Abstract
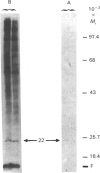
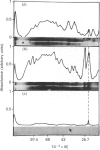
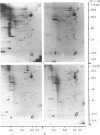
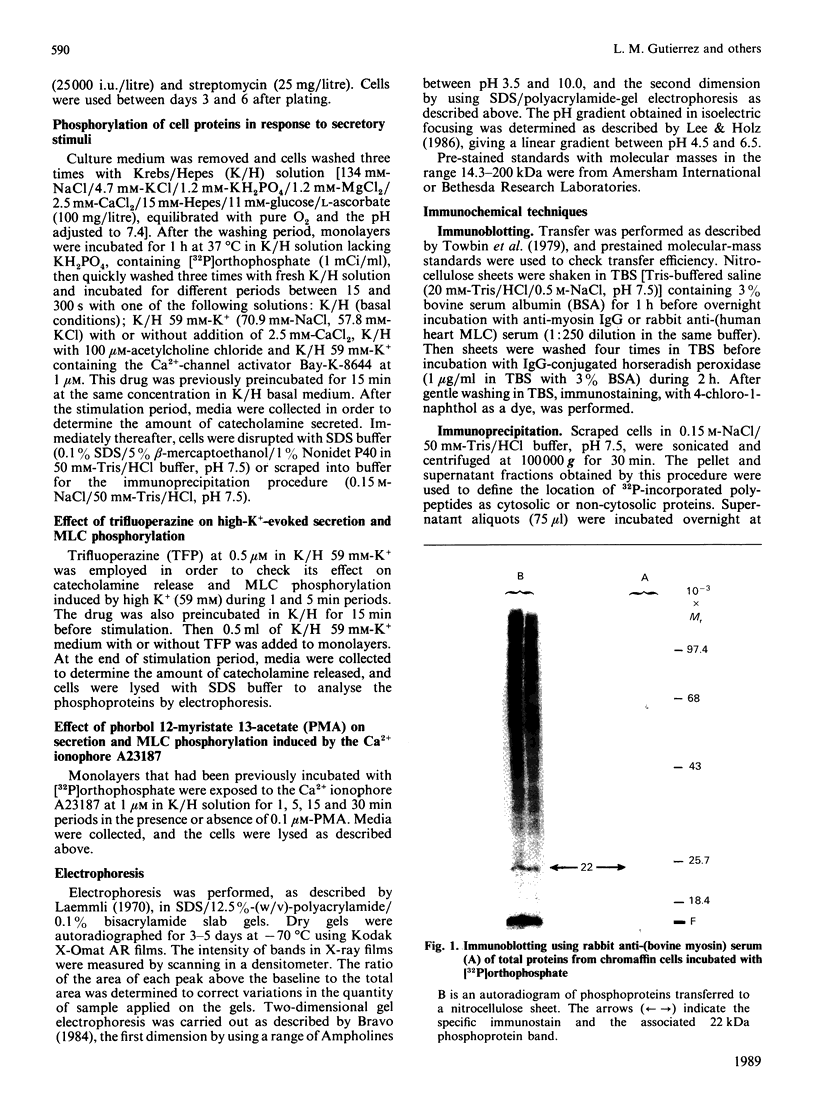
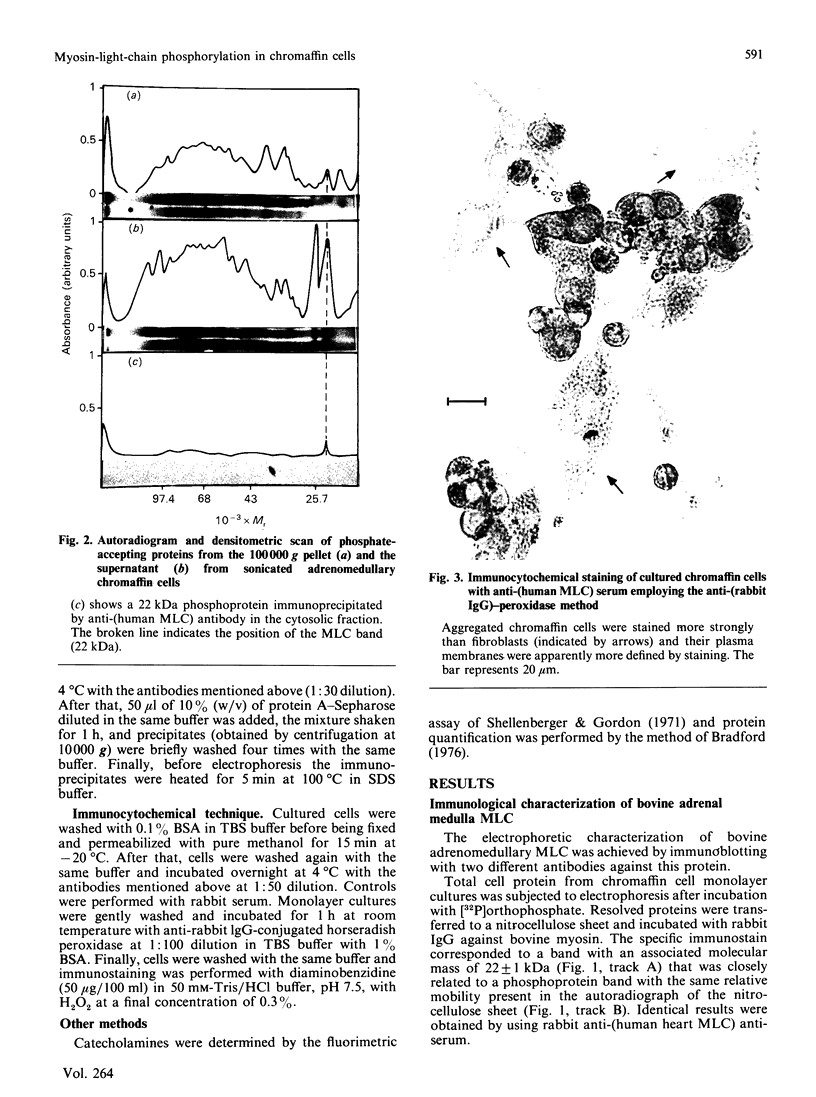
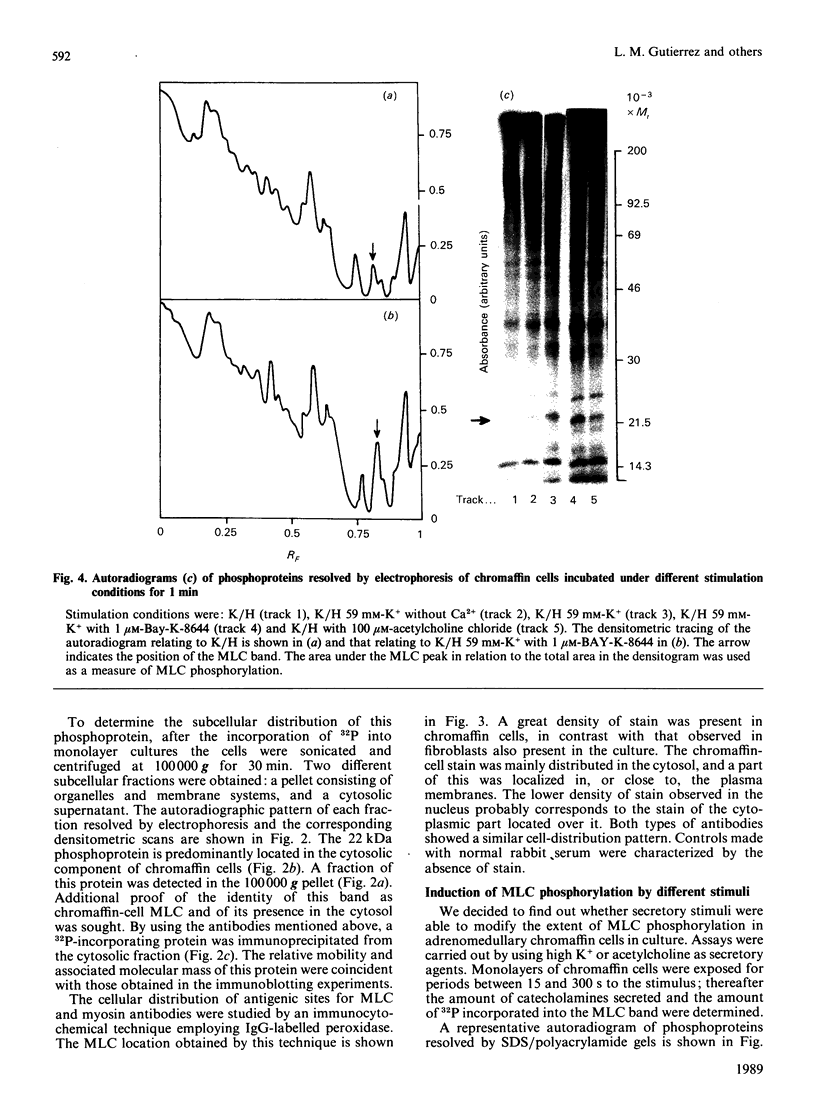
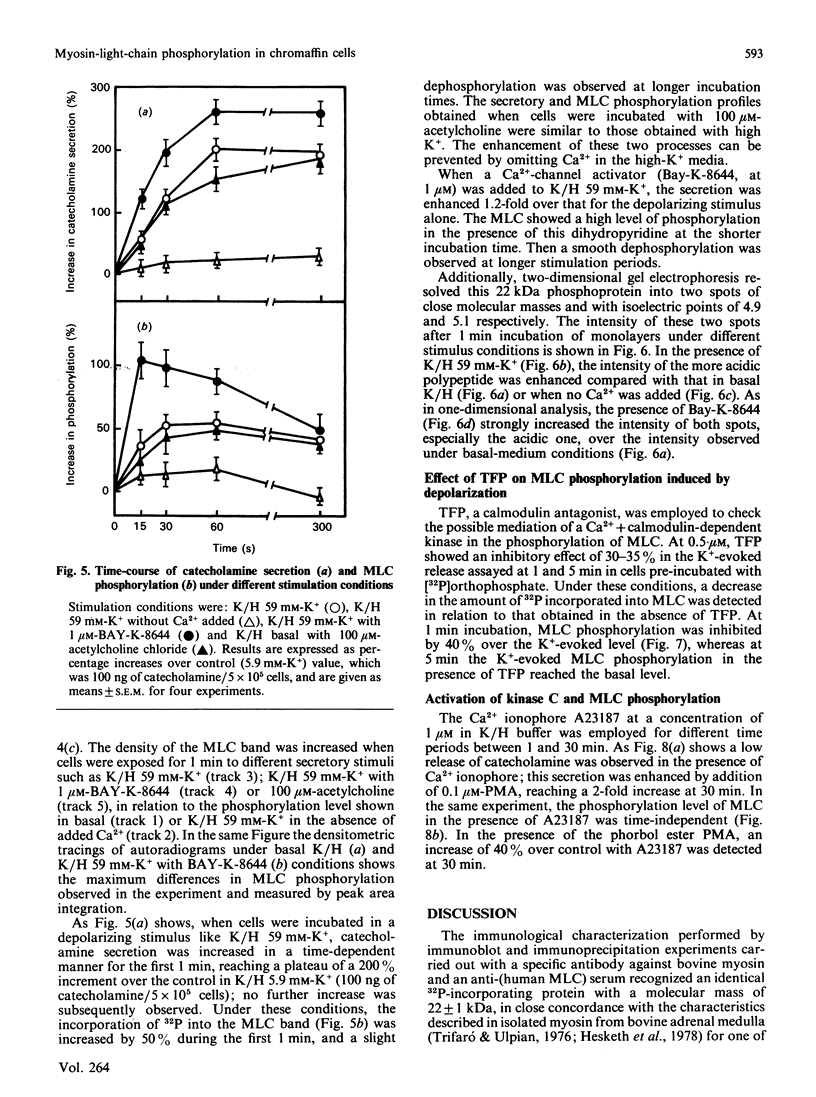
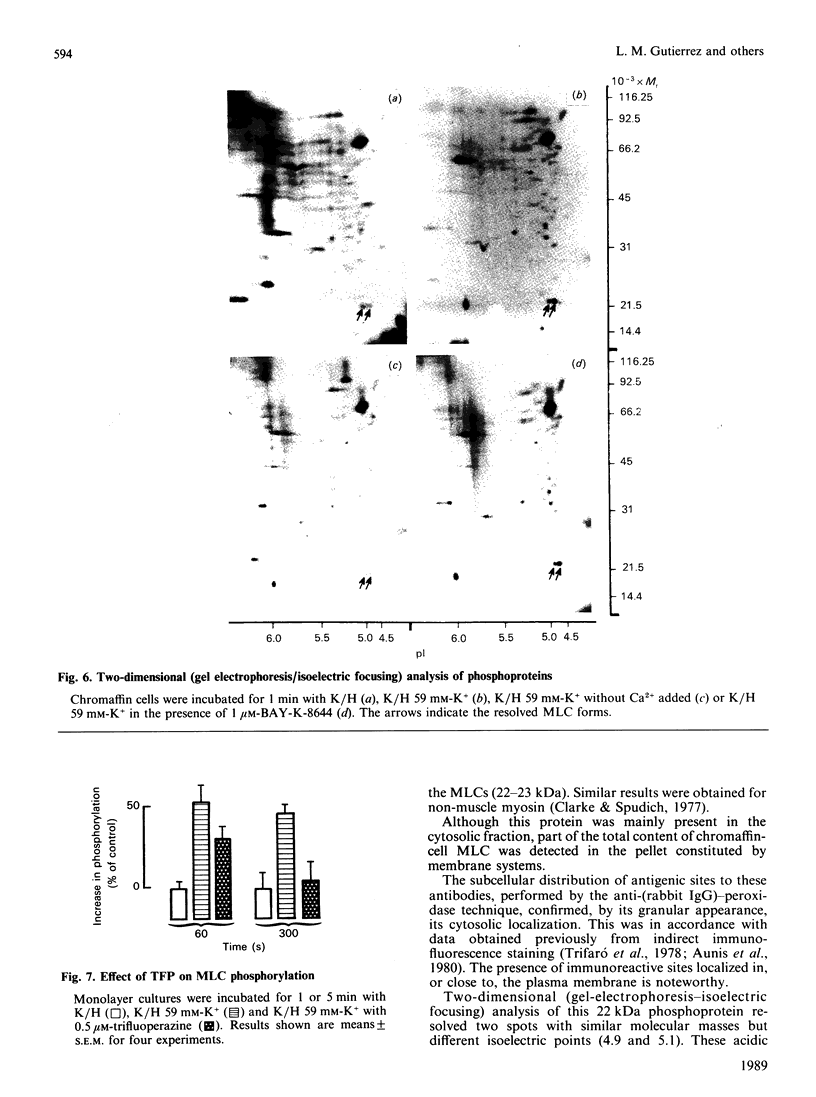
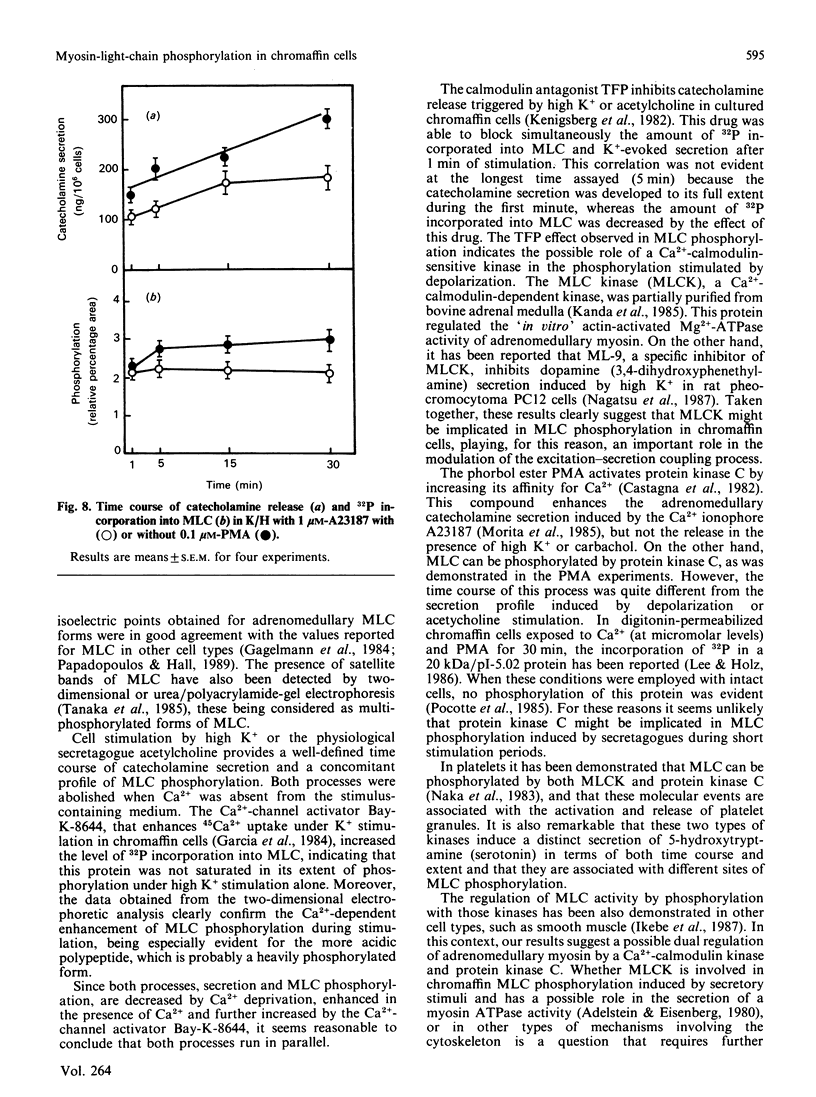
The myosin-light-chain (MLC) phosphorylation accompanying catecholamine release in chromaffin cells was investigated with the objective of assessing the possible role of this contractile protein in catecholamine secretion. The electrophoretic characteristics of adrenomedullary MLC were determined by immunochemical techniques using two different specific antibodies. The identified 22 kDa phosphoprotein was mainly present in the cytosol, as demonstrated by ultracentrifugation and immunocytochemical analysis. A part of this protein was located on, or close to, the plasma membrane. Cell stimulation by secretagogues resulted in a Ca2(+)-dependent 32P incorporation into MLC, the time course of this process being related to catecholamine release. These findings were supported by a two-dimensional gel-electrophoretic analysis by which means this protein was resolved into two acidic forms. A role for Ca2(+)-calmodulin and Ca2(+)-phospholipid kinases in adrenomedullary MLC phosphorylation is reported. The results obtained suggest a regulatory role for such a protein in the underlying exocytotic event.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Almazan G., Aunis D., García A. G., Montiel C., Nicolás G. P., Sánchez-García P. Effects of collagenase on the release of [3H]-noradrenaline from bovine cultured adrenal chromaffin cells. Br J Pharmacol. 1984 Apr;81(4):599–610. doi: 10.1111/j.1476-5381.1984.tb16124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy C. M., Kirshner N. Phosphorylation of adrenal medulla cell proteins in conjunction with stimulation of catecholamine secretion. J Neurochem. 1981 Mar;36(3):847–854. doi: 10.1111/j.1471-4159.1981.tb01671.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Geisow M. J. Phosphoproteins of the adrenal chromaffin granule membrane. J Neurochem. 1982 Nov;39(5):1387–1396. doi: 10.1111/j.1471-4159.1982.tb12582.x. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- Côté A., Doucet J. P., Trifaró J. M. Phosphorylation and dephosphorylation of chromaffin cell proteins in response to stimulation. Neuroscience. 1986 Oct;19(2):629–645. doi: 10.1016/0306-4522(86)90286-1. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo R. J., Freedman S. D. Calcium dependent neurotransmitter release and protein phosphorylation in synaptic vesicles. Biochem Biophys Res Commun. 1978 Jan 13;80(1):183–192. doi: 10.1016/0006-291x(78)91121-x. [DOI] [PubMed] [Google Scholar]
- Gagelmann M., Rüegg J. C., Di Salvo J. Phosphorylation of the myosin light chains and satellite proteins in detergent-skinned arterial smooth muscle. Biochem Biophys Res Commun. 1984 May 16;120(3):933–938. doi: 10.1016/s0006-291x(84)80196-5. [DOI] [PubMed] [Google Scholar]
- García A. G., Sala F., Reig J. A., Viniegra S., Frías J., Fontériz R., Gandía L. Dihydropyridine BAY-K-8644 activates chromaffin cell calcium channels. Nature. 1984 May 3;309(5963):69–71. doi: 10.1038/309069a0. [DOI] [PubMed] [Google Scholar]
- Gutierrez L. M., Ballesta J. J., Hidalgo M. J., Gandia L., García A. G., Reig J. A. A two-dimensional electrophoresis study of phosphorylation and dephosphorylation of chromaffin cell proteins in response to a secretory stimulus. J Neurochem. 1988 Oct;51(4):1023–1030. doi: 10.1111/j.1471-4159.1988.tb03063.x. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Lynham J. A. Relationship between phosphorylation of blood platelet proteins and secretion of platelet granule constituents. I. Effects of different aggregating agents. Biochem Biophys Res Commun. 1977 Jul 25;77(2):714–722. doi: 10.1016/s0006-291x(77)80037-5. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L., Kulczycki A., Jr, Parker C. W. Phosphorylation of the IgE receptor from ionophore A23187 stimulated intact rat mast cells. Biochem Biophys Res Commun. 1981 Feb 12;98(3):815–822. doi: 10.1016/0006-291x(81)91184-0. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Hartshorne D. J., Elzinga M. Phosphorylation of the 20,000-dalton light chain of smooth muscle myosin by the calcium-activated, phospholipid-dependent protein kinase. Phosphorylation sites and effects of phosphorylation. J Biol Chem. 1987 Jul 15;262(20):9569–9573. [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Kanda K., Sobue K., Kakiuchi S. Phosphorylation of myosin light chain and the actin-activated ATPase activity of adrenal medullary myosin. J Biochem. 1985 Mar;97(3):961–964. doi: 10.1093/oxfordjournals.jbchem.a135138. [DOI] [PubMed] [Google Scholar]
- Kenigsberg R. L., Côté A., Trifaró J. M. Trifluoperazine, a calmodulin inhibitor, blocks secretion in cultured chromaffin cells at a step distal from calcium entry. Neuroscience. 1982;7(9):2277–2286. doi: 10.1016/0306-4522(82)90138-5. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Kesteven N. T. Evoked transient intracellular free Ca2+ changes and secretion in isolated bovine adrenal medullary cells. Proc R Soc Lond B Biol Sci. 1983 May 23;218(1211):177–199. doi: 10.1098/rspb.1983.0033. [DOI] [PubMed] [Google Scholar]
- Krueger B. K., Forn J., Greengard P. Depolarization-induced phosphorylation of specific proteins, mediated by calcium ion influx, in rat brain synaptosomes. J Biol Chem. 1977 Apr 25;252(8):2764–2773. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. A., Holz R. W. Protein phosphorylation and secretion in digitonin-permeabilized adrenal chromaffin cells. Effects of micromolar Ca2+, phorbol esters, and diacylglycerol. J Biol Chem. 1986 Dec 25;261(36):17089–17098. [PubMed] [Google Scholar]
- Morita K., Brocklehurst K. W., Tomares S. M., Pollard H. B. The phorbol ester TPA enhances A23187--but not carbachol- and high K+-induced catecholamine secretion from cultured bovine adrenal chromaffin cells. Biochem Biophys Res Commun. 1985 Jun 14;129(2):511–516. doi: 10.1016/0006-291x(85)90181-0. [DOI] [PubMed] [Google Scholar]
- Nagatsu T., Suzuki H., Kiuchi K., Saitoh M., Hidaka H. Effects of myosin light-chain kinase inhibitor on catecholamine secretion from rat pheochromocytoma PC12h cells. Biochem Biophys Res Commun. 1987 Mar 30;143(3):1045–1048. doi: 10.1016/0006-291x(87)90357-3. [DOI] [PubMed] [Google Scholar]
- Naka M., Nishikawa M., Adelstein R. S., Hidaka H. Phorbol ester-induced activation of human platelets is associated with protein kinase C phosphorylation of myosin light chains. Nature. 1983 Dec 1;306(5942):490–492. doi: 10.1038/306490a0. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Tanaka T., Hidaka H. Ca2+-calmodulin-dependent phosphorylation and platelet secretion. Nature. 1980 Oct 30;287(5785):863–865. doi: 10.1038/287863a0. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V., Hall P. F. Isolation and characterization of protein kinase C from Y-1 adrenal cell cytoskeleton. J Cell Biol. 1989 Feb;108(2):553–567. doi: 10.1083/jcb.108.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocotte S. L., Frye R. A., Senter R. A., TerBush D. R., Lee S. A., Holz R. W. Effects of phorbol ester on catecholamine secretion and protein phosphorylation in adrenal medullary cell cultures. Proc Natl Acad Sci U S A. 1985 Feb;82(3):930–934. doi: 10.1073/pnas.82.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellenberger M. K., Gordon J. H. A rapid, simplified procedure for simultaneous assay of norepinephrine, dopamine, and 5-hydroxytryptamine from discrete brain areas. Anal Biochem. 1971 Feb;39(2):356–372. doi: 10.1016/0003-2697(71)90426-x. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Yamazaki K., Sobue K. Correlation between multiple phosphorylation of gizzard myosin light chains and actin-activated myosin ATPase activity. J Biochem. 1985 Jun;97(6):1823–1826. doi: 10.1093/oxfordjournals.jbchem.a135244. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifaró J. M., Ulpian C. Isolation and characterization of myosin from the adrenal medulla. Neuroscience. 1976 Dec;1(6):483–488. doi: 10.1016/0306-4522(76)90100-7. [DOI] [PubMed] [Google Scholar]
- Trifaró J. M., Ulpian C., Preiksaitis H. Anti-myosin stains chromaffin cells. Experientia. 1978 Dec 15;34(12):1568–1571. doi: 10.1007/BF02034678. [DOI] [PubMed] [Google Scholar]
- Wise B. C., Costa E. Ca2+ and phospholipid-dependent protein kinase activity and phosphorylation of endogenous proteins in bovine adrenal medulla. J Neurochem. 1985 Jul;45(1):227–234. doi: 10.1111/j.1471-4159.1985.tb05497.x. [DOI] [PubMed] [Google Scholar]