Abstract
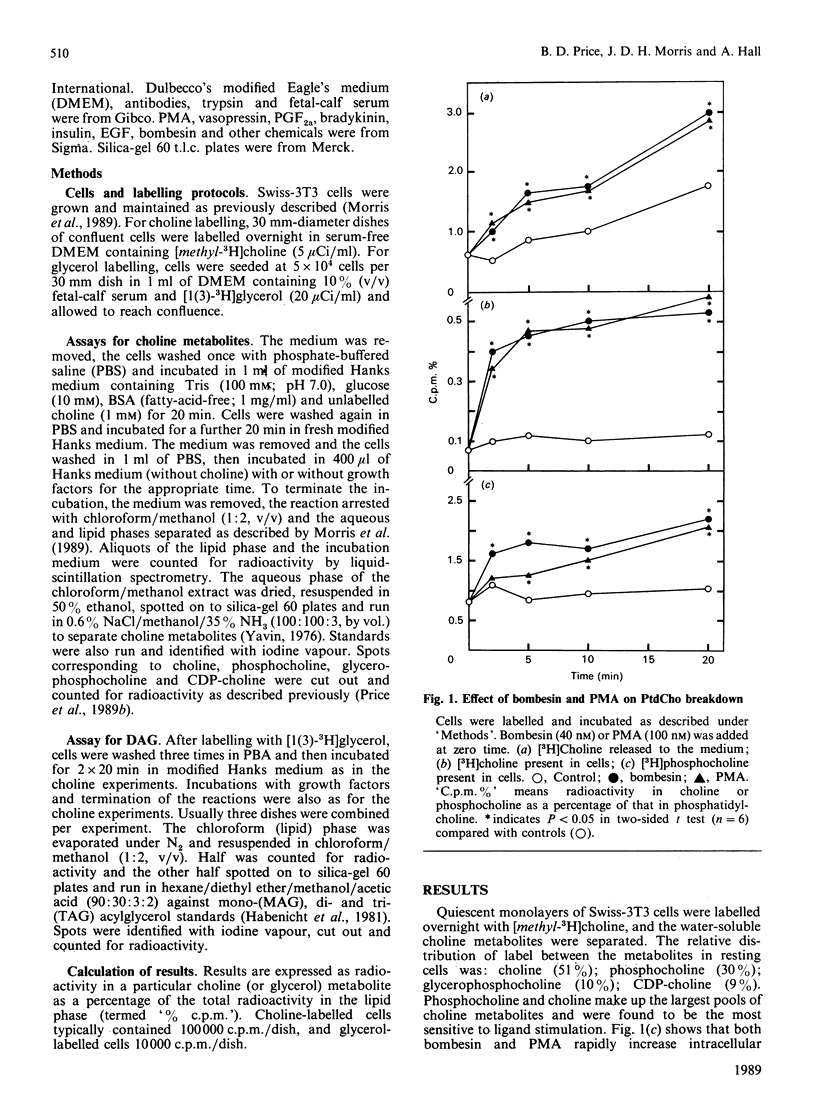
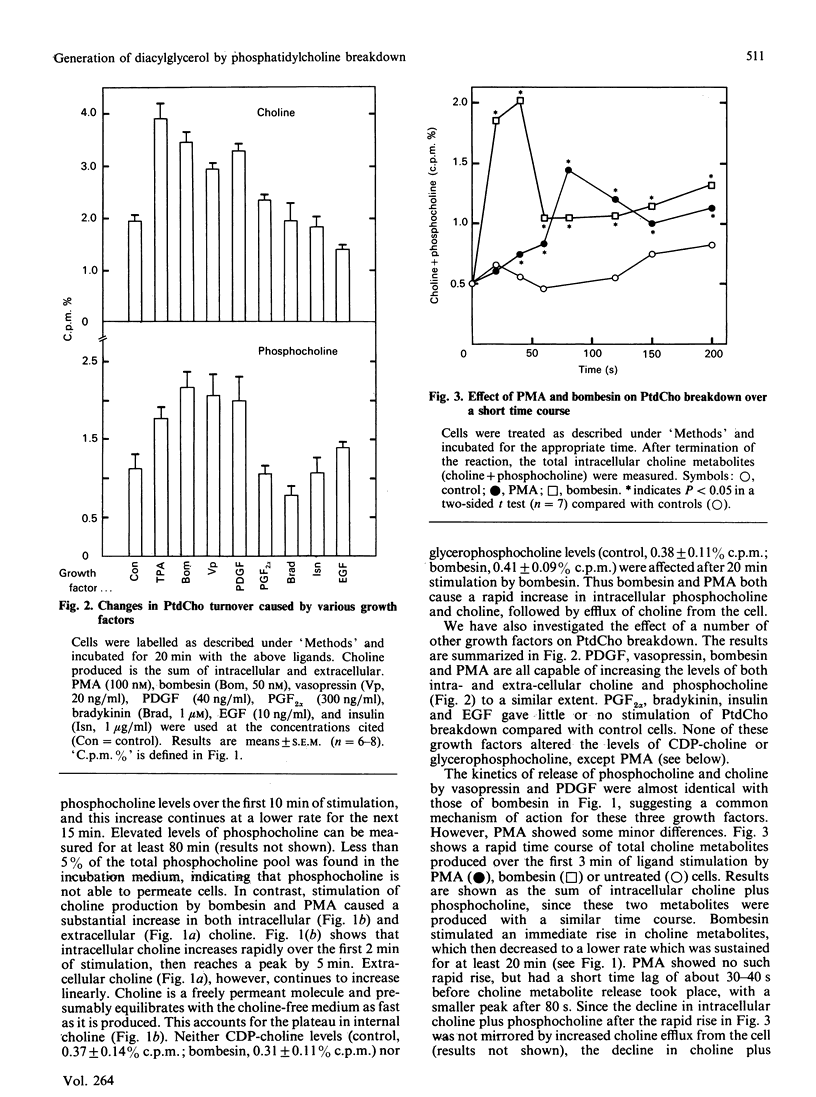
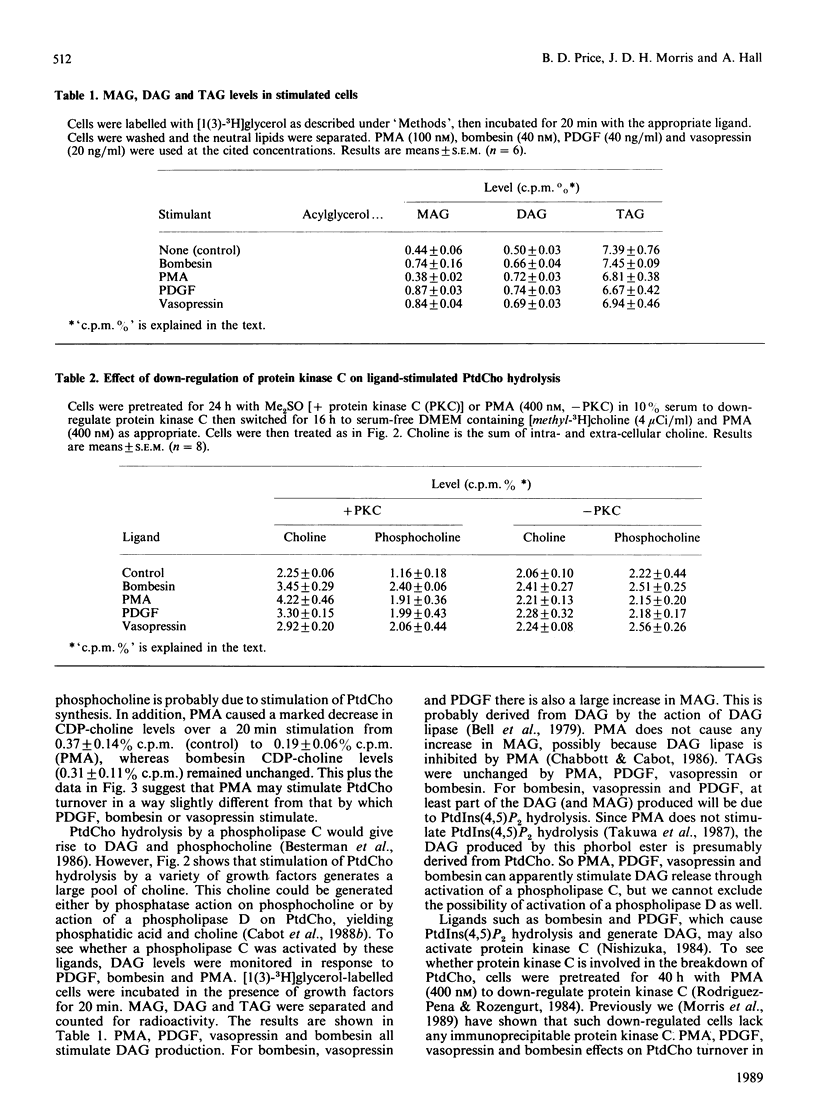
The effect of a number of growth factors on phosphatidylcholine (PtdCho) turnover in Swiss-3T3 cells was studied. Phorbol 12-myristate 13-acetate (PMA), bombesin, platelet-derived growth factor (PDGF) and vasopressin rapidly stimulated PtdCho hydrolysis, diacylglycerol (DAG) production, and PtdCho synthesis. Insulin and prostaglandin F2 alpha (PGF2 alpha) stimulated PtdCho synthesis, but not its breakdown, whereas epidermal growth factor (EGF) and bradykinin were without effect. Stimulation of PtdCho hydrolysis by the above ligands resulted in increased production of phosphocholine and DAG (due to phospholipase C activity) and significant amounts of choline, suggesting activation of a phospholipase D as well. CDP-choline and glycerophosphocholine levels were unchanged. Down-regulation of protein kinase C with PMA (400 nM, 40 h) abolished the stimulation of PtdCho hydrolysis and PtdCho synthesis by PMA, bombesin, PDGF and vasopressin, but not the stimulation of PtdCho synthesis by insulin and PGF2 alpha. PtdCho hydrolysis therefore occurs predominantly by activation of protein kinase C (either by PMA or PtdIns hydrolysis) leading to elevation of DAG levels derived from non-PtdIns(4,5)P2 sources. PtdCho synthesis occurs by both a protein kinase C-dependent pathway (stimulated by PMA, PDGF, bombesin and vasopressin) and a protein kinase C-independent pathway (stimulated by insulin and PGF2 alpha). DAG production from PtdCho hydrolysis is not the primary signal to activate protein kinase C, but may contribute to long-term activation of this kinase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman J. M., Duronio V., Cuatrecasas P. Rapid formation of diacylglycerol from phosphatidylcholine: a pathway for generation of a second messenger. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6785–6789. doi: 10.1073/pnas.83.18.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop W. R., Bell R. M. Functions of diacylglycerol in glycerolipid metabolism, signal transduction and cellular transformation. Oncogene Res. 1988 Feb;2(3):205–218. [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M., Hamon M. H., Laurie M. S., Corps A. N. Protein kinase C-mediated negative-feedback inhibition of unstimulated and bombesin-stimulated polyphosphoinositide hydrolysis in Swiss-mouse 3T3 cells. Biochem J. 1987 Aug 1;245(3):631–639. doi: 10.1042/bj2450631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabot M. C., Welsh C. J., Cao H. T., Chabbott H. The phosphatidylcholine pathway of diacylglycerol formation stimulated by phorbol diesters occurs via phospholipase D activation. FEBS Lett. 1988 Jun 6;233(1):153–157. doi: 10.1016/0014-5793(88)81374-7. [DOI] [PubMed] [Google Scholar]
- Cabot M. C., Welsh C. J., Zhang Z. C., Cao H. T., Chabbott H., Lebowitz M. Vasopressin, phorbol diesters and serum elicit choline glycerophospholipid hydrolysis and diacylglycerol formation in nontransformed cells: transformed derivatives do not respond. Biochim Biophys Acta. 1988 Mar 4;959(1):46–57. doi: 10.1016/0005-2760(88)90148-8. [DOI] [PubMed] [Google Scholar]
- Chabbott H., Cabot M. C. Phorbol diesters inhibit enzymatic hydrolysis of diacylglycerols in vitro. Proc Natl Acad Sci U S A. 1986 May;83(10):3126–3130. doi: 10.1073/pnas.83.10.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L., Parker P. J., Rhee L., Yang-Feng T. L., Chen E., Waterfield M. D., Francke U., Ullrich A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science. 1986 Aug 22;233(4766):859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- Daniel L. W., Waite M., Wykle R. L. A novel mechanism of diglyceride formation. 12-O-tetradecanoylphorbol-13-acetate stimulates the cyclic breakdown and resynthesis of phosphatidylcholine. J Biol Chem. 1986 Jul 15;261(20):9128–9132. [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Hesketh T. R., Morris J. D., Moore J. P., Metcalfe J. C. Ca2+ and pH responses to sequential additions of mitogens in single 3T3 fibroblasts: correlations with DNA synthesis. J Biol Chem. 1988 Aug 25;263(24):11879–11886. [PubMed] [Google Scholar]
- Kolesnick R. N., Paley A. E. 1,2-Diacylglycerols and phorbol esters stimulate phosphatidylcholine metabolism in GH3 pituitary cells. Evidence for separate mechanisms of action. J Biol Chem. 1987 Jul 5;262(19):9204–9210. [PubMed] [Google Scholar]
- Kolesnick R. N. Thyrotropin-releasing hormone and phorbol esters induce phosphatidylcholine synthesis in GH3 pituitary cells. Evidence for stimulation via protein kinase C. J Biol Chem. 1987 Oct 25;262(30):14525–14530. [PubMed] [Google Scholar]
- Lacal J. C., Moscat J., Aaronson S. A. Novel source of 1,2-diacylglycerol elevated in cells transformed by Ha-ras oncogene. Nature. 1987 Nov 19;330(6145):269–272. doi: 10.1038/330269a0. [DOI] [PubMed] [Google Scholar]
- Liscovitch M., Blusztajn J. K., Freese A., Wurtman R. J. Stimulation of choline release from NG108-15 cells by 12-O-tetradecanoylphorbol 13-acetate. Biochem J. 1987 Jan 1;241(1):81–86. doi: 10.1042/bj2410081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A. C., Paterson H. F., Morris J. D., Hall A., Marshall C. J. p21H-ras-induced morphological transformation and increases in c-myc expression are independent of functional protein kinase C. EMBO J. 1989 Apr;8(4):1099–1104. doi: 10.1002/j.1460-2075.1989.tb03479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M. L., Mack K. F., Richardson C. N., Glomset J. A. Regulation of diacylglycerol kinase reaction in Swiss 3T3 cells. Increased phosphorylation of endogenous diacylglycerol and decreased phosphorylation of didecanoylglycerol in response to platelet-derived growth factor. J Biol Chem. 1988 Jan 25;263(3):1575–1583. [PubMed] [Google Scholar]
- Morris J. D., Price B., Lloyd A. C., Self A. J., Marshall C. J., Hall A. Scrape-loading of Swiss 3T3 cells with ras protein rapidly activates protein kinase C in the absence of phosphoinositide hydrolysis. Oncogene. 1989 Jan;4(1):27–31. [PubMed] [Google Scholar]
- Muir J. G., Murray A. W. Bombesin and phorbol ester stimulate phosphatidylcholine hydrolysis by phospholipase C: evidence for a role of protein kinase C. J Cell Physiol. 1987 Mar;130(3):382–391. doi: 10.1002/jcp.1041300311. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Paddon H. B., Vance D. E. Tetradecanoyl-phorbol acetate stimulates phosphatidylcholine biosynthesis in HeLa cells by an increase in the rate of the reaction catalyzed by CTP:phosphocholine cytidylyltransferase. Biochim Biophys Acta. 1980 Dec 5;620(3):636–640. doi: 10.1016/0005-2760(80)90156-3. [DOI] [PubMed] [Google Scholar]
- Pai J. K., Siegel M. I., Egan R. W., Billah M. M. Phospholipase D catalyzes phospholipid metabolism in chemotactic peptide-stimulated HL-60 granulocytes. J Biol Chem. 1988 Sep 5;263(25):12472–12477. [PubMed] [Google Scholar]
- Pelech S. L., Paddon H. B., Vance D. E. Phorbol esters stimulate phosphatidylcholine biosynthesis by translocation of CTP:phosphocholine cytidylyltransferase from cytosol to microsomes. Biochim Biophys Acta. 1984 Oct 4;795(3):447–451. doi: 10.1016/0005-2760(84)90171-1. [DOI] [PubMed] [Google Scholar]
- Price B. D., Morris J. D., Marshall C. J., Hall A. Scrape-loaded p21ras down-regulates agonist-stimulated inositol phosphate production by a mechanism involving protein kinase C. Biochem J. 1989 May 15;260(1):157–161. doi: 10.1042/bj2600157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Rosoff P. M., Savage N., Dinarello C. A. Interleukin-1 stimulates diacylglycerol production in T lymphocytes by a novel mechanism. Cell. 1988 Jul 1;54(1):73–81. doi: 10.1016/0092-8674(88)90181-x. [DOI] [PubMed] [Google Scholar]
- Slivka S. R., Meier K. E., Insel P. A. Alpha 1-adrenergic receptors promote phosphatidylcholine hydrolysis in MDCK-D1 cells. A mechanism for rapid activation of protein kinase C. J Biol Chem. 1988 Sep 5;263(25):12242–12246. [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Rasmussen H. A tumour promoter, 12-O-tetradecanoylphorbol 13-acetate, increases cellular 1,2-diacylglycerol content through a mechanism other than phosphoinositide hydrolysis in Swiss-mouse 3T3 fibroblasts. Biochem J. 1987 May 1;243(3):647–653. doi: 10.1042/bj2430647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden C. H., Friedkin M. Regulation of phosphatidylcholine biosynthesis by mitogenic growth factors. Biochim Biophys Acta. 1984 Mar 7;792(3):270–280. doi: 10.1016/0005-2760(84)90194-2. [DOI] [PubMed] [Google Scholar]
- Whetton A. D., Monk P. N., Consalvey S. D., Huang S. J., Dexter T. M., Downes C. P. Interleukin 3 stimulates proliferation via protein kinase C activation without increasing inositol lipid turnover. Proc Natl Acad Sci U S A. 1988 May;85(10):3284–3288. doi: 10.1073/pnas.85.10.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavin E. Regulation of phospholipid metabolism in differentiating cells from rat brain cerebral hemispheres in culture. Patterns of acetylcholine phosphocholine, and choline phosphoglycerides labeling from (methyl-14C)choline. J Biol Chem. 1976 Mar 10;251(5):1392–1397. [PubMed] [Google Scholar]


