Abstract
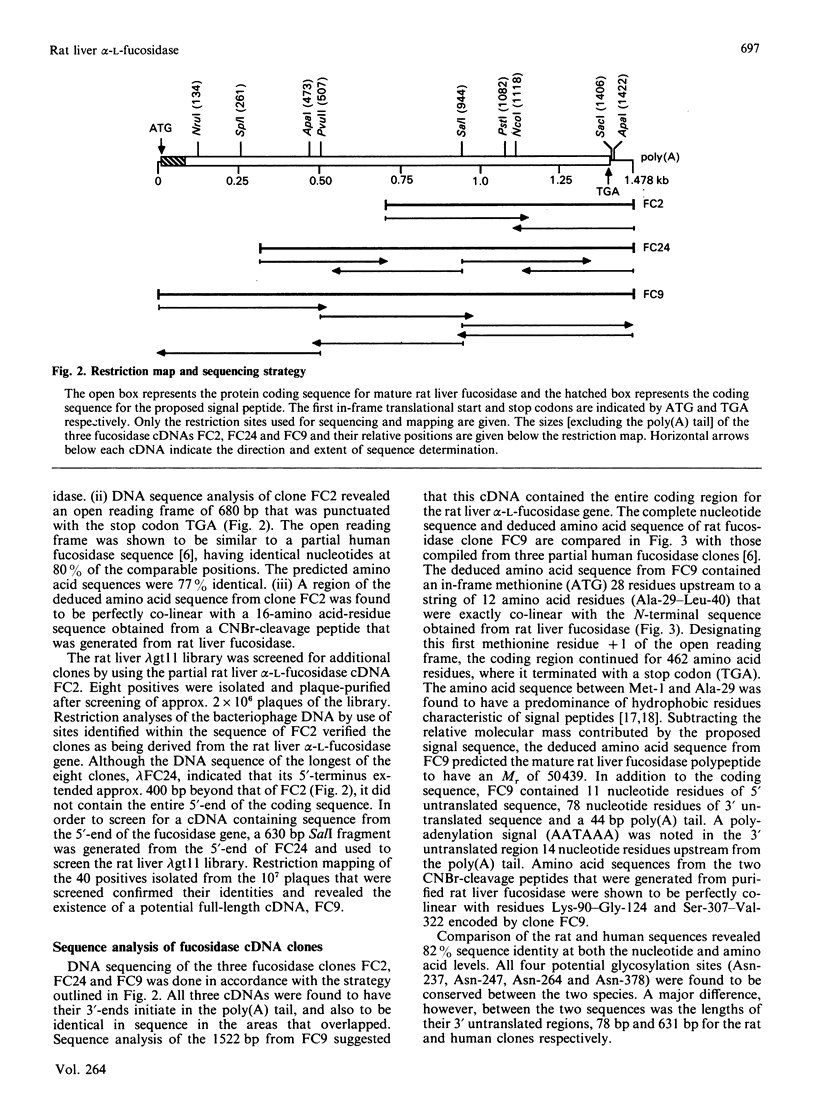
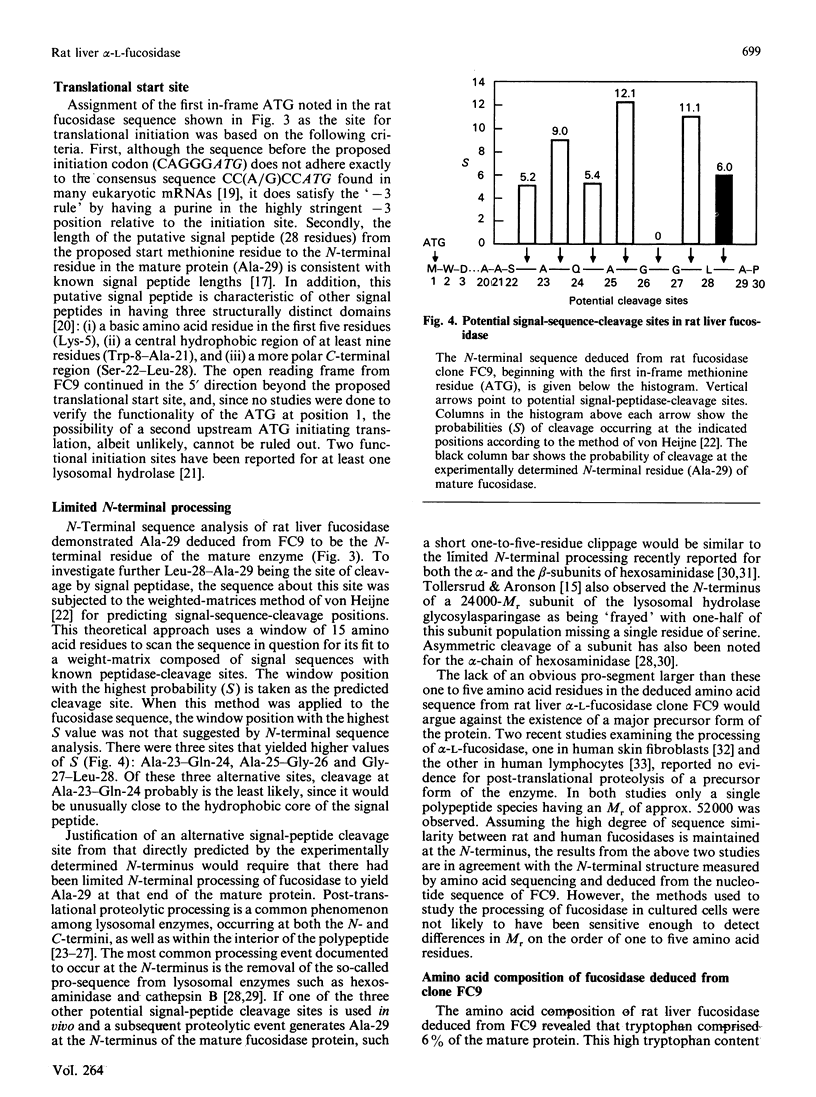
cDNA clones for alpha-L-fucosidase were isolated from a rat liver lambda gt11 expression library by using both monospecific polyclonal antibodies against the affinity-purified enzyme and biotinylated rat liver fucosidase cDNA sequences as probes. The largest clone, lambda FC9, contained a 1522 bp full-length cDNA insert (FC9) that encoded the 434-amino acid-residue subunit (Mr 50439) of rat liver alpha-L-fucosidase. A putative signal peptide 28 amino acid residues in length preceded the sequence for the mature protein. In addition, FC9 specified for 11 nucleotide residues of 5' untranslated sequence, 78 nucleotide residues of 3' untranslated sequence and a poly(A) tail. The deduced amino acid sequence from FC9 in conjunction with the experimentally determined N-terminus of the mature enzyme suggested that rat liver fucosidase did not contain a pro-segment. However, there was the possibility of limited N-terminal processing (one to five amino acid residues) having occurred after removal of the predicted signal peptide. Amino acid sequences deduced from FC9 were co-linear with amino acid sequences measured at the N-terminus of purified fucosidase and on two of its CNBr-cleavage peptides. An unusual aspect of rat liver alpha-L-fucosidase protein structure obtained from the FC9 data was its high content of tryptophan (6%). The coding sequence from FC9 showed 82% sequence identity with that from a previously reported incomplete human fucosidase sequence [O'Brien, Willems, Fukushima, de Wet, Darby, DiCioccio, Fowler & Shows, (1987) Enzyme 38, 45-53].
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argade S. P., Hopfer R. L., Strang A. M., van Halbeek H., Alhadeff J. A. Structural studies on the carbohydrate moieties of human liver alpha-L-fucosidase. Arch Biochem Biophys. 1988 Oct;266(1):227–247. doi: 10.1016/0003-9861(88)90254-8. [DOI] [PubMed] [Google Scholar]
- Bapat B., Ethier M., Neote K., Mahuran D., Gravel R. A. Cloning and sequence analysis of a cDNA encoding the beta-subunit of mouse beta-hexosaminidase. FEBS Lett. 1988 Sep 12;237(1-2):191–195. doi: 10.1016/0014-5793(88)80199-6. [DOI] [PubMed] [Google Scholar]
- Barker C., Dell A., Rogers M., Alhadeff J. A., Winchester B. Canine alpha-L-fucosidase in relation to the enzymic defect and storage products in canine fucosidosis. Biochem J. 1988 Sep 15;254(3):861–868. doi: 10.1042/bj2540861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes A. K., Wynn C. H. Homology of lysosomal enzymes and related proteins: prediction of posttranslational modification sites including phosphorylation of mannose and potential epitopic and substrate binding sites in the alpha- and beta-subunits of hexosaminidases, alpha-glucosidase, and rabbit and human isomaltase. Proteins. 1988;4(3):182–189. doi: 10.1002/prot.340040305. [DOI] [PubMed] [Google Scholar]
- Bishop D. F., Calhoun D. H., Bernstein H. S., Hantzopoulos P., Quinn M., Desnick R. J. Human alpha-galactosidase A: nucleotide sequence of a cDNA clone encoding the mature enzyme. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4859–4863. doi: 10.1073/pnas.83.13.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. J., San Segundo B., McCormick M. B., Steiner D. F. Nucleotide and predicted amino acid sequences of cloned human and mouse preprocathepsin B cDNAs. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7721–7725. doi: 10.1073/pnas.83.20.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCioccio R. A., Brown K. S. Biosynthesis, processing, and extracellular release of alpha-L-fucosidase in lymphoid cell lines of different genetic origins. Biochem Genet. 1988 Jun;26(5-6):401–420. doi: 10.1007/BF02401794. [DOI] [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Carboxyl-terminal proteolytic processing during biosynthesis of the lysosomal enzymes beta-glucuronidase and cathepsin D. Biochemistry. 1983 Oct 25;22(22):5201–5205. doi: 10.1021/bi00291a021. [DOI] [PubMed] [Google Scholar]
- Faust P. L., Kornfeld S., Chirgwin J. M. Cloning and sequence analysis of cDNA for human cathepsin D. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4910–4914. doi: 10.1073/pnas.82.15.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Kincaid R. L., Nightingale M. S. A rapid non-radioactive procedure for plaque hybridization using biotinylated probes prepared by random primed labeling. Biotechniques. 1988 Jan;6(1):42–49. [PubMed] [Google Scholar]
- Klapper M. H. The independent distribution of amino acid near neighbor pairs into polypeptides. Biochem Biophys Res Commun. 1977 Oct 10;78(3):1018–1024. doi: 10.1016/0006-291x(77)90523-x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuranda M. J., Aronson N. N., Jr A di-N-acetylchitobiase activity is involved in the lysosomal catabolism of asparagine-linked glycoproteins in rat liver. J Biol Chem. 1986 May 5;261(13):5803–5809. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leibold D. M., Robinson C. B., Scanlin T. F., Glick M. C. Lack of proteolytic processing of alpha-L-fucosidase in human skin fibroblasts. J Cell Physiol. 1988 Dec;137(3):411–420. doi: 10.1002/jcp.1041370304. [DOI] [PubMed] [Google Scholar]
- Little L. E., Lau M. M., Quon D. V., Fowler A. V., Neufeld E. F. Proteolytic processing of the alpha-chain of the lysosomal enzyme, beta-hexosaminidase, in normal human fibroblasts. J Biol Chem. 1988 Mar 25;263(9):4288–4292. [PubMed] [Google Scholar]
- Mahuran D. J., Neote K., Klavins M. H., Leung A., Gravel R. A. Proteolytic processing of pro-alpha and pro-beta precursors from human beta-hexosaminidase. Generation of the mature alpha and beta a beta b subunits. J Biol Chem. 1988 Apr 5;263(10):4612–4618. [PubMed] [Google Scholar]
- Marck C. 'DNA Strider': a 'C' program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988 Mar 11;16(5):1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Taubenberger A., Westphal M., Noegel A., Gerisch G. A developmentally regulated gene product from Dictyostelium discoideum shows high homology to human alpha-L-fucosidase. FEBS Lett. 1989 Mar 27;246(1-2):185–192. doi: 10.1016/0014-5793(89)80280-7. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Kato K. In vitro biosynthesis of the lysosomal cathepsin H. Biochem Biophys Res Commun. 1987 Jul 15;146(1):159–164. doi: 10.1016/0006-291x(87)90705-4. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Willems P. J., Fukushima H., de Wet J. R., Darby J. K., Di Cioccio R., Fowler M. L., Shows T. B. Molecular biology of the alpha-L-fucosidase gene and fucosidosis. Enzyme. 1987;38(1-4):45–53. doi: 10.1159/000469189. [DOI] [PubMed] [Google Scholar]
- Opheim D. J., Touster O. The purification and characterization of rat liver lysosomal alpha-L-fucosidase. J Biol Chem. 1977 Jan 25;252(2):739–743. [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Powell P. P., Kyle J. W., Miller R. D., Pantano J., Grubb J. H., Sly W. S. Rat liver beta-glucuronidase. cDNA cloning, sequence comparisons and expression of a chimeric protein in COS cells. Biochem J. 1988 Mar 1;250(2):547–555. doi: 10.1042/bj2500547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quon D. V., Proia R. L., Fowler A. V., Bleibaum J., Neufeld E. F. Proteolytic processing of the beta-subunit of the lysosomal enzyme, beta-hexosaminidase, in normal human fibroblasts. J Biol Chem. 1989 Feb 25;264(6):3380–3384. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Elledge S., Sweetser D., Young R. A., Davis R. W. Lambda gt 11: gene isolation with antibody probes and other applications. Methods Enzymol. 1987;154:107–128. doi: 10.1016/0076-6879(87)54073-3. [DOI] [PubMed] [Google Scholar]
- Sorge J. A., West C., Kuhl W., Treger L., Beutler E. The human glucocerebrosidase gene has two functional ATG initiator codons. Am J Hum Genet. 1987 Dec;41(6):1016–1024. [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollersrud O. K., Aronson N. N., Jr Purification and characterization of rat liver glycosylasparaginase. Biochem J. 1989 May 15;260(1):101–108. doi: 10.1042/bj2600101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White W. J., Jr, Schray K. J., Alhadeff J. A. Studies on the catalytic residues at the active site of human liver alpha-L-fucosidase. Biochim Biophys Acta. 1985 Jul 1;829(3):303–310. doi: 10.1016/0167-4838(85)90237-7. [DOI] [PubMed] [Google Scholar]
- Willems P. J., Darby J. K., DiCioccio R. A., Nakashima P., Eng C., Kretz K. A., Cavalli-Sforza L. L., Shooter E. M., O'Brien J. S. Identification of a mutation in the structural alpha-L-fucosidase gene in fucosidosis. Am J Hum Genet. 1988 Nov;43(5):756–763. [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]