Abstract
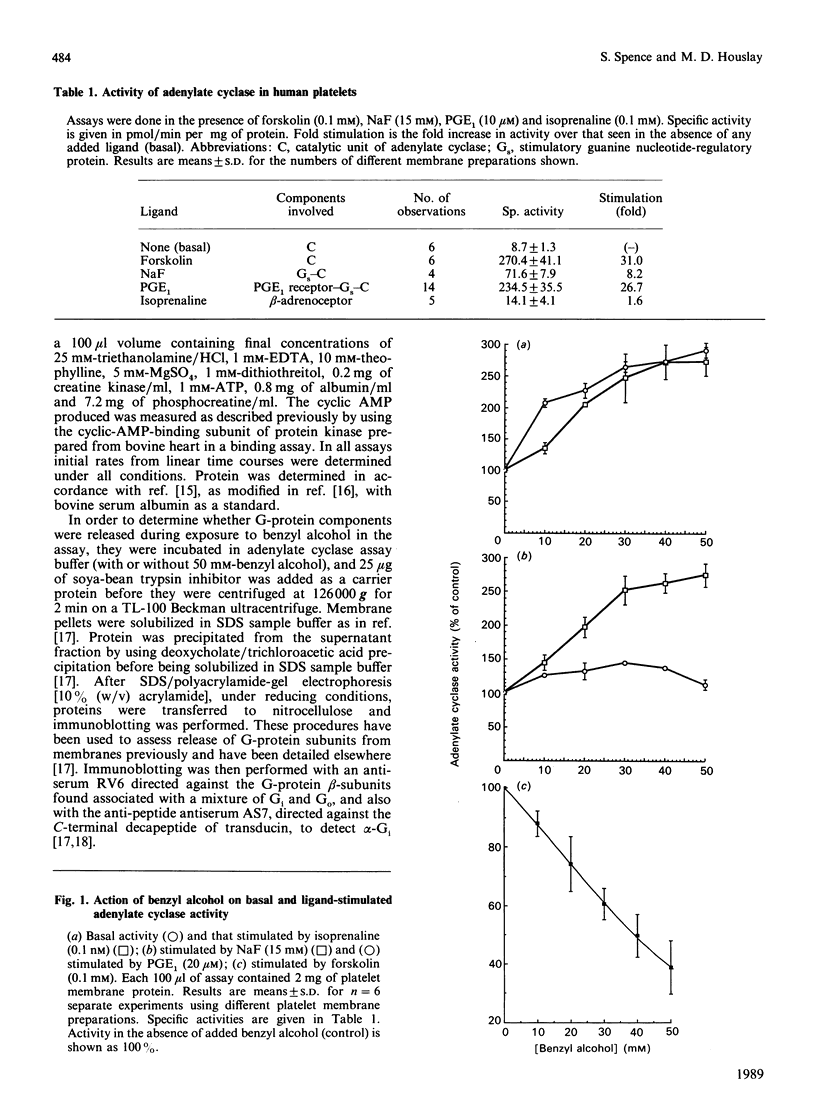
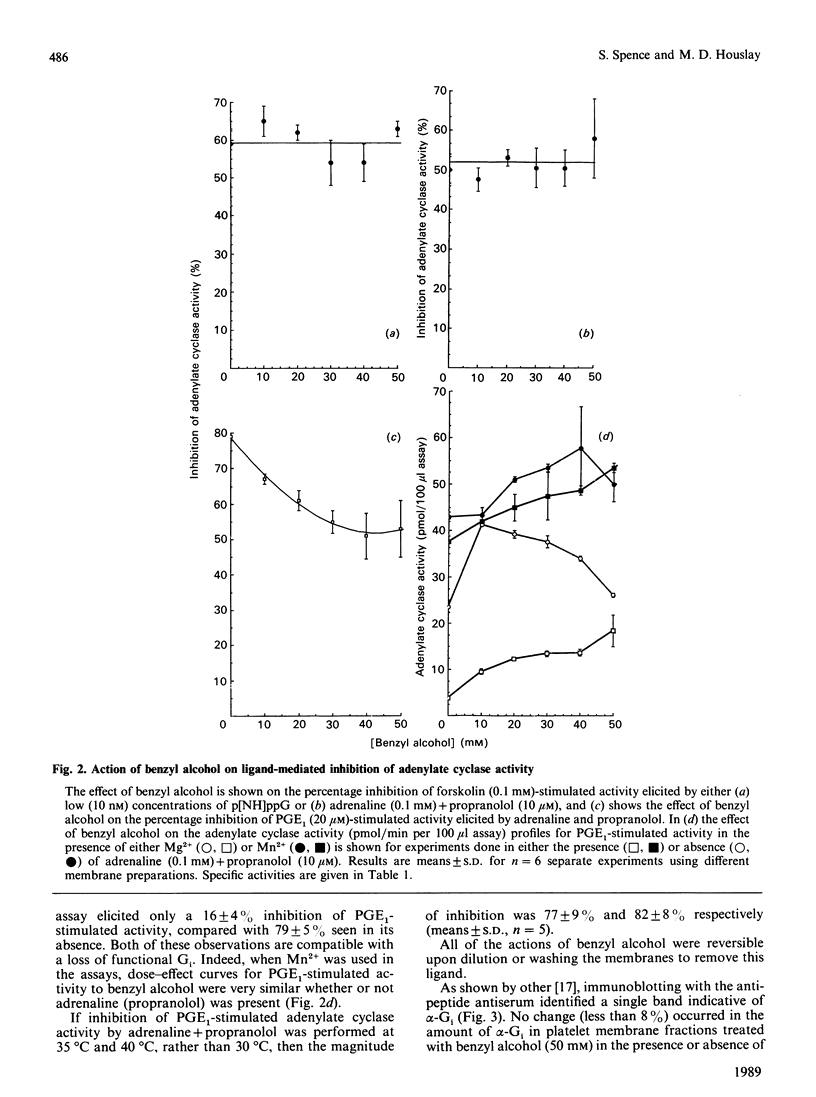
Treatment of human platelets with concentrations of benzyl alcohol up to 50 mM augmented adenylate cyclase activity when it was assayed in the basal state and when stimulated by prostaglandin E1 (PGE1), isoprenaline or NaF. Benzyl alcohol antagonized the stimulatory effect exerted on the catalytic unit of adenylate cyclase by the diterpene forskolin. Benzyl alcohol did not modify the magnitude of the inhibitory response when the catalytic unit of adenylate cyclase was inhibited by using either low concentrations of guanosine 5'-[beta gamma-imido]triphosphate, which acts selectively on the inhibitory guanine nucleotide-regulatory protein Gi, or during alpha 2-adrenoceptor occupancy, by using adrenaline (+ propranolol). Some 34% of the potent inhibitory action of adrenaline on PGE1-stimulated adenylate cyclase was obliterated in a dose-dependent fashion (concn. giving 50% inhibition = 12.5 mM) by benzyl alcohol, with the residual inhibitory action being apparently resistant to the action of benzyl alcohol at concentrations up to 50 mM. Treatment of membranes with benzyl alcohol did not lead to the release of either the alpha-subunit of Gi or G-protein subunits. The alpha 2-adrenoceptor-mediated inhibition of adenylate cyclase was abolished when assays were performed in the presence of Mn2+ rather than Mg2+ and, under such conditions, dose-effect curves for the action of benzyl alcohol on PGE1-stimulated adenylate cyclase activity were similar whether or not adrenaline (+propranolol) was present. We suggest that (i) alpha 2-adrenoceptor- and Gi-mediated inhibition of PGE1-stimulated adenylate cyclase may have two components, one of which is sensitive to inhibition by benzyl alcohol, and (ii) the Gi-mediated inhibition of forskolin-stimulated adenylate cyclase exhibits predominantly the benzyl alcohol-insensitive component.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brasitus T. A., Schachter D. Lipid dynamics and lipid-protein interactions in rat enterocyte basolateral and microvillus membranes. Biochemistry. 1980 Jun 10;19(12):2763–2769. doi: 10.1021/bi00553a035. [DOI] [PubMed] [Google Scholar]
- Colley C. M., Metcalfe J. C. The localisation of small molecules in lipid bilayers. FEBS Lett. 1972 Aug 15;24(3):241–246. doi: 10.1016/0014-5793(72)80364-8. [DOI] [PubMed] [Google Scholar]
- Dipple I., Houslay M. D. The activity of glucagon-stimulated adenylate cyclase from rat liver plasma membranes is modulated by the fluidity of its lipid environment. Biochem J. 1978 Jul 15;174(1):179–190. doi: 10.1042/bj1740179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander G., Le Grimellec C., Giocondi M. C., Amiel C. Benzyl alcohol increases membrane fluidity and modulates cyclic AMP synthesis in intact renal epithelial cells. Biochim Biophys Acta. 1987 Oct 2;903(2):341–348. doi: 10.1016/0005-2736(87)90224-0. [DOI] [PubMed] [Google Scholar]
- Gawler D., Milligan G., Spiegel A. M., Unson C. G., Houslay M. D. Abolition of the expression of inhibitory guanine nucleotide regulatory protein Gi activity in diabetes. Nature. 1987 May 21;327(6119):229–232. doi: 10.1038/327229a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gordon L. M., Mobley P. W., Esgate J. A., Hofmann G., Whetton A. D., Houslay M. D. Thermotropic lipid phase separations in human platelet and rat liver plasma membranes. J Membr Biol. 1983;76(2):139–149. doi: 10.1007/BF02000614. [DOI] [PubMed] [Google Scholar]
- Gordon L. M., Sauerheber R. D., Esgate J. A., Dipple I., Marchmont R. J., Houslay M. D. The increase in bilayer fluidity of rat liver plasma membranes achieved by the local anesthetic benzyl alcohol affects the activity of intrinsic membrane enzymes. J Biol Chem. 1980 May 25;255(10):4519–4527. [PubMed] [Google Scholar]
- Gordon L. M., Whetton A. D., Rawal S., Esgate J. A., Houslay M. D. Perturbations of liver plasma membranes induced by Ca2+ are detected using a fatty acid spin label and adenylate cyclase as membrane probes. Biochim Biophys Acta. 1983 Mar 23;729(1):104–114. doi: 10.1016/0005-2736(83)90461-3. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M., Desjardins J. V. Inhibition of adenylate cyclase by adenosine analogues in preparations of broken and intact human platelets. Evidence for the unidirectional control of platelet function by cyclic AMP. Biochem J. 1978 Oct 15;176(1):83–95. doi: 10.1042/bj1760083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henis Y. I., Rimon G., Felder S. Lateral mobility of phospholipids in turkey erythrocytes. Implications for adenylate cyclase activation. J Biol Chem. 1982 Feb 10;257(3):1407–1411. [PubMed] [Google Scholar]
- Heyworth C. M., Houslay M. D. Challenge of hepatocytes by glucagon triggers a rapid modulation of adenylate cyclase activity in isolated membranes. Biochem J. 1983 Jul 15;214(1):93–98. doi: 10.1042/bj2140093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. D., Hanoune J., Birnbaumer L. Guanine nucleotide inhibition of cyc- S49 mouse lymphoma cell membrane adenylyl cyclase. J Biol Chem. 1982 Dec 25;257(24):14723–14725. [PubMed] [Google Scholar]
- Hoffman B. B., Yim S., Tsai B. S., Lefkowitz R. J. Preferential uncoupling by manganese of alpha adrenergic receptor mediated inhibition of adenylate cyclase in human platelets. Biochem Biophys Res Commun. 1981 May 29;100(2):724–731. doi: 10.1016/s0006-291x(81)80235-5. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Bojanic D., Gawler D., O'Hagan S., Wilson A. Thrombin, unlike vasopressin, appears to stimulate two distinct guanine nucleotide regulatory proteins in human platelets. Biochem J. 1986 Aug 15;238(1):109–113. doi: 10.1042/bj2380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D., Bojanic D., Wilson A. Platelet activating factor and U44069 stimulate a GTPase activity in human platelets which is distinct from the guanine nucleotide regulatory proteins, Ns and Ni. Biochem J. 1986 Mar 15;234(3):737–740. doi: 10.1042/bj2340737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D., Dipple I., Elliott K. R. Guanosine 5'-triphosphate and guanosine 5'-[beta gamma-imido]triphosphate effect a collision coupling mechanism between the glucagon receptor and catalytic unit of adenylate cyclase. Biochem J. 1980 Mar 15;186(3):649–658. doi: 10.1042/bj1860649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D., Metcalfe J. C., Warren G. B., Hesketh T. R., Smith G. A. The glucagon receptor of rat liver plasma membrane can couple to adenylate cyclase without activating it. Biochim Biophys Acta. 1976 Jun 17;436(2):489–494. doi: 10.1016/0005-2736(76)90210-8. [DOI] [PubMed] [Google Scholar]
- Houslay M. D. Regulation of adenylate cyclase (EC 4.6.I.I) activity by its lipid environment. Proc Nutr Soc. 1985 Jul;44(2):157–165. doi: 10.1079/pns19850034. [DOI] [PubMed] [Google Scholar]
- Huang R. D., Smith M. F., Zahler W. L. Inhibition of forskolin-activated adenylate cyclase by ethanol and other solvents. J Cyclic Nucleotide Res. 1982;8(6):385–394. [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Minuth M., Schultz G. Inhibition of adenylate cyclase. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:137–150. [PubMed] [Google Scholar]
- Jakobs K. H., Lasch P., Minuth M., Aktories K., Schultz G. Uncoupling of alpha-adrenoceptor-mediated inhibition of human platelet adenylate cyclase by N-ethylmaleimide. J Biol Chem. 1982 Mar 25;257(6):2829–2833. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lenox R. H., Ellis J., Van Riper D., Ehrlich Y. H. Alpha 2-adrenergic receptor-mediated regulation of adenylate cyclase in the intact human platelet. Evidence for a receptor reserve. Mol Pharmacol. 1985 Jan;27(1):1–9. [PubMed] [Google Scholar]
- MacIntyre D. E., Pollock W. K. Platelet-activating factor stimulates phosphatidylinositol turnover in human platelets. Biochem J. 1983 May 15;212(2):433–437. doi: 10.1042/bj2120433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G., Davies S. A., Houslay M. D., Wakelam M. J. Identification of the pertussis and cholera toxin substrates in normal and N-ras transformed NIH3T3 fibroblasts and an assessment of their involvement in bombesin-stimulation of inositol phospholipid metabolism. Oncogene. 1989 May;4(5):659–663. [PubMed] [Google Scholar]
- Milligan G., Mullaney I., Unson C. G., Marshall L., Spiegel A. M., McArdle H. GTP analogues promote release of the alpha subunit of the guanine nucleotide binding protein, Gi2, from membranes of rat glioma C6 BU1 cells. Biochem J. 1988 Sep 1;254(2):391–396. doi: 10.1042/bj2540391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. Techniques used in the identification and analysis of function of pertussis toxin-sensitive guanine nucleotide binding proteins. Biochem J. 1988 Oct 1;255(1):1–13. doi: 10.1042/bj2550001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham L., Houslay M. D. The activity of dopamine-stimulated adenylate cyclase from rat brain stratum is modulated by temperature and the bilayer-fluidizing agent, benzyl alcohol. Biochem J. 1982 Jul 15;206(1):89–95. doi: 10.1042/bj2060089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Waelbroeck M., Chatelain P., Camus J. C., Christophe J. Inhibition of forskolin-stimulated cardiac adenylate cyclase activity by short-chain alcohols. FEBS Lett. 1983 Apr 5;154(1):205–208. doi: 10.1016/0014-5793(83)80904-1. [DOI] [PubMed] [Google Scholar]
- Schauf C., Agin D. Cooperative effect of certain anaesthetics on the lobster giant axon. Nature. 1969 Feb 22;221(5182):768–769. doi: 10.1038/221768a0. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Guanosine 5'-(beta, gamma-imido)triphosphate inhibition of forskolin-activated adenylate cyclase is mediated by the putative inhibitory guanine nucleotide regulatory protein. J Biol Chem. 1982 Oct 10;257(19):11591–11596. [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Whetton A. D., Needham L., Dodd N. J., Heyworth C. M., Houslay M. D. Forskolin and ethanol both perturb the structure of liver plasma membranes and activate adenylate cyclase activity. Biochem Pharmacol. 1983 May 15;32(10):1601–1608. doi: 10.1016/0006-2952(83)90334-9. [DOI] [PubMed] [Google Scholar]
- de Foresta B., Rogard M., le Maire M., Gallay J. Effects of temperature and benzyl alcohol on the structure and adenylate cyclase activity of plasma membranes from bovine adrenal cortex. Biochim Biophys Acta. 1987 Dec 11;905(2):240–256. doi: 10.1016/0005-2736(87)90452-4. [DOI] [PubMed] [Google Scholar]



