Abstract
Genetically heterogeneous UM-HET3 mice born in 2020 were used to test possible lifespan effects of alpha-ketoglutarate (AKG), 2,4-dinitrophenol (DNP), hydralazine (HYD), nebivolol (NEBI), 16α-hydroxyestriol (OH_Est), and sodium thiosulfate (THIO), and to evaluate the effects of canagliflozin (Cana) when started at 16 months of age. OH_Est produced a 15% increase (p = 0.0001) in median lifespan in males but led to a significant (7%) decline in female lifespan. Cana, started at 16 months, also led to a significant increase (14%, p = 0.004) in males and a significant decline (6%, p = 0.03) in females. Cana given to mice at 6 months led, as in our previous study, to an increase in male lifespan without any change in female lifespan, suggesting that this agent may lead to female-specific late-life harm. We found that blood levels of Cana were approximately 20-fold higher in aged females than in young males, suggesting a possible mechanism for the sex-specific disparities in its effects. NEBI was also found to produce a female-specific decline (4%, p = 0.03) in lifespan. None of the other tested drugs provided a lifespan benefit in either sex. These data bring to 7 the list of ITP-tested drugs that induce at least a 10% lifespan increase in one or both sexes, add a fourth drug with demonstrated mid-life benefits on lifespan, and provide a testable hypothesis that might explain the sexual dimorphism in lifespan effects of the SGLT2 inhibitor Cana.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-024-01176-2.
Keywords: Alpha-ketoglutarate, SGLT2 inhibitor Canagliflozin, Lifespan
Introduction
Each year the Interventions Testing Program (ITP) tests candidate geroprotector drugs, suggested by members of the scientific community, to see if drugs can extend the lifespan of genetically heterogeneous male or female mice [1]. In the 19 years from 2004 to 2023, the ITP initiated 107 full lifespan studies, of which 67 involved a single agent given in food at a single concentration, 36 involved drugs at varying concentrations and starting at different ages, and 4 involved pairs of drugs used together. Of these 107 lifespan studies, 84 had been completed (i.e., all mice had died) as of December 2023, and 77 had been published in peer-reviewed journals. The most recent publication reported on studies initiated in 2018 and 2019 showed a significant extension of male lifespan by meclizine and astaxanthin and an absence of benefit from fisetin, SG1002 (a hydrogen sulfide donor), dimethyl fumarate, mycophenolic acid, and 4‑phenylbutyrate [2].
The current paper now reports lifespan results for mice born in 2020 (“C2020 mice”). One of the newly tested agents, 16-hydroxyestriol (OH_Est), was found to produce significant lifespan extension in male, but not female, mice, with a 15% increase in median lifespan. A second agent, sodium thiosulfate (THIO), a sulfur donor, was found to increase male lifespan by 5%, which just failed to meet the traditional significance criterion at p = 0.06 by the log-rank test. THIO did not affect the lifespan of female mice. In addition, the SGLT2 inhibitor Canagliflozin (Cana), previously shown to extend male lifespan [3] and diminish multiple forms of mid-life pathology [4] if initiated at age 7 months, produced a substantial (14%) increase in male lifespan if started at 16 months. Four other tested drugs, i.e., alpha-ketoglutarate (AKG, started at 18 months), 2,4-dinitrophenol (DNP), hydralazine (HYD, started at 6 or 16 months), and nebivolol (NEBI), were found not to extend lifespan in either sex.
OH_Est was included in the C2020 test group to pursue a hypothesis about the sex-specific lifespan benefit induced by 17-α-estradiol (17aE2), which increases median survival by 20% in males only [5, 6], and leads to male-specific improvements in some tests of physical fitness [7]. A metabolomic screen of hepatic responses to 17aE2 [8] showed that estriol-3-sulfate and 16-oxoestradiol 3-sulfate were increased approximately 100-fold in the livers of 17aE2-treated male mice but were minimally changed in females. Estriol-3-sulfate and 16-oxoestradiol 3-sulfate are both products of the conversion of 17aE2 to estriol [9], of which estriol-3-sulfate is the main sulfated form [10]. This indicates that 17-α-estradiol undergoes sex-specific metabolism to other estrogens in mice and suggests that estriol might be involved in some of the male-specific anti-aging benefits of 17aE2 treatment. We, therefore, speculated that treating mice directly with estriol might produce a lifespan benefit similar to that seen in 17aE2-treated males, but do so in both sexes.
The strategy of repurposing FDA-approved drugs has significantly contributed to new therapy development. Hydralazine, first synthesized in the 1940s, was initially recognized as a potent vasodilator for treating hypertension. In the 1980s, hydralazine was repurposed for heart failure treatment, and, in the 2000s, found application in cancer epigenetic therapy [11]. Consideration of hydralazine as a possible anti-aging drug has been stimulated by data showing effects on molecular pathways associated with longevity. In C. elegans, hydralazine has been shown to extend lifespan by activating NRF2/SKN-1 signaling and by enhancing mitochondrial function via sirtuin activation [12, 13].
Leakage of protons (H+) across the mitochondria, bypassing ATP synthase, is thought to convert as much as 25% of caloric intake to heat and could contribute to slower aging by reducing free radical production [14]. 2, 4,-dinitrophenol (DNP) may mimic the potential benefit of proton leak at low doses [15]. DNP was used early in the twentieth century as a treatment for obesity but was found to produce toxic effects, sometimes fatal, at the high doses employed [16]. More recently, low doses of DNP were found to produce a small but significant increase in mean lifespan when administered, in drinking water, to female outbred Swiss-Webster mice [17]. The dose used was estimated to provide between 30 and 105 µg/kg body weight per day, equivalent to approximately 0.18 to 0.63 ppm if given in food. The authors of this earlier study attributed the beneficial effects of DNP to enhanced cellular respiration and noted potentially beneficial changes in serum glucose and triglycerides, along with lower body weight in their treated mice. Mitochondrial uncoupling, indicated by elevation of the mitochondrial uncoupling protein UCP1, has been demonstrated in many varieties of slow-aging mice, including both genetic mutants and those treated with anti-aging drugs [18–20], and transgenic augmentation of UCP1 in skeletal muscle can lead to increased mouse lifespan [21], suggesting potential benefits from oral administration of uncoupling agents like DNP.
Rapamycin has the strongest effects on lifespan of all ITP-tested drugs and extends a healthy lifespan in both sexes of mice but may have side effects that complicate long-term use in healthy people. This creates a good rationale for testing other inhibitors of mTORC1 that may differ in their mechanism of action, target specificity, and side effect profile. Meclizine, for example, emerged from a screen of 1600 clinically used drugs for inhibition of mTORC1 activity [22] and subsequently proved to be able to extend the lifespan of male UM-HET3 mice to a significant extent [2]. NEBI emerged from a parallel screen and has been found to bind to mTOR at a site different from that modulated by rapamycin, inhibiting mTORC1 by displacement of the raptor component of the mTORC1 complex (Cortopassi et al., unpublished). NEBI seems to inhibit mTORC1, in arterial tissue, without inhibition of mTORC2 [23]. NEBI has an established profile of safety in clinical use as an antihypertensive agent in elderly humans and was included in the C2020 protocol as a candidate anti-mTOR geroprotective agent.
The Krebs cycle metabolite α-ketoglutarate (AKG) has been reported to extend lifespan when administered to C. elegans [24]. The authors of this C. elegans study suggested that AKG acted via increased production of NAD, and thence through sirtuin-mediated enhancement of peroxisome biogenesis and function, including increased beta-oxidation of fatty acids. Analyses of tissues from multiple models of slowed aging have implicated shifts in fatty-acid beta-oxidation and peroxisomal pathways as a potential shared mechanism for lifespan benefits in both mutant and drug-treated mice [25, 26], and both Snell dwarf and GHR-knockout mice have elevations of both mRNA and protein for many enzymes involved in mitochondrial and peroxisomal beta-oxidation [27]. In a separate study [28], AKG was administered in food to C57BL/6 mice at a dose of 2% w/v (20,000 ppm) from the age of 540 days (18 months) and led to small increases in median lifespan that reached statistical significance in female but not in male mice. In two independent female cohorts, the median lifespan, calculated from birth, was increased by 6.3% or by 3.3% (p < 0.05 in each case), and in males, the median lifespan was increased by 4.2% or by 3.7% in two distinct cohorts (not significant). These promising results with inbred mice prompted the ITP to evaluate AKG using UM-HET3 mice, in the C2020 protocol, starting at 18 months of age, and to study AKG from a younger age in the C2021 mice (to be reported separately).
Work from the group of the late James Mitchell implicated the internal production of hydrogen sulfide, H2S, as a potential mediator of lifespan extension and stress resistance in several mouse models of calorie and amino acid restriction [29, 30]. H2S can also extend the lifespan of C. elegans [31]. The mechanism of action is unclear but might involve post-translational modification of protein cysteine residues, i.e., persulfidation, also called sulfhydration. In animal models, exogenous H2S has shown some protective effects against myocardial injury and impairment of glucose homeostasis [32, 33]. Sodium thiosulfate (THIO) was proposed as a water-soluble, inexpensive, orally available source of elemental sulfur that had been shown to increase the persulfidation of aortic proteins when given intravenously to mice (Mitchell, unpublished). THIO is used clinically, in humans, to treat cyanide toxicity, to treat calcium stones in the urinary tract [34], and to reduce the toxicity of the chemotherapy drug cisplatin [35]. This established safety profile, both in rodents and in humans, suggested that THIO might lead to lifespan benefits, perhaps via supplementation of endogenous levels of H2S, if given to ITP mice.
The C2020 study also included an evaluation of the SGLT2 inhibitor, Cana, started at 16 months of age. Prior work had noted significant lifespan benefits when rapamycin, acarbose, or 17aE2 was initiated in mice 16 to 20 months of age [5, 36, 37]. Considered as a percentage extension of the median, treatment with rapamycin or 17aE2 was as effective started later in life as in groups given the drugs starting in young adulthood, while the effect of acarbose was diminished by approximately half. The practical and theoretical importance of evaluating agents for efficacy when started in middle age prompted us to include a late-start Cana group in the C2020 protocol.
This report presents the results of ITP lifespan experiments initiated on this set of agents using mice in C2020.
Methods
Mice and husbandry
These have been described in detail previously [3, 38] and will be summarized only briefly. The study used UM-HET3 mice, the progeny of CByB6F1 mothers (JAX stock 100009), and C3D2F1 fathers (JAX stock 100004). Mice from second and subsequent litters, produced over a 6-month interval at each site, were weaned into cages containing 3 males or 4 females. Approximately equal numbers of mice were weaned at each of the three test sites: the Jackson Laboratory (TJL), the University of Michigan (UM), and the University of Texas Health Science Center at San Antonio (UT). Table 1 shows the doses and starting ages of drugs tested using mice born in 2020, the “C2020 cohort.”
Table 1.
Doses, abbreviations, and start ages of agents evaluated in C2020 mice
| Abbreviation | Agent | Nominal dose (ppm) | Start age (months) | Note |
|---|---|---|---|---|
| AKG_18 | Alpha-ketoglutarate | 20,000 | 18 | |
| Cana_16 | Canagliflozin | 180 | 16 | UM, UT only |
| Cana_6 | Canagliflozin | 180 | 6 | TJL only |
| DNP | 2,4-Dinitrophenol | 3 | 6 | |
| HYD | Hydralazine | 30 | 6 | |
| HYD_16 | Hydralazine | 30 | 16 | |
| NEBI | Nebivolol | 60 | 6 | |
| OH_Est | 16-α-hydroxyestriol | 5 | 12 | |
| THIO | Sodium thiosulfate | 10,000 | 6 |
Each site used the same supplier for bedding, and food was prepared centrally and shipped simultaneously to all three sites, but each site used its own local water supply. Cages were inspected daily, and mice were weighed at 6, 12, 18, and 24 months of age. Mice found dead were noted, and mice judged to be near death, using a checklist, were euthanized for humane reasons and considered for statistical purposes as having died on the date of euthanasia. Mice that were removed from the study because of fighting or for reasons unrelated to aging and disease (examples: chip failure, water bottle leakage, escape) were considered alive at the time of removal and lost to follow-up thereafter. These “removed” mice are included in the Kaplan–Meier survival statistics but not in estimates of median or 90th percentile survival. “Removed” mice made up 4% of all males and 0.7% of females. Specific pathogen-free status was assessed quarterly at each site using a mixture of serologic and molecular methods—all such tests were negative at each site through the course of the study. All the experiments were approved by the respective animal care committees at each of the three sites.
Assessment of drug concentrations in food and tissue
These are given in the Supplementary Information.
Statistical analyses
These are unchanged from previous ITP papers. The primary hypothesis of drug effectiveness was tested by a log-rank test stratified by site and tested separately for each sex. P-values presented are nominal, i.e., they are not adjusted for multiple comparisons, and readers should evaluate the evidence for our conclusions with this in mind. An important secondary hypothesis for each drug is that the drug increases the proportion of mice still alive at the 90th percentile age of the joint survival distribution combining control mice and those treated with the tested drug. This is the Wang-Allison (“WA”) test [39], using the Fisher Exact test version, and is taken as a surrogate for drug effects on maximum lifespan or, more accurately, on survival to exceptionally old ages. Statistical tables show median and p90 (90th percentile age), as well as p-values for log-rank and WA tests. We also show survival curves for each drug at each site for each sex to provide insights into site-specific variation, although the low statistical power for each of these subsets of the C2020 cohort is too small to avoid Type II errors.
Analysis of drug effects on body weight was done by one-way ANOVA, separately for each sex at each age. When the ANOVA F-test statistic implied (at p < 0.05) that not all groups were the same in weight, a Sidak post-hoc test was used to evaluate all pair-wise tests among groups, and the graphic shows only differences between a drug and the sex-matched control group at the same age. Differences between males and females in levels of Canagliflozin in various tissues were tested using Student’s t-test.
Data sharing
All data in the paper will be made available, at the time of publication, within the Mouse Phenome Data Base, https://phenome.jax.org/projects/ITP1.
Results
Extended lifespan in male mice exposed to Cana from 16 months to OH_Est or to THIO
Table 1 lists the nine cohorts of mice that were evaluated, along with a double-size simultaneous control group (“Cont_20”), in mice born in 2020. The original plan had been to test mice receiving Cana from age 16 months, but because of a technical error, this group of mice was placed on Cana at 6 months at TJL. Thus, the Cana_6 group consists only of mice housed at TJL, and the Cana_16 group consists of mice housed at UM or UT. Hydralazine (HYD) was evaluated in two independent cohorts, one of which got HYD from 6 months, and the other from 16 months of age.
Table 2 shows survival statistics for mice in each group, including controls, separately for each sex and pooled across sites (except for Cana_6, from TJL only). The “Count” column shows the number of mice initially entered into each group. The “% Change in Median” column shows a change in median survival for the pooled mice; it is similar, but not identical, to the median survival averaged across sites (not shown). The log-rank p-value column represents the result of the log-rank test, stratified by site, so that expected and observed deaths are compared to those of the control group at the same site. The “p90” column is the age reached by the longest-lived 10% of the mice. The column for “WA p-value” shows the result of the Wang-Allison statistic [39], using the Fisher Exact test variation, and stratified by site, so that expected and observed numbers of mice reaching the 90th percentile of the joint survival table are calculated separately for each site and then combined for the p-value calculation. Mice that were removed from the survival table because of fighting or accidental death are included for the log-rank test but not for any of the other statistics tabulated.
Table 2.
Survival statistics for C2020 ITP mice
| Rx | Count | Live | Median | % Change in median | Log-rank p-value | Note | p90 | % change in p90 | WA p-value |
|---|---|---|---|---|---|---|---|---|---|
| Females | |||||||||
| AKG_Late | 132 | 0 | 914 | 0.9 | 0.59 | 1058 | − 4.8 | 0.16 | |
| Cana_16 | 92 | 0 | 851 | − 6.1 | 0.03 | A, B | 1020 | − 8.2 | 0.23 |
| Cana_6 | 44 | 0 | 894 | − 1.3 | 0.90 | C | 1155 | 4.0 | 0.79 |
| Cont_20 | 272 | 2 | 906 | 1111 | |||||
| DNP | 132 | 4 | 874 | − 3.5 | 0.89 | 1107 | − 0.4 | 0.99 | |
| HYD | 132 | 1 | 891 | − 1.7 | 0.19 | 1043 | − 6.1 | 0.16 | |
| HYD_16 | 132 | 1 | 889 | − 1.9 | 0.48 | 1117 | 0.5 | 0.60 | |
| NEBI | 132 | 1 | 868 | − 4.2 | 0.03 | A | 1059 | − 4.7 | 0.16 |
| OH_Est | 136 | 0 | 847 | − 6.5 | 0.002 | A | 1061 | − 4.5 | 0.16 |
| THIO | 132 | 1 | 898 | − 0.9 | 0.76 | 1111 | 0.0 | 0.99 | |
| Males | |||||||||
| AKG_Late | 153 | 3 | 851 | 3.0 | 0.58 | 1069 | − 3.3 | 0.50 | |
| Cana_16 | 102 | 4 | 940 | 13.8 | 0.004 | B | 1153 | 4.2 | 0.04 |
| Cana_6 | 60 | 1 | 899 | 8.8 | 0.02 | C | 1156 | 4.5 | 0.09 |
| Cont_20 | 302 | 3 | 826 | 1106 | |||||
| DNP | 150 | 1 | 862 | 4.4 | 0.80 | 1099 | − 0.6 | 0.86 | |
| HYD | 153 | 2 | 867 | 5.0 | 0.26 | 1113 | 0.6 | 0.62 | |
| HYD_16 | 153 | 2 | 862 | 4.4 | 0.81 | 1076 | − 2.7 | 0.19 | |
| NEBI | 150 | 1 | 798 | − 3.4 | 0.62 | 1096 | − 0.9 | 0.50 | |
| OH_Est | 156 | 3 | 951 | 15.1 | 0.0001 | 1157 | 4.6 | 0.03 | |
| THIO | 153 | 2 | 867 | 5.0 | 0.06 | 1148 | 3.8 | 0.41 | |
Notes: A: decline in lifespan; B: UM and UT only; C: TJL only. Count: number of mice initially enrolled in lifespan study. Live = number of mice alive when the table was compiled (February 9, 2024)
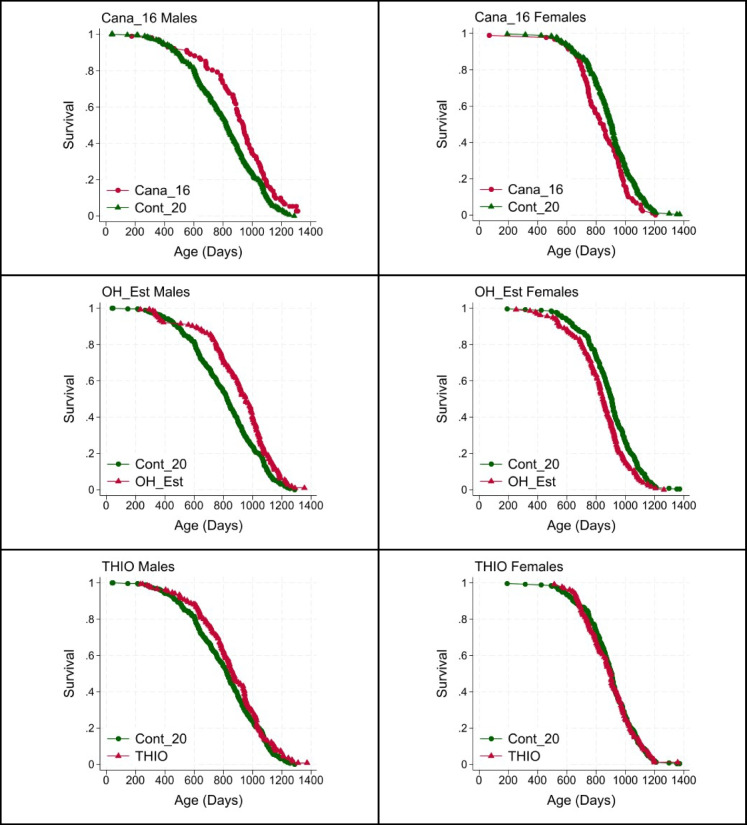
Two of the tested agents, Cana_16 and OH_Est, led to a significant p-value in the log-rank test, our pre-specified primary outcome test, but did so in male mice only. A third, THIO, led to an increase in male lifespan (p = 0.06) that was close to the traditional significance criterion. Figure 1 shows survival curves, for males and for females separately, for each of these interventions. The increase in median survival was 14%, 15%, and 5%, respectively, in male mice. Cana_16 and OH_Est also led to a significant increase in the proportion of male mice alive at the 90th percentile, with p = 0.04 and p = 0.03, respectively, by the WA test. Interestingly, Cana_16 and OH_Est produced a statistically significant decline in lifespan (i.e., an increase in hazard rate) in female mice, consistent with the supposition that these agents might lead to a mixture of beneficial and harmful effects in female animals. In addition, NEBI was found to have a significantly harmful effect on lifespan in female mice (p = 0.03 by log-rank test). Supplemental Fig. 1 shows survival curves for males at each site, for the Cana_16, OH_Est, and THIO treatment groups. Each of these site-specific subsets of the data has much lower statistical power than the data set pooled across sites, as shown in Table 2.
Fig. 1.
Survival plots for Cana_16, OH_Est, and THIO males (left) and females (right). Table 2 shows counts, medians, and p90 values, and p-values for log-rank and Wang-Allison statistical tests. Data are pooled across the three ITP sites, except that Cana_16 data are from UM and UT only
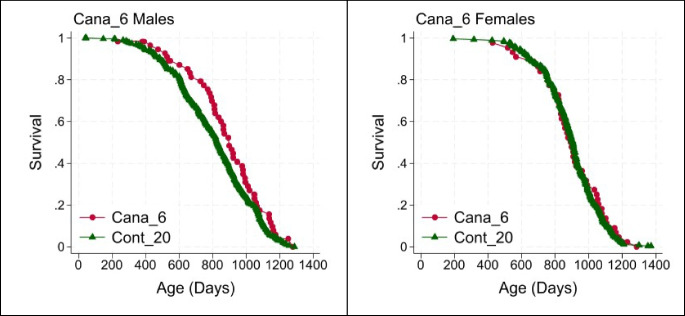
Canagliflozin started at 6 months of age was tested only at TJL and can be seen as a replication of our previous study of Cana [3]. Figure 2 shows the survival curves. Confirming our earlier study, Cana started at this early age led to a significant lifespan benefit in males (9% increase in median, log-rank p = 0.02, WA p = 0.09) and produced neither beneficial nor harmful effect in treated female mice.
Fig. 2.
Survival plots for males (left) and females (right) exposed to Cana from 6 months of age (TJL only). Table 2 shows counts, medians, and p90 values, and p-values for log-rank and Wang-Allison statistical tests
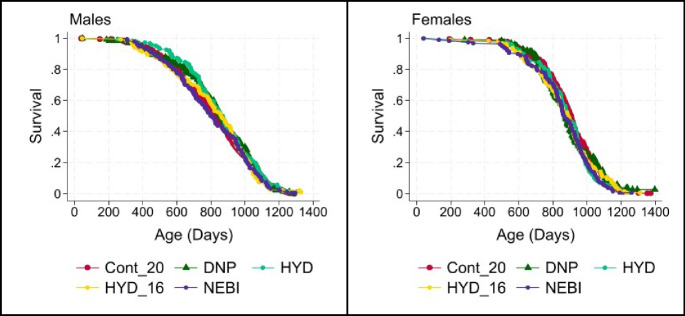
None of the other agents tested led to a significant increase in lifespan by the log-rank test or by the WA statistic. Figure 3 shows the survival curves for each of these ineffective agents.
Fig. 3.
Survival plots for males (left) and females (right) exposed to agents that did not significantly alter survival in either sex. Table 2 shows counts, medians, and p90 values, and p-values for log-rank and Wang-Allison statistical tests
Effects on body weight
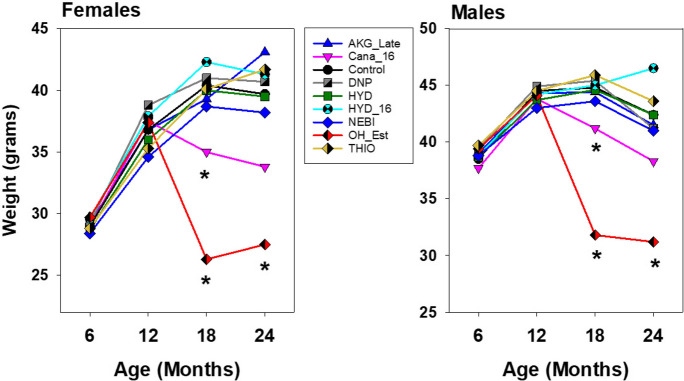
Figure 4 shows the mean levels of body weight for each treatment group, for each sex, at ages 6, 12, 18, and 24 months. Cana, started at 16 months, leads to a decline in weight in each sex when measured at 18 months. This effect is noted at 24 months as well, although variance is increased at 24 months and the drug effect on weight is no longer significant (Sidak test after one-way ANOVA) at that age. Mice given OH_Est from 12 months of age are also significantly lighter than controls at ages 18 and 24 months, for both sexes. None of the other drugs led to a significant alteration in body weight at any age tested.
Fig. 4.
Mean body weights for treated mice in C2020. Asterisks indicate values that differed significantly from untreated control mice of the same sex at the same age by Sidak post-hoc test, following one-way ANOVA done separately for each sex. Note that the Y-axis scale differs between the two panels
Increased Cana levels in female and in older mice
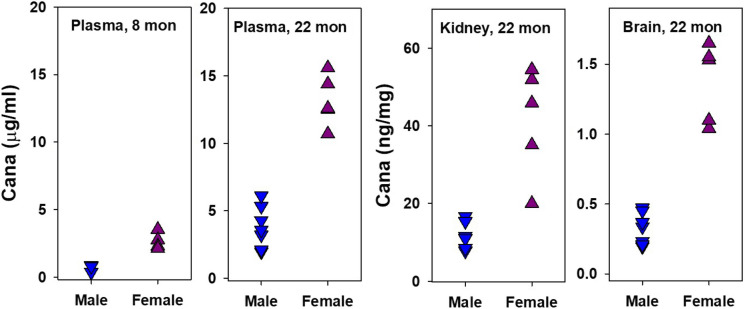
Because Cana started at 16 months was beneficial to males but harmful to females, we measured levels of Cana in the plasma, brain, and kidney of male and female mice euthanized at 22 months, in which Cana had been initiated at 16 months. For comparison, we also evaluated plasma from young adult (4 months) mice that had received Cana for a 17-week pilot study and were thus approximately 8 months old at the time of euthanasia. The results are shown in Fig. 5. There are several points of note. In the plasma of young mice, and in the plasma and tissue of 22-month-old mice, levels of Cana are approximately threefold higher in females than in males, significant by t-test at p < 0.001 in each case. In addition, levels in plasma rise, in both sexes, approximately fivefold, between 8 and 22 months of age. Thus plasma levels in 22-month-old females are 19.7-fold higher than in 8-month-old male mice. It seems plausible to suspect that these high levels seen in older females might lead to toxic effects that could mask hypothetical benefits of Cana in female mice.
Fig. 5.
Cana levels in plasma and tissues. Each symbol represents an individual mouse. Plasma values of young and old mice are shown as µg/ml, and kidney and brain tissue values of old mice are shown as ng/mg tissue. In each case, the effect of sex is significant at p < 0.001. Numbers of mice in the 8 columns are as follows: 3, 4, 8, 5, 8, 5, 8, and 5. Minimum detectable levels were 0.1 µg/ml for plasma and 0.11 ng/ml for tissue. Note differences in the Y-axis scale among the panels. The data for 22-month-old mice were previously shown, in another format, in (3)
Tests for levels of other drugs in the plasma of treated mice
Levels of test drugs were evaluated in the plasma of young adult mice given each agent for a period of 8 weeks. Plasma was taken from ad libitum-fed mice between 8 and 10 am, just prior to euthanasia, frozen, and then sent to the ITP Pharmacology group for assessment. DNP was present in the plasma of both male (169 ± 47 ng/ml; mean ± SD) and female (194 ± 53) mice; there was no significant difference between the sexes. NEBI was also detected in both sexes: males at 88 ± 4 and females at 41 ± 26 ng/ml; for NEBI, the difference between males and females was significant by t-test at p < 0.001. Measurements of THIO were inconsistent: with a minimal detection limit of 0.5 µg/ml, THIO was detected in only 1 of 6 male and 2 of 8 female mice. HYD was detected in each of 8 female mice (6.2 ± 1.5 ng/ml), but only 2 of 6 tested males had measurable HYD, with a detection limit of 5 ng/ml. Our efforts to devise a reliable method to quantify AKG in plasma were unsuccessful.
Discussion
This report adds another agent to the list of those shown by the ITP to increase mouse lifespan to a significant degree, i.e., 16-hydroxyestriol. Our data also suggest that THIO may also provide lifespan benefits for males, although the results (at p = 0.06) just failed to reach the traditional significance level. We also report that Cana extends mouse lifespan dramatically, in males, even when started at 16 months of age (at UM and UT), and we replicate (at TJL) the prior observation that Cana extends male lifespan when started in young adults. The Wang-Allison calculation shows that all three of these interventions—Cana at 16 months, Cana at 6 months, and OH_Est at 12 months—increase the fraction of mice still alive at the 90th percentile age. Each of these beneficial effects is seen in male mice only, for reasons we do not understand. The C2020 data also provide the first examples, in the ITP experience, of statistically significant harmful effects on lifespan, including effects of NEBI, OH-Est, and late-start Cana in female mice.
The observation that plasma Cana levels in older females are nearly 20 times higher than those seen in young males suggests a potential explanation for the male-specific benefit of this agent: levels in aged females could potentially lead to harmful effects that counterbalance hypothetical benefits in female mice. Sex-specific differences in plasma Cana levels were also noted in [40]. Cana initiated at 6 months is neither harmful nor beneficial to female HET3 mice [3], but in the current study, Cana initiated at 16 months led to a 6% decline in the median lifespan of females, significant at p = 0.03 by the log-rank test. This disparity is consistent with the idea that hypothetical benefits that might accrue in females at younger ages are then countered by harmful effects at ages above 16 months, ages at which blood levels of this drug are particularly high in females. The basis for this disparity is unknown but is likely to involve age- and sex-specific differences in drug conjugation and excretion.
We are now testing the hypothesis that high levels of Cana in aged females conceal potential benefits by setting up two new lifespan studies, one in which Cana is administered at a lower dose (60 ppm, one-third of the 180 ppm used for this and previous work), in the hopes of avoiding toxic effects in later life, and one in which Cana will be given to mice as young adults, but then removed from the diet at 20 months and older ages. It is noteworthy that Cana inhibits mTORC1 action in the liver, kidney, and muscle taken from 22-month-old male mice that have received Cana (180 ppm) from 6 months of age; this inhibitory effect is either absent or is significantly lower in female mice at the same Cana dose [41]. This male-specific effect of Cana affects both the phosphorylation of one mTORC1 substrate, S6, and the absolute levels of a second substrate, 4EBP1, at both mRNA and protein level. The protein kinase cascade, through which GH and insulin signals control protein translation via MEK1, Erk1/2, MNK1/2, and phosphorylation of eIF4E, is also reduced in aged male mice only [41]. These sex-specific biochemical changes imply that not all of the sexual dimorphism in responses to Cana reflect female-specific toxicity, but more data at earlier ages and at lower Cana doses will help to sort out beneficial from harmful responses to this agent. Similarly, multiple forms of age-sensitive pathological change are blunted by Cana in male mice only [4], an observation difficult to explain by models of female-specific drug toxicity.
The extent of lifespan extension seen in males given OH_Est, a 15% increase in median, is similar to that produced by 17aE2, 19% at its optimal tested dose [5, 6, 37]. It is plausible that testing OH_Est over a wider range of doses might produce increases in longevity that exceed 15%. The original speculation that 17aE2 would improve male lifespan by mimicking hormonal effects present in normal, untreated females was refuted by the data showing that the lifespan of 17aE2-treated males exceeded that of treated or untreated females to a significant degree. We speculated that estriol, metabolites of which were dramatically increased in plasma by 17aE2 in males only, might be the active metabolite by which 17aE2 altered lifespan and age-sensitive health outcomes, but the absence of any benefit in OH_Est-treated females makes this seem unlikely. At this point, we have no evidence pointing either to a molecular target, or cellular locus, for the beneficial effects of either 17aE2 or OH_Est in males, or for the harmful effects documented here for OH_Est in females. The effects of 17aE2 on glucose tolerance and physical function in male mice are dependent on the presence of male gonads [7], and it would be of interest to determine whether the sex-specific responses to OH_Est are also dependent on gonadal hormones in one or both sexes. Male mice treated with 17aE2 do show a pattern of change in fat browning, fat UCP1, anti-inflammatory changes in fat-associated macrophage subsets, muscle production of FNDC5 and irisin, liver synthesis of GPLD1 via cap-independent translation, and increases of brain BDNF and doublecortin that are characteristic of long-lived mutant mice and of males and females given rapamycin or acarbose [18, 20, 42–44]. Some of these changes—in mTORC1, cap-independent translation, Erk1/2 protein kinase cascade, and GPLD1 protein in the liver—are triggered by 17aE2 only in males but not seen in 17aE2-treated females. We speculate that alteration of these multi-tissue aging rate indicators may also be sex-specific in responses to OH_Est and are testing this idea at present. A good deal of additional work will be needed to formulate and test hypotheses about the specific cell types and specific steroid receptors that are sensitive to 17aE2, OH_Est, and their metabolites in male and female mice, and to work out the balance between beneficial and harmful effects differentially produced between the sexes. Expression of estrogen receptor ERα is required for 17aE2 to elicit metabolic benefits in male mice fed a high-fat diet, and, therefore, this receptor may also be a good candidate for involvement in survival and health benefits after estrogen treatment in male mice [45].
The lifespan effects in males treated with THIO are suggestive, though not statistically significant. For males, p = 0.06 in our primary analysis, i.e., pooled across sites. Median survival was increased by 5% in males. THIO showed no indication of benefits for female mice at the dose used. An earlier protocol attempted to produce therapeutic levels of H2S through the use of another sulfur-containing agent, SG1002, but this agent did not produce lifespan extension regardless of the age at which it was initiated [2]. Evaluation of THIO effects at higher or lower doses may be justified because it is unlikely that the dose used in any initial lifespan study will prove to be optimal when compared to a wider range of doses.
Our data on AKG fail to confirm the published work in which this agent produced a small but significant lifespan benefit in female C57BL/6 mice [28]. In the study using B6 mice, the increase in median, estimated from birth, ranged from 3.3 to 6.3% across the two male and two female cohorts studied. Using UM-HET3 mice, we saw only a 3.0% increase in males and a 0.9% increase in females; neither effect was significant by log-rank test. We used the same dose of AKG as in the published study and started at the same age (540 days). Our experiment utilized 132 females and 153 males in the treated group, and a double-sized control group, and was thus not underpowered compared to the published study of B6 mice, which used only 45 mice per cohort. It is possible that the differences in outcome reflect the genetic background of the tested animals or environmental differences between the ITP colonies and those used for the previously published study. The ITP is also testing AKG in the C2021 cohort, starting at an earlier age. It will be of interest to see if other groups will or will not be able to document lifespan benefits in mice given AKG orally, either in UM-HET3 mice or in other stocks.
We also failed to detect any lifespan benefit from NEBI, DNP, or HYD. It is possible that one or more of these agents might have led to increased lifespan using a different protocol, for example, at a different dose, starting at a different age, or with treatment restricted to some age interval, although the most plausible interpretation is that none of these agents can slow mouse aging. Both DNP and NEBI were detected in the plasma of every mouse tested in an initial pilot experiment; NEBI levels were higher in males than in females. HYD was detected in each of 8 female mice in a pilot group, but in only 2 of 6 pilot males; it is possible that a higher dose might have reached therapeutic levels more consistently in both sexes.
To evaluate the potential effects of DNP on mitochondrial free radical production, we measured free and esterified isoprostanes (IsoP) in the plasma, heart, liver, brain, and muscle of 22-month-old mice that had been exposed to DNP since 6 months of age. We expected we might see an increase with age in IsoP and that this increase would be blunted in the DNP-treated mice. The data, however (Supplemental Fig. 2), showed no significant effect of age (4 to 6 months vs 22 months) or of drug treatment. The absence of a significant age effect of DNP on isoprostanes may reflect the absence of an age effect, in UM-HET3 mice, prior to 22 months. It is possible that ages beyond 22 months might lead to increased IsoP levels in UM-HET3 mice, and we do not know if DNP exposure might mitigate against increases in IsoP levels in older animals.
Tissues from the mice used in C2020 are available to researchers through the NIA Collaborative Interactions Program and can be used to test specific hypotheses about drug effects on gene expression, age-sensitive changes in tissue architecture or cellular composition, or related issues. A detailed histopathology study of Cana_16 and OH_Est mice is now underway and may provide additional clues as to the ability of these agents to retard tissue-specific aspects of aging, or to the basis of the accelerated mortality seen in females exposed to either drug.
The current study brings to 9 the number of ITP-tested agents that have led to a significant increase in lifespan in mice of one or both sexes and brings to 7 the number of agents that increase median survival by 10% or more. For three drugs, rapamycin, acarbose, and 17aE2, follow-up studies showed that different doses could produce more dramatic lifespan extension than that reported in the initial study, and it is possible that higher or lower doses of Cana, THIO, or OH_Est might also achieve larger increases in lifespan than those seen in the current study.
The ITP program has laid to rest the previously widespread assumption that the aging process is too complex to be slowed by pharmacologic intervention and provided strong support for the idea that many forms of age-dependent disease and disability can be delayed in parallel by interventions targeting aging per se. The substantial and growing repertoire of effective anti-aging interventions provides a foundation for seeking shared, common pathways by which different drugs, diets, and genes might slow aging [18]. Success in identifying these hypothetical shared pathways could provide new targets for developing more effective anti-aging interventions that may eventually find a place in human preventive medicine.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to Lindsey Burger, Micah Bush, Robert Dilg, Lori Roberts, and Jacob Sheets for animal husbandry at UM; Pamela Krason, Vicki Ingalls, Nelson Durgin, and Leonor Robidoux for expert animal care at TJL; Vanessa Calderon and Victoria DeLeon for expert assistance at UT; and technical assistance from Alexey Tomilov. We thank NIH representative Jennifer Fox for advice and collaboration.
Author contribution
DEH, NR, RS, and RAM are the principal investigators at the three collaborating institutions and are responsible for project design, supervision of technical personnel, interpretation of results, and preparation of manuscript drafts. BCG and MLC ran the Pharmacology Core and helped with the manuscript. ABS, JFN, RK, CK, and SL advised on experimental design and interpretation and helped with the manuscript. PR supervised laboratory procedures and data collection at The Jackson Laboratory site and organized diet preparations for all three sites. EF supervised laboratory personnel and data collection at the UTHSCSA site. GM conducted the isoprostane analyses. MH coordinated sample preparation across the three sites. NK assisted in statistical analyses. GAC, ID, MG, JGG, and JRM proposed the drugs used in the C2020 study.
Funding
This work was supported by NIH grants AG022308 (DEH), AG022303 (RAM), AG022307 and AG013319 (RS), and AG062817 (GAC).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
James R. Mitchell passed away during the course of this study.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/15/2024
A Correction to this paper has been published: 10.1007/s11357-024-01283-0
References
- 1.Macchiarini F, Miller RA, Strong R, Rosenthal N, Harrison DE. NIA interventions testing program: a collaborative approach for investigating interventions to promote healthy aging. In: Musi N, Hornsby PJ, editors. Handbook of the Biology of Aging. 9th ed. London (UK): Academic Press; 2021. [Google Scholar]
- 2.Harrison DE, Strong R, Reifsnyder P, Rosenthal N, Korstanje R, Fernandez E, et al. Astaxanthin and meclizine extend lifespan in UM-HET3 male mice; fisetin, SG1002 (hydrogen sulfide donor), dimethyl fumarate, mycophenolic acid, and 4-phenylbutyrate do not significantly affect lifespan in either sex at the doses and schedules used. Geroscience. 2023;46(1):795–816. 10.1007/s11357-023-01011-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller RA, Harrison DE, Allison DB, Bogue M, Debarba L, Diaz V, et al. Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight. 2020;5(21):e140019. 10.1172/jci.insight.140019. [DOI] [PMC free article] [PubMed]
- 4.Snyder JM, Casey KM, Galecki A, Harrison DE, Jayarathne H, Kumar N, et al. Canagliflozin retards age-related lesions in heart, kidney, liver, and adrenal gland in genetically heterogenous male mice. Geroscience. 2022;45(1):385–97. 10.1007/s11357-022-00641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15(5):872–84. 10.1111/acel.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13(2):273–82. 10.1111/acel.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garratt M, Leander D, Pifer K, Bower B, Herrera JJ, Day SM, et al. 17-alpha estradiol ameliorates age-associated sarcopenia and improves late-life physical function in male mice but not in females or castrated males. Aging Cell. 2019;18(2):e12920. 10.1111/acel.12920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garratt M, Lagerborg KA, Tsai YM, Galecki A, Jain M, Miller RA. Male lifespan extension with 17-alpha estradiol is linked to a sex-specific metabolomic response modulated by gonadal hormones in mice. Aging Cell. 2018;11(4):e12786. 10.1111/acel.12786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pascualini JR, Kincl FA. Biosythesis and metabolism of different hormones in the fetal and placental compartments Production, concentration and metabolism during pregnancy. Amsterdam: Pergamon; 1985. p. 73–172. [Google Scholar]
- 10.Tanaka T, Suguro N, Kubodera A. Specific antisera for the radioimmunoassay of estriol 3-sulfate. Steroids. 1984;43(3):235–42. 10.1016/0039-128X(84)90042-4 [DOI] [PubMed] [Google Scholar]
- 11.Burcham PC. Carbonyl scavengers as pharmacotherapies in degenerative disease: hydralazine repurposing and challenges in clinical translation. Biochem Pharmacol. 2018;154:397–406. 10.1016/j.bcp.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 12.Dehghan E, Zhang Y, Saremi B, Yadavali S, Hakimi A, Dehghani M, et al. Hydralazine induces stress resistance and extends C. elegans lifespan by activating the NRF2/SKN-1 signalling pathway. Nat Commun. 2017;8(1):2223. 10.1038/s41467-017-02394-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dehghan E, Goodarzi M, Saremi B, Lin R, Mirzaei H. Hydralazine targets cAMP-dependent protein kinase leading to sirtuin1/5 activation and lifespan extension in C. elegans. Nat Commun. 2019;10(1):4905. 10.1038/s41467-019-12425-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolfe DF, Brand MD. Contribution of mitochondrial proton leak to skeletal muscle respiration and to standard metabolic rate. Am J Physiol. 1996;271(4 Pt 1):C1380–9. 10.1152/ajpcell.1996.271.4.C1380 [DOI] [PubMed] [Google Scholar]
- 15.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47(4):333–43. 10.1016/j.freeradbiomed.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 16.Tainter ML, Cutting WC, Stockton AB. Use of dinitrophenol in nutritional disorders: a critical survey of clinical results. Am J Public Health Nations Health. 1934;24(10):1045–53. 10.2105/AJPH.24.10.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Caldeira Silva CC, Cerqueira FM, Barbosa LF, Medeiros MH, Kowaltowski AJ. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell. 2008;7(4):552–60. 10.1111/j.1474-9726.2008.00407.x [DOI] [PubMed] [Google Scholar]
- 18.Miller RA, Li X, Garcia G. Aging rate indicators: speedometers for aging research in mice. Aging Biol. 2023;1(1):20230003. 10.59368/agingbio.20230003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, McPherson M, Hager M, Lee M, Chang P, Miller RA. Four anti-aging drugs and calorie-restricted diet produce parallel effects in fat, brain, muscle, macrophages, and plasma of young mice. Geroscience. 2023;45(4):2495–510. 10.1007/s11357-023-00770-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Frazier JA, Spahiu E, McPherson M, Miller RA. Muscle-dependent regulation of adipose tissue function in long-lived growth hormone-mutant mice. Aging (Albany NY). 2020;12(10):8766–89. 10.18632/aging.103380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gates AC, Bernal-Mizrachi C, Chinault SL, Feng C, Schneider JG, Coleman T, et al. Respiratory uncoupling in skeletal muscle delays death and diminishes age-related disease. Cell Metab. 2007;6(6):497–505. 10.1016/j.cmet.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 22.Allen SA, Tomilov A, Cortopassi GA. Small molecules bind human mTOR protein and inhibit mTORC1 specifically. Biochem Pharmacol. 2018;155:298–304. 10.1016/j.bcp.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Zhang F, Liu Y, Yin S, Pang X, Li Z, et al. Nebivolol alleviates aortic remodeling through eNOS upregulation and inhibition of oxidative stress in l-NAME-induced hypertensive rats. Clin Exp Hypertens. 2017;39(7):628–39. 10.1080/10641963.2017.1306539 [DOI] [PubMed] [Google Scholar]
- 24.Wu N, Ma YC, Gong XQ, Zhao PJ, Jia YJ, Zhao Q, et al. The metabolite alpha-ketobutyrate extends lifespan by promoting peroxisomal function in C. elegans. Nat Commun. 2023;14(1):240. 10.1038/s41467-023-35899-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burns AR, Wiedrick J, Feryn A, Maes M, Midha MK, Baxter DH, et al. Proteomic changes induced by longevity-promoting interventions in mice. Geroscience. 2023;46(2):1543–60. 10.1007/s11357-023-00917-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe K, Wilmanski T, Baloni P, Robinson M, Garcia GG, Hoopmann MR, et al. Lifespan-extending interventions induce consistent patterns of fatty acid oxidation in mouse livers. Commun Biol. 2023;6(1):768. 10.1038/s42003-023-05128-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elmansi AM, Miller RA. Coordinated transcriptional upregulation of oxidative metabolism proteins in long-lived endocrine mutant mice. Geroscience. 2023;45(5):2967–81. 10.1007/s11357-023-00849-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AsadiShahmirzadi A, Edgar D, Liao CY, Hsu YM, Lucanic M, AsadiShahmirzadi A, et al. Alpha-ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell Metab. 2020;32(3):447-56 e6. 10.1016/j.cmet.2020.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hine C, Kim HJ, Zhu Y, Harputlugil E, Longchamp A, Matos MS, et al. Hypothalamic-pituitary axis regulates hydrogen sulfide production. Cell Metab. 2017;25(6):1320-33 e5. 10.1016/j.cmet.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160(1–2):132–44. 10.1016/j.cell.2014.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller DL, Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104(51):20618–22. 10.1073/pnas.0710191104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92(2):791–896. 10.1152/physrev.00017.2011 [DOI] [PubMed] [Google Scholar]
- 33.Ravindran S, Kurian GA. Effect of sodium thiosulfate postconditioning on ischemia-reperfusion injury induced mitochondrial dysfunction in rat heart. J Cardiovasc Transl Res. 2018;11(3):246–58. 10.1007/s12265-018-9808-y [DOI] [PubMed] [Google Scholar]
- 34.Auriemma M, Carbone A, Di Liberato L, Cupaiolo A, Caponio C, De Simone C, et al. Treatment of cutaneous calciphylaxis with sodium thiosulfate: two case reports and a review of the literature. Am J Clin Dermatol. 2011;12(5):339–46. 10.2165/11587060-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 35.Brock PR, Maibach R, Childs M, Rajput K, Roebuck D, Sullivan MJ, et al. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med. 2018;378(25):2376–85. 10.1056/NEJMoa1801109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66(2):191–201. 10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison DE, Strong R, Reifsnyder P, Kumar N, Fernandez E, Flurkey K, et al. 17-a-estradiol late in life extends lifespan in aging UM-HET3 male mice; nicotinamide riboside and three other drugs do not affect lifespan in either sex. Aging Cell. 2021;20(5):e13328. 10.1111/acel.13328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strong R, Miller RA, Cheng CJ, Nelson JF, Gelfond J, Allani SK, et al. Lifespan benefits for the combination of rapamycin plus acarbose and for captopril in genetically heterogeneous mice. Aging Cell. 2022;21(12):e13724. 10.1111/acel.13724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C, Li Q, Redden DT, Weindruch R, Allison DB. Statistical methods for testing effects on “maximum lifespan.” Mech Ageing Dev. 2004;125(9):629–32. 10.1016/j.mad.2004.07.003 [DOI] [PubMed] [Google Scholar]
- 40.Jayarathne HSM, Debarba LK, Jaboro JJ, Ginsburg BC, Miller RA, Sadagurski M. Neuroprotective effects of canagliflozin: lessons from aged genetically diverse UM-HET3 mice. Aging Cell. 2022;21(7):e13653. 10.1111/acel.13653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang E, Dinesh A, Jadhav S, Miller RA, Garcia GG. Canagliflozin shares common mTOR and MAPK signaling mechanisms with other lifespan extension treatments. Life Sci. 2023;328:121904. 10.1016/j.lfs.2023.121904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, McPherson M, Hager M, Lee M, Chang P, Miller RA. Four anti-aging drugs and calorie-restricted diet produce parallel effects in fat, brain, muscle, macrophages, and plasma of young mice. Geroscience. 2023;45(4):2495–510. 10.1007/s11357-023-00770-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Shi X, McPherson M, Hager M, Garcia GG, Miller RA. Cap-independent translation of GPLD1 enhances markers of brain health in long-lived mutant and drug-treated mice. Aging Cell. 2022;21(9):e13685. 10.1111/acel.13685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, McPherson M, Hager M, Fang Y, Bartke A, Miller RA. Transient early life growth hormone exposure permanently alters brain, muscle, liver, macrophage, and adipocyte status in long-lived Ames dwarf mice. FASEB J. 2022;36(7):e22394. 10.1096/fj.202200143R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mann SN, Hadad N, Nelson Holte M, Rothman AR, Sathiaseelan R, Ali Mondal S, et al. Health benefits attributed to 17alpha-estradiol, a lifespan-extending compound, are mediated through estrogen receptor alpha. eLife. 2020;9:e59616. 10.7554/eLife.59616. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.