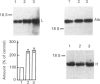
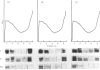
Abstract
In rats with chronic dietary iron overload, a higher amount of liver ferritin L-subunit mRNA was found mainly engaged on polysomes, whereas in control rats ferritin L-subunit mRNA molecules were largely stored in ribonucleoprotein particles. On the other hand, ferritin H-subunit mRNA was unchanged by chronic iron load and remained in the inactive cytoplasmic pool. In agreement with previous reports, in rats acutely treated with parenteral iron, only the ferritin L-subunit mRNA increased in amount, whereas both ferritin subunit mRNAs shifted to polysomes. This may indicate that, whereas in acute iron overload the hepatocyte operates a translation shift of both ferritin mRNAs to confront rapidly the abrupt entry of iron into the cell, during chronic iron overload it responds to the slow iron influx by translating a greater amount of L-subunit mRNA to synthesize isoferritins more suitable for long-term iron storage.
Full text
PDF
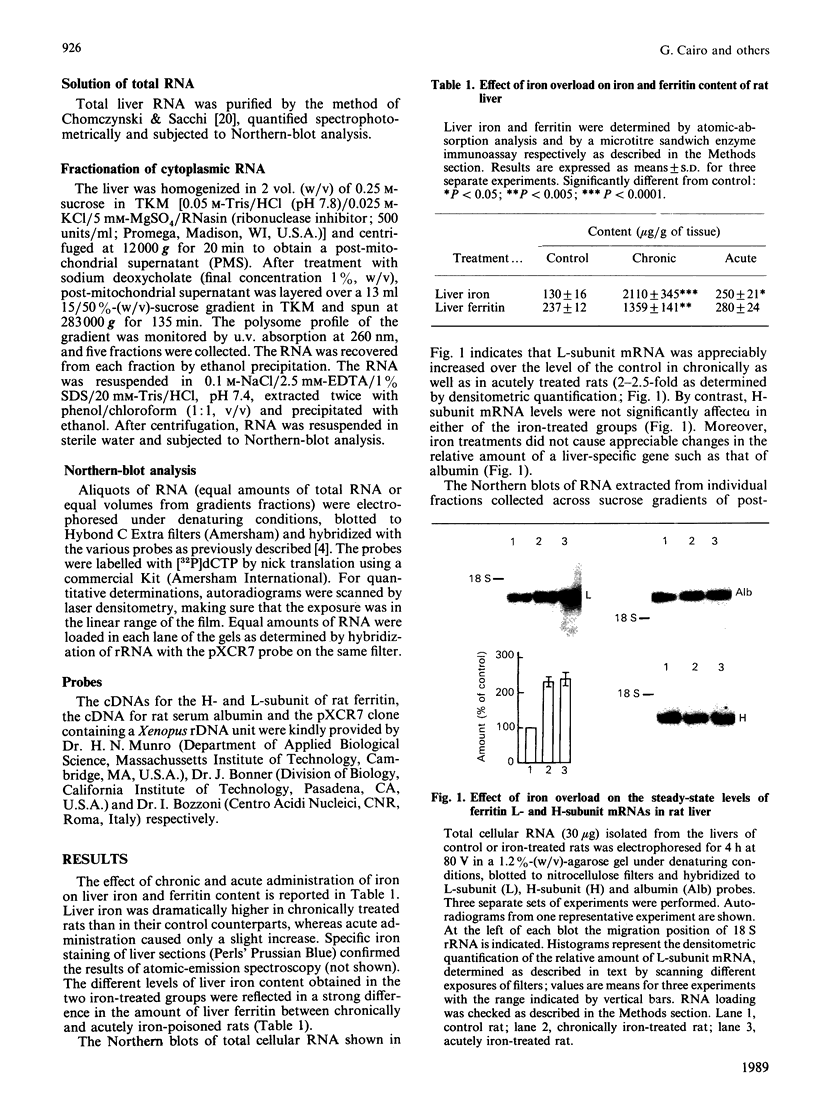
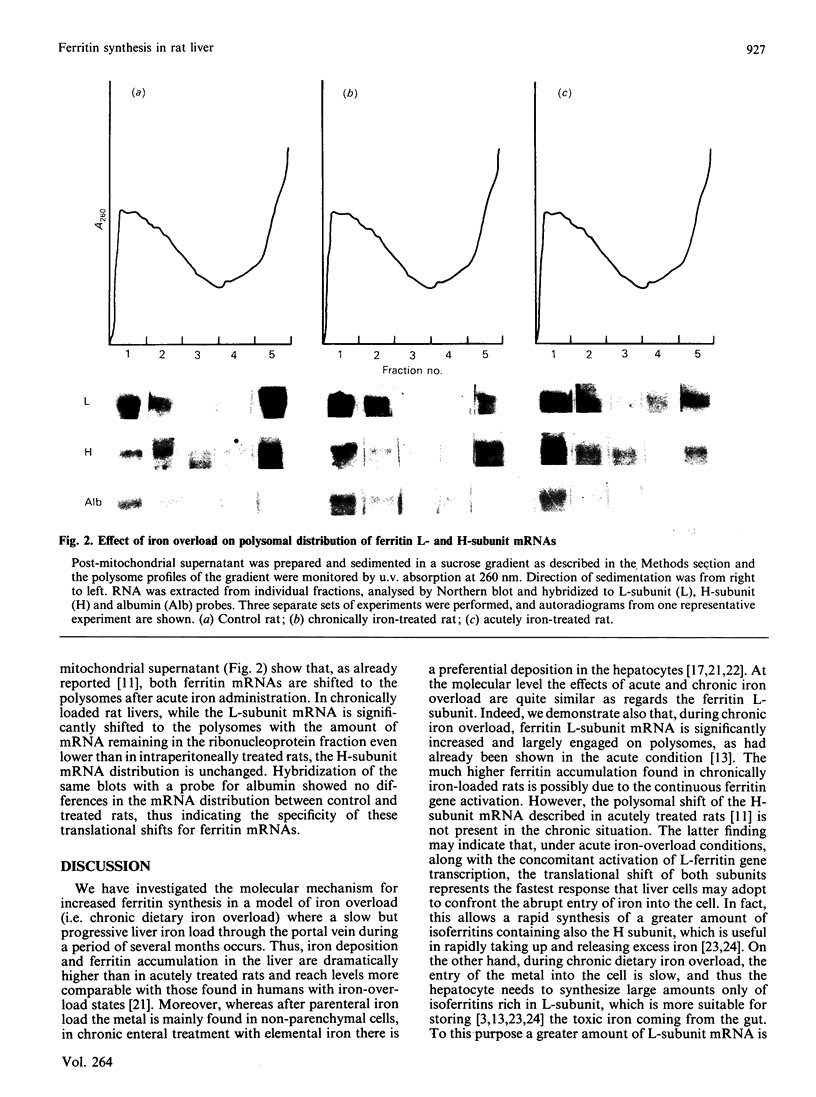

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz N., Munro H. N. Both subunits of rat liver ferritin are regulated at a translational level by iron induction. Nucleic Acids Res. 1986 Jan 24;14(2):915–927. doi: 10.1093/nar/14.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz N., Munro H. N. Iron regulates ferritin mRNA translation through a segment of its 5' untranslated region. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon B. R., Healey J. F., Brittenham G. M., Park C. H., Nunnari J., Tavill A. S., Bonkovsky H. L. Hepatic microsomal function in rats with chronic dietary iron overload. Gastroenterology. 1986 Jun;90(6):1844–1853. doi: 10.1016/0016-5085(86)90251-9. [DOI] [PubMed] [Google Scholar]
- Bacon B. R., Tavill A. S., Brittenham G. M., Park C. H., Recknagel R. O. Hepatic lipid peroxidation in vivo in rats with chronic iron overload. J Clin Invest. 1983 Mar;71(3):429–439. doi: 10.1172/JCI110787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett M. L., Halliday J. W., Powell L. W. Genetic hemochromatosis. Semin Liver Dis. 1984 Aug;4(3):217–227. doi: 10.1055/s-2008-1041772. [DOI] [PubMed] [Google Scholar]
- Bomford A., Conlon-Hollingshead C., Munro H. N. Adaptive responses of rat tissue isoferritins to iron administration. Changes in subunit synthesis, isoferritin abundance, and capacity for iron storage. J Biol Chem. 1981 Jan 25;256(2):948–955. [PubMed] [Google Scholar]
- Cairo G., Bardella L., Schiaffonati L., Arosio P., Levi S., Bernelli-Zazzera A. Multiple mechanisms of iron-induced ferritin synthesis in HeLa cells. Biochem Biophys Res Commun. 1985 Nov 27;133(1):314–321. doi: 10.1016/0006-291x(85)91877-7. [DOI] [PubMed] [Google Scholar]
- Campbell C. H., Solgonick R. M., Linder M. C. Translational regulation of ferritin synthesis in rat spleen: effects of iron and inflammation. Biochem Biophys Res Commun. 1989 Apr 28;160(2):453–459. doi: 10.1016/0006-291x(89)92454-6. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Casey J. L., Koeller D. M., Rouault T. A., Harford J. B., Klausner R. D. A model for the structure and functions of iron-responsive elements. Gene. 1988 Dec 10;72(1-2):201–208. doi: 10.1016/0378-1119(88)90145-x. [DOI] [PubMed] [Google Scholar]
- Kohgo Y., Yokota M., Drysdale J. W. Differential turnover of rat liver isoferritins. J Biol Chem. 1980 Jun 10;255(11):5195–5200. [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S., Bannister J. V., Williams R. J. Structure and composition of ferritin cores isolated from human spleen, limpet (Patella vulgata) hemolymph and bacterial (Pseudomonas aeruginosa) cells. J Mol Biol. 1986 Mar 20;188(2):225–232. doi: 10.1016/0022-2836(86)90307-4. [DOI] [PubMed] [Google Scholar]
- Mattia E., den Blaauwen J., Ashwell G., van Renswoude J. Multiple post-transcriptional regulatory mechanisms in ferritin gene expression. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1801–1805. doi: 10.1073/pnas.86.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren G. D., Muir W. A., Kellermeyer R. W. Iron overload disorders: natural history, pathogenesis, diagnosis, and therapy. Crit Rev Clin Lab Sci. 1983;19(3):205–266. doi: 10.3109/10408368309165764. [DOI] [PubMed] [Google Scholar]
- Pechet G. S. Parenteral iron overload. Organ and cell distribution in rats. Lab Invest. 1969 Jan;20(1):119–126. [PubMed] [Google Scholar]
- Rocchi E., Gibertini P., Cassanelli M., Pietrangelo A., Borghi A., Ventura E. Serum ferritin in the assessment of liver iron overload and iron removal therapy in porphyria cutanea tarda. J Lab Clin Med. 1986 Jan;107(1):36–42. [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Caughman S. W., Harford J. B., Klausner R. D. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988 Sep 2;241(4870):1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- Schiaffonati L., Rappocciolo E., Tacchini L., Bardella L., Arosio P., Cozzi A., Cantu G. B., Cairo G. Mechanisms of regulation of ferritin synthesis in rat liver during experimental inflammation. Exp Mol Pathol. 1988 Apr;48(2):174–181. doi: 10.1016/0014-4800(88)90054-8. [DOI] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Wagstaff M., Worwood M., Jacobs A. Properties of human tissue isoferritins. Biochem J. 1978 Sep 1;173(3):969–977. doi: 10.1042/bj1730969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden W. E., Daniels-McQueen S., Brown P. H., Gaffield L., Russell D. A., Bielser D., Bailey L. C., Thach R. E. Translational repression in eukaryotes: partial purification and characterization of a repressor of ferritin mRNA translation. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9503–9507. doi: 10.1073/pnas.85.24.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K., Munro H. N. Induction of ferritin subunit synthesis by iron is regulated at both the transcriptional and translational levels. J Biol Chem. 1988 Jun 25;263(18):8938–8942. [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]