Abstract
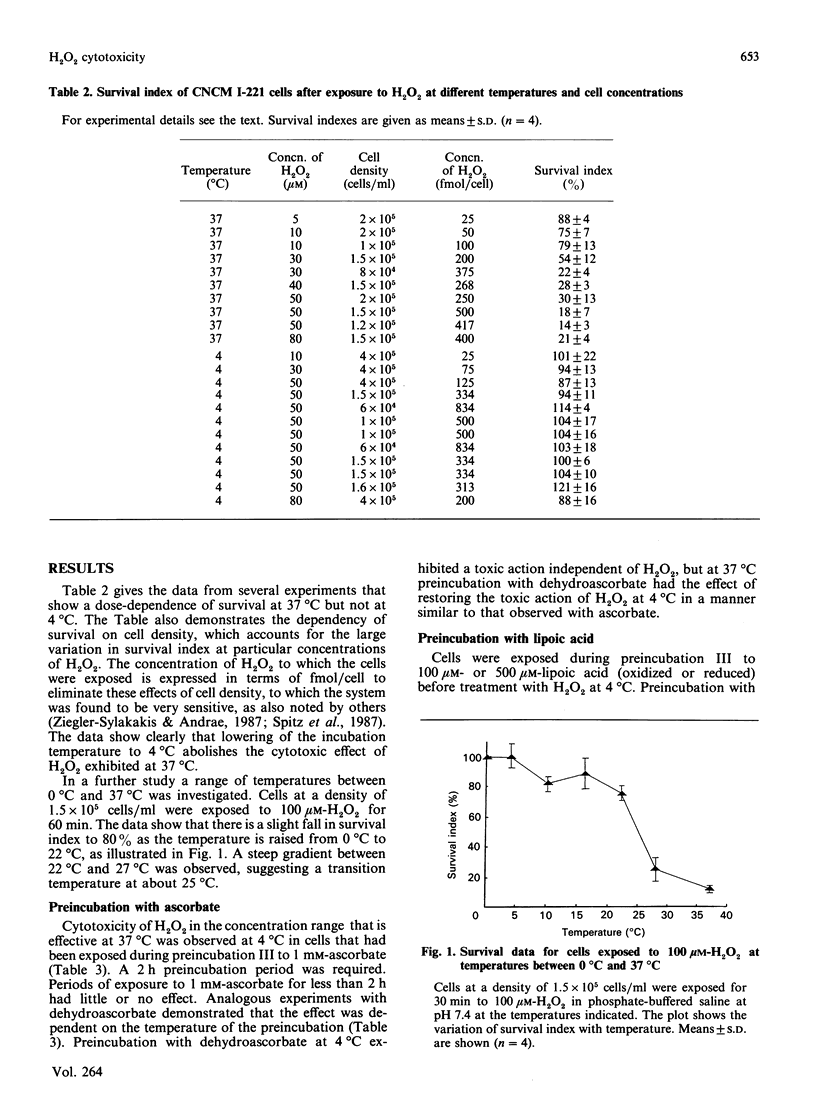
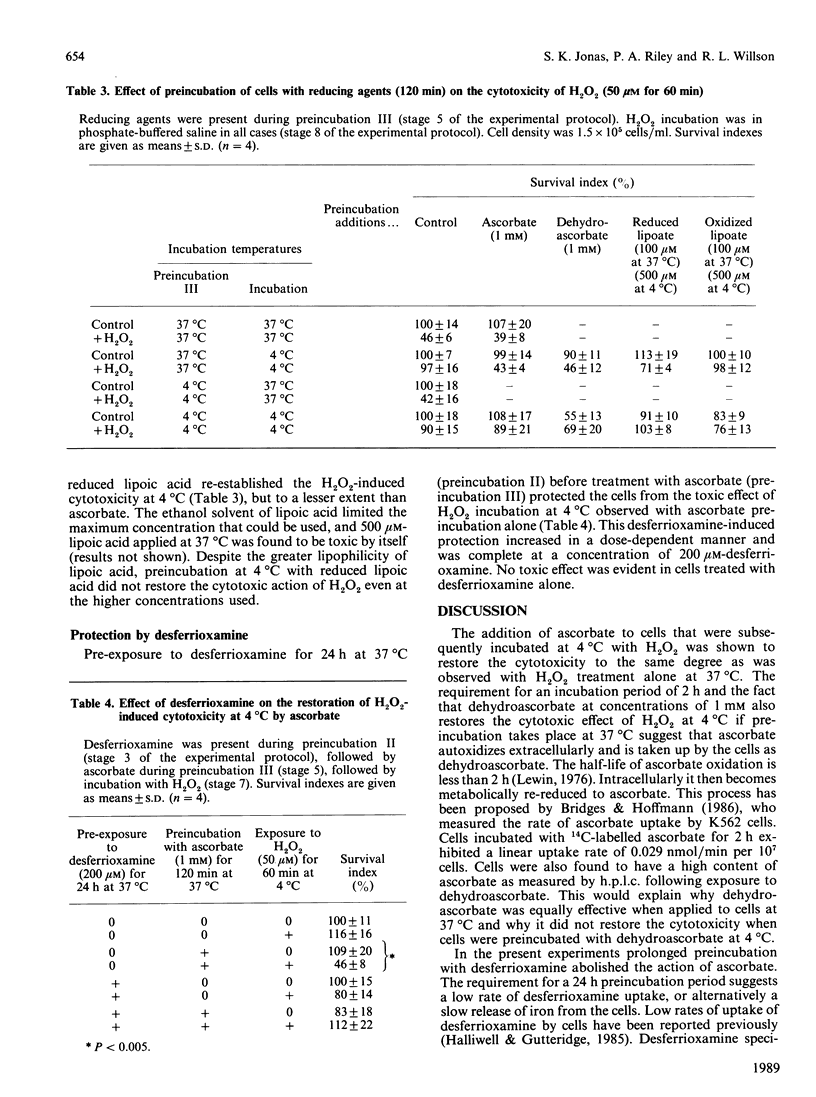
The principal mechanism of H2O2 toxicity is thought to involve the generation of hydroxyl (HO.) radicals through its interactions with Fe2+ ions by the Fenton reaction. Of particular interest has been the demonstration by Ward, Blakely & Joner [(1985) Radiat. Res. 103, 383-392] that the cytotoxicity of H2O2 is diminished at low temperature. We have now examined this phenomenon further with a mammalian epithelial cell line (CNCMI-221). Resistance of these cells to 100 microM-H2O2 added extracellularly exhibits a transition in the temperature range between 27 degrees C and 22 degrees C. We have found that the low-temperature resistance to cytotoxic concentrations of H2O2 is abolished by preincubation of cells with reductants such as ascorbate or reduced lipoic acid. This implies that the low-temperature resistance to H2O2 cytotoxicity may be due to inhibition of cellular reductive processes. The restoration of the cytotoxic action of H2O2 at 4 degrees C by ascorbate is prevented by pre-exposure of cells to desferrioxamine. This is evidence that transition-metal ions (such as iron ions) are involved in the cytotoxicity and is consistent with a mechanism of cell damage that depends on the Fenton reaction and a metal ion in the reduced state. Restoration of H2O2 cytotoxicity at low temperature by ascorbate is consistent with the artificial production of an intracellular reducing environment that at normal temperatures is sustained by cellular metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridges K. R., Hoffman K. E. The effects of ascorbic acid on the intracellular metabolism of iron and ferritin. J Biol Chem. 1986 Oct 25;261(30):14273–14277. [PubMed] [Google Scholar]
- Dallegri F., Ballestrero A., Frumento G., Adami R., Patrone F. Erythrocyte lysis by monocytes: investigations on the mechanism and role of the target cell hydrogen peroxide catabolizing pathways. J Clin Lab Immunol. 1987 Jun;23(2):95–99. [PubMed] [Google Scholar]
- Garlick P. B., Davies M. J., Hearse D. J., Slater T. F. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ Res. 1987 Nov;61(5):757–760. doi: 10.1161/01.res.61.5.757. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M., Blake D. Metal ions and oxygen radical reactions in human inflammatory joint disease. Philos Trans R Soc Lond B Biol Sci. 1985 Dec 17;311(1152):659–671. doi: 10.1098/rstb.1985.0171. [DOI] [PubMed] [Google Scholar]
- Hoffmann M. E., Mello-Filho A. C., Meneghini R. Correlation between cytotoxic effect of hydrogen peroxide and the yield of DNA strand breaks in cells of different species. Biochim Biophys Acta. 1984 Apr 5;781(3):234–238. doi: 10.1016/0167-4781(84)90088-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann M. E., Meneghini R. Action of hydrogen peroxide on human fibroblast in culture. Photochem Photobiol. 1979 Jul;30(1):151–155. doi: 10.1111/j.1751-1097.1979.tb07128.x. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Chin S. M., Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988 Apr 29;240(4852):640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- Jacobs G. P., Samuni A., Czapski G. The contribution of endogenous and exogenous effects to radiation-induced damage in the bacterial spore. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Jun;47(6):621–627. doi: 10.1080/09553008514550861. [DOI] [PubMed] [Google Scholar]
- Kyle M. E., Nakae D., Sakaida I., Miccadei S., Farber J. L. Endocytosis of superoxide dismutase is required in order for the enzyme to protect hepatocytes from the cytotoxicity of hydrogen peroxide. J Biol Chem. 1988 Mar 15;263(8):3784–3789. [PubMed] [Google Scholar]
- Larramendy M., Mello-Filho A. C., Martins E. A., Meneghini R. Iron-mediated induction of sister-chromatid exchanges by hydrogen peroxide and superoxide anion. Mutat Res. 1987 May;178(1):57–63. doi: 10.1016/0027-5107(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Link E. M., Riley P. A. Role of hydrogen peroxide in the cytotoxicity of the xanthine/xanthine oxidase system. Biochem J. 1988 Jan 15;249(2):391–399. doi: 10.1042/bj2490391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello Filho A. C., Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984 Feb 24;781(1-2):56–63. doi: 10.1016/0167-4781(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Rubin R., Farber J. L. Mechanisms of the killing of cultured hepatocytes by hydrogen peroxide. Arch Biochem Biophys. 1984 Feb 1;228(2):450–459. doi: 10.1016/0003-9861(84)90010-9. [DOI] [PubMed] [Google Scholar]
- Samuni A., Aronovitch J., Godinger D., Chevion M., Czapski G. On the cytotoxicity of vitamin C and metal ions. A site-specific Fenton mechanism. Eur J Biochem. 1983 Dec 1;137(1-2):119–124. doi: 10.1111/j.1432-1033.1983.tb07804.x. [DOI] [PubMed] [Google Scholar]
- Spitz D. R., Dewey W. C., Li G. C. Hydrogen peroxide or heat shock induces resistance to hydrogen peroxide in Chinese hamster fibroblasts. J Cell Physiol. 1987 Jun;131(3):364–373. doi: 10.1002/jcp.1041310308. [DOI] [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Endogenous defenses against the cytotoxicity of hydrogen peroxide in cultured rat hepatocytes. J Biol Chem. 1985 Jan 10;260(1):86–92. [PubMed] [Google Scholar]
- Sunderman F. W., Jr, Barber A. M. Finger-loops, oncogenes, and metals. Claude Passmore Brown memorial lecture. Ann Clin Lab Sci. 1988 Jul-Aug;18(4):267–288. [PubMed] [Google Scholar]
- Ward J. F., Blakely W. F., Joner E. I. Mammalian cells are not killed by DNA single-strand breaks caused by hydroxyl radicals from hydrogen peroxide. Radiat Res. 1985 Sep;103(3):383–392. [PubMed] [Google Scholar]
- Williams R. J. Zinc: what is its role in biology? Endeavour. 1984;8(2):65–70. doi: 10.1016/0160-9327(84)90040-1. [DOI] [PubMed] [Google Scholar]
- Ziegler-Skylakakis K., Andrae U. Mutagenicity of hydrogen peroxide in V79 Chinese hamster cells. Mutat Res. 1987 Sep;192(1):65–67. doi: 10.1016/0165-7992(87)90127-8. [DOI] [PubMed] [Google Scholar]


