Abstract
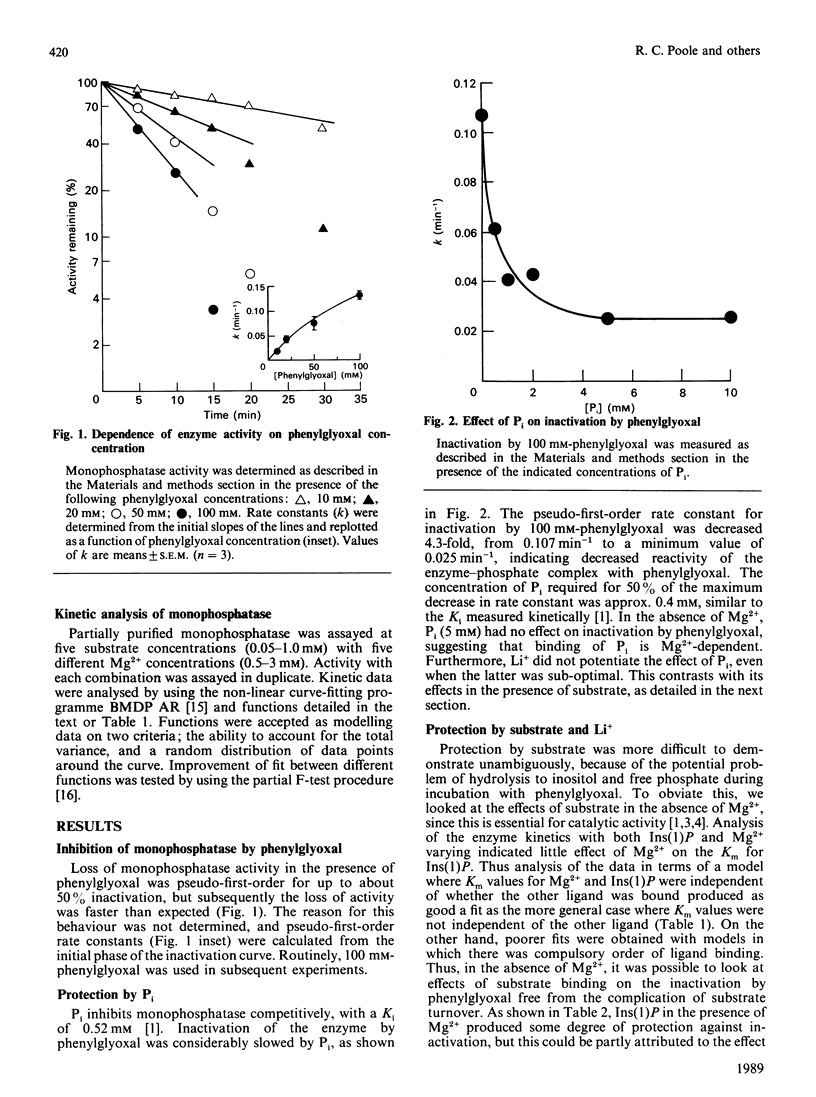
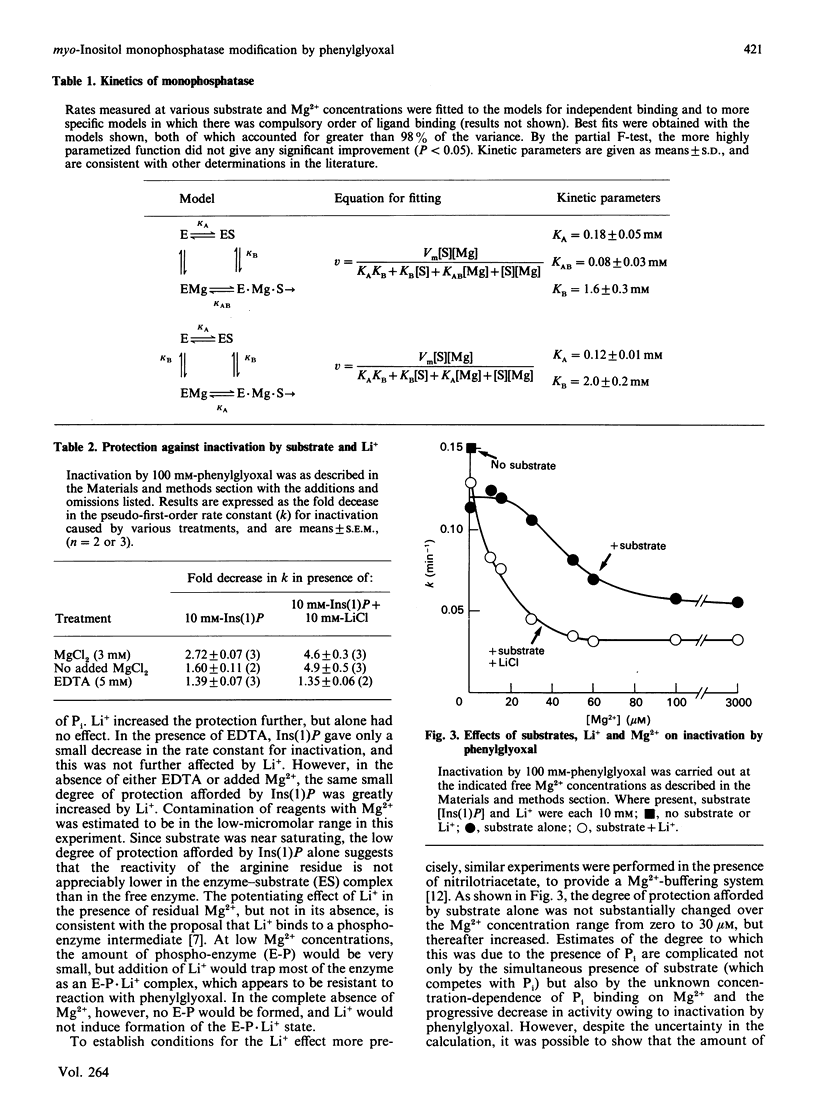
myo-Inositol monophosphatase is inhibited by the arginine-specific reagent phenylglyoxal. The rate of inactivation is decreased in the presence of Pi, a competitive inhibitor of the enzyme. The effect of Pi is dependent on the presence of Mg2+, but is unaffected by Li+, an uncompetitive inhibitor. In the absence of Mg2+, the substrate, Ins(1)P, binds to the enzyme but is not converted into products, and affords only a small degree of protection against inactivation by phenylglyoxal. Li+ had no further effect under these conditions, but in the presence of Mg2+ caused a marked potentiation of the protective effect of substrate alone. In the absence of substrate, Li+ had no effect on activation by phenylglyoxal. Incorporation of 14C-labelled phenylglyoxal showed that inactivation was associated with modification of a single arginine residue per monomer in the dimeric enzyme. These findings support a mechanism in which Li+ inhibits monophosphatase by trapping a phosphorylated enzyme intermediate and preventing its hydrolysis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee N. S., Ragan C. I., Watling K. J., Aspley S., Jackson R. G., Reid G. G., Gani D., Shute J. K. The purification and properties of myo-inositol monophosphatase from bovine brain. Biochem J. 1988 Feb 1;249(3):883–889. doi: 10.1042/bj2490883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Knowles J. R. Enzyme-catalyzed phosphoryl transfer reactions. Annu Rev Biochem. 1980;49:877–919. doi: 10.1146/annurev.bi.49.070180.004305. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Bansal V. S., Inhorn R. C., Ross T. S., Lips D. L. Inositol phosphates: synthesis and degradation. J Biol Chem. 1988 Mar 5;263(7):3051–3054. [PubMed] [Google Scholar]
- Ragan C. I., Watling K. J., Gee N. S., Aspley S., Jackson R. G., Reid G. G., Baker R., Billington D. C., Barnaby R. J., Leeson P. D. The dephosphorylation of inositol 1,4-bisphosphate to inositol in liver and brain involves two distinct Li+-sensitive enzymes and proceeds via inositol 4-phosphate. Biochem J. 1988 Jan 1;249(1):143–148. doi: 10.1042/bj2490143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto K., Okada M., Matsuda Y., Nakagawa H. Purification and properties of myo-inositol-1-phosphatase from rat brain. J Biochem. 1985 Aug;98(2):363–370. doi: 10.1093/oxfordjournals.jbchem.a135290. [DOI] [PubMed] [Google Scholar]


