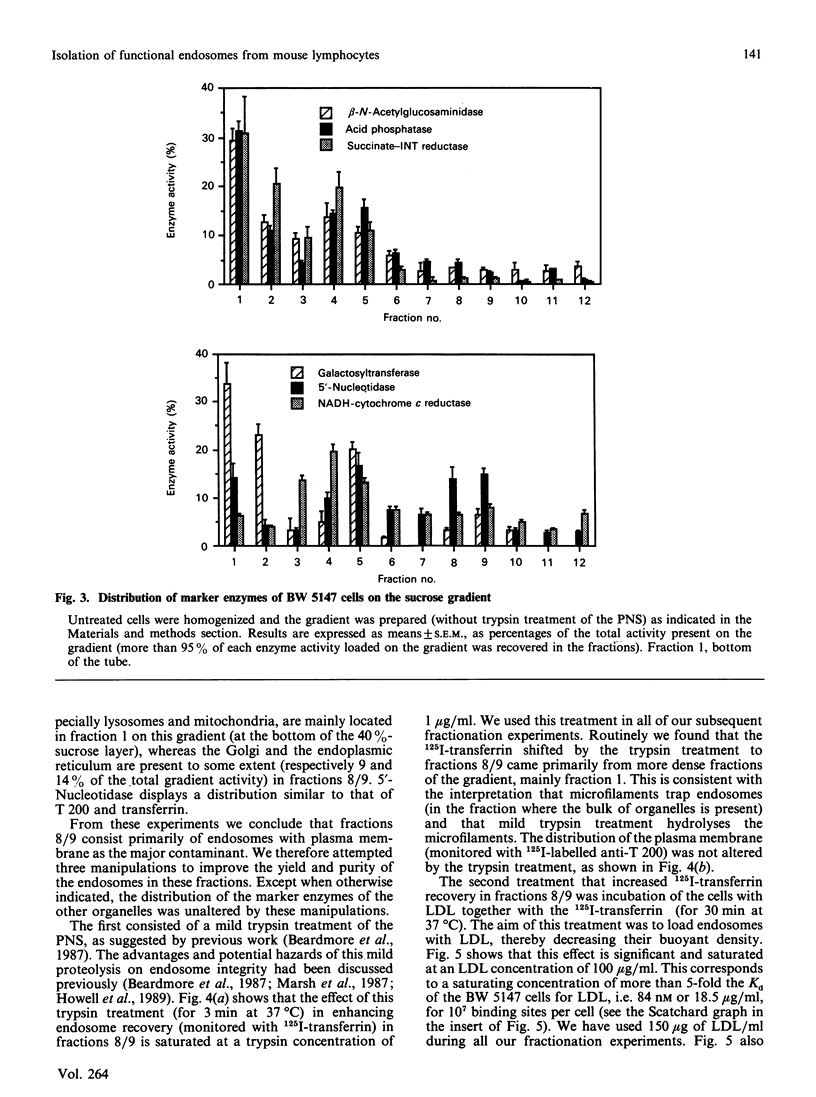
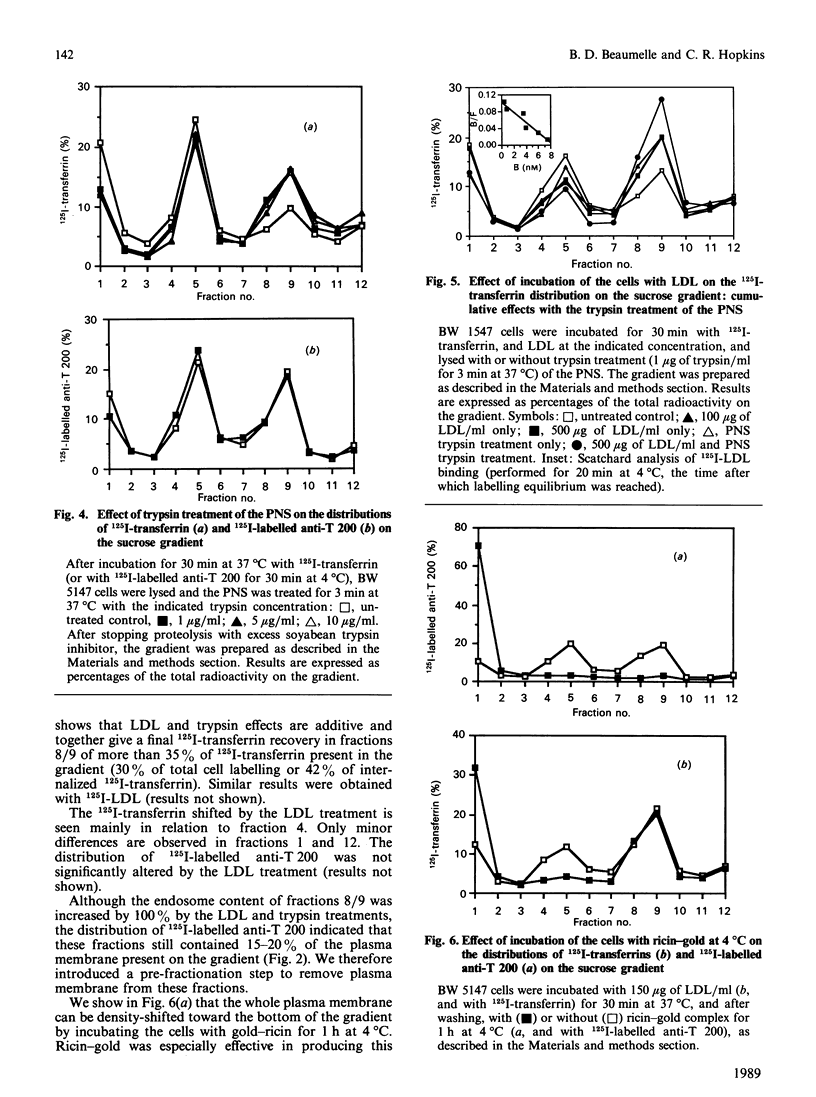
- Johnson G. L., Bourne H. R. Influence of cholera toxin on the regulation of adenylate cyclase by GTP. Biochem Biophys Res Commun. 1977 Sep 23;78(2):792–798. doi: 10.1016/0006-291x(77)90249-2. [DOI] [PubMed] [Google Scholar]
- Khan M. N., Savoie S., Bergeron J. J., Posner B. I. Characterization of rat liver endosomal fractions. In vivo activation of insulin-stimulable receptor kinase in these structures. J Biol Chem. 1986 Jun 25;261(18):8462–8472. [PubMed] [Google Scholar]
- Lesley J., Domingo D. L., Schulte R., Trowbridge I. S. Effect of an anti-murine transferrin receptor-ricin A conjugate on bone marrow stem and progenitor cells treated in vitro. Exp Cell Res. 1984 Feb;150(2):400–407. doi: 10.1016/0014-4827(84)90583-4. [DOI] [PubMed] [Google Scholar]
- Marsh M., Schmid S., Kern H., Harms E., Male P., Mellman I., Helenius A. Rapid analytical and preparative isolation of functional endosomes by free flow electrophoresis. J Cell Biol. 1987 Apr;104(4):875–886. doi: 10.1083/jcb.104.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga L. S., Diaz R., Stahl P. D. Plasma membrane-derived vesicles containing receptor-ligand complexes are fusogenic with early endosomes in a cell-free system. J Biol Chem. 1988 Nov 25;263(33):17213–17216. [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Merion M., Schlesinger P., Brooks R. M., Moehring J. M., Moehring T. J., Sly W. S. Defective acidification of endosomes in Chinese hamster ovary cell mutants "cross-resistant" to toxins and viruses. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5315–5319. doi: 10.1073/pnas.80.17.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S. C., Hubbard A. L. Receptor-mediated endocytosis of asialoglycoproteins by rat hepatocytes: receptor-positive and receptor-negative endosomes. J Cell Biol. 1986 Mar;102(3):932–942. doi: 10.1083/jcb.102.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullock B. M., Luzio J. P., Hinton R. H. Preparation of a low-density species of endocytic vesicle containing immunoglobulin A. Biochem J. 1983 Sep 15;214(3):823–827. doi: 10.1042/bj2140823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsnes S., Moskaug J. O., Stenmark H., Sandvig K. Diphtheria toxin entry: protein translocation in the reverse direction. Trends Biochem Sci. 1988 Sep;13(9):348–351. doi: 10.1016/0968-0004(88)90105-3. [DOI] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Disposition of T200 glycoprotein in the plasma membrane of a murine lymphoma cell line. J Biol Chem. 1980 Feb 25;255(4):1662–1669. [PubMed] [Google Scholar]
- Pastan I., Willingham M. C., FitzGerald D. J. Immunotoxins. Cell. 1986 Dec 5;47(5):641–648. doi: 10.1016/0092-8674(86)90506-4. [DOI] [PubMed] [Google Scholar]
- Quintart J., Courtoy P. J., Baudhuin P. Receptor-mediated endocytosis in rat liver: purification and enzymic characterization of low density organelles involved in uptake of galactose-exposing proteins. J Cell Biol. 1984 Mar;98(3):877–884. doi: 10.1083/jcb.98.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- Schmid S. L., Fuchs R., Male P., Mellman I. Two distinct subpopulations of endosomes involved in membrane recycling and transport to lysosomes. Cell. 1988 Jan 15;52(1):73–83. doi: 10.1016/0092-8674(88)90532-6. [DOI] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S. Interspecies spleen-myeloma hybrid producing monoclonal antibodies against mouse lymphocyte surface glycoprotein, T200. J Exp Med. 1978 Jul 1;148(1):313–323. doi: 10.1084/jem.148.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Myers D. E., Ledbetter J. A., Swaim S. E., Gajl-Peczalska K. J., Vallera D. A. Use of colony assays and anti-T cell immunotoxins to elucidate the immunobiologic features of leukemic progenitor cells in T-lineage acute lymphoblastic leukemia. J Immunol. 1988 Mar 15;140(6):2103–2111. [PubMed] [Google Scholar]
- Vidal M., Sainte-Marie J., Philippot J. R., Bienvenue A. Comparison of the internalization efficiency of LDL and transferrin receptors on L2C guinea pig lymphocytes. FEBS Lett. 1986 Feb 17;196(2):242–246. doi: 10.1016/0014-5793(86)80255-1. [DOI] [PubMed] [Google Scholar]
- Walker G., Kerrick W. G., Bourguignon L. Y. The role of caldesmon in the regulation of receptor capping in mouse T-lymphoma cell. J Biol Chem. 1989 Jan 5;264(1):496–500. [PubMed] [Google Scholar]
- Wall D. A., Hubbard A. L. Receptor-mediated endocytosis of asialoglycoproteins by rat liver hepatocytes: biochemical characterization of the endosomal compartments. J Cell Biol. 1985 Dec;101(6):2104–2112. doi: 10.1083/jcb.101.6.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G., Woodman P., Pypaert M., Smythe E. Cell-free assays and the mechanism of receptor-mediated endocytosis. Trends Biochem Sci. 1988 Dec;13(12):462–465. doi: 10.1016/0968-0004(88)90229-0. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Hanover J. A., Dickson R. B., Pastan I. Morphologic characterization of the pathway of transferrin endocytosis and recycling in human KB cells. Proc Natl Acad Sci U S A. 1984 Jan;81(1):175–179. doi: 10.1073/pnas.81.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman P. G., Warren G. Fusion between vesicles from the pathway of receptor-mediated endocytosis in a cell-free system. Eur J Biochem. 1988 Apr 5;173(1):101–108. doi: 10.1111/j.1432-1033.1988.tb13972.x. [DOI] [PubMed] [Google Scholar]
- Youle R. J., Uckun F. M., Vallera D. A., Colombatti M. Immunotoxins show rapid entry of diphtheria toxin but not ricin via the T3 antigen. J Immunol. 1986 Jan;136(1):93–98. [PubMed] [Google Scholar]
- van Deurs B., Pedersen L. R., Sundan A., Olsnes S., Sandvig K. Receptor-mediated endocytosis of a ricin-colloidal gold conjugate in vero cells. Intracellular routing to vacuolar and tubulo-vesicular portions of the endosomal system. Exp Cell Res. 1985 Aug;159(2):287–304. doi: 10.1016/s0014-4827(85)80003-3. [DOI] [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]