Abstract
Novel chimeric antigen receptor (CAR) T-cell approaches are needed to improve therapeutic efficacy in solid tumors. High-risk neuroblastoma is an aggressive pediatric solid tumor that expresses cell-surface GPC2 and GD2 with a tumor microenvironment infiltrated by CD16a-expressing innate immune cells. Here we engineer T-cells to express a GPC2-directed CAR and simultaneously secrete a bispecific innate immune cell engager (BiCE) targeting both GD2 and CD16a. In vitro, GPC2.CAR-GD2.BiCE T-cells induce GPC2-dependent cytotoxicity and secrete GD2.BiCE that promotes GD2-dependent activation of antitumor innate immunity. In vivo, GPC2.CAR-GD2.BiCE T-cells locally deliver GD2.BiCE and increase intratumor retention of NK-cells. In mice bearing neuroblastoma patient-derived xenografts and reconstituted with human CD16a-expressing immune cells, GD2.BiCEs enhance GPC2.CAR antitumor efficacy. A CAR.BiCE strategy should be considered for tumor histologies where antigen escape limits CAR efficacy, especially for solid tumors like neuroblastoma that are infiltrated by innate immune cells.
Subject terms: Paediatric cancer, Translational research, Cancer immunotherapy
GPC2 and GD2 have been described as immunotherapeutic targets in neuroblastoma. Here the authors engineer T cells to simultaneously express a GPC2-directed CAR and a bispecific innate immune cell engager targeting GD2 and CD16a, showing antitumor activity in neuroblastoma preclinical models.
Introduction
T-cells can be directed to tumor cells by expressing a chimeric antigen receptor (CAR)1. CAR T-cell therapies have shown unprecedented clinical responses against hematological malignancies2, but their efficacy against solid tumors remains limited. Solid tumors can escape CAR therapeutic pressure by inducing antigen downregulation, via inherent antigen heterogeneity or through the immunosuppressive tumor microenvironment (TME)3. To circumvent these barriers to sustained efficacy, next-generation CARs have been engineered to modify T-cell intracellular signaling to enable greater recognition of antigen-low tumors and maintain persistence4, target more than one tumor cell surface antigen simultaneously5 or locally deliver additional therapeutic agents to remodel the TME6–8. Since innate immune cell activation is required for optimal T-cell performance9,10, CAR T-cell-mediated delivery of cytokines11, nanobodies6 or bacterial factors12 that boost innate immune cell sustenance within the TME are especially attractive. Other therapeutic strategies that exploit innate immunity include bispecific engagers built from antibody fragments that target both tumor-associated antigens and activate receptors on natural killer (NK) cells or macrophages13, but their therapeutic combination with CAR T-cells has not been explored.
High-risk neuroblastoma is a pediatric tumor arising from neural crest progenitors that has a dismal prognosis despite intense multimodal cytotoxic therapies14. High-risk neuroblastoma tumors are in part characterized by a T-cell-excluded TME, but alternatively harbor a substantial infiltration of innate immune cells including macrophages, NK-cells and other myeloid cell populations15. Immunotherapy with monoclonal antibodies (mAb) against the disialoganglioside GD2, a mammalian glycolipid of neuroectodermal origin16, is now part of the standard of care for neuroblastoma patients17,18, with a mechanism of action reliant on engaging innate immune cells via their cell surface Fc-receptors (e.g., FcγRII and FcγRIII) to release cytotoxic granules and induce tumor cell killing19. Recently, adoptive CAR T-cell therapies against GD2 have also achieved objective responses in early-phase clinical trials20–23. However, targeting GD2 can be accompanied by off-tumor on-target toxicities due to GD2 expression on mature tissues (e.g., central nervous system, peripheral nerves16,24,25); and some tumors progress despite treatment with current GD2 immunotherapies23,26. Thus, identifying additional tumor-restricted antigens as well as improving the safety and efficacy of GD2-based therapies with more localized treatment approaches are critical to improve the treatment of children with high-risk neuroblastoma.
Our group recently identified glypican-2 (GPC2) as a cell surface immunotarget differentially expressed on neuroblastoma tumors with no expression on vital normal tissues27. GPC2 is a signaling co-receptor heparan sulfate proteoglycan that promotes neuroblastoma tumorigenesis and is transcriptionally regulated by MYCN27,28. We and others have identified several high-affinity and specific GPC2 binders and developed safe and effective therapies targeting this molecule, including antibody-drug conjugates28,29 and CAR T-cells30–33, one of which is currently being tested clinically (NCT05650749). However, although optimally tuned GPC2 CAR T-cells have shown potent antitumor activities in preclinical models, tumors can downregulate GPC2 under persistent CAR pressure facilitating tumor relapse30. In turn, bispecific CARs against GPC2 and other neuroblastoma-associated antigens such as B7-H3 have been shown to be feasible and might have important clinical implications34, but are solely reliant on inducing adoptive T-cell antitumor immunity. Furthermore, therapeutic co-targeting of GPC2 and GD2 has not been explored in neuroblastoma or other tumors expressing both antigens.
In this study, we present a CAR-based dual-targeting therapeutic approach for high-risk neuroblastoma. We hypothesized that T-cells could be engineered to express a GPC2.CAR and secret a bispecific innate immune cell engager (or BiCE) that targets GD2 and FcγRIIIa (or CD16a) to activate bystander NK-cells and macrophages in the TME to facilitate antitumor innate immunity. Herein we evaluate the safety and efficacy of this approach and present data with important implications for next-generation CAR T-cell design.
Results
GPC2.CAR-treated neuroblastoma cells have lower cell surface expression of GPC2 but maintain GD2 and upregulate innate immune cell ligands
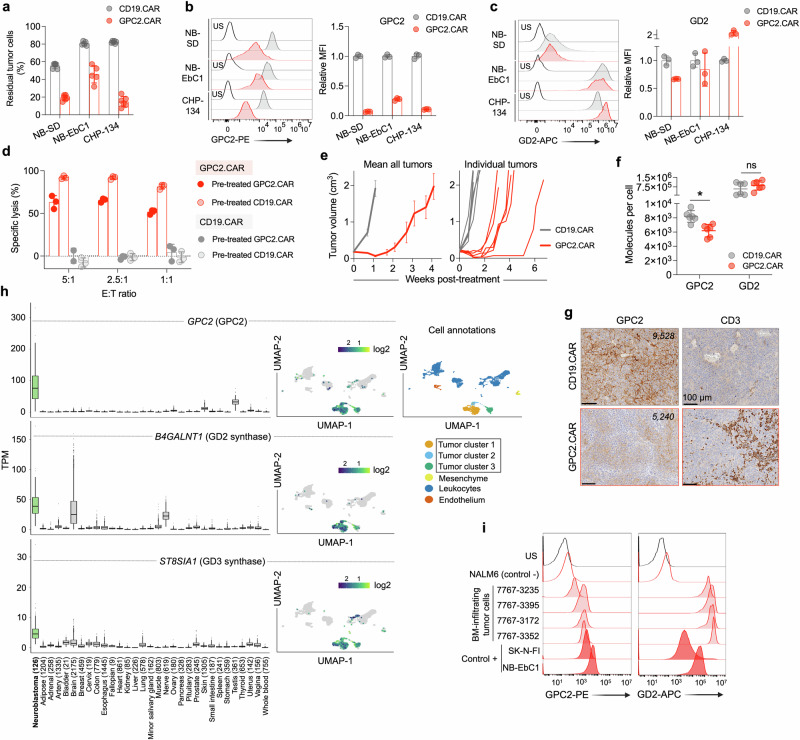
We previously showed that GPC2.CAR T-cells have potent antitumor effects in neuroblastoma preclinical models; however, a subset of tumors circumvent CAR pressure by the downregulation of cell surface GPC230. To validate these findings that used a retroviral GPC2 CAR derived from the D19 single-chain variable fragment (scFv), here we first generated a GPC2.CAR lentiviral vector using an alternative D3 scFv27,28, with a CD28 transmembrane and CD28 and CD3ζ signaling domains. After 24 hours of CAR T-cell co-incubation with target cells, D3-based GPC2 but not control CD19.CARs evoked potent neuroblastoma cell killing with substantial T-cell activation measured by IFN‐γ and IL-2 release (Supplementary Fig. 1a–c). However, stable GPC2.CAR pressure for 4 days utilizing a low 1:2.5 E:T ratio resulted in the persistence of residual tumor cells (Fig. 1a). Characterization of these cells revealed substantially reduced cell surface GPC2 but maintained expression of other neuroblastoma molecules such as GD2 (Fig. 1b, c), and a decreased response to GPC2.CAR re-exposure in vitro (Fig. 1d). Similarly, in mice bearing GPC2+ NB-EbC1 cell line xenografts, GPC2.CAR T-cells induced rapid tumor regressions (Fig. 1e); however, most of the tumors relapsed after 2-3 weeks with lower GPC2, but similar GD2 cell surface levels compared to control CD19 CAR-treated tumors (Fig. 1f, g and Supplementary Fig. 1d).
Fig. 1. GPC2.CAR-treated tumor cells downregulate GPC2 but maintain expression of GD2.
a Quantification of residual neuroblastoma cells after GPC2 or CD19 CAR T-cell co-incubation for 4 days [effector:tumor (E:T) ratio of 1:2.5]. Means and SDs are shown (n = 6 technical replicates). b (left) Flow cytometry histograms of GPC2 expression on neuroblastoma cells after GPC2 or CD19 CAR T-cell co-incubation in (a). (right) Fold-change GPC2 cell surface expression on GPC2.CAR vs. CD19.CAR-treated cells. Means and SDs are shown (n = 3 technical replicates). c (left) Flow cytometry histograms of GD2 expression on neuroblastoma cells after GPC2 or CD19 CAR T-cell co-incubation in (a). (right) Fold-change GD2 expression on GPC2.CAR vs. CD19.CAR-treated cells. Means and SDs are shown (n = 3 technical replicates). d CAR T-cell (CD19.CAR or GPC2.CAR) re-exposure assay after 24 hours of co-incubation with CHP-134 cells that were previously exposed to GPC2 or CD19.CAR T cells for 4 days at a 1:2.5 E:T ratio as shown in (a). Means and SDs are shown (n = 3 technical replicates). e (left) Serial tumor volumes of NB-EbC1 xenografts treated with CAR T-cells (CD19.CAR, n = 6 and GPC2.CAR, n = 8). Means and SEM are shown. (right) Individual volumes of tumors used to quantify GPC2 and GD2 expression by flow cytometry in (f). f Quantification of GPC2 and GD2 cell surface molecules from tumors in (e). *P = 0.004 (Mann Whitney t-test; two-tailed). Means and SDs are shown (n = 6). g GPC2 and CD3 immunohistochemistry (IHC) from a representative tumor from both control CD19.CAR and GPC2.CAR-treated mice in (e). Scale bars are indicated. Cell surface molecules determined by flow cytometry of the same tumors are indicated. h (left panels) Plots displaying GPC2, B4GALNT1 and ST8SIA1 expression in high-risk neuroblastoma patient tumors (n = 126) compared to normal tissue RNA sequencing data (n = 16,233 samples across 31 unique normal tissues, n = 9–2175 samples per tissue). N for each tissue is indicated. Box plots extend from first to third-quartile, horizontal line is the median and error bars represent the 1.5 interquartile range from the first-and third-quartile. (right panels) Single-cell RNA-seq plots showing single-cell expression of GPC2, B4GALNT1 and ST8SIA1 (6442 cells35). A UMAP defining the main cell identities is shown. i GPC2 and GD2 flow histograms from bone marrow-infiltrating neuroblastoma cells (n = 4). NALM6 cells were used as a negative control, and NB-EbC1 and SK-N-FI cells were used as positive controls. Gating strategies are shown in Supplementary Fig. 1e. US unstained, MFI mean fluorescence intensity. Represented data has been validated with at least 2 independent experiments. Source data are provided as a Source Data file.
Next, considering a dual GPC2 and GD2 targeting approach, we queried the expression of GPC2, and B4GALNT1 and ST8SIA1 [encoding GD2 and GD3 synthases, respectively, and both required for optimal GD2 surface exposure16]; in human neuroblastoma tumors compared to 30 different normal human tissues. GPC2 expression was high [mean of 48.7 Transcripts Per Million (TPM)] and tumor-restricted (Fig. 1h, left), as previously described27, whereas B4GALNT1 and ST8SIA1 were expressed in neuroblastomas (mean 36.8 and 9.2 TPM, respectively) but also in several neural-derived healthy tissues (Fig. 1h, left). We also examined the expression of GPC2, B4GALNT1 and ST8SIA1 in single-cell RNA-sequencing (scRNA-seq) data containing 6,442 cells from 4 neuroblastoma tumors at diagnosis35. Cell identity analysis identified 4 main clusters including endothelial, mesenchymal, immune and tumor cell populations, with only the tumor clusters expressing appreciable amounts of GPC2, B4GALNT1 and ST8SIA1 (Fig. 1h, right). To further study dual GPC2 and GD2 protein expression in primary neuroblastoma samples, we also obtained bone marrow (BM) aspirates from neuroblastoma patients (Supplementary Table 1). By flow cytometry, BM-infiltrating tumor cells (defined as CD45-/CD13-/NCAM1+; Supplementary Fig. 1e) were detected at significant levels in 4 cases and showed GPC2 and GD2 cell surface levels comparable to representative neuroblastoma cell lines (Fig. 1i).
Finally, since cytotoxic T-cell killing has been shown to induce immunogenic cell death and activate bystander endogenous immune cells including NK-cells36–38, we next studied whether GPC2.CAR T-cell pressure upregulated NK-cell activating ligands on neuroblastoma cells. Indeed, after 4 days of exposure to CAR T-cells, neuroblastoma cells that showed lower GPC2 levels and maintained robust GD2 on their surface also had increased levels of ULBP-1 and MICA/B (ligands for NKG2D; Supplementary Fig. 1f, g) and B7-H6 (ligand for NKp30; Supplementary Fig. 1h). In summary, we observed that neuroblastoma cells under GPC2.CAR T-cell pressure displayed lower GPC2, maintained GD2 and upregulated expression of NK-cell ligands, supporting engineering T-cells to target both GPC2 and GD2 while simultaneously exploiting the antitumor properties of NK and other innate immune cells in the neuroblastoma TME.
High-risk neuroblastoma tumors are infiltrated by CD16a-expressing innate immune cells
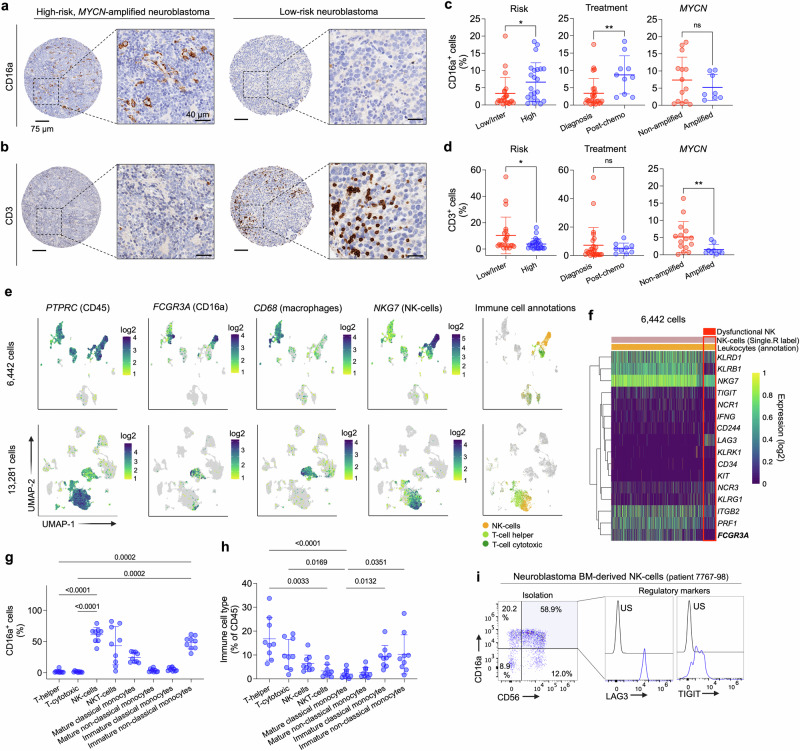
Previous studies have profiled the immune cell signatures in the human neuroblastoma TME and showed that high-risk tumors harbored an enriched infiltration of innate immune cells15. To further validate these studies, we next used a tumor microarray (TMA) containing 64 neuroblastoma tumors with different clinical features to evaluate both CD16a (Fig. 2a) and CD3 (Fig. 2b) expression, aiming to quantify the abundance of tumor-infiltrating innate immune cells and T-cells, respectively. In this neuroblastoma patient cohort, we observed that CD16a-positive cells infiltrated tumors at higher levels after treatment and in tumors from high-risk neuroblastoma patients independent of MYCN amplification (Fig. 2c), whereas CD3 showed increased staining in low- and intermediate-risk and MYCN non-amplified tumors, independent of previous treatment (Fig. 2d). To elucidate which specific CD16a-expressing innate immune cells were infiltrating the neuroblastoma TME, we next used 2 single-cell RNA-sequencing (scRNA-seq) datasets; one containing 6,442 cells from 4 neuroblastoma tumors at diagnosis (Fig. 2e, top) as described above and the other containing 13,281 cells from 16 pre-treated tumors, with 13 from high-risk patients (Fig. 2e, bottom)35. The immune cell clusters were first identified by the expression of the PTPRC gene (encoding CD45) and immune cell identities were further confirmed by Single-R, a reference-based tool for scRNA-seq data annotation39. Among PTPRC-expressing leukocytes, FCGR3A (encoding CD16a) was expressed in 2 different primary immune cell populations also expressing CD68 (macrophages) or NKG7 (NK-cells), but not in T-cells. In addition, since NK-cells infiltrating neuroblastoma tumors might display dysfunctional properties40, we further characterized the NK-cell cluster by gene expression profiling and identified a heterogeneous NK-cell population, including a small subgroup of NK-cells with an inhibitory phenotype expressing the regulatory marker LAG3, lacking PRF1 (encoding perforin), IFNG (IFN-γ) or NCR1 (activating receptor) and with lower FCGR3A (Fig. 2f). In addition, considering that the bone marrow is a major reservoir of metastatic neuroblastoma cells41, we also defined the abundance of CD16a-expressing immune cells in this tumor compartment by flow cytometry (Supplementary Fig. 2). CD16a-positive immune cells were detected in all neuroblastoma patient-derived bone marrow samples analyzed (Supplementary Table 1; n = 9; mean CD16a+ cells in total CD45+ cell population = 27%). NK-cells (defined as CD45+, CD3-, CD56+) and a subpopulation of cells defined as immature non-classical monocytes (CD45+, CD11b+, CD15-, CD14dim, HLA-DRdim) were the immune cell types expressing the highest levels of CD16a in the neuroblastoma-infiltrating BMs (Fig. 2g). NKT-cells (CD45+, CD3+, CD56+) and mature classical monocytes (CD45+, CD11b+, CD15-, CD14+, HLA-DR+) also expressed CD16a (Fig. 2g) but were detected at lower levels in the BM (Fig. 2h). Similar to the primary tumors analyzed by scRNA-seq, some NK-cells isolated from the BM niche of neuroblastoma patients expressed high levels of the inhibitory markers LAG3 and TIGIT (Fig. 2i). In summary, we demonstrated that the high-risk neuroblastoma TME and a principal metastatic niche (bone marrow) are infiltrated by a broad range of innate immune cell populations expressing CD16a.
Fig. 2. High-risk neuroblastoma tumors harbor a substantial infiltration of CD16a-expressing innate immune cells.
a CD16a IHC of representative high-risk (MYCN amplified; left) and low-risk (right) neuroblastoma tumors, respectively. Scales bars are indicated. b CD3 IHC of the same tumors shown in (a). Scales bars are indicated. c Percentage of CD16a+ cells in tumors stratified by International Neuroblastoma Risk Group (INRG) (low/intermediate; n = 22 vs. high; n = 23), previous chemotherapy treatment [diagnosis; n = 25 vs. post-chemo (only high-risk); n = 10] and MYCN amplification (non-amplified; n = 14 vs. amplified; n = 8) included in the TMA. *P = 0.038 and **P = 0.004 (Unpaired t-test; two-tailed). Means and SDs are shown. d Percentage of CD3+ cells in tumors stratified by INRG (low/intermediate; n = 22 vs. high; n = 24), previous chemotherapy treatment [diagnosis; n = 26 vs. post-chemo (only high-risk); n = 9] and MYCN amplification (non-amplified; n = 15 vs. amplified; n = 9) included in the TMA. *P = 0.034 and **P = 0.027 (Unpaired t-test; two-tailed). MS neuroblastomas were excluded from the analyses in (c and d). Means and SDs are shown. e Expression of PTPRC (encoding CD45), FCGR3A (encoding CD16a), CD68 (defining macrophages) and NKG7 (defining NK-cells) in 2 neuroblastoma single cell datasets (6442 and 13,281 cells, respectively). SingleR analysis was also used to label different cell types [NK-cells and T-cells (helper and cytotoxic); right]. f Heatmap showing single-cell expression profiles (n = 16 NK-related genes) in the subset of NK-cells identified in dataset in (e) (top). Red box indicates the subpopulation of NK-cells with potential dysfunctional properties. FCGR3A is highlighted in bold. g Percentage of CD16a-positive cells in immune cell subsets present in neuroblastoma-infiltrated bone marrows. P values are shown in graph (One-way ANOVA plus Dunn’s multiple comparison test). Means and SDs are shown (n = 9). h Immune cell types present in neuroblastoma-infiltrated bone marrows. Percentage of total CD45 cells is indicated. P values are shown in graph (One-way ANOVA plus Dunn’s multiple comparison test). Means and SDs are shown (n = 9). Gating strategies are shown in Supplementary Fig. 2. i (left) Dot plots showing co-expression of CD56 and CD16a on NK-cells isolated from a neuroblastoma-infiltrating patient BM specimen. (right) Flow cytometry histograms showing TIGIT and LAG3 levels on BM-derived NK-cells. For further clinical information see Supplementary Table 2. US unstained, inter intermediate, chemo chemotherapy. Source data are provided as a Source Data file.
Development and characterization of bicistronic GPC2 CAR vectors secreting GD2 bispecific innate immune cell engagers (BiCE)
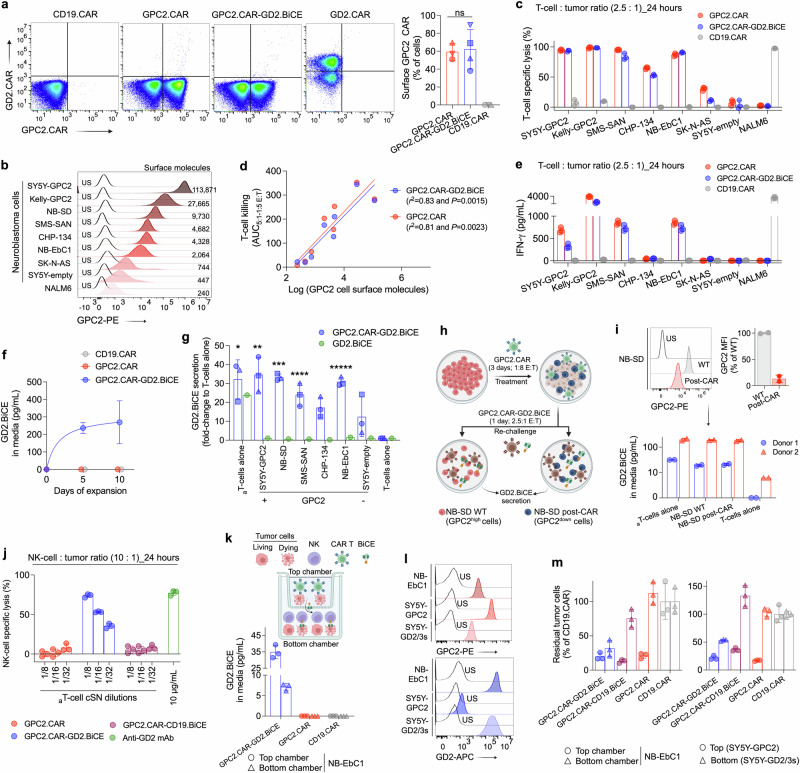
To address GPC2 and GD2 dual immunotherapeutic targeting while simultaneously exploiting the abundance of innate immune cells in the neuroblastoma TME, we designed a GPC2.CAR (identical to GPC2.CAR backbone described above) that secretes a BiCE against GD2 and CD16a (Fig. 3a, b). The BiCE regions were encoded from an scFv either against GD2 (GPC2.CAR-GD2.BiCE) or CD19 (control; GPC2.CAR-CD19.BiCE) linked to a single-domain antibody (sdAb) against human CD16a, previously characterized by our group42. BiCE sequences were preceded by an Igκ leader peptide to enhance cellular secretion and followed by a polyhistidine-tag (His-tag) for ease of detection and purification. We first confirmed robust secretion of GD2.BiCE from transfected HEK293T cells by His-tag western blot (Fig. 3c). By flow cytometry, GD2.BiCE selectively bound GD2+ neuroblastoma cells (but not GD2- leukemia cells) (Fig. 3d) in a concentration-dependent manner (Supplementary Fig. 3a) that directly correlated to neuroblastoma cell surface GD2 levels (Fig. 3e). GD2.BiCE bound to GD2-isogenic, but not wild-type (WT), HEK293T cells (Supplementary Fig. 3b), and binding was substantially abrogated when GD2 was blocked with the anti-GD2 chimeric antibody dinutuximab (Supplementary Fig. 3c). Additionally, in dual GD2-CD16a binding assays, GD2.BiCE was able to cross-link APC-tagged human recombinant CD16a protein and GD2+ NB-EbC1 neuroblastoma cells (Fig. 3f). Further, the anti-GD2 scFv idiotype 1A7 tagged with PE bound to CD16a+ human primary NK-cells (but not CD16a- T-cells) after incubation with GD2.BiCE (Fig. 3g). When co-cultured together with primary NK-cells from multiple healthy donors, supernatant from GPC2.CAR-GD2.BiCE-transfected HEK293T cells (but not control supernatant from GPC2.CAR-CD19.BiCE or from GPC2 CARs alone) evoked potent neuroblastoma cell killing similar to that observed with dinutuximab (Fig. 3h). This GD2.BiCE-mediated NK-cell cytotoxicity was dependent on GD2 expression (Supplementary Fig. 3d, e), and correlated with NK-cell:tumor ratio and GD2.BiCE concentration (Supplementary Fig. 3f, g). In addition, GD2.BiCE induced NK-cell activation (surface co-expression of CD69 and CD107a; Fig. 3i, and release of IFN-γ; Fig. 3j) and NK-cell polyfunctionality quantified using IsoPlexis single-cell proteomics technology, which is able to identify the unique, single NK-cells able to secret multiple cytokines simultaneously43 (Fig. 3k and Supplementary Fig. 3h, i). To confirm that the NK-cell induced cytotoxicity was dependent on CD16a engagement, we next demonstrated that only immortalized NK92 cells engineered to overexpress CD16a [but not CD16alow wild-type (WT) NK92 cells] efficiently killed GD2+ neuroblastoma cells when co-incubated with GD2.BiCE (Fig. 3l and Supplementary Fig. 3j), again with similar cytotoxicity to dinutuximab. Importantly, we also tested whether GD2.BiCE could engage NK-cells directly isolated from neuroblastoma-involved BM aspirates from 4 different neuroblastoma patients with confirmed tumor infiltration (Supplementary Table 2). Supernatant from GPC2.CAR-GD2.BiCE-transfected HEK293T cells activated neuroblastoma patient BM-isolated NK-cells (Fig. 3m, left), with signs of tumor cell killing in vitro (Fig. 3m, right). Finally, considering the potential role of other CD16a+ innate immune cells, we also demonstrated that GD2.BiCE induced phagocytosis of green-fluorescent protein (GFP)-tagged GD2+ neuroblastoma cells in co-culture assays with human monocyte-differentiated macrophages (Fig. 3n). In summary, we designed a bicistronic GPC2 CAR construct dictating expression of a GD2-directed BiCE that potently activates antitumor innate immunity in vitro.
Fig. 3. Development and characterization of bicistronic CAR vectors secreting bispecific innate immune cell engagers (BiCE).
a Graphical illustration of T-cell GPC2.CAR expression and GD2.BiCE secretion. b Schematic representation of transgenes for two BiCE-secreting GPC2.CAR constructs targeting GD2 and CD19 (GPC2.CAR-GD2.BiCE and GPC2.CAR-CD19.BiCE, respectively), as well as single GPC2.CAR, CD19.CAR and GD2.BiCE constructs. c Detection of GD2 BiCE by western blot (His-tag) in cSN from HEK293T cells transfected with GPC2.CAR-GD2.BiCE vector. d Binding assays detecting secondary His-tag expression on tumor cells incubated with cSN from HEK293T cells transfected with different constructs as indicated. e Correlation between GD2 BiCE binding and GD2 cell surface expression in NB cell lines. Spearman correlation (two-tailed). f Illustration (left) and histograms (right) of binding assay evaluating human recombinant CD16a protein binding to tumor cells incubated with different CAR construct cSNs. g Illustration (left) and histograms (right) of binding assay evaluating 1A7 binding to human primary NK-cells or T-cells incubated with different CAR construct cSNs. 1, control medium; 2, GPC2.CAR; 3, GPC2.CAR-CD19.BiCE; and 4, GPC2.CAR-GD2.BiCE in (d, f, and g). h NK-cell-mediated specific lysis of NB-EbC1 cells in the presence of cSNs (5 ng/mL of His-tagged BiCE), or dinutuximab (10 µg/mL) as a positive control. *P = 0.0009 and **P = 0.0028 (One-way ANOVA plus Tukey’s multiple comparison test). Means and SDs are shown (n = 3 independent donors). i Co-expression of CD107a and CD69 by flow cytometry in NK-cells from the cytotoxicity experiment in (h). *P = 0.0003 (One-way ANOVA plus Tukey’s multiple comparison test). Means and SDs are shown (n = 3 independent donors). j IFN-γ levels measured by ELISA in NK-cell supernatants from the cytotoxicity experiment in (h). *P = 0.0028 (One-way ANOVA plus Tukey’s multiple comparison test). Means and SDs are shown (n = 3 independent donors). k Polyfunctionality pie charts indicating the percentage of single NK-cells secreting 1 or more cytokine when cultured alone or in the presence of NB-EbC1 cells together with GD2 BiCE, CD19 BiCE or dinutuximab as in (h). l NK92 WT vs. NK92-CD16a cell cytotoxicity against NB-EbC1 cells co-incubated with different cSNs or dinutuximab (10 µg/mL) as a positive control after 24 hours at a 10:1 E:T ratio. Means and SDs are shown (n = 3 technical replicates). m (left) Percentage of CD107a+ NK-cells isolated from either neuroblastoma-infiltrating BM aspirates or peripheral blood of healthy donors after 24 hours of co-incubation with NB-EbC1 cells and cSNs (from GPC2.CAR-GD2.BiCE or GPC2.CAR-CD19.BiCE; 5 ng/mL). *P = 0.0139 and **P < 0.0001 (Paired, two-way ANOVA plus Šídák’s multiple comparisons test). (right) Quantification of residual tumor cells (CD45-/GD2+) in the same samples. A total of 4 BMs were utilized and shown with different symbols. The degree of opacity indicates the E:T ratio utilized for each BM sample (10:1, 5:1 and 2.5:1; lower to higher opacity). The same E:T ratios were utilized for healthy donor blood NK-cells to facilitate comparison between groups. n (left) Representative contour plots of phagocytosis assay with GFP-labeled NB-EbC1 cells exposed to different cSNs or dinutuximab (10 µg/mL) together with macrophages. Macrophages alone are shown to define GFP+/CD11b+ cells. (right) Quantification of NB-EbC1 phagocytosis. Means and SDs are shown (n = 3 technical replicates). TM transmembrane, neg negative, cSN concentrated supernatant, BM bone marrow, HD healthy donor. Represented data has been validated with at least 2 independent experiments. Figure 3a, f and g created with BioRender.com, and released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license. Source data are provided as a Source Data file.
GPC2.CAR-GD2.BiCE-transduced T-cells induce GPC2.CAR-mediated cytotoxicity and secrete GD2.BiCE to activate bystander antitumor innate immunity
We next addressed whether a lentiviral system could efficiently transfer sufficient CAR.BiCE bicistronic constructs to human primary T-cells to induce CAR and BiCE-directed cytotoxicity. Transduction efficiency using multiple healthy human T-cell donors measured by surface GPC2.CAR expression was similar for GPC2.CAR-GD2.BiCE and GPC2.CARs (Fig. 4a). T-cell phenotypic analysis at the end of T-cell expansion (day 12-15) indicated the presence of central and effector memory populations and low levels of markers of T-cell exhaustion in both bicistronic and single CARs (Supplementary Fig. 4a, b). The CAR-induced target cell cytotoxicity of GPC2.CAR-GD2.BiCE versus GPC2.CAR T-cells was compared in a panel of 8 luciferase-tagged neuroblastoma and control cell lines with heterogeneous levels of cell surface GPC2 (240-113,871 GPC2 molecules per cell; Fig. 4b). In co-culture assays, both GPC2.CAR-GD2.BiCE and GPC2.CAR T-cells induced substantial and comparable GPC2-dependent cytotoxicity of neuroblastoma cells (Fig. 4c, d and Supplementary Fig. 4c). Further, tumor GPC2 selectively activated GPC2.CAR-GD2.BiCE T-cells to induce the release of IFN-γ and IL-2, although at decreased levels compared to single GPC2.CARs (Fig. 4e and Supplementary Fig. 4d), possibly due to the lower CAR mean fluorescence intensity (MFI) observed for the bicistronic CAR construct (Supplementary Fig. 4e), as observed previously44.
Fig. 4. CAR.BiCE-transduced T-cells induce GPC2 CAR-mediated cytotoxicity and secrete GD2 BiCE to activate bystander innate immunity in vitro.
a (left) Cell surface expression of GPC2 CAR on primary human T-cells transduced with single CAR and bicistronic vectors at the end of T-cell expansion. (Right) Percent of CAR-positive cells for each vector (n = 4 donors for CAR.BiCE; n = 3 donors for single CARs). Means and SDs are shown. b Flow cytometry histograms showing GPC2 expression in luciferase-tagged tumor cell lines (n = 8 neuroblastoma and n = 1 leukemia as a negative control). Mean cell surface molecules are indicated. c CAR T-cell cytotoxicity assay for 7 neuroblastoma cell lines (ordered from high to low GPC2 expression) and negative control NALM6 (leukemia) cells. The cytotoxicity of a second T-cell donor is shown in Supplementary Fig. 4c. Means and SDs are shown (n = 3 technical replicates). d Correlation between GPC2.CAR and GPC2.CAR-GD2.BiCE T-cell cytotoxicity (measured as AUC of all tested E:T ratios; AUC5:1-1:5) vs. GPC2 expression on neuroblastoma cell lines. Spearman correlation (two-tailed). e IFN-γ release in culture supernatants of killing assays described in c. Means and SDs are shown (n = 3 technical replicates). f GD2.BiCE secretion in supernatants of human primary T-cells transduced with indicated CARs during T-cell expansion of 3 different donors represented as mean ± SD. g Fold-change GD2.BiCE secretion measured by ELISA of GPC2.CAR-GD2.BiCE and GD2.BiCE T-cells compared to T-cells alone. CAR.BiCE or BiCE T-cells co-incubated with αCD3/αCD28 beads plus cytokines [activated (a)T-cells alone] or GPC2-expressing neuroblastoma cells (n = 6; ordered from high to low GPC2 expression). *P = 0.0003, **P = 0.0001, ***P = 0.0002, ****P = 0.004 and *****P = 0.0004 (Two-way ANOVA plus Dunnett’s multiple comparison test compared to T-cells alone). Means and SDs are shown (n = 3 independent donors). h Graphical diagram showing GPC2.CAR treatment and subsequent re-challenge with GPC2.CAR-GD2.BiCE T-cells. i (upper panel) GPC2 expression in wild-type (WT) and GPC2.CAR-treated NB-SD cells (left) and % change in GPC2 MFI post-CAR treatment (right). Means and SDs are shown (n = 2). (lower panel) GD2.BiCE secretion in WT and GPC2.CAR pre-treated NB-SD cells after re-exposure to GPC2.CAR-GD2.BiCE T-cells. Two different donors of CAR.BiCE T-cells were used and means and SDs of technical replicates are shown (n = 2). aT-cell alone, activated T-cells alone with αCD28/αCD3 beads and IL-7/IL-15 cytokines. j NK-cell-mediated specific lysis of NB-EbC1 cells incubated with different dilutions of CAR.BiCE T-cell cSNs and human primary NK-cells. Dinutuximab was used as positive control. Means and SDs are shown (n = 3 technical replicates). k (upper graph) Graphical scheme of the Transwell assay. (lower graph) GD2.BiCE quantification in supernatants from top and bottom Transwell chambers. Means and SDs are shown (n = 3 technical replicates). l Flow cytometry histograms showing GPC2 and GD2 expression in the tumor cell lines utilized for the Transwell assays in (k). m Quantification of residual tumor cells in top (circles) and bottom chambers (triangles) using indicated CARs in top and NK-cells in the bottom chambers. Left graph shows NB-EbC1 (GPC2+/GD2+) in top and bottom chambers as target cells. Right graph shows SY5Y-GPC2 in the top (GPC2+/GD2low) and SY5Y-GD2/GD3 synthase in the bottom (GPC2low/GD2+) as target cells. Means and SDs are shown (n = 3 technical replicates). MFI mean fluorescence intensity, NS not significant, US unstained, AUC area under the curve, down downregulated. Represented data has been validated with at least 2 independent experiments. Figure 4h and k created with BioRender.com, released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license. Source data are provided as a Source Data file.
To accurately quantify GD2.BiCE in T-cell supernatants, we developed a sandwich ELISA with a limit of quantification of 1.25 pg/mL (Supplementary Fig. 4f). We next confirmed that only T-cells transduced with GPC2.CAR-GD2.BiCE constructs efficiently secreted GD2.BiCE during expansion (Fig. 4f). Co-culturing of GPC2.CAR-GD2.BiCE T-cells with 6 different GPC2-expressing neuroblastoma cells or αCD3/αCD28 beads plus IL-15/IL-7 cytokines [activated (a)T-cells alone] increased GD2.BiCE secretion 12 to 35-fold compared to T-cells alone (Fig. 4g), which was not the result of differential T-cell proliferation at this early 24-hour timepoint (Supplementary Fig. 4g). For these studies, we also utilized a single GD2.BiCE construct without the GPC2.CAR component and observed that GD2.BiCE secretion was only enhanced in the presence of beads and cytokines, but not when co-cultured with GPC2+ target cells (Fig. 4g), suggesting a T-cell activation-specific enhancement of GD2.BiCE secretion. No significant changes were observed in CAR cell surface expression after T-cell stimulation (Supplementary Fig. 4h), and by qPCR, expression of BiCE and CAR transgenes were comparable (Supplementary Fig. 4i), confirming a specific increase only in GD2.BiCE protein secretion upon T-cell activation. As further validation of this phenomenon, in a time-course experiment co-culturing GPC2.CAR-GD2.BiCE T-cells with NB-EbC1 target cells for 7 days, we observed that GD2.BiCE secretion was associated with IFN-γ release at early timepoints, again independent of the T-cell proliferation that occurred later (days 5-7; Supplementary Fig. 4j, k). Importantly, we observed that previous exposure of neuroblastoma cells to GPC2.CARs resulting in reduction of cell surface GPC2 did not affect GD2.BiCE secretion upon re-challenge with GPC2.CAR-GD2.BiCE T-cells (Fig. 4h, i), confirming maintained GD2.BiCE secretion even when engaging antigen-downregulated or lower-expressing tumor cells. Next, we observed that GD2.BiCE containing supernatants from activated GPC2.CAR-GD2.BiCE T-cells co-incubated with human primary NK-cells induced selective and robust killing of GD2-expressing NB-EbC1 cells (Fig. 4j). Finally, we evaluated whether GPC2.CAR-GD2.BiCE T-cells could induce bystander NK-cell activation in situ using Transwell assays, where CAR T-cells were plated with neuroblastoma cells in the top chambers and NK-cells together with neuroblastoma cells in the bottom chambers, allowing diffusion of GD2.BiCE between chambers (Fig. 4k). GPC2.CAR, GPC2.CAR-CD19.BiCE or GPC2.CAR-GD2.BiCE T-cells induced similar killing of GPC2+ neuroblastoma cells in the top chambers, but only GPC2.CAR-GD2.BiCE efficiently crosslinked tumor and NK-cells in the bottom chambers to induce NK-cell-mediated killing of GPC2+/GD2+ NB-EbC1 or GPC2low/GD2+ SY5Y cells (Fig. 4l, m). Taken together, GPC2.CAR-GD2.BiCE T-cells maintain comparable GPC2.CAR-specific cytotoxicity to GPC2.CAR T-cells alone and simultaneously secret GD2.BiCE that targets GD2-expressing cells to induce bystander antitumor innate immunity.
GPC2.CAR-GD2.BiCE T-cells locally release GD2.BiCE and enhance intratumor NK-cell retention in vivo
We next used mice bearing GPC2+/GD2+ neuroblastoma patient-derived xenografts (PDX; COG-N-561x) to address whether in vivo administration of GPC2.CAR-GD2.BiCE T-cells could i) selectively deliver GD2.BiCE to the tumor bed and ii) enhance intratumor retention of human CD16+ NK-cells. First, we quantified GD2.BiCE concentrations in tumors and murine normal tissues after one intravenous dose of 10 million CAR+ T-cells and compared these data to the tissue biodistribution of the chimeric monoclonal GD2 antibody dinutuximab given at a clinically translatable regimen45 (Fig. 5a). GD2.BiCE concentrations were significantly higher in the tumor (11.03 ± 4.09 pg per mg of tissue protein; mean ± SD) compared to mouse normal tissues (0.94 ± 0.95 pg/mg; mean ± SD; Fig. 5b), and no GD2.BiCE was detected in mouse plasma (Supplementary Fig. 5a). GD2.BiCE biodistribution was significantly more tumor restricted than that of dinutuximab (120.87 ± 37.74 ng/mg in tumor vs. 160.04 ± 104.70 ng/mg in mouse normal tissues; mean ± SD; Fig. 5c), which also showed significant levels in mouse plasma (Supplementary Fig. 5b). Tumor-specific GD2.BiCE release was higher at early (day 5) compared to later timepoints (day 7) post T-cell infusion (Supplementary Fig. 5c). In GPC2.CAR-GD2.BiCE T-cell-treated mice, we also identified a substantial infiltration of activated human T-cells in tumors (Fig. 5d, e and Supplementary Fig. 5d), validating robust CAR T-cell targeting to GPC2+ neuroblastoma PDXs. We additionally observed infiltration of human T-cells in the mouse lung and spleen (Fig. 5d, e and Supplementary Fig. 5d), consistent with normal trafficking of human T cells in NSG mice46. However, only measurable levels of GD2.BiCE were found in the tumor (Fig. 5b), together validating our in vitro observation that T-cell activation enhances GD2.BiCE secretion (Fig. 4g and Supplementary Fig. 4g–k). Further, in plasma samples from GPC2.CAR-GD2.BiCE T-cell-treated mice, we detected high levels of human cytokines related to T-cell activation and cytotoxicity using multiplexed bulk proteomics (Supplementary Fig. 5e). To understand the effect of GD2.BiCE on intratumor NK-cell abundance, we next intravenously infused COG-N-561x PDX-bearing animals with either 10 million GPC2.CAR-GD2.BiCE or control GPC2.CAR-CD19.BiCE T-cells, followed by intravenous (recruitment) or intratumor injection (retention) of luciferase-labeled CD16-overexpressing NK92 cells 4 days later (Fig. 5f and Supplementary Fig. 5f). NK92 tumor recruitment and retention was monitored by serial luciferase imaging (Fig. 5g and Supplementary Fig. 5g). In the recruitment study, intravenous NK92 cells rapidly homed and were captured into the lungs at 3 hours post injection with minimal signal in the tumor tissue at later timepoints in both groups (Supplementary Fig. 5g). However, in the retention study, NK92 luciferase signal was higher at early timepoints (24 hours) and was significantly prolonged (up to 96 hours) in GPC2.CAR-GD2.BiCE compared to GPC2.CAR-CD19.BiCE treated animals (Fig. 5g, h). The difference in intratumor NK92 cell retention was not the result of differences in T-cell proliferation which was similar for both treatment cohorts (Supplementary Fig. 5h), validating a specific role for GD2.BiCE in the noted differences in NK92 cell tumor retention kinetics. Altogether, these studies demonstrate that GPC2.CAR-GD2.BiCE T-cells locally release GD2.BiCE in the tumor bed to promote GD2.BiCE-mediated intratumor retention of NK-cells.
Fig. 5. CAR.BiCE T-cells locally release GD2.BiCE and enhance accumulation of NK-cells in the tumor bed.
a Schematic in vivo protocol for the biodistribution/pharmacokinetic study of GD2.BiCE compared to the GD2 antibody dinutuximab in mice. b GD2.BiCE levels (pg per mg of total tissue protein) in tumors and mouse normal tissues after CAR.BiCE T-cell infusion (n = 13; except brainstem n = 12). Means and SDs are shown. ****P < 0.0001 (One-way ANOVA plus Dunnett’s multiple comparison test). c GD2 mAb levels (ng per mg of total tissue protein) in the same tissues as b, harvested at day 1 (circles), 2 (squares) and 3 (triangles) after the last dose of dinutuximab (tumor n = 9; spleen=10; lung, heart, cortex n = 11; remaining organs n = 12). Means and SDs are shown. *P = 0.016 and **P = 0.025, #P = 0.0001, ##P < 0.0001 and ###P = 0.0040. (*; tumor vs. normal, #; normal vs. tumor) (One-way ANOVA plus Dunnett’s multiple comparison test). d Quantification of human CD3-positive cells in IHC-stained samples from the biodistribution study in (a). Means and SEMs are shown (n = 3). Circles, squares and triangles indicate 5, 6 and 7 day timepoints, respectively, after GPC2.CAR-GD2.BiCE T-cell infusion in (b and d). e CD3 IHC from tissues from a representative case from biodistribution assay of GPC2.CAR-GD2.BiCE T-cell-treated mice in (a). Scale bars are indicated. f Schematic in vivo protocol for the pharmacodynamics NK92 cell tumor accumulation study. g Serial IVIS imaging of mice bearing neuroblastoma PDXs after intratumoral NK92-CD16a-luc injection and intravenous infusion of GPC2.CAR-GD2.BiCE (n = 5) or GPC2.CAR-CD19.BiCE T-cells (n = 4). h (left) Serial quantification of NK92 cell retention in GPC2.CAR-GD2.BiCE and GPC2.CAR-CD19.BiCE-treated animals from g. Mean, SEM and substrate inhibition model curves are shown. (right) AUC3-96h quantification from previous curves. Mean and SD are shown. *P = 0.015 (Mann–Whitney t-test). PDX patient-derived xenograft, ip intraperitoneal, iv intravenous, it intratumor, AUC area under the curve, luc luciferase, h hours. Source data are provided as a Source Data file.
GD2.BiCEs enhance the efficacy of GPC2.CARs in neuroblastoma PDX mouse models reconstituted with human innate immune cells
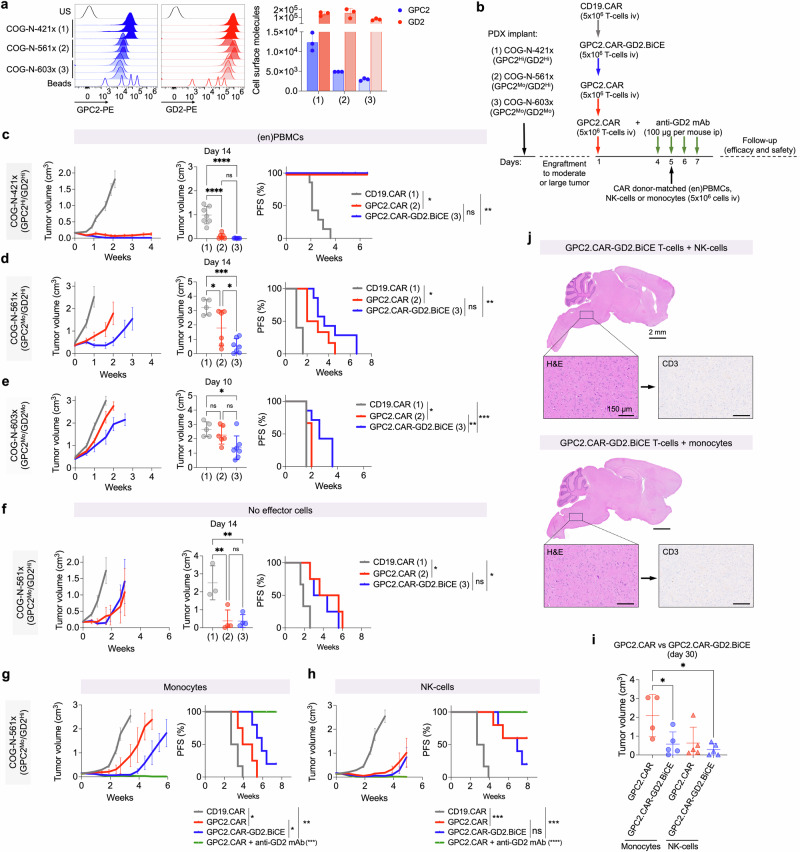
We next evaluated whether GD2.BiCE could enhance the in vivo efficacy of GPC2.CARs in neuroblastoma PDX models engrafted with human innate immune cells. We selected COG-N-421x, COG-N-561x and COG-N-603x neuroblastoma PDX models for these in vivo assays because they had varied levels of cell surface GPC2 and GD2 (Fig. 6a). Since GD2.BiCE does not cross-react with mouse CD16 (Supplementary Fig. 6a), we also reconstituted NSG mice with human CD16a-expressing innate immune cells.
Fig. 6. GD2.BiCEs enhance GPC2.CAR efficacy in human neuroblastoma PDX mouse models reconstituted with human innate immune cells.
a Flow cytometry histograms showing GPC2 and GD2 expression (left) and cell surface molecules (right) on neuroblastoma COG-N-421x (1), COG-N-561x (2) and COG-N-603x (3) PDX models. Means and SDs are shown (n = 3). b Schematic representation for the in vivo protocol used for efficacy studies in (c–h). (en)PBMCs, CD16a-enriched PBMCs. c COG-N-421x average tumor growth curves (left), tumor volume at day 14 (middle; ****P < 0.0001; One-way ANOVA plus Tukey’s multiple comparison test), and progression-free survival (PFS; right; *P = 0.0003 and **P = 0.0001; log-rank test). N = 7, 6 and 7 for CD19.CAR, GPC2.CAR and GPC2.CAR-GD2.BiCE, respectively, in the presence of (en)PBMCs as effector cells. Means and SEMs are shown. d COG-N-561x average tumor growth curves (left), tumor volume at day 14 (middle; *P = 0.04 and ***P = 0.0002; One-way ANOVA plus Tukey’s multiple comparison test) and PFS (right; *P = 0.0011 and **P = 0.0005; log-rank test). N = 5, 6 and 7 for CD19.CAR, GPC2.CAR and GPC2.CAR-GD2.BiCE, respectively, in the presence of (en)PBMCs as effector cells. Means and SEMs are shown. e COG-N-603x average tumor growth curves (left), tumor volume at day 10 (middle; *P = 0.0156; One-way ANOVA plus Tukey’s multiple comparison test) and PFS (right; *P = 0.029, **P = 0.021 and ***P = 0.0051; log-rank test). N = 5, 6 and 7 for CD19.CAR, GPC2.CAR and GPC2.CAR-GD2.BiCE, respectively, in the presence of enriched PBMCs as effector cells. Means and SEMs are shown. f COG-N-561x average tumor growth curves (left), tumor volume at day 14 (middle; **P = 0.006; One-way ANOVA plus Tukey’s multiple comparison test) and PFS (right; *P = 0.0288; log-rank test). N = 3, 4 and 4 for CD19.CAR, GPC2.CAR and GPC2.CAR-GD2.BiCE, respectively, without the infusion of immune effector cells. Means and SEMs are shown. g COG-N-561x average tumor growth curves (left) and PFS [right; *P < 0.05, **P = 0.0009 and ***P < 0.0001 (GPC2.CAR + GD2 mAb vs. all groups); log-rank test]. N = 6, 5, 6 and 5 for CD19.CAR, GPC2.CAR, GPC2.CAR-GD2.BiCE and GPC2.CAR + GD2 mAb, respectively, with monocytes as immune effector cells. Means and SEMs are shown. h COG-N-561x average tumor growth curves (left) and PFS [right; ***P = 0.0009 and ****P < 0.0001 (GPC2.CAR + GD2 mAb vs. all groups); log-rank test]. N = 6, 6, 6 and 6 for CD19.CAR, GPC2.CAR, GPC2.CAR-GD2.BiCE and GPC2.CAR + GD2 mAb, respectively, with NK-cells as immune effector cells. Means and SEMs are shown. i Tumor volumes comparing treatment groups in (g and h) at day 30 post CAR T-cell infusion. (*P < 0.05; one-way ANOVA plus Tukey’s multiple comparison test; mean ± SEM; n = 4 GPC2.CAR+ monocytes; n = 5 all other groups). j Brain histology (H&E) and T-cell infiltration (CD3 IHC) of the brains of mice bearing subcutaneous COG-N-561x PDX models treated with systemic GPC2.CAR-GD2.BiCE T-cells and either NK-cells (top) or monocytes (bottom) as effector cells. Scale bars are indicated. Brainstem regions are shown in higher magnification. Representative image of one independent mouse per group. US unstained, HI high, MO moderate, iv intravenous, ip intraperitoneal. Source data are provided as a Source Data file.
In the first set of in vivo efficacy studies, we infused a modest amount of CAR+ T-cells (5 million) on day 1, followed by a second infusion of 5 million donor-matched CD16a-enriched PBMCs (Fig. 6b) on day 5, at which point we identified substantial intratumor GD2.BiCE release (Supplementary Fig. 5c). These PBMCs were previously manipulated to deplete T-and B-cells, obtaining an enriched CD16a+ cell population of 70% (compared to the 20% observed in total PBMCs) that was composed of monocytes (59.7%) and NK-cells (36.0%; Supplementary Fig. 6b). In the COG-N-421x PDX model (GPC2Hi/GD2Hi), both GPC2.CAR-GD2.BiCE and GPC2.CAR T-cells induced complete responses (CR) in all treated mice with moderate starting tumor volumes (0.14-0.2 cm3), extending animal survival up to 6 weeks post-T-cell infusion (Fig. 6c). However, in lower GPC2-expressing COG-N-561x PDXs (GPC2Mo/GD2Hi) with larger tumor volumes at study enrollment (0.2-0.5 cm3), GPC2.CAR-GD2.BiCE T-cells induced enhanced tumor regression versus GPC2.CARs alone and resulted in similar progression-free survival (PFS; Fig. 6d). Finally, in a PDX model expressing moderate levels of GPC2 and GD2 (COG-N-603x; GPC2Mo/GD2Mo) with large starting PDX volumes (0.3-0.6 cm3), both CD19.CAR and single GPC2.CAR-treated animals displayed rapid tumor progression, whereas GPC2.CAR-GD2.BiCE T-cells significantly improved tumor control and extended PFS (Fig. 6e). No clinically apparent toxicities were observed in either CAR or CAR.BiCE treatment groups and serial body weights were comparable between both GPC2 CARs and control cohorts in these studies (Supplementary Fig. 6c).
In a second set of in vivo efficacy studies, we selected the COG-N-561x PDX model (GPC2Mo/GD2Hi) to 1) define which innate effector cell (NK-cells, monocytes) would lead to the most enhanced efficacy of GPC2.CAR-GD2.BiCE compared to GPC2 CAR T-cells alone and 2) evaluate whether GPC2.CAR-GD2.BiCE CAR T-cells could induce similar antitumor activity compared to combining single GPC2 CARs with GD2-targeting dinutuximab. First, we observed that CAR and CAR.BiCE T-cells displayed similar activity without the infusion of effector cells (Fig. 6f). Second, when monocytes were used solely as effector cells, GPC2.CAR-GD2.BiCE had increased antitumor activity compared to GPC2.CARs (Fig. 6g), whereas NK-cells increased the antitumor activity of both single CARs and bicistronic constructs (Fig. 6h). Indeed, GPC2.CAR-GD2.BiCE T-cells plus either NK-cells or monocytes and GPC2.CARs alone plus NK-cells both displayed superior antitumor activity compared to GPC2.CARs plus monocytes at day 30 (Fig. 6i). Third, the combination of GPC2.CAR plus anti-GD2 mAb also led to significant, long-term antitumor responses, further supporting the dual targeting of GPC2 and GD2 in neuroblastoma (Fig. 6g, h). Serial body weights were again comparable between all groups (Supplementary Fig. 6d).
Finally, because GD2 is expressed in the normal CNS, GD2-based therapies with intrinsic ability to cross the blood brain barrier might cause neurological toxicities24, although reassuringly recent clinical experience infusing GD2.CARs locally in the brain showed no significant on-target, off-tumor effects47. Nonetheless, here we evaluated brain histology after systemic GPC2.CAR-GD2.BiCE T-cell and either NK-cell or monocyte infusion which did not demonstrate the presence of any measurable amount of human CAR T cells or any histological signs of CAR.BiCE induced neurotoxicity (Fig. 6j).
In summary, these studies utilizing aggressive and clinically relevant human neuroblastoma PDX models reconstituted with human innate cells validate the in vivo safety and antitumor efficacy of this CAR.BiCE therapeutic strategy.
Discussion
Overcoming the barriers of antigen heterogeneity, cancer cell plasticity and the immunosuppressive TME are critical aspects to maximizing CAR T-cell therapeutic potential in solid tumors. In this study, we tested a strategy to address these obstacles by generating CAR T-cells that induce direct cytotoxicity against GPC2-expressing tumor cells via a GPC2.CAR and simultaneously secrete a BiCE that targets a second tumor antigen, GD2. GPC2.CAR T-cell secretion of GD2.BiCE can circumvent antigen-dependent or -independent resistance to GPC2 CARs by promoting new GD2+ neuroblastoma cell-CD16a+ NK-cell/macrophage synapses that activate antitumor innate immunity. Indeed, we found that when infused into mice harboring aggressive and clinically relevant human neuroblastoma PDX models, GPC2.CAR-GD2.BiCE T-cells locally delivered GD2.BiCEs to the tumor bed, promoted NK-cell intratumor retention and displayed enhanced antitumor activity compared to single GPC2.CARs.
Target antigen modulation has emerged as one of the most common mechanisms of disease progression after CAR T-cell therapy48. For example, a recent survey across CD19 CAR pediatric Phase 1/2 clinical trials showed that up to 25% of children with B-ALL had a CD19-negative relapse47, occurring through a diverse set of mechanisms49–52. Similar rates of antigen-low relapses occur with CD22-directed therapies53 and may be even higher in solid tumors54–56. For example, 70% of glioblastoma patients infused with EGFRvIII CAR T-cells had EGFRvIII loss after CAR T-cell treatment54. In addition, we have shown that cell surface GPC2 is substantially reduced under CAR pressure in neuroblastoma preclinical models with both our prioritized D3-scFv-based CARs studied here and with similar GPC2 CAR constructs previously30. Whether this lower GPC2 expression is due to antigen downregulation or selection of antigen-low cells in a heterogeneous tumor cell population remains to be fully elucidated and is an active area of investigation in our laboratory, but together support the necessity to target multiple neuroblastoma antigens to enable durable CAR T-cell efficacy in this disease.
Understanding neuroblastoma immunobiology is also critical to rationally designing new combination immunotherapies. Using a clinically annotated tissue microarray, we observed that high-risk and post-treatment neuroblastoma tumors were poorly infiltrated by T-cells but contained larger amounts of innate immune cells expressing CD16a, which were better defined as macrophages and NK-cells using single-cell RNA-sequencing data. Although a small subset of potential dysfunctional NK-cells infiltrating these primary tumors have decreased FCGR3A expression, most intratumoral NK-cells in this dataset express high levels of FCGR3A. This immunophenotypic observation led us to engineer CARs that secrete BiCEs to capitalize on these CD16a-expressing cell populations while also inducing enhanced tumor T-cell infiltration via a GPC2 CAR component. In other diseases, BiTE-secreting CARs have been utilized to activate bystander endogenous T-cells that have been efficacious in preclinical models7 and clinically57, but there is less rationale for their use in T-cell-excluded pediatric tumors such as high-risk neuroblastoma. Further, we observed that GPC2.CAR pressure directly upregulated NK-cell activating ligands on neuroblastoma cells further supporting the design of novel CAR-based strategies that enhance the antitumor properties of bystander NK-cells. However, it is possible that IFN-γ secretion by CAR T-cell activation might also up-regulate MHC class I molecules that may ultimately inhibit NK-cell function58, suggesting that further dissection of this complex cascade of events will need to be studied. Also, despite being usually defined as immunosuppressive59, the strong myeloid compartment of neuroblastoma tumors can be reinvigorated to evoke antitumor functions29,60. In turn, we observed that GD2.BiCEs induced macrophage-mediated phagocytosis of neuroblastoma cells at higher levels than observed for the anti-GD2 antibody dinutuximab, a molecule that itself has also been shown to have macrophage checkpoint inhibitory properties60.
For the BiCE component of our bicistronic CAR construct, we utilized a CD16a-directed sdAb rather than a full scFv, reducing the molecular size of the molecule to facilitate solid tumor penetration61, CAR vector design and robust secretion by T-cells. We also demonstrate that our GD2.BiCE design induced NK-cell activation, polyfunctionality and tumor-killing properties as well as macrophage-mediated tumor phagocytosis at levels comparable to the FDA-approved anti-GD2 antibody dinutuximab. Further, NK-cells isolated directly from the neuroblastoma bone marrow niche could be engaged and activated by GD2.BiCE when co-incubated with GD2-positive neuroblastoma cell lines ex vivo. Since neuroblastoma-associated NK-cells in the primary tumor and metastatic BM niche might display dysfunctional properties41, our data suggest that this phenotype can be reverted by a CAR.BiCE approach, although clinical studies are required to further confirm these findings. For example, innate immune cell immunophenotyping in pre- and post-bone marrow samples collected during CAR.BiCE T-cell therapy would be optimal to study BiCE-mediated engagement in the neuroblastoma bone marrow metastatic niche. Other studies have proven that trifunctional antibody formats incorporating the IL-15 cytokine62 or co-engaging CD16a together with the NK-cell activating cell-surface glycoprotein NKp4663,64 may further enhance innate immune cell antitumor functions in vivo. Although engineering such additional arms to this bicistronic construct could enhance the therapeutic potency of this new tumor-immune cell synapse, T-cell-derived cytokines naturally released from CAR activation may also enhance innate immune cell performance in our current approach.
CAR T-cell secretion of GD2.BiCE was significantly increased upon T-cell activation mediated either via CAR-GPC2 synapse or with αCD3/28 beads and cytokines. These data suggest a similar mechanism to previous studies where IL-15 secretion was enhanced upon antigen-induced stimulation in IL-15-armored CARs11,65,66. Here we propose that CAR-GPC2 engagement activates T-cells to rapidly boost their cellular secretion machinery including the GD2.BiCE molecules that are designed under a Igκ leader peptide without the need of further logic gating strategies25,67. The threshold of GPC2 expression required to enhance GD2.BiCE secretion was below that required for GPC2.CAR-mediated killing though, as we observed increased GD2.BiCE secretion when exposing CAR.BiCE T-cells to CAR-resistant, GPC2low SY5Y cells, validating that measurable GD2.BiCE secretion still occurs when CAR.BiCE T-cells encounter tumor cells with lower antigen expression. Further, CAR.BiCE T-cells efficiently secreted GD2.BiCE even when challenged with antigen-downregulated neuroblastoma cells previously exposed to GPC2.CARs. Importantly, the CAR T-cell activation-enhanced GD2.BiCE secretion we observed dictates the local delivery of GD2 BiCEs in vivo, potentially avoiding any off-tumor on-target effects commonly observed with GD2-based therapies24. In our biodistribution studies, we observed that mice treated with dinutuximab showed high GD2 mAb concentrations in tumors but also in normal murine tissues. Although the route of administration, dosage and kinetics of dinutuximab are not comparable to those for GD2.BiCEs released by CAR T-cells and thus total tissue concentrations are difficult to compare across modalities, our results overall support the enhanced specificity and safety of using GPC2.CARs to locally deliver GD2-targeted therapies. In these studies, we also observed that infused T-cells infiltrated GPC2-positive PDXs and also the mouse spleen due to the intrinsic human T-cell tropism in NSG mice46, whereas GD2.BiCE was only detected at significant levels in the tumor. Previous pharmacokinetic studies have demonstrated the short half-life of CD16a-containing NK-cell engagers68, which could provide additional support for the lack of any substantial GD2.BiCE concentrations in healthy tissues that do not have significant infiltration and activation of GPC2 CAR T-cells. Importantly, although we did not observe clinical or histological signs of toxicities in the CNS, we propose that the small subset of neuroblastoma patients with CNS involvement at the time of relapse should be excluded in the first stage of any future clinical trial given the possibility of GD2-related toxicities, as tumor-involved CNS could activate GPC2 CAR T-cells to release GD2.BiCE affecting the surrounding healthy CNS tissues. However, considering the recent clinical experience infusing GD2.CARs (containing the same 14g2a binder as our GD2.BiCE) locally in the brain with no significant on-target, off-tumor toxicities in patients with midline gliomas47, low levels of GD2.BiCE in the CNS seem unlikely to result in any clinically significant toxicities.
We performed in vivo efficacy studies in immunodeficient NSG mice bearing different human neuroblastoma PDX models infused with CAR.BiCE T-cells plus either donor-matched CD16a-enriched innate immune cells, NK-cells or monocytes as effector cells for GD2.BiCEs. The main advantage of using such flank human neuroblastoma PDX models is that they best recapitulate the human tumor expression of GPC2 and GD2 and allowed validation of the enhanced efficacy of human GPC2.CAR-GD2.BiCE T-cells compared to the single GPC2.CARs. Also, our studies using different innate immune cells as effector cells allowed us to determine that monocytes acted most synergistically with the bicistronic construct compared to the single CAR. In this regard, we also observed that NK-cells enhanced the efficacy of single GPC2 CARs compared to the monocyte group, which validates previous studies demonstrating the immunoregulatory role of NK-cells in enhancing CAR T-cell antitumor activity10. Interestingly, GPC2.CAR combined with dinutuximab plus either NK-cells or monocytes induced durable antitumor responses which should also be further studied.
Several limitations of our in vivo models might underestimate the therapeutic benefit of the GPC2.CAR-GD2.BiCE compared to GPC2.CAR T-cells alone. First, the inability of our human CD16a sdAb to bind mouse Fc-receptors did not allow us to study whether the human CAR.BiCE constructs engaged endogenous murine innate immune cells. Second, it is well described that, after injection of human PBMCs in immunodeficient mice, innate immune cells poorly persist in the mouse blood and infiltrate mouse tissues69, potentially limiting their tumor trafficking in our models. Thus, other murine models should also be considered for testing the efficacy and safety of GPC2.CAR-GD2.BiCE T-cells in future work. For example, a humanized mouse model encoding cytokines important for innate immune cell development could support the differentiation and function of monocytes/macrophages and NK-cells derived from human CD34+ progenitor cells that may be able to more faithfully infiltrate the engrafted human tumor xenografts70. Another alternative strategy could be using syngeneic murine neuroblastoma models that resemble immunological properties of human neuroblastomas and are infiltrated by endogenous mouse NK-cells and macrophages71. Here, paired fully murine CAR.BiCE constructs would need to be engineered and, although the utilized GPC2 and GD2 scFvs can bind mouse neuroblastoma cells29, binders for mouse Fc-receptors including CD16.1 (FcγRIII) and CD16.2 (FcγRIV) that can engage mouse NK-cells and macrophages, respectively72,73, would also have to be identified and validated. Finally, the use of immunocompetent mouse strains in which all murine Fcγ receptors are deleted and replaced by human Fcγ homologs encoded as transgenes could also be an alternative experimental system to test the efficacy of CAR.BiCE T-cells with the human CD16a binder74, although in this case it would need to be tested against neuroblastoma murine tumors that poorly recapitulate heterogeneous antigen expression75.
In summary, we developed CAR T-cells secreting BiCEs that target two neuroblastoma-associated antigens simultaneously and activate bystander innate immune cells that showed enhanced antitumor activity in preclinical models by synergizing both adaptive and innate antitumor immunity. Together these studies lay the foundation for the clinical translation of this therapeutic approach for children with neuroblastoma who desperately need new safe and effective targeted therapies.
Methods
Ethics statement
Patient samples were obtained under approved Institutional Review Board (IRB) protocols [tumor microarray (TMA); 18-015545 and cryopreserved bone marrow samples; 10-007767], and the study was conducted in accordance with the U.S. Common Rule. Healthy donors provided informed consent through the University of Pennsylvania Immunology Core.
Animal experiments were conducted using protocols approved by the CHOP Institutional Animal Care and Use Committee (IACUC; Protocols #643 and #1464) with adherence to the NIH guide for the Care and Use of Laboratory Animals accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Patient samples
A neuroblastoma tumor microarray (TMA) was constructed with duplicate punches from formalin-fixed paraffin-embedded human neuroblastoma tumors with diverse clinical features archived at the Children’s Hospital of Philadelphia (CHOP). A total of 64 unique neuroblastoma specimens were included in the TMA, typically in duplicate. Per the neuroblastoma INRG staging system, 31 tumors were classified as high-risk, 25 as intermediate-risk and 8 as low-risk. For the high-risk tumors, 12 harbored MYCN amplification and 19 tumors were MYCN non-amplified. Regarding previous treatments, 34 tumors were collected at the time of diagnosis (pre-treatment), 24 were collected post-chemotherapy, typically at the time of local control surgery (post-treatment), and 6 were collected at the time of relapse. All tumors were reviewed by a pediatric pathologist and 1-4 samples (0.6 mm cores) of representative tissue from each case and normal control tissue were included in the TMA using a manual arrayer (Beecher Instruments, Inc). TMA creation and annotation with clinical co-variate data was approved by the CHOP IRB.
Fresh bone marrow samples from high-risk neuroblastoma patients with different clinical features (n = 15; Supplementary Tables 1 and 2) were collected after written, signed and dated informed consent provided by their parents/guardians and biobanked at CHOP [Center for Childhood Cancer Research (CCCR) Biorepository and Registry].
Bulk and single-cell gene expression analysis
TARGET gene expression data for high-risk neuroblastoma tumors was downloaded from (https://treehousegenomics.soe.ucsc.edu/public-data/). The dataset used for analysis was “Tumor Compendium v11 Public PolyA (April 2020)” and only TARGET neuroblastoma identifications were selected as they also had paired risk stratification data available. Gene expression TPM data were inverse log transformed. To compare with human normal tissue expression, gene expression data in TPM for diverse normal tissues was downloaded from the GTEx portal (https://gtexportal.org/home/datasets). Different subcategories of the same tissue type were collapsed to their respective tissue for ease of viewing. In total, we compared the gene expression of 30 unique tissue types (n = 15,989 total samples) from GTEx to 126 high-risk neuroblastoma tumors from the TARGET project. Boxplots were generated by ggplot and reshape2 R libraries in R 4.3.0.
Single-cell RNA-sequencing datasets (6,442 single cells; 4 neuroblastoma tumors at diagnosis and 13,281 single cells; 16 pre-treated neuroblastoma tumors) were previously described35. RDS files for these neuroblastoma single-cell RNA-sequencing data were downloaded from (https://www.neuroblastomacellatlas.org/). These Seurat objects were further scrutinized for certain gene expression patterns in already defined clusters. The clusters were defined previously35 using expression of lineage specific markers and further corroborated by running “quickMarkers” function in SoupX R package76. FeaturePlot from the Seurat package77 was used to plot UMAPs. SingleR was run on the 2 neuroblastoma datasets using the Database Immune Cell Expression Data (DICE) that comprises 1561 bulk RNA-seq samples of sorted cell populations as a reference dataset. Prior to running SingleR, both datasets were filtered to remove high mitochondrial content and cells with low RNA count.
Patient-derived xenograft models
To generate NB-EbC1 xenografts, a total of 5×106 NB-EbC1 cells were injected subcutaneously in NSG-MHC I/II DKO (024216; Jackson Labs) using 100 µL of Matrigel (Corning). To generate neuroblastoma PDX models, 3 × 3 mm3 fresh tumor fragments of COG-N-421x, COG-N-561x or COG-N-603x tumors (characterized previously in78) were engrafted subcutaneously in the flanks of 6-week-old immunodeficient female NSG mice (NOD-scid IL2Rgammanull; 005557; Jackson Labs). Mouse weights and tumor volumes were measured at least twice weekly, and tumor volumes were calculated as volume = /100. Tumor volumes equal or larger than 2 cm3 at the last measurement were considered endpoint. Mice, unless otherwise noted, were treatment naive and maintained in cages of up to 5 mice under barrier conditions with ready access to feed and water.
Cell culture
Human neuroblastoma NB-SD, CHP-134, NB-EbC1, SMS-SAN, SH-EP, SK-N-FI, NB69, NB-LS, LAN-5, Kelly, SY5Y, SK-N-AS, SMS-SAN and control NALM6 cell lines were obtained from the CHOP cell line repository, and the NK92 wild-type cell line was obtained from the Wistar Institute (Chi Van Dang laboratory). HEK293T and NK92-GFP-CD16 176V cell lines were obtained from ATCC (Catalog No. CRL-3216 and PTA-8836, respectively). All tumor cell lines and HEK293T cells were cultured in Roswell Park Memorial Institute (RPMI) containing 10% FBS, 2 mM L-Glutamine, and 1% streptomycin/penicillin (RPMIc). NK92 cells were cultured in Mem α (no nucleosides) containing 12.5% horse serum, 12.5% FBS, 0.2 mM inositol, 0.1 mM 2-mercaptoethanol, 0.02 mM folic acid and human recombinant IL-15 (5 ng/mL). All cell lines were grown under humidified conditions in 5% CO2 at 37 °C and samples were regularly tested for cell identity and mycoplasma contamination.
Primary human peripheral blood mononuclear cells (PBMCs), T-cells, NK-cells and monocytes were obtained from healthy donors through the Human Immunology Core at the University of Pennsylvania (Philadelphia, PA).
Patient-derived NK-cells were isolated from tumor-infiltrating bone marrow samples from 5 different patients using a human NK Cell Isolation Kit (130-092-657; Miltenyi Biotec) according to the manufacturer’s instructions.
DNA constructs
All CAR, BiCE and CAR.BiCE constructs were generated using a lentiviral construct backbone containing an EF1α promoter. Single GPC2.CAR was designed using a CD8α leader, followed by the single-chain variable fragment (scFv) of the GPC2 D3 antibody27–29 with a VL-VH orientation with (Gly4Ser)x3 linker79, a CD28 hinge and transmembrane domain and CD28 and CD3-ζ co-stimulatory domains. Control CD19.CAR was designed using a CD8α leader, followed by a scFv derived from the FMC63 antibody, a CD8 hinge and transmembrane domain and a 4-1BB and CD3-ζ co-stimulatory domains80. Control GD2.CAR was designed using a IGHV leader sequence, followed by the scFv derived from the 14g2a monoclonal antibody, a CD8 hinge a transmembrane domain and 4-1BB and CD3-ζ signaling endodomains81. For bicistronic GPC2.CAR-BiCE vectors, GPC2.28.28.28ζ amino-acid sequence was identical to the single GPC2.CAR and followed by a P2A sequence, an Igκ leader sequence and the GD2 or CD19.BiCE molecule. GD2.BiCE molecule was composed of a GD2-targeted scFv (14g2a antibody; VL-linker-VH orientation), a human CD16a-targeted single antibody domain (sdAb; D6)42,82 and followed by a His-tag. CD19.BiCE was constructed identically to the GD2.BiCE but with the CD19-specific scFv (FMC63). In BiCE molecules, the linker GSTSGSGKPGSGEGSTKG was utilized between both scFv VL and VH, and scFv and sdAb, as it was previously reported to reduce protein aggregation83. To generate GD2.BiCE vector alone, the GPC2.CAR cassette was removed from GPC2.CAR-GD2.BiCE and replaced with an in-frame mock sequence using standard restriction enzyme cloning with AsiSI and Bsu36I. All sequences were codon optimized and synthesized by Twist Bioscience or Integrated DNA Technologies (IDT). Final constructs were transformed in One Shot™ Stbl3™ Chemically Competent E. Coli cells (ThermoFisher) and bacterial cultures were grown overnight at 37 °C while shaking, subsequently pelleted and DNA was extracted using a ZymoPURE II Plasmid Maxiprep Kit (Zymo Research). All constructs were verified with Sanger sequencing.
Lentiviral vector pLV-Puro-CMV-mB4galnt1 (encoding GD2 synthase) was kindly provided by Dr. Paul Sondel, whereas pLV-Bsd-CMV-mSt8sia1-IRES-Luc vector (encoding GD3 synthase and firefly luciferase) was designed through Vector Builder and both were used to induce high levels of surface ganglioside GD2 on target cells, as previously reported84. pLV-EF1α-hCD16a vector was obtained from Genecopoeia (pLV156-A7846) to overexpress human CD16a in NK92 cells. Addgene vectors pLenti-PGK-V5-LUC-Neo (w623-2), pLenti-CMV-puro-Luc (w168-1) and pLenti-CMV-GFP-Puro (658-5) were a gift from Eric Campeau85 and were used to tag tumor cells with firefly luciferase or GFP, respectively. Vectors for GPC2 overexpression were previously described27,28.
BiCE production, detection, purification and quantification
HEK293T cells (1 × 106 cells) were plated and transfected with the BiCE or CAR.BiCE vectors using FuGENE 6 (Promega) according to the manufacturer’s instructions. BiCE-containing supernatants were collected 24–96 hours after transfection and concentrated using Amicon® Ultra-15 Centrifugal Filter Units (10 kDa; Merck Millipore). To detect BiCE, a total of 30 µg of total protein from supernatants (SN) or concentrated supernatants (cSN) from CAR.BiCE-transfected HEK293T cells were separated on 4-12% Bis-Tris gels (Life Technologies), transferred to a PVDF membrane, blocked in 5% non-fat milk in Tris-buffered saline and Tween-20 (TBS-T) and blotted using standard protocols. Membranes were incubated at 4 °C overnight in rabbit HRP anti-6X His tag® antibody (ab1187; Abcam; 1:5000), washed x 3 in TBS-T and then developed with a chemiluminescent reagent (SuperSignal West Femto, Thermo Fisher Scientific). To purify His-tagged BiCE, a MagneHis™ Protein Purification System (Promega; V8500) was utilized. To rapidly quantify HEK293T-derived BiCE, we used the competitive His Tag ELISA Detection Kit (Genescript; L00436).
BiCE binding assays
In a first set of experiments, a total of 1×106 cells from high to low GD2 expression (HEK293T-GD2/GD3 synthase, NB-EbC1, SMS-SAN, LAN-5, NB-LS, NB69, SK-N-FI, SH-EP, NALM6 and HEK293T) were exposed to 50 µL of concentrated supernatants (cSN) from the different vectors (GPC2.CAR, GPC2.CAR-CD19.BiCE and GPC2.CAR-GD2.BiCE) for 30 min on ice, washed with PBS 1x, subsequently stained with phycoerythrin (PE)-tagged anti-His-tag antibody (362603; Biolegend; 1:20) for 30 min and then analyzed by flow cytometry. In competition binding assays, GD2 was previously blocked with 5 or 0.5 µg/mL of dinutuximab (Unituxin®; United Therapeutics) before exposing cells to BiCE-containing cSN.
In a second set of experiments, recombinant human Fc gamma RIIIA/CD16a (4325-FC-050), mouse FcgR4/CD16-2 (1974-CD-050) and mouse Fc gamma RIII/CD16 (1960-FC-050) proteins were obtained from R&D Systems and conjugated with Allophycocyanin (APC) using the APC Conjugation Kit - Lightning-Link® (Abcam; ab201807) according to the manufacturer’s protocol. Then, GD2+ NB-EbC1 cells were exposed to cSN from the different constructs, washed, stained with 5 µg/mL of APC-tagged recombinant human CD16a or mouse CD16 proteins and analyzed by flow cytometry.
In a third set of experiments, mouse recombinant anti-GD2 scFv (14g2a) idiotype 1A7 (Creative Biolabs; FAMAB-0073-CN) was conjugated with PE/R-Phycoerythrin Conjugation Kit - Lightning-Link® (Abcam; ab102918) according to the manufacturer’s protocol. A total of 1×106 human primary NK or T-cells were then exposed to cSN from different constructs, washed, stained with PE-tagged 1A7 idiotype at 5 µg/mL and analyzed by flow cytometry.
Lentiviral production and titration, transductions and CAR T-cell manufacturing
Replication-deficient lentiviruses were produced using a third-generation lentiviral system. The day before transfection, 10 million HEK293T cells were plated in T175 flasks. The day of transfection, 80 µl Lipofectamine 2000 (Life Technologies, Invitrogen) was added to 2.3 mL room temperature Opti-MEM medium (Gibco). Concurrently, 18 µg pRSV.Rev (Rev), 18 µg pMDLg/p.RRE (gag, pol and RRE), 7 µg pMD2.G (VSV-G envelope) and 15 µg of transfer plasmid were added to 2.3 mL of room temperature Opti-MEM medium. Lipofectamine and DNA-containing Opti-MEM aliquots were mixed, incubated for 5 min and added to HEK293T cells. Virus supernatants were collected 24 and 48 hours later, filtered with 0.45 μm nitrocellulose membranes and concentrated using ultracentrifugation (28,000 rpm for 2 hours at 4 °C). Concentrated lentiviral particles were stored at −80 °C.
For lentivirus titration, primary T-cells (CD4 and CD8; 1:1 ratio) were thawed and activated for 24 hours with Dynabeads™ Human T-Expander CD3/CD28 (Thermo Fisher Scientific) at a 3:1 bead: T-cell ratio with human recombinant IL-15 and IL-7 (PeproTech; 5 ng/mL) in AIM-V medium supplemented with 5% FBS, 2 mM L-Glutamine, 0.1 M HEPES buffer and 1% streptomycin/penicillin at a density of 1×106 cells per mL. On day 2, 0.1×106 activated T-cells were plated in 96-well plates and concentrated lentiviral particles were added at different dilutions (from 1:3 to 1:384). Four days after transduction, beads were magnetically removed and T-cells were analyzed by flow cytometry to determine CAR expression.
For CAR T-cell manufacturing, primary T-cells were activated as above, transduced at day 2 with the virus to achieve the optimal transduction efficiency based on the associated titration and cultured with the beads until days 5–7 at a density of 1 × 106 cells per mL. Then, beads were magnetically removed, and primary T-cells were placed in a vented Erlenmeyer flasks at a density of 0.25 × 106 cells per mL of media and cultured while shaking at 125 rpm until day 12–15. At that time, cells were collected, cell viability and CAR expression determined and cells were viably frozen.
For NK92, HEK293T or tumor cells, concentrated lentiviral particles were added in the presence of polybrene (8 µg/mL; Sigma) to increase transduction efficiency, removed after 24 hours and cells were replenished with their corresponding media supplemented with antibiotic (puromycin, neomycin or blasticidin S depending on the transfer plasmid) at an optimized concentration for bulk selection. For HEK293T and SY5Y cell lines transduced with GD2 and GD3 synthase vectors, cells were first selected with the corresponding antibiotics and then flow sorted for the highest GD2 surface-expressing cell population.
NK-cell and CAR T-cell cytotoxicity assays and cytokine Enzyme-Linked Immunosorbent Assay (ELISA)
For cytotoxicity assays using human primary NK-cells and the NK92 cell line, luciferase-tagged tumor cells (SMS-SAN, NB-EbC1, CHP-134, SY5Y and NALM6) were plated in 96-well plates for 24 hours (5,000-20,000 cells per well) and then exposed to concentrated supernatants (cSN) from single (GPC2.CAR) or bicistronic constructs (GPC2.CAR-GD2.BiCE or GPC2.CAR-CD19.BiCE) containing 0.2-50 ng/mL of His-tagged BiCE, or anti-GD2 mAb dinutuximab at 10 µg/mL. Following addition of BiCE or mAb, NK-cells were added at different E:T ratios (10:1, 5:1, 2:1 and 1:1) for 24 hours. When using CAR T-cells for cytotoxicity assays, luciferase-tagged tumors cells (Kelly-GPC2, SY5Y-GPC2, NB-EbC1, SMS-SAN, CHP-134, SK-N-AS, SY5Y-empty and NALM6) were also plated in 96-well plates for 24 hours (5,000-20,000 cells per well) and then CAR T-cells (CD19.CAR, GPC2.CAR or GPC2.CAR-GD2-BiCE) were added at different E:T ratios (5:1, 2.5:1, 1:1, 1:2.5 and 1:5) for 24 hours. For both NK-cell and CAR T-cell cytotoxicity assays, ONE-Glo™ Luciferase Assay System (Promega) was used to determine cell viability on a Promega™ GloMax® Plate Reader. Specific killing (%) was determined using the following formula: In both NK-cell and CAR T-cell cytotoxicity assays, supernatants were collected at 24 hours to measure IL-2 and/or IFN-γ secretion using Human IL-2 and IFN-gamma DuoSet ELISA (R&D Systems), respectively, following the manufacturer’s protocol.
For Transwell-based cytotoxicity assays, we used 12-well plates containing a Transwell permeable support with 0.4 µm pore polyester membrane (Sigma) allowing for diffusion of BiCE through the semi-impermeable membrane. First, NB-EbC1 tumor cells were plated in both top (25,000 cells in 0.5 mL of RPMIc) and bottom (25,000 cells in 1 mL of RPMIc) chambers for 24 hours. Alternatively, SY5Y-GPC2 cells were plated in the top (25,000 cells in 0.5 mL) and SY5Y-GD2 in the bottom (50,000 cells in 1 mL) chambers, also for 24 hours. In both experiments, CAR T-cells (CD19.CAR, GPC2.CAR, GPC2.CAR-CD19.BiCE or GPC2.CAR-GD2.BiCE) were added to the top chambers at a 10:1 E:T ratio, whereas primary NK-cells were added in the bottom chambers at a 5:1 E:T ratio. After 24 hours, cells from each well were collected and stained with CD45-FITC and CD3-APC to quantify tumor cells (CD45-/CD3-), T-cells (CD45+/CD3+) and NK-cells (CD45+/CD3-) by flow cytometry. The percentage of residual tumor cells was determined relative to CD19.CAR-treated conditions in both top and bottom wells.
Macrophage-mediated phagocytosis assays
To generate macrophages, human monocytes were cultured at 1 × 106 cell/mL density in 75 cm2 flasks with RPMIc with human recombinant M-CSF (25 ng/mL; Peprotech; 300–25) for 7 days. For phagocytosis assays, GFP-labeled NB-EbC1 cells were plated in 6-well plates overnight (50,000 cells/well), exposed to cSNs from GPC2.CAR-GD2.BiCE (5 ng/mL His-tagged BiCE), GPC2.CAR or dinutuximab (10 µg/mL) and macrophages were added at a tumor: macrophage ratio of 1:1. After 24 hours, cells were stained with human CD11b-PE antibody (clone ICRF44 301306; Biolegend; 1:20) and analyzed by flow cytometry. Percentage of phagocytosis was determined by quantifying live GFP-FITC and CD11b-PE positive macrophages.
Single-cell and bulk cytokine proteomics
We studied NK-cell polyfunctionality using single-cell proteomics. To this end, we co-cultured human primary NK-cells with NB-EbC1 cells at a 5:1 E:T ratio in the presence of GD2.BiCE or CD19.BiCE (50 ng/mL) or dinutuximab (10 µg/mL) for 24 hours. After 24 hours of co-culture, NK-cells were separated from tumor cells by positive isolation using CD45 magnetic beads (130-045-801; Miltenyi Biotec), NK-cells were loaded on a Single-Cell Secretome Adaptive Immune: Human NK-cell chip (Isoplexis; ISOCODE-3L03-4) and run on an IsoSpark System (IsoPlexis) using the manufacturer’s protocols.
We also unbiasedly quantified human cytokines present in the plasma of GPC2.CAR-GD2.BiCE T-cell-treated mice in a multiplexed manner. Here, we loaded a total of 11 µL of mouse plasma onto a CodePlex Secretome: Adaptive Immune chip (Isoplexis; CODEPLEX-2L01-1) which was run on an IsoSpark System (IsoPlexis) according to the manufacturer’s protocol.
Flow cytometry
Single cell suspensions from human primary immune cells, CAR T-cells, tumors, control cell lines and neuroblastoma patient-derived xenografts were stained with LIVE/DEADTM Fixable Violet Dead Cell Stain (Invitrogen; 1:3000), incubated with the indicated primary antibody and run on a Beckman CytoFLEX S cytometer (Beckam Coulter). Cell surface GPC2 expression was measured as described previously29. PE or APC-tagged mouse anti-human Ganglioside GD2 antibody (clone 14g2a; Biolegend; 1:20) was used to measure GD2 surface expression on human tumor cells. Cell surface molecules of GPC2 and GD2 were determined using the BD Quantibrite™ Beads PE Fluorescence Quantitation Kit (BD Bioscience). Anti-human antibodies APC-MICA/MICB (clone 6D4; 320907; BioLegend; 1:20), PE-ULBP-1 (clone 170818; FAB1380P; R&D Systems; 1:20) and PE-B7-H6 (clone 875001; FAB7144P; R&D Systems; 1:20) were used to study NK-cell ligand expression in tumor cells after GPC2 CAR T-cell pressure.
Immune cell phenotyping, exhaustion and activation was also studied by flow cytometry with the following anti-human antibodies: CD45-APC, PE or FITC (clone J33, Beckman Coulter; 1:10), CD3-APC, PE or FITC (clone UCHT1; Beckman Coulter; 1:10), CD19-PE (clone HIB19; 982402; BioLegend; 1:20), CD16a-AlexaFluor®647 (clone 1001049; FAB4325R; R&D Systems; 1:20), CCR7-FITC (clone G043H7; 353215; BioLegend; 1:20), CD45RO-APC (clone UCHL1; 304210; BioLegend; 1:20), PD1-APC (clone J105; eBioscience™; 1:20), LAG3-PE (clone 3DS223H; eBioscience™; 1:20), TIGIT-PE (clone 741182; FAB7898P; R&D Systems; 1:20), CD69-FITC (clone FN50; 310903; BioLegend; 1:20) and CD107a-PE (clone H4A3; 328607; BioLegend; 1:20). For T-cell proliferation, we stained cells with CellTrace™ CFSE Cell Proliferation Kit (C34554; ThermoFisher) using the manufacturer’s protocol and FITC signal was determined by flow cytometry. GPC2 CAR expression was determined using APC- or PE-conjugated human recombinant GPC2 protein (R&D Systems; 2304-GP-050; 1:500), GD2 CAR expression was determined using PE- or APC-conjugated idiotype 1A7 (1:200) and CD19 CAR expression was determined using PE-tagged protein L (58036 S; Cell Signaling; 1:20).
For patient-derived bone marrow samples, cryopreserved peripheral blood mononuclear cells previously isolated using Ficoll® Paque Plus (Sigma) were thawed, divided into 3 tubes (each one containing at least 300,000 live cells) and stained with antibody panels for lymphoid, myeloid or tumor compartments. The lymphoid panel consisted of the following antibodies: Zombie Aqua Fixable Viability Kit (77143; Biolegend; 1:3000), CD56-PE/Cyanine7 (clone HCD56; 318318; Biolegend; 1:20), CD8-Brilliant Violet 421™ (clone SK1; 344748; Biolegend; 1:20), CD4-PerCP/Cyanine5.5 (clone OKT4; 317428; Biolegend; 1:20), CD3-APC/Cyanine7 (clone SK7; 344818; Biolegend; 1:20), CD16a-AlexaFluor®647 and CD45-FITC. The myeloid panel consisted of the following antibodies: Zombie Aqua Fixable Viability Kit, HLA-DR Brilliant Violet 785™ (clone L243; 307642; Biolegend; 1:20), CD11b-PE/Cyanine7 (clone ICRF44; 301322; Biolegend; 1:20), CD14-APC/Cyanine7 (clone 63D3; 367108; Biolegend; 1:20), CD15-PerCP/Cyanine5.5 (clone W6D3; 323020; Biolegend; 1:20), CD68-BD Horizon™ BV421 (clone Y1/82 A; 564943; BD Biosciences; 1:20), CD16a-AlexaFluor®647 and CD45-FITC. Both lymphoid and myeloid panels were analyzed on a BD LSRII Cytometer (BD Biosciences). The tumor panel consisted of the following antibodies: LIVE/DEADTM Fixable Violet Dead Cell Stain, CD45-Pacific Blue™ (clone HI30; 304029; Biolegend; 1:20), Human Aminopeptidase N/CD13 Alexa Fluor® 405 (clone 986002; AB38152V, R&D Systems; 1:20), CD56 (NCAM)-Alexa Fluor® 488 (clone MEM-188; 304611; Biolegend; 1:20) and GPC2-PE and GD2-APC, as described above. The tumor panel was run on a Beckman CytoFLEX S cytometer as described above and only bone marrow samples containing at least 200 tumor cell events were considered for antigen expression.
Detection of T-cell secreted GD2.BiCE and dinutuximab by ELISA
To detect GD2.BiCE in T-cell supernatants and other biological matrices, we developed a highly specific and sensitive sandwich ELISA method. To this end, uncoated Nunc™ MaxiSorp™ ELISA plates (423501; BioLegend) were coated with 5 µg/mL of the idiotype 1A7 diluted in coating buffer (DY006; R&D Systems) overnight at room temperature (RT). The next day, plates were washed with PBS 1x containing 0.05% Tween-20 (Biorad) (x4), blocked for 3 hours at RT with PBS 1x containing 10% of FBS, washed again and incubated with samples and standards (purified His-tagged BiCE; 80, 40, 20, 10, 5, 2.5 and 1.25 pg/mL) diluted in blocking reagent overnight at 4 °C. The next day, plates were washed and incubated with rabbit anti-6X His-tag® antibody (horseradish peroxidase, HRP) (ab1187; Abcam; 1:5000) for 3 hours at RT. For dinutuximab detection, ELISA plates were coated with 5 µg/mL of goat anti-human IgG Fc (ab97221; Abcam), blocked with PBS 1x with 2% bovine serum albumin (BSA), incubated with samples and standards (dinutuximab at 250, 139, 77, 42, 24, 13 and 7 ng/mL) and detected with HRP-tagged 1A7 idiotype (1:1000). HRP Conjugation Kit - Lightning-Link® was obtained from Abcam (ab102890). Both GD2.BiCE and dinutuximab ELISA plates were developed with TMB substrate reagent (DY999B; R&D Systems) and optical density (OD) was determined in a microreader plate at 450 nm, subtracting 560 nm background values.
In vitro, levels of GD2.BiCE were quantified by ELISA in supernatants from GPC2.CAR-GD2.BiCE-transduced T-cells during i) T-cell expansion, ii) after co-culture with neuroblastoma cells (2.5:1 E:T ratio), T-cells re-stimulated with αCD3/αCD28 beads plus cytokines or T-cells alone for 24 hours and iii) in supernatants from the Transwell assays described above. In vivo, T-cell secreted GD2.BiCE and dinutuximab were quantified by ELISA in the biodistribution experiments detailed below.
RT-qPCR
Total RNA was extracted using Maxwell® RSC simplyRNA Tissue Kit (AS1340; Promega) with a Maxwell® RSC Instrument (Promega) using the manufacturer’s instructions. Complementary DNA (cDNA) was generated using the qScriptTM cDNA SuperMix (QuantaBio). A total of 2 ng of cDNA was used to determine CAR and BiCE transgene, CD3e (Hs01062241_m1) and HPRT1 (Hs03929096_g1) expression using TaqMan™ Gene Expression Master Mix in a QuantStudio™ 6 Flex Real-Time PCR System (ThermoFisher Scientific). Primers and probes (5’ FAM, 3’ TAMRA) for CAR and BiCE transgene regions were designed using PrimeTimeTM qPCR Primer Assays (IDT). Sequences of CAR primers are the following: Forward 5’-GAAGCGGAACCGAGTTTACA-3’, probe 5’- ACTTCGCCACATACTATTGCCAGCA-3’ and reverse 5’- CTGGCCAAATGTGATAGGGTAG-3’. Sequences of BiCE primers are the following: Forward 5’- TCCAATTACGGTATGTCTTGGG-3’, probe 5’- TACGACAAGCTCCCGGTAAGGGA-3’ and reverse 5’- GTGACTTGAGGGATGGATTGT-3’. Since total RNA was extracted from a cell population of mixed target tumor cells and T-cells, Ct values of the CD3e gene (specific for T-cells but not present in tumor target cells) were subtracted from CAR and BiCE transgene Ct values to determine the ∆Ct. ∆∆Ct was calculated relative to T-cells alone.
Immunohistochemistry (IHC)
Tumors and/or normal murine tissues were collected from euthanized animals, fixed with 10% formalin for a minimum of 24 hours and embedded in paraffin. Formalin-fixed paraffin embedded (FFPE) tissues were cut on 5 µm slides and stained on a Bond Max automated staining system using the Bond Refine polymer staining kit (Leica Biosystems) according to the standard protocol. Rabbit polyclonal anti-human CD3 (Agilent; A0452; 1:100) and monoclonal anti-human CD16a (C-terminal; SP189; ab227665; Abcam; 1:100) were used as primary antibodies. GPC2 IHC staining was performed as previously described27. Stained slides were digitally scanned at 20x magnification on an Aperio CS-O slide scanner (Leica Biosystems) and representative pictures taken with the Aperio ImageScope program v12.2.2.5015. Quantification of positive cells by IHC was performed using QuPath software.
In vivo BiCE biodistribution
We studied whole-body biodistribution of GD2.BiCE after injection of CAR.BiCE T-cells and compared to the biodistribution of a clinically relevant regimen of dinutuximab45. Briefly, a total of n = 30 mice were engrafted with COG-N-561x PDX tumors and randomized in Group 1 (GPC2.CAR-GD2.BiCE; n = 13) and Group 2 (dinutuximab; n = 12) with tumor volumes ranging from 0.30–0.40 cm3 (day 36 post-engraftment). On day 1, Group 1 was injected intravenously with 1 × 107 GPC2.CAR-GD2.BiCE CAR+ T-cells diluted in 100 µL of PBS 1x, whereas Group 2 was treated with four consecutive doses (day 1-4) of dinutuximab intraperitoneally at 100 µg per mouse, which is approximately equivalent to the four consecutive infusions at 17.5 mg/m2 that patients receive in the clinic45. At days 5, 6 and 7, n = 4-5 mice per timepoint in each group were bled (50 µL) through the retroorbital plexus to collect plasma and then sacrificed to harvest PDX tumors and healthy tissues [liver, kidney, spleen, lung, heart and central nervous system tissues (cortex, cerebellum, and brainstem)]. Tissues were fragmented into 5×5 mm3 pieces and then either directly snap-frozen in liquid nitrogen or processed for IHC as described above. For sample processing, snap-frozen tissues were homogenized with 350 µL of cell lysis buffer (Cell Signaling Technology; #9803; 1:10) containing 1 mmol/L of PMSF (Cell Signaling Technology, #8553) using a TissueRuptor II (Qiagen), samples were rotated for 15 minutes at 4 °C and centrifuged at 14,000 × g at 4 °C for 10 minutes to collect protein-containing supernatants. Protein concentration was quantified by Bio-Rad Bradford protein assays. GD2.BiCE and dinutuximab tissue concentrations were determined by ELISA as detailed above. Biodistribution data is presented as pg of GD2.BiCE or ng of dinutuximab relative to the amount of total protein in the tissue lysate measured in mg. Human T-cell infiltration and activation within mouse tissues was evaluated by IHC and western blot as detailed above. For western blot, we used the following antibodies: rabbit CD3ζ (Cell Signaling; 88083; 1:800), rabbit CD3ζ phospho Y142 (Abcam; ab68235; 1:1000), rabbit Zap70 (Cell Signaling; 3165 S; 1:1000), rabbit phosho-Zap70 (Tyr319)/Syk (Tyr352) (Cell Signaling; 2701; 1:800) and rabbit β-actin (Cell Signaling; 4967 S; 1:5000).
In vivo NK-cell tumor retention
We studied NK92 cell intratumor retention in mice bearing COG-N-561x PDX tumors previously infused with CAR.BiCE-transduced T-cells. Here, we first engrafted a total of n = 20 mice with COG-N-561x PDXs and randomized to Group 1 (GPC2.CAR-GD2.BiCE; n = 10) and Group 2 (GPC2.CAR-CD19.BiCE; n = 8) with tumor volumes ranging from 0.26-0.30 cm3 (day 20 post-engraftment). On day 1, Groups 1 and 2 were infused intravenously with 1 × 107 CAR+ GPC2.CAR-GD2.BiCE or GPC2.CAR-CD19.BiCE T-cells per mice, respectively. Five days later, a total of 5 × 106 NK92 cells engineered to express CD16, GFP and firefly-luciferase (NK92-CD16-GFP/luc) were injected intravenously (n = 9) or intratumorally (n = 9) in all CAR.BiCE-treated mice in 50 µL of PBS 1x. At 3, 24, 72 and 96 hours post-NK92 cell injection, animals were imaged using Xenogen IVIS-Spectrum system to track the luciferase signal of NK92-CD16-GFP/luc cells intratumorally in both groups. Average bioluminiscence counts were quantified per individual tumor at each experimental timepoint and normalized to the first timepoint (3 hours), which had a similar NK92 cell signal between the 2 groups. Area under the curve (AUC) for all timepoints (3-96 hours; AUC3-96h) was used to estimate total NK92 cell tumor retention.
In the mice from the NK92 cell study, we also measured in vivo CAR.BiCE T-cell proliferation. Approximately 50 µL of blood was extracted as described above, erythrocytes were lysed with Ammonium Chloride Solution (STEMCELL Technologies) and PBMCs were stained with anti-human APC-CD3 and FITC-CD45 antibodies to detect human T-cells by flow cytometry. Invitrogen™ CountBright™ Plus Absolute Counting Beads (C36950; Thermo Scientific) were used to quantify total human T-cells per µL of blood.
In vivo efficacy studies
First, we studied the efficacy of single GPC2.CARs compared to CD19.CARs in mice bearing NB-EbC1 xenografts, aiming to determine antitumor activity and quantify antigen loss upon tumor relapse. For this in vivo study, we selected the NB-EbC1 tumor model due to the consistent antigen loss in the same cells under GPC2 CAR T-cell pressure in vitro. Mice were randomized to 2 groups: GPC2.CAR (n = 8) or CD19.CAR (n = 6) when tumor volumes ranged from 0.15–0.2 cm3 and each group received an intravenous infusion of 10 × 106 CAR+ T-cells in 100 µL of PBS 1x. Tumor that reached experimental endpoint equal or larger than 2 cm3 at the last measurement were harvested and processed for viable single-cell suspensions (flow cytometry and cryopreservation), snap-frozen or were FFPE.
Second, we addressed whether GPC2.CAR-GD2.BiCE T cells could outperform the antitumor activity of single GPC2.CAR T-cells in three neuroblastoma PDX models: COG-N-421x (donor 1), and COG-N-561x and COG-N-603x (donor 2). To this end, a total of n = 25 mice were engrafted per PDX model and randomized to 3 groups: Group 1 (CD19.CAR; n = 5-6), Group 2 (GPC2.CAR; n = 6) and Group 3 (GPC2.CAR-GD2.BiCE; n = 7), when tumors volumes ranged from 0.15–0.5 cm3. On the first day of treatment, each group received an intravenous infusion of 5 × 106 CAR+ T-cells in 100 µL of PBS 1x. Five days later, all groups received an intravenous infusion of 5 × 106 PBMCs isolated from the same healthy donor as the engineered T-cells and previously enriched for innate immune cells. Innate immune cell enrichment was obtained by staining total PBMCs with CD3 (T-cells) and CD19 (B-cells) with PE-conjugated antibodies to then enable anti-PE MicroBeads (130-048-801; Miltenyi) to negatively isolate CD19-/CD3- PBMCs.
Third, we used the PDX model COG-N-561x to evaluate whether the GPC2.CAR-GD2.BiCE construct could increase GPC2.CAR efficacy after a second cell infusion of donor-matched NK-cells or monocytes (5×106 cells IV; day 5; donor 3). In that experiment we also included a group in which we treated mice with GPC2.CAR T-cells (day 1) combined with a clinically relevant regimen of dinutuximab (day 4, 5, 6 and 7), also in the presence of NK-cells or monocytes given at day 5. Control CD19.CARs were not infused with monocytes or NK-cells due to lack of cellular product for all groups. Here, we also compared the efficacy of GPC2.CAR vs. GPC2.CAR-GD2.BiCE constructs without the presence of immune effector cells. Any CAR T-cell-treated mouse that developed xenogeneic graft-vs-host disease (GvHD) defined as weight loss of 20% accompanied by hunched posture, labored breathing or poor general body condition were removed from these studies.
Statistical analysis
All statistical analyzes were performed using GraphPad Prism software except for the gene expression analyzes, which were performed using R software. Student’s t-test and Mann-Whitney t-test (for nonnormally distributed data) were used for nonpaired comparisons of two groups. Wilcoxon signed-rank test was used to compare paired groups. One-way ANOVAs and Tukey’s multiple comparison t-test were applied to determine differences between multiple groups with one independent variable. Two-way ANOVA and Šídák’s multiple comparison test were applied to determine differences between multiple groups with two or more independent variables. Median survivals were calculated using the Kaplan-Meier method, and curves were compared using the log-rank test. The threshold for significance (α) was set at 0.05 unless otherwise specified.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
Kristopher R. Bosse is a Damon Runyon Physician-Scientist supported (in part) by the Damon Runyon Cancer Research Foundation (PST-07-16). This work was supported in part by a St. Baldrick’s-Stand Up to Cancer Dream Team Translational Research Grant (SU2C-AACR-DT-27-17). Stand Up to Cancer is a division of the Entertainment Industry Foundation. Research Grants are administered by the American Association for Cancer Research, the Scientific Partner of SU2C. This work was also supported by the Alex’s Lemonade Stand Foundation (G.P.-P, A.J.W. and K.R.B.), by NCI K08 CA230223 (K.R.B.) and CA266914 (A.J.W.), NCI R37 CA282041 (K.R.B.), NCI U54 CA232568 (K.R.B.), a Children’s Hospital of Philadelphia Cell and Gene Therapy Collaborative Seed Grant (K.R.B.), the EVAN Foundation (K.R.B.), Solving Kids’ Cancer (K.R.B.), Fishin’ For The Cure (K.R.B.), Pierce Phillips Charity (K.R.B.), Catherine Elizabeth Blair Memorial Foundation (K.R.B.) and the Ronan Thompson Foundation (K.R.B.). We thank the DNA sequencing and Human Immunology core facilities at the University of Pennsylvania for their work on plasmid verification and human immune cell isolation, respectively. We also would like to thank the Pathology and Small Animal Imaging facility cores at the Children’s Hospital of Philadelphia. We would like to acknowledge the Center for Childhood Cancer Research (CCCR) Biorepository and Registry for providing the bone marrow specimens from neuroblastoma patients and Drs. Amy Erbe and Paul Sondel for providing the GD2 synthase vector.
Author contributions
Conceptualization: G.P.-P., K.R.B. Methodology: G.P.-P., M.G.H, T.H., W.L., K.R.B. Investigation: G.P.-P., B.M., A.M.G., J.H., L.G.-G., S.M., S.L., P.M.S., M.Y., W.L., K.R.B. Formal analysis: G.P.-P., P.M., R.S., F.A., K.R.B. Visualization: G.P.-P., K.R.B. Funding acquisition: G.P.-P., K.R.B. Resources: D.M., M.T., L.G-d-G., A.J.W., T.S., K.M.B., D.S.D., W.L., K.R.B. Supervision: G.P.-P., K.R.B. Writing – original draft: G.P.-P., K.R.B. Writing – review & editing: All authors.
Peer review
Peer review information
Nature Communications thanks John Anderson, Rosa Nguyen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
High-risk neuroblastoma TARGET gene expression data were downloaded from (https://treehousegenomics.soe.ucsc.edu/public-data), whereas human normal tissue gene expression data were downloaded from the GTEx portal (https://gtexportal.org/home/datasets). RDS files for neuroblastoma single-cell RNA-sequencing data were downloaded from (https://www.neuroblastomacellatlas.org). All other data are available in the Article, Supplementary Information or the Source Data file that is provided with this paper. Source data are provided with this paper.
Competing interests
G.P.-P. and K.R.B. report having a pending patent directly related to this work entitled Dual targeting of pediatric malignancies through CAR T cells secreting Bispecific Innate Immune Cell Engagers (BICEs) (International Patent Application No. PCT/US2022/080935). M.Y., D.S.D., W.L. and K.R.B. hold patents for the discovery and development of other immunotherapies for cancer, including patents related to GPC2 and CD16a-directed immunotherapies. M.Y. is a co-founder of Hula Therapeutics. K.R.B. receives royalties and research funding from Tmunity/Kite Pharma and ConjugateBio for research on GPC2-directed immunotherapies. K.R.B. is on the ConjugateBio Scientific Advisory Board. K.M.B. has received research funding from Syndax. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-51337-2.
References
- 1.June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med379, 64–73 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude, S. L. et al. Tisagenlecleucel in children and young adults with b-cell lymphoblastic leukemia. N. Engl. J. Med378, 439–448 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou, A. J., Chen, L. C. & Chen, Y. Y. Navigating CAR-T cells through the solid-tumour microenvironment. Nat. Rev. Drug Discov.20, 531–550 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Majzner, R. G. et al. Tuning the antigen density requirement for CAR T-cell activity. Cancer Discov.10, 702–723 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirabayashi, K. et al. Dual targeting car-t cells with optimal costimulation and metabolic fitness enhance antitumor activity and prevent escape in solid tumors. Nat. Cancer2, 904–918 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie, Y. J. et al. Improved antitumor efficacy of chimeric antigen receptor t cells that secrete single-domain antibody fragments. Cancer Immunol. Res8, 518–529 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi, B. D. et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol.37, 1049–1058 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Rafiq, S. et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol.36, 847–856 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulch, M. et al. A cross-talk between CAR T cell subsets and the tumor microenvironment is essential for sustained cytotoxic activity. Sci. Immunol.6, eabd4344 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Bachiller, M. et al. NK cells enhance CAR-T cell antitumor efficacy by enhancing immune/tumor cells cluster formation and improving CAR-T cell fitness. J. Immunother. Cancer9, e002866 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanitis, E. et al. Optimized gene engineering of murine CAR-T cells reveals the beneficial effects of IL-15 coexpression. J. Exp. Med218, e20192203 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin, C., Ma, J., Ramachandran, M., Yu, D. & Essand, M. CAR T cells expressing a bacterial virulence factor trigger potent bystander antitumour responses in solid cancers. Nat. Biomed. Eng.6, 830–841 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demaria, O., Gauthier, L., Debroas, G. & Vivier, E. Natural killer cell engagers in cancer immunotherapy: Next generation of immuno-oncology treatments. Eur. J. Immunol.51, 1934–1942 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Maris, J. M. Recent advances in neuroblastoma. N. Engl. J. Med362, 2202–2211 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa, A. et al. Single-cell transcriptomics reveals shared immunosuppressive landscapes of mouse and human neuroblastoma. J. Immunother. Cancer10, e004807 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossig, C., Kailayangiri, S., Jamitzky, S. & Altvater, B. Carbohydrate targets for CAR T cells in solid childhood cancers. Front Oncol.8, 513 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu, A. L. et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med363, 1324–1334 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mody, R. et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol.18, 946–957 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung, N. K. & Dyer, M. A. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat. Rev. Cancer13, 397–411 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Straathof, K. et al. Antitumor activity without on-target off-tumor toxicity of GD2-chimeric antigen receptor T cells in patients with neuroblastoma. Sci. Transl. Med12, eabd6169 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Louis, C. U. et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood118, 6050–6056 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heczey, A. et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol. Ther.25, 2214–2224 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Bufalo, F. et al. GD2-CART01 for relapsed or refractory high-risk neuroblastoma. N. Engl. J. Med388, 1284–1295 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Richman, S. A. et al. High-affinity GD2-specific CAR T cells induce fatal encephalitis in a preclinical neuroblastoma model. Cancer Immunol. Res6, 36–46 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moghimi, B. et al. Preclinical assessment of the efficacy and specificity of GD2-B7H3 SynNotch CAR-T in metastatic neuroblastoma. Nat. Commun.12, 511 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keyel, M. E. & Reynolds, C. P. Spotlight on dinutuximab in the treatment of high-risk neuroblastoma: development and place in therapy. Biologics13, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosse, K. R. et al. Identification of GPC2 as an oncoprotein and candidate immunotherapeutic target in high-risk neuroblastoma. Cancer Cell32, 295–309.e212 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raman, S. et al. A GPC2 antibody-drug conjugate is efficacious against neuroblastoma and small-cell lung cancer via binding a conformational epitope. Cell Rep. Med2, 100344 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pascual-Pasto, G. et al. GPC2 antibody-drug conjugate reprograms the neuroblastoma immune milieu to enhance macrophage-driven therapies. J. Immunother. Cancer10, e004704 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heitzeneder, S. et al. GPC2-CAR T cells tuned for low antigen density mediate potent activity against neuroblastoma without toxicity. Cancer Cell40, 53–69.e59 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, N. et al. CAR T cells targeting tumor-associated exons of glypican 2 regress neuroblastoma in mice. Cell Rep. Med2, 100297 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, N., Fu, H., Hewitt, S. M., Dimitrov, D. S. & Ho, M. Therapeutically targeting glypican-2 via single-domain antibody-based chimeric antigen receptors and immunotoxins in neuroblastoma. Proc. Natl Acad. Sci. USA114, E6623–E6631 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun, M. et al. Preclinical optimization of a GPC2-targeting CAR T-cell therapy for neuroblastoma. J. Immunother. Cancer11, e005881 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian, M. et al. An optimized bicistronic chimeric antigen receptor against GPC2 or CD276 overcomes heterogeneous expression in neuroblastoma. J. Clin. Invest132, e155621 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kildisiute, G. et al. Tumor to normal single-cell mRNA comparisons reveal a pan-neuroblastoma cancer cell. Sci. Adv.7, eabd3311 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minute, L. et al. Cellular cytotoxicity is a form of immunogenic cell death. J. Immunother. Cancer8, e000325corr1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sen Santara, S. et al. The NK cell receptor NKp46 recognizes ecto-calreticulin on ER-stressed cells. Nature616, 348–356 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uzhachenko, R. V. & Shanker, A. CD8(+) T lymphocyte and nk cell network: circuitry in the cytotoxic domain of immunity. Front Immunol.10, 1906 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aran, D. et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol.20, 163–172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wienke, J. et al. Integrative analysis of neuroblastoma by single-cell RNA sequencing identifies the NECTIN2-TIGIT axis as a target for immunotherapy. Cancer Cell10.1016/j.ccell.2023.12.008 (2023). [DOI] [PMC free article] [PubMed]
- 41.Fetahu, I. S. et al. Single-cell transcriptomics and epigenomics unravel the role of monocytes in neuroblastoma bone marrow metastasis. Nat. Commun.14, 3620 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li, W., Yang, H. & Dimitrov, D. S. Identification of high-affinity anti-CD16A allotype-independent human antibody domains. Exp. Mol. Pathol.101, 281–289 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vicioso, Y. et al. NF-kappaB c-Rel is dispensable for the development but is required for the cytotoxic function of NK cells. Front Immunol.12, 652786 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez-Marquez, P. et al. CAR density influences antitumoral efficacy of BCMA CAR T cells and correlates with clinical outcome. Sci. Adv.8, eabo0514 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen, R. et al. Interleukin-15 enhances anti-GD2 antibody-mediated cytotoxicity in an orthotopic PDX model of neuroblastoma. Clin. Cancer Res25, 7554–7564 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sellmyer, M. A. et al. Imaging CAR T cell trafficking with eDHFR as a PET reporter gene. Mol. Ther.28, 42–51 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Majzner, R. G. et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature603, 934–941 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Labanieh, L. & Mackall, C. L. CAR immune cells: design principles, resistance and the next generation. Nature614, 635–648 (2023). [DOI] [PubMed] [Google Scholar]
- 49.Bagashev, A. et al. CD19 alterations emerging after CD19-directed immunotherapy cause retention of the misfolded protein in the endoplasmic reticulum. Mol. Cell Biol.38, e00383–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sotillo, E. et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov.5, 1282–1295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacoby, E. et al. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat. Commun.7, 12320 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamieh, M. et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature568, 112–116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paul, M. R., Wong, V., Aristizabal, P. & Kuo, D. J. Treatment of recurrent refractory pediatric pre-b acute lymphoblastic leukemia using inotuzumab ozogamicin monotherapy resulting in cd22 antigen expression loss as a mechanism of therapy resistance. J. Pediatr. Hematol. Oncol.41, e546–e549 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Rourke, D. M. et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med9, eaaa0984 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hegde, M. et al. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J. Clin. Invest126, 3036–3052 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anurathapan, U. et al. Kinetics of tumor destruction by chimeric antigen receptor-modified T cells. Mol. Ther.22, 623–633 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi, B. D. et al. Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma. N. Engl. J. Med390, 1290–1298 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vivier, E., Tomasello, E., Baratin, M., Walzer, T. & Ugolini, S. Functions of natural killer cells. Nat. Immunol.9, 503–510 (2008). [DOI] [PubMed] [Google Scholar]
- 59.Frosch, J., Leontari, I. & Anderson, J. Combined effects of myeloid cells in the neuroblastoma tumor microenvironment. Cancers (Basel)13, 1743 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Theruvath, J. et al. Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication. Nat. Med28, 333–344 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jain, R. K. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res50, 814s–819s (1990). [PubMed] [Google Scholar]
- 62.Vallera, D. A. et al. IL15 trispecific killer engagers (TriKE) make natural killer cells specific to cd33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin. Cancer Res22, 3440–3450 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gauthier, L. et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell177, 1701–1713.e1716 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Gauthier, L. et al. Control of acute myeloid leukemia by a trifunctional NKp46-CD16a-NK cell engager targeting CD123. Nat. Biotechnol.41, 1296–1306 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krenciute, G. et al. Transgenic expression of IL15 improves antiglioma activity of IL13Ralpha2-CAR T cells but results in antigen loss variants. Cancer Immunol. Res5, 571–581 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoyos, V. et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia24, 1160–1170 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roybal, K. T. et al. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell164, 770–779 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu. J., Fu, J., Zhang, M. & Liu, D. AFM13: a first-in-class tetravalent bispecific anti-CD30/CD16A antibody for NK cell-mediated immunotherapy. J Hematol Oncol.8, 96 (2015). [DOI] [PMC free article] [PubMed]
- 69.Rongvaux, A. et al. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu Rev. Immunol.31, 635–674 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rongvaux, A. et al. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol.32, 364–372 (2014). [DOI] [PMC free article] [PubMed]
- 71.Webb, E. R. et al. Immune characterization of pre-clinical murine models of neuroblastoma. Sci. Rep.10, 16695 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bruhns, P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood119, 5640–5649 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Mechetina, L. V., Najakshin, A. M., Alabyev, B. Y., Chikaev, N. A. & Taranin, A. V. Identification of CD16-2, a novel mouse receptor homologous to CD16/Fc gamma RIII. Immunogenetics54, 463–468 (2002). [DOI] [PubMed] [Google Scholar]
- 74.Smith, P., DiLillo, D. J., Bournazos, S., Li, F. & Ravetch, J. V. Mouse model recapitulating human Fcgamma receptor structural and functional diversity. Proc. Natl Acad. Sci. USA109, 6181–6186 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McNerney, K. O. et al. TH-MYCN tumors, but not tumor-derived cell lines, are adrenergic lineage, GD2+, and responsive to anti-GD2 antibody therapy. Oncoimmunology11, 2075204 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Young, M. D. & Behjati, S. SoupX removes ambient RNA contamination from droplet-based single-cell RNA sequencing data. Gigascience9, giaa151 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell184, 3573–3587.e3529 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rokita, J. L. et al. Genomic profiling of childhood tumor patient-derived xenograft models to enable rational clinical trial design. Cell Rep.29, 1675–1689.e1679 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Foster, J. B. et al. Development of GPC2-directed chimeric antigen receptors using mRNA for pediatric brain tumors. J. Immunother. Cancer10, e004450 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Imai, C. et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia18, 676–684 (2004). [DOI] [PubMed] [Google Scholar]
- 81.Mount, C. W. et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nat. Med24, 572–579 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li, W. et al. One-domain CD4 fused to human anti-CD16 antibody domain mediates effective killing of HIV-1-infected cells. Sci. Rep.7, 9130 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whitlow, M. et al. An improved linker for single-chain Fv with reduced aggregation and enhanced proteolytic stability. Protein Eng.6, 989–995 (1993). [DOI] [PubMed] [Google Scholar]
- 84.Voeller, J. et al. Combined innate and adaptive immunotherapy overcomes resistance of immunologically cold syngeneic murine neuroblastoma to checkpoint inhibition. J. Immunother. Cancer7, 344 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Campeau, E. et al. A versatile viral system for expression and depletion of proteins in mammalian cells. PLoS One4, e6529 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
High-risk neuroblastoma TARGET gene expression data were downloaded from (https://treehousegenomics.soe.ucsc.edu/public-data), whereas human normal tissue gene expression data were downloaded from the GTEx portal (https://gtexportal.org/home/datasets). RDS files for neuroblastoma single-cell RNA-sequencing data were downloaded from (https://www.neuroblastomacellatlas.org). All other data are available in the Article, Supplementary Information or the Source Data file that is provided with this paper. Source data are provided with this paper.