Abstract
Nuclear receptor action is mediated in part by the nuclear receptor corepressor 1 (NCOR1) and the silencing mediator of retinoic acid and thyroid hormone receptor (SMRT). NCOR1 and SMRT regulate metabolic pathways that govern body mass, insulin sensitivity, and energy expenditure, representing an understudied area in the realm of metabolic health and disease. Previously, we found that NCOR1 and SMRT are essential for maintaining metabolic homeostasis and their knockout (KO) leads to rapid weight loss and hypoglycemia, which is not survivable. Because of a potential defect in glucose absorption, we sought to determine the role of NCOR1 and SMRT specifically in intestinal epithelial cells (IECs). We used a postnatal strategy to disrupt NCOR1 and SMRT throughout IECs in adult mice. These mice were characterized metabolically and underwent metabolic phenotyping, body composition analysis, and glucose tolerance testing. Jejunal IECs were isolated and profiled by bulk RNA sequencing. We found that the postnatal KO of NCOR1 and SMRT from IECs leads to rapid weight loss and hypoglycemia with a significant reduction in survival. This was accompanied by alterations in glucose metabolism and activation of fatty acid oxidation in IECs. Metabolic phenotyping confirmed a reduction in body mass driven by a loss of body fat without altered food intake. This appeared to be mediated by a reduction of key intestinal carbohydrate transporters, including SGLT1, GLUT2, and GLUT5. Intestinal NCOR1 and SMRT act in tandem to regulate glucose levels and body weight. This in part may be mediated by regulation of intestinal carbohydrate transporters.
Keywords: nuclear receptor corepressor, carbohydrate transport, intestinal metabolism
Nuclear receptor corepressor 1 (NCOR1) and silencing mediator of retinoic acid and thyroid hormone receptor (SMRT; also known as NCOR2) are critical regulators of metabolism required for maintaining metabolic homeostasis. They recruit a multiprotein complex, including histone deacetylase 3 (HDAC3), to repress nuclear receptor gene expression via histone deacetylation in the presence of low levels of NR ligand (1-4). Determining the systemic roles of NCOR1 and SMRT has been difficult because embryonic knockout (KO) of either NCOR1 or SMRT is lethal (5, 6). Embryonic KO of NCOR1 causes hematologic and neurologic abnormalities, while embryonic KO of SMRT leads to impaired cardiac ventricular development, suggesting that these corepressors have independent roles despite being highly homologous (5, 6). To circumvent this, numerous models have been developed to study NCOR1 and SMRT in both a systemic and tissue-specific manner. Global interruption of NCOR1-HDAC3 binding leads to lower body weight, decreased whole-body fat mass, insulin sensitization, and an altered oscillatory pattern of key metabolic genes (7). Similar findings, with lower weight driven by a reduction in fat and increased energy expenditure, are seen when NCOR1 binding to the thyroid hormone receptor (TR) is globally inhibited (8). In contrast, global KO of SMRT leads to body weight gain, insulin resistance, an increase in hepatic lipogenesis, and hepatic steatosis (9).
NCOR1 and SMRT have also been studied in a tissue-specific manner with systemic consequences noted. Genetic alterations of hepatic NCOR1 have shown that in the context of thyroid hormone (TH) action, decreased cellular availability of NCOR1 leads to an enhanced response to TH, and its loss is associated with the activation of hepatic lipogenesis (10, 11). Muscle-specific KO of NCOR1 improves exercise endurance and increases skeletal muscle size with de-repression of peroxisome proliferator-activated receptor (PPAR) β/δ action, altering mitochondrial function and leading to a preponderance of lipid oxidation (12). In adipocytes, KO of NCOR1 derepresses PPARγ action, leading to improved hepatic, fat, and muscle insulin sensitivity and rendering mice resistant to a high-fat diet (13). The KO of SMRT from mouse skeletal muscle cells leads to an upregulation of muscle-specific β-oxidation markers via activation of PPARβ/δ, ERRα, and MEF2 (14). Thus, NCOR1 and SMRT have key roles, both globally and in a tissue-specific manner, that act to regulate body weight, multiple metabolic pathways, and insulin sensitivity.
Recently, we showed that the double KO of NCOR1 and SMRT in adult mice is not survivable and rapidly leads to weight loss, hypoglycemia, and hypothermia (15). Further characterization of the hypoglycemia pointed to impaired glucose absorption from the intestine. We found several abnormalities throughout the intestine of these mice, including a reduced expression of Slc5a1 (solute carrier family 5 member 1, aka sodium-dependent glucose cotransporter 1; SGLT1), Slc2a2 (glucose transporter 2; GLUT2), and Slc2a5 (GLUT5), which are critical transporters of glucose, galactose, and fructose in the small intestine (15-19). SGLT1 is of particular interest, given that its KO in mice mimics the blunted response to oral glucose administration seen in our NCOR1/SMRT global KO mice (16). Indeed, the phenotype of SGLT1 KO mice resembles congenital glucose-galactose reabsorption syndrome in humans, where mutations in SGLT1 cause an inability to absorb glucose and galactose, leading to failure to thrive and osmotic diarrhea in newborns (20-22).
Thus, we hypothesized that intestinal NCOR1 and SMRT act to regulate intestinal carbohydrate transporters, and consequently, glucose levels and body mass. To test this hypothesis, we generated a tamoxifen-inducible KO model of NCOR1 and SMRT in IECs to examine their role in regulating glucose levels and other metabolic pathways. Strikingly, the loss of NCOR1 and SMRT from IECs regulates survival, likely through the regulation of glucose absorption via downregulation of expression of key glucose and fructose transporters in IECs.
Materials and Methods
Animals
NCOR1loxP/loxP SMRTloxP/loxP female mice were bred to male mice carrying a tamoxifen-inducible cre recombinase under control of the villin 1 promoter (B6.Cg-Tg(Vil1-cre/ERT2)23Syr/J; a gift from Dr Sylvie Robine, also available via Jackson Lab, JAX stock No. 020282) (23). Subsequent generations were bred to generate NCOR1loxP/loxP SMRTloxP/loxP villin 1 Cre+/− and NCOR1loxP/loxP SMRTloxP/loxP villin 1 Cre−/− mice for experiments.
All experiments were approved by the Weill Cornell Medicine Institutional Animal Care and Use Committee. Mice were housed in a temperature-controlled, 12-hour light/dark cycle and supplied with food and water ad libitum. Mice were treated with a previously employed strategy of tamoxifen via intraperitoneal (i.p.) administration. Body temperature was checked using a lubricated 1-inch (25 mm) metal probe gently inserted rectally (Thermalert TH-5, physitemp). Mice were euthanized by CO2 asphyxiation followed by cardiac puncture in accordance with humane animal use guidelines. Tissues were rapidly collected, and flash-frozen in liquid nitrogen before storage at −80 °C unless collected for staining. IECs were collected by first sectioning the small intestine into duodenum, jejunum, and ileum. Each section was flushed with cold phosphate-buffered saline and the intestine was opened longitudinally with luminal side facing up. IECs were physically separated from the lamina propria using a clean glass slide, immediately placed in a microcentrifuge tube and flash-frozen (24, 25).
Real-time Quantitative Polymerase Chain Reaction
RNA STAT-60 (Fisher Scientific) was used to extract RNA from the liver and 500 ng of RNA was reverse-transcribed into complementary DNA using the SuperScript VILO kit (Invitrogen). The RNeasy Lipid Tissue Mini Kit (Qiagen) was used to extract RNA from IECs. Quantitative polymerase chain reaction (qPCR) was performed in duplicate using QuantStudio systems. Power SYBR Green Master Mix TaqMan Universal PCR Master Mix and TaqMan gene expression assays were purchased from ThermoFisher. Relative messenger RNA (mRNA) levels were calculated using standard-curve methods and normalized to the level of cyclophilin.
Glucose Tolerance Tests
Glucose was dissolved in sterile saline and 1-mg/g body weight glucose was administered either orally by gavage or by i.p. injection after an 8-hour fast. Blood glucose levels were checked using a glucometer (OneTouch Ultra, Johnson & Johnson) immediately before glucose administration, as well as 15, 30, 60, 90, and 120 minutes after initial dosing.
Histologic and Morphometric Analysis
Paraffin-embedded sections were used for routine hematoxylin and eosin staining. Sections of small intestine were isolated and flushed with cold phosphate-buffered saline. Villus height from Swiss-rolled jejunum was assessed using ImageJ Fiji (26-28).
Metabolic Phenotyping
Comprehensive metabolic monitoring was performed using a Promethion Metabolic Screening System (Promethion High-Definition Multiplexed Respirometry System for Mice; Sable Systems International). Briefly, mice were individually housed in metabolic cages located within ambient temperature and light-controlled environmental enclosures (22 °C, 12-hour light/dark cycle) (DB034-LT Laboratory Incubator; Darwin Chambers Company). Mice were housed on pine chip bedding, without nestlets, and provided ad libitum access to food and acidified water. Energy expenditure was assessed via indirect calorimetry and calculated using the Weir equation (3.941 kcal/L × VO2 + 1.106 kcal/L × VCO2) and normalized to lean body mass (29, 30). Respirometry values were determined every 5 minutes; the dwell time for each cage was 30 seconds, with baseline cage sampling frequency of 30 seconds occurring every 4 cages. Rates of oxygen consumption and carbon dioxide production were acquired with a sampling frequency of 1 second. Food and water intake and body mass were assessed gravimetrically. Distance traveled (activity) was determined by XY position displacements that are represented by beam breaks (PedMeters and AllMeters).
Oxygen Bomb Calorimetry
All fecal pellets were collected from the bottom of metabolic cages, one mouse per cage, dried at −60 °C, and total mass was recorded. Bomb calorimetry was performed in accordance with the manufacturer's directions. Three samples from each mouse were weighed and tested. Data were analyzed as previously described using an oxygen bomb calorimeter (Parr 6765 Combination Calorimeter, Parr Instrument Company) (31).
RNA Sequencing
RNA sequencing (RNA-seq) was performed on the NovaSeq 6000 platform by the Genomics Resources Core Facility at Weill Cornell Medicine. Total RNA from IECs was isolated using RNeasy Lipid Tissue Mini Kit (Qiagen). The quality of RNA was checked, and all samples were confirmed to have an RNA integrity number greater than 8. Paired-end libraries were synthesized by using a TruSeq RNA Sample Preparation kit (Illumina).
Base cells were converted to FASTQ using the illumina package bcl2fastq, and removal of adapters and low-quality reads was performed using fastp (version 0.21.0).
FASTQ files were aligned to mouse genome build mm10 using STAR (version 2.7.9a) (32). Ensembl-Gene-level counts for nonmitochondrial genes were generated using featureCounts (Subread package, version 1.6.2) and Ensembl annotation build 100 (uniquely aligned proper pairs, same strand). FASTQ quality was assessed using FastQC (version 0.11.7), and alignment quality was assessed using RSeQC (version 3.0.0). Variance-stabilizing transformation was accomplished using the varianceStabilizingTransformation function in the DESeq2 R package (version 1.23.10) (33). Differential expression was assessed using the Wald test implemented in the DESeq2 R package, and correction for multiple hypothesis testing was accomplished using the Benjamini-Hochberg false discovery rate (FDR). All analyses were performed using the R environment for statistical computing (version 4.1.2).
Gene Set Enrichment Analysis (GSEA) (version 2.2.1) was used to identify biological terms, pathways, and processes that are coordinately upregulated or downregulated within each pairwise comparison (34). The Entrez Gene identifiers of the human homologues of all genes in the Ensembl Gene annotation (identified using the NCBI “gene orthologs’ table, retrieved March 26, 2023) were ranked by Wald statistic. Ensembl Genes matching multiple mouse Entrez Gene identifiers, and mouse genes with multiple human homologues (or vice versa), were excluded prior to ranking, so that the ranked list represents only those human Entrez Gene IDs that match exactly one mouse Ensembl Gene. This ranked list was then used to perform preranked GSEA analyses (default parameters with random seed 1234) using the Entrez Gene versions of the H (Hallmark), C2 CP (Biocarta, KEGG, PID, Reactome, WikiPathways), C3 (transcription factor and microRNA motif), and C5 (Gene Ontology, GO) gene sets obtained from the Molecular Signatures Database (MSigDB), version 7.5.1. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE268594 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE268594 reviewer token ovqfksuwfpcvrul) with further analyzed results deposited in Mendeley (35, 36).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism, 9.3.0. All gene expression and hormonal analyses were performed on group numbers indicated in figure legends. The differences in mRNA, hormone levels, and body composition were analyzed by t test. Body weight, blood glucose, body temperature, and glucose tolerance test (GTTs) were analyzed by 2-way analysis of variance with repeated measures followed by post hoc Bonferroni multiple comparisons test. Data are expressed as mean ±SEM and P values less than .05 were considered statistically significant.
Results
Postnatal Deletion of NCOR1 and SMRT From Intestinal Epithelial Cells Leads to Sustained Weight Loss, Hypoglycemia, and Increased Mortality
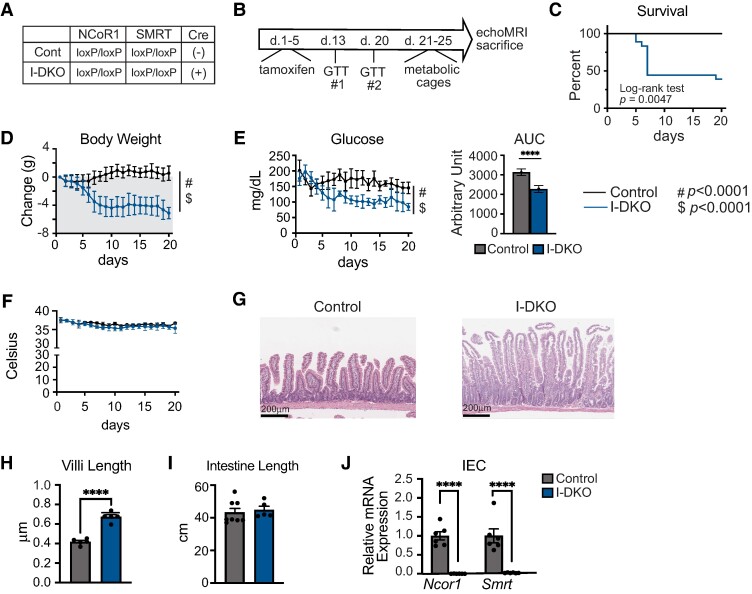
To study the actions of NCOR1 and SMRT in IECs, we crossed NCOR1loxP/loxP SMRTloxP/loxP female mice with male NCOR1loxP/loxP SMRTloxP/loxP mice expressing tamoxifen-inducible Cre under the control of the villin 1 promoter (B6.Cg-Tg(Vil1-cre/ERT2)23Syr/J), which has been shown to have activity for up to 60 days, despite continual renewal of intestinal epithelium, without an adverse effect on body weight or other metabolic parameters (37). This process generated control mice and NCOR1/SMRT IEC double knockout (I-DKO) mice (Fig. 1A). At age 8 to 9 weeks, adult male mice underwent i.p. tamoxifen injection at a dose of 15 mg/100 g body weight for 5 days as previously described (9). Mice were monitored daily for body weight, glucose levels, and temperature for 20 days until placement in metabolic cages on day 21 (Fig. 1B). Remarkably, the DKO of NCOR1 and SMRT in IECs is lethal in more than 50% of male mice, with most of these animals succumbing within 10 days of initiation of tamoxifen administration (Fig. 1C). A similar pattern was observed in female mice that underwent an identical protocol of tamoxifen administration (Supplementary Fig. S1A) (36). In the surviving I-DKO mice, reduced body weight and lower glucose levels were seen throughout the experiment, beginning at day 6 for body weight (Fig. 1D) and day 8 for glucose levels with corresponding area under the curve for daily glucose levels from day 1 to day 20 (Fig. 1E) (P < .05, 2-way analysis of variance with repeated measures followed by Bonferroni correction for multiple comparisons). There was no difference in body temperature between control and I-DKO mice (Fig. 1F). Hematoxylin and eosin staining of the jejunum revealed no grossly significant abnormalities of intestinal architecture between control and I-DKO mice (Fig. 1G), which is similar to observations when HDAC3 is deleted in IECs (37). However, further analysis showed an increase in villi length in I-DKO mice compared to control mice (Fig. 1H), but no differences were seen in the length of intestine between the groups (Fig. 1I). Female I-DKO mice also had significant reductions in body weight and blood glucose, but not temperature levels (Supplementary Fig. S1B-S1D) (36). We next confirmed that tamoxifen administration and subsequent Cre activation led to a reduction in Ncor1 and Smrt gene expression in jejunal IECs of male mice (Fig. 1J) and female mice (Supplementary Fig. S1E) (36). Appropriately, there was no reduction in Ncor1 and Smrt levels seen in the liver of I-DKO mice compared to controls (Supplementary Fig. S2A) (36). The male I-DKO mice had a higher thyrotropin level compared to control mice but no changes were seen in thyroxine levels, and the majority of known TH targets in the liver were no different between groups (Supplementary Fig. S1B and S1C) (36). This contrasts with the global deletion of NCOR1 and SMRT, where there is a reduction in thyroxine levels and known TH targets (15).
Figure 1.
Postnatal deletion of NCOR1 and SMRT in intestinal epithelial cells leads to sustained weight loss, hypoglycemia, and increased mortality. A) Shown are the genotypes that were studied, control and NCOR1/SMRT I-DKO mice. B) Experimental timeline. Tamoxifen was administered to male mice at age 8 to 9 weeks for 5 days and mice then underwent glucose tolerance test followed by metabolic phenotyping and EchoMRI. C) Percentage of male control and I-DKO mice that survived to 20 days (n = 7 control mice, 7 [out of 18] I-DKO mice survived). D) Body weight; E) blood glucose with area under the curve analysis; and F) body temperature from the surviving mice (n = 7 control, 7 I-DKO). G) Hematoxylin and eosin staining from representative isolated jejunum. H) Villi length (n = 5 per genotype). I) Intestine length (n = 7 control, 5 I-DKO). J) Quantitative polymerase chain reaction for Ncor1 and Smrt was performed on RNA extracted from intestinal epithelial cells (IECs) (n = 6 per genotype). Data are shown as mean ± SEM, 2-way analysis of variance for time (#P < .0001) and genotype ($P < .0001) for survival, body weight, glucose, and temperature analysis, otherwise unpaired t test (****P < .0001).
NCOR1/SMRT Intestinal Epithelial Cell Double Knockout Mice Have a Blunted Response to Oral Glucose Administration
To further evaluate the hypoglycemia associated with loss of NCOR1 and SMRT throughout the intestine, control and I-DKO mice underwent both intraperitoneal (i.p.) and oral glucose tolerance tests (intraperitoneal glucose tolerance test [IPGTT] and oral glucose tolerance test [OGTT], respectively) as described in earlier. Control and I-DKO mice were randomly assigned to either oral or i.p. administration of glucose on day 13 after initial administration of tamoxifen (see Fig. 1B for experiment timeline). The same protocol was followed on day 20, except method of glucose administration was switched (ie, day 13 was oral administration, day 20 was i.p. administration) to minimize time as a factor relating to any differences between groups. As seen in Fig. 2A, oral glucose administration resulted in significantly reduced peak glucose levels in I-DKO mice compared to control mice throughout the duration of the test. Although the I-DKO mice had significantly lower glucose levels prior to the start of the IPGTT, glucose levels in all but one of the I-DKO mice reached above 300 mg/dL and were not different between the groups by 90 minutes after i.p. administration (see Fig. 2A).
Figure 2.
I-DKO mice have a blunted response to oral glucose administration. A) Glucose was administered by oral gavage (OGTT) or via intraperitoneal injection (IPGTT) (n = 14 control, 9 I-DKO for both tests). B) Quantitative polymerase chain reaction (qPCR) of key carbohydrate transporters performed in intestinal epithelial cell (IEC) messenger RNA (mRNA). C) qPCR of genes involved in gluconeogenesis (G6pc, Pck1) and fatty acid metabolism (Ppara, Cpt1a) performed in intestinal epithelial cell (IEC) mRNA. D) qPCR of genes involved in hepatic gluconeogenesis performed in liver mRNA. N = 6 per genotype for qPCR. Two-way analysis of variance with post hoc Bonferroni multiple comparisons test was used for glucose tolerance tests; unpaired t test was used for qPCR analysis (*P < .05; **P < .01; ***P < .001; ****P < .0001).
Since intestinal carbohydrate transporters are critical in reabsorbing glucose from the proximal small intestine, we next assessed the expression of Slc5a1 (SGLT1), Slc2a2 (GLUT2), and Slc2a5 (GLUT5) in jejunal IECs. Compared to control mice, I-DKO mice were found to have reduced expression of all 3 transporters (Fig. 2B) in the male mice. This was also true in the female mice (Supplementary Fig. S1F) (36). Since the intestine plays a role in gluconeogenesis, we also assessed the gluconeogenic-related genes G6pc and Pck1. Interestingly, while G6pc expression was significantly increased in I-DKO mice compared to controls, Pck1 expression was significantly lower in the I-DKO mice (Fig. 2C, left panel). However, there appeared to be activation of genes involved in fatty acid oxidation (FAO) in I-DKO IECs (see Fig. 2C, right panel). Because the liver plays a large role in glucose metabolism, we investigated potential compensatory effects resulting from the lower glucose levels in intestinal NCOR1 and SMRT KO. Therefore, we assessed key genes associated with hepatic gluconeogenesis, G6pc, Pck1, and Fbp1, which were no different between control and I-DKO mice (Fig. 2D).
Metabolic Phenotyping Demonstrates Systemic Abnormalities in NCOR1/SMRT Intestinal Epithelial Cell Double Knockout Mice
Control and I-DKO male mice then underwent metabolic phenotyping to better determine what factors contributed to the reduced body weight and hypoglycemia observed in the I-DKO mice. Body composition analysis showed that the decreased body mass in surviving I-DKO mice was driven by reductions both in lean and fat mass (Fig. 3A). This decrease in body mass was not associated with a change in food intake, digestive efficiency, or caloric absorption, although there was a significant reduction in water intake in I-DKO mice (Fig. 3B). Energy expenditure was lower in the I-DKO mice over 24 hours and, when normalized to lean body mass, was significantly lower in the dark cycle in the I-DKO mice compared to control mice (Fig. 3C, left, middle panel). Total distance traveled was also significantly lower in the I-DKO mice compared to control mice (Fig. 3C, right panel). While respiratory exchange ratio was unchanged in I-DKO mice compared to control mice, both oxygen consumption and carbon dioxide production were significantly lower in I-DKO mice compared to controls (Fig. 3D).
Figure 3.
Metabolic phenotyping confirms systemic abnormalities in I-DKO mice. A) Body mass, lean mass, and fat mass as measured by EchoMRI. B) Food intake/24 hours, water intake/24 hours, digestive efficiency, and caloric absorption. C) Energy expenditure/24 hours, light/dark energy expenditure, and distance traveled/24 hours. D) Respiratory exchange ratio, O2 consumption and CO2 production. (n = 8 control, 6 I-DKO). Energy expenditure normalized to lean body mass by analysis of covariance. Unpaired t test (*P < .05; **P < .01; ****P < .0001).
RNA Sequencing Analysis Shows a Reduction in Carbohydrate Transporter Expression and Activation of Fatty Acid Oxidation in NCOR1/SMRT Intestinal Epithelial Cell Double Knockout Mice
After the completion of metabolic phenotyping and body composition analysis, control and I-DKO mice were euthanized, IECs were collected, and RNA was extracted as described earlier for bulk RNA-seq. There was very strong and significant differential gene expression between the IECs of the I-DKO and control animals (Fig. 4A), with the upregulation or downregulation of select genes confirmed by qPCR (Fig. 4B). As expected, the expression both of Ncor1 and Smrt was strongly reduced in the I-DKO mice compared to controls, and among the most highly upregulated genes were Scd1 and Acot1, which have critical roles in fatty acid metabolism (see Fig. 4A). GSEA revealed that pathways and biological processes were associated with carbohydrate transport, digestive processes, water, and sodium transport, and the brush-border membrane were coordinately downregulated in the I-DKO mice compared to controls (Fig. 4C). These gene sets include the carbohydrate transporter Slc5a1 (which is represented across all 5 selected gene sets), Slc2a2, Slc2a5, and key aquaporins including Aqp1, Aqp3, and Aqp7. We also used qPCR to confirm the significant downregulation of the leading-edge genes from Gene Ontology set GO:0015144 (carbohydrate transmembrane transporter activity) in I-DKO mice (Supplementary Fig. S3A and 3B) (36). Conversely, pathways and biological processes associated with fatty acid β-oxidation, metabolism, transport, and biosynthesis were coordinately upregulated in the I-DKO mice, including critical genes for fatty acid metabolism such as Acaa1b, Cpt1α, and Pparα (Fig. 4D), indicating a potential switch from carbohydrates to lipids as a primary energy source.
Figure 4.
RNA sequencing analysis shows a reduction in carbohydrate transporters and activation of fatty acid oxidation in I-DKO mice. A) Volcano plot of bulk RNA sequencing analysis of intestinal epithelial cells (IECs) from control and I-DKO mice. Genes with false discovery rate (FDR) q less than 0.05 and fold change greater than 1 are shown in red (right) and blue (left) for upregulated and downregulated genes, respectively, with top differentially expressed genes highlighted. B) Quantitative polymerase chain reaction (qPCR) from isolated IEC messenger RNA (mRNA) of the 5 most significantly upregulated and downregulated genes (n = 6 per group, unpaired t test. **P < .01; ***P < .001; ****P < .0001). C) Heat map of the union set of leading-edge genes from 5 gene sets associated with digestive processes with significant coordinate upregulation (FDR q < .01) in I-DKO vs control mice. Top to bottom: GO:0034219 (“carbohydrate transmembrane transport”), GO:0007586 (“digestion”), GO:0006833 (“water transport”), GO:0006814 (“sodium ion transport”), and GO:0031526 (“brush border membrane,” BBM). D) Heat map of the union set of leading-edge genes from 5 gene sets associated with fatty acid metabolic pathways with significant coordinate downregulation (FDR q < 0.01) in I-DKO vs control mice. Top to bottom: GO:0006635 (“fatty acid β-oxidation”), Hallmark “fatty acid metabolism”, KEGG hsa00071 (“fatty acid metabolism”), WP357 (“fatty acid biosynthesis), WP5061 (“fatty acid transporters”). Gene set sources are as follows: H, Hallmark; K, KEGG; WP, WikiPathways; BP, GO biological process; and CC, GO cellular component. Rows correspond to individual samples (3 control, 3 I-DKO) and columns correspond to genes. Columns are sorted left to right first by gene set and then in descending order by the magnitude of the Wald statistic. Variance-stabilizing transformation gene expression values were z-score–normalized to a mean of zero and SD of 1 across all samples in each column, with blue, white, and red indicating final z scores of less than or equal to 2, 0, and greater than or equal to 2, respectively. qPCR unpaired t test (**P < .01; ***P < .001; ****P < .0001).
Discussion
Herein, we show that intestinal loss both of NCOR1 and SMRT in adult mice leads to sustained weight loss and hypoglycemia with less than 50% survival. Strikingly, the phenotype seen in I-DKO mice is very close to that observed in our mouse model of global NCOR1 and SMRT loss at age 9 weeks. Taken together, these data suggest that the combined role of NCOR1 and SMRT is essential for glucose absorption and normal metabolic function in the small intestine, and that their loss leads to significant mortality. Furthermore, these data indicate that the roles of NCOR1 and SMRT are independent of HDAC3, whose global deletion in adulthood does not lead to an increase in mortality (15).
The hypoglycemic phenotype observed with the global NCOR1/SMRT deletion is replicated in the I-DKO mice, as seen by the significant reduction in peak glucose levels attained after oral glucose administration. We hypothesize that this is likely mechanistically related to the reduction in Scl5a1 and Scl2a2 expression. Indeed, SGLT1 is the known apical transporter of glucose into the IEC, and the expression of GLUT2 at the basolateral membrane allows the glucose to then enter the bloodstream (16, 38, 39). Previous work in murine models has shown that SGLT1 is essential for glucose absorption, and without it, mice cannot survive on a regular chow diet and cannot absorb glucose (16). Furthermore, SGLT1 is required for glucose-mediated release of glucagon-like peptide 1 and gastric inhibitory peptide (16, 40). In humans, biallelic loss-of-function mutations in SGLT1 lead to glucose-galactose malabsorption, a neonatal disorder that is fatal if not effectively treated with a fructose-based diet (21, 22). More recently, mendelian randomization studies of SGLT1 gene variants suggest that decreased SGLT1 function may be protective in the context of diabetes and obesity (41). Furthermore, SGLT1 has been implicated in the pathogenesis of cardiovascular disease and its inhibition noted as a possible treatment option (42-44). Thus, understanding how these transporters are regulated could shed light on pathways that have therapeutic potential for numerous conditions.
In addition to hypoglycemia, I-DKO mice also experienced significant weight loss that was not secondary to a reduction in food intake or a change in energy expenditure or activity level. This differed from the phenotype observed in mice with a global KO of NCOR1 and SMRT, in which reduction in body mass was correlated with a reduction in food intake and a paradoxical increase in the caloric density of the fecal matter. Given the role of glucose absorption via SGLT1 and the regulation of incretin expression, it is likely that the loss of body weight is multifactorial and beyond the sensitivity of the techniques employed in this study. Further analysis is necessary to determine the exact mechanism behind the metabolic changes.
Analysis of gene expression in IECs from I-DKO mice revealed a reduction not only in the expression of SGLT1 and GLUT2, but also that of additional carbohydrate transporters, including GLUT5 (Slc2a5) and GLUT7 (Slc2A7). This suggests that the NCOR1/SMRT pathway governs a key regulatory node controlling glucose absorption and potentially glucose or nutrient sensing in the small intestine. Little is known about signaling pathways that may regulate the expression of these transporters, or why NCOR1/SMRT would play such a fundamental role. In contrast to the downregulation of glucose transport, IECs from I-DKO mice showed a striking upregulation of FAO, suggesting that the cells were responding to a decrease in glucose absorption by switching to lipids as a primary energy source. Interestingly, an increase in FAO in IECs has been associated with increased intestinal stem cell function, which may partially explain the increased villi length seen in the I-DKO mice. Further exploration of association using this model will be critical in the analysis of cell renewal and function in the small intestine.
HDAC3 is a critical part of the corepressor complex and is the enzymatic component that catalyzes the removal of acetyl groups from lysine residues of histone proteins, leading to chromatin condensation (45). The physical interaction of HDAC3 with NCOR1 or SMRT is required for its activity (46). Previously, we showed that the global KO of both NCOR1 and SMRT functioned independently of its ability to recruit or regulate HDAC3 levels (15). Intestinal HDAC3 KO mice have some overlapping characteristics with the I-DKO mice, including activation of PPAR signaling and Scd2 expression and an overall increase in FAO (47). This is strikingly like the broad derepression of genes related to fatty acid metabolism and transport observed when NCOR1 is deleted throughout the intestine, with some overlap seen in the gene expression changes of the I-DKO mice in the present study (48). Weight loss and a decrease in adiposity are also observed in the intestinal HDAC3 KO, but with a much slower onset (11 weeks post tamoxifen administration) (37, 47). Furthermore, whereas the loss of intestinal NCOR1 and SMRT is lethal, the loss of intestinal HDAC3 is not. So, while metabolic changes occur with either the loss of NCOR1 or HDAC3 in the small intestine, unique and distinct characteristics are associated with the loss of both NCOR1 and SMRT from IECs. Indeed, the ability of NCOR1 and SMRT to regulate body weight and glucose levels via their actions in IECs through the inhibition of glucose transport suggest that identification of critical pathways controlling carbohydrate transporters may offer therapeutic potential in the treatment of metabolic disease.
Acknowledgments
We thank Johan Auwerx for the NCoR1loxp/loxp, and the Weill Cornell Medicine Metabolic Phenotyping Center.
Abbreviations
- FAO
fatty acid oxidation
- FDR
false discovery rate
- GLUT2
glucose transporter 2
- GSEA
Gene Set Enrichment Analysis
- HDAC3
histone deacetylase 3
- I-DKO
NCOR1/SMRT IEC double knockout
- IECs
intestinal epithelial cells
- i.p.
intraperitoneal
- IPGTT
intraperitoneal glucose tolerance test
- KO
knockout
- mRNA
messenger RNA
- NCOR1
nuclear receptor corepressor 1
- OGTT
oral glucose tolerance test
- PPAR
peroxisome proliferator-activated receptor
- qPCR
quantitative polymerase chain reaction
- RNA-seq
RNA sequencing
- SGLT1
Slc5a1, solute carrier family 5 member 1
- SMRT
silencing mediator of retinoic acid and thyroid hormone receptor
- TH
thyroid hormone
Contributor Information
Megan J Ritter, Department of Medicine, Section of Endocrinology, Diabetes, Nutrition and Weight Management, Boston University Chobanian and Avedisian School of Medicine, Boston, MA 02118, USA; Division of Endocrinology, Diabetes and Metabolism, Joan and Sanford I. Weill Department of Medicine, New York, NY 10021, USA.
Izuki Amano, Department of Medicine, Section of Endocrinology, Diabetes, Nutrition and Weight Management, Boston University Chobanian and Avedisian School of Medicine, Boston, MA 02118, USA; Division of Endocrinology, Diabetes and Metabolism, Joan and Sanford I. Weill Department of Medicine, New York, NY 10021, USA; Department of Integrative Physiology, Gunma University Graduate School of Medicine, Maebashi, 371-8511, Japan.
Anne H van der Spek, Division of Endocrinology, Diabetes and Metabolism, Joan and Sanford I. Weill Department of Medicine, New York, NY 10021, USA; Department of Endocrinology, Amsterdam Gastroenterology Endocrinology Metabolism, University of Amsterdam UMC, 1105 AZ Amsterdam, the Netherlands.
Adam C Gower, Boston University Clinical and Translational Science Institute, Boston, MA 02118, USA.
Hendrik J Undeutsch, Department of Medicine, Section of Endocrinology, Diabetes, Nutrition and Weight Management, Boston University Chobanian and Avedisian School of Medicine, Boston, MA 02118, USA; Division of Endocrinology, Diabetes and Metabolism, Joan and Sanford I. Weill Department of Medicine, New York, NY 10021, USA.
Victor A P Rodrigues, Department of Medicine, Section of Endocrinology, Diabetes, Nutrition and Weight Management, Boston University Chobanian and Avedisian School of Medicine, Boston, MA 02118, USA.
Hanix E Daniel, Division of Endocrinology, Diabetes and Metabolism, Joan and Sanford I. Weill Department of Medicine, New York, NY 10021, USA.
Anthony N Hollenberg, Department of Medicine, Section of Endocrinology, Diabetes, Nutrition and Weight Management, Boston University Chobanian and Avedisian School of Medicine, Boston, MA 02118, USA; Division of Endocrinology, Diabetes and Metabolism, Joan and Sanford I. Weill Department of Medicine, New York, NY 10021, USA.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grant Nos. DK056123 to A.N.H. and DK056123—21S1 to M.J.R.), JSPS KAKENHI Fostering Joint International Research (A) (21KK0274) (to I.A.), The Netherlands Organisation for Health Research and Development Rubicon grant, and the Dr. Catharine van Tussenbroek Fund (to A.H.V.S.). The bioinformatics analysis of this project (to A.C.G.) was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (through BU-CTSI grant No. 1UL1TR001430).
Disclosures
The authors have nothing to disclose.
Data Availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Horlein AJ, Naar AM, Heinzel T, et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377(6548):397‐404. [DOI] [PubMed] [Google Scholar]
- 2. Emiliani S, Fischle W, Van Lint C, Al-Abed Y, Verdin E. Characterization of a human RPD3 ortholog, HDAC3. Proc Natl Acad Sci USA. 1998;95(6):2795‐2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mottis A, Mouchiroud L, Auwerx J. Emerging roles of the corepressors NCoR1 and SMRT in homeostasis. Genes Dev. 2013;27(8):819‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stanya KJ, Kao HY. New insights into the functions and regulation of the transcriptional corepressors SMRT and N-CoR. Cell Div. 2009;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jepsen K, Hermanson O, Onami TM, et al. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell. 2000;102(6):753‐763. [DOI] [PubMed] [Google Scholar]
- 6. Jepsen K, Solum D, Zhou T, et al. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature. 2007;450(7168):415‐419. [DOI] [PubMed] [Google Scholar]
- 7. Alenghat T, Meyers K, Mullican SE, et al. Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature. 2008;456(7224):997‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Astapova I, Vella KR, Ramadoss P, et al. The nuclear receptor corepressor (NCoR) controls thyroid hormone sensitivity and the set point of the hypothalamic-pituitary-thyroid axis. Mol Endocrinol. 2011;25(2):212‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shimizu H, Lu Y, Vella KR, et al. Nuclear corepressor SMRT is a strong regulator of body weight independently of its ability to regulate thyroid hormone action. PLoS One. 2019;14(8):e0220717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Astapova I, Lee LJ, Morales C, Tauber S, Bilban M, Hollenberg AN. The nuclear corepressor, NCoR, regulates thyroid hormone action in vivo. Proc Natl Acad Sci USA. 2008;105(49):19544‐19549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mendoza A, Tang C, Choi J, et al. Thyroid hormone signaling promotes hepatic lipogenesis through the transcription factor ChREBP. Sci Signal. 2021;14(709):eabh3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamamoto H, Williams EG, Mouchiroud L, et al. NCoR1 is a conserved physiological modulator of muscle mass and oxidative function. Cell. 2011;147(4):827‐839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li P, Fan W, Xu J, et al. Adipocyte NCoR knockout decreases PPARgamma phosphorylation and enhances PPARgamma activity and insulin sensitivity. Cell. 2011;147(4):815‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shimizu H, Horibata Y, Amano I, et al. Nuclear corepressor SMRT acts as a strong regulator of both beta-oxidation and suppressor of fibrosis in the differentiation process of mouse skeletal muscle cells. PLoS One. 2022;17(12):e0277830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ritter MJ, Amano I, Imai N, Soares De Oliveira L, Vella KR, Hollenberg AN. Nuclear receptor CoRepressors, NCOR1 and SMRT, are required for maintaining systemic metabolic homeostasis. Mol Metab. 2021;53:101315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gorboulev V, Schurmann A, Vallon V, et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61(1):187‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koepsell H. Glucose transporters in the small intestine in health and disease. Pflugers Arch. 2020;472(9):1207‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakamura C, Ishizuka N, Yokoyama K, et al. Regulatory mechanisms of glucose absorption in the mouse proximal small intestine during fasting and feeding. Sci Rep. 2023;13(1):10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishimura K, Fujita Y, Ida S, et al. Glycaemia and body weight are regulated by sodium-glucose cotransporter 1 (SGLT1) expression via O-GlcNAcylation in the intestine. Mol Metab. 2022;59:101458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alruwaili NW, Alshdayed F. Fructose metabolism and its effect on glucose-galactose malabsorption patients: a literature review. Diagnostics (Basel). 2023;13(2):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hosnut FO, Janecke AR, Sahin G, et al. SLC5A1 variants in turkish patients with congenital glucose-galactose malabsorption. Genes (Basel). 2023;14(7):1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martin MG, Turk E, Lostao MP, Kerner C, Wright EM. Defects in Na+/glucose cotransporter (SGLT1) trafficking and function cause glucose-galactose malabsorption. Nat Genet. 1996;12(2):216‐220. [DOI] [PubMed] [Google Scholar]
- 23. Marjou FE, Janssen KP, Chang BH, et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39(3):186‐193. [DOI] [PubMed] [Google Scholar]
- 24. Quaroni A. In: Hochman J, ed. Advanced Drug Delivery Reviews. vol. 22. Elsevier Science Publishers, B.V.; 1996:3‐52. [Google Scholar]
- 25. Ghaleb AM, McConnell BB, Kaestner KH, Yang VW. Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Kruppel-like factor 4 gene. Dev Biol. 2011;349(2):310‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moolenbeek C, Ruitenberg EJ. The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab Anim. 1981;15(1):57‐59. [DOI] [PubMed] [Google Scholar]
- 28. Bialkowska AB, Ghaleb AM, Nandan MO, Yang VW. Improved Swiss-rolling technique for intestinal tissue preparation for immunohistochemical and immunofluorescent analyses. J Vis Exp. 2016;113:54161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109(1–2):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tschop MH, Speakman JR, Arch JR, et al. A guide to analysis of mouse energy metabolism. Nat Methods. 2011;9(1):57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grobe JL. Comprehensive assessments of energy balance in mice. Methods Mol Biol. 2017;1614:123‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545‐15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ritter M. Data from: Corepressors NCOR1 and SMRT Regulate Metabolism via Intestinal Regulation of Carbohydrate Transport. National Center for Biotechnology Information; 2024. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE268594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ritter M. 2024. Data from: “Nuclear Receptor Corepressors NCOR1 and SMRT Regulate Metabolism via Intestinal Regulation of Carbohydrate Transport”. Mendeley Data. https://data.mendeley.com/datasets/rry567r92p/1. Date of deposit 26 July 2024. [DOI] [PMC free article] [PubMed]
- 37. Davalos-Salas M, Montgomery MK, Reehorst CM, et al. Deletion of intestinal Hdac3 remodels the lipidome of enterocytes and protects mice from diet-induced obesity. Nat Commun. 2019;10(1):5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gouyon F, Caillaud L, Carriere V, et al. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2-null mice. J Physiol. 2003;552(Pt 3):823‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thorens B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia. 2015;58(2):221‐232. [DOI] [PubMed] [Google Scholar]
- 40. Oguma T, Nakayama K, Kuriyama C, et al. Intestinal sodium glucose cotransporter 1 inhibition enhances glucagon-like peptide-1 secretion in normal and diabetic rodents. J Pharmacol Exp Ther. 2015;354(3):279‐289. [DOI] [PubMed] [Google Scholar]
- 41. Dobbie LJ, Cuthbertson DJ, Hydes TJ, Alam U, Zhao SS. Mendelian randomisation reveals sodium-glucose cotransporter-1 inhibition's potential in reducing non-alcoholic fatty liver disease risk. Eur J Endocrinol. 2023;188(6):K33‐K37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dasari D, Bhat A, Mangali S, et al. Canagliflozin and dapagliflozin attenuate glucolipotoxicity-induced oxidative stress and apoptosis in cardiomyocytes via inhibition of sodium-glucose cotransporter-1. ACS Pharmacol Transl Sci. 2022;5(4):216‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao M, Li N, Zhou H. SGLT1: a potential drug target for cardiovascular disease. Drug Des Devel Ther. 2023;17:2011‐2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ng KM, Lau YM, Dhandhania V, et al. Empagliflozin ammeliorates high glucose induced-cardiac dysfuntion in human iPSC-derived cardiomyocytes. Sci Rep. 2018;8(1):14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ning L, Rui X, Bo W, Qing G. The critical roles of histone deacetylase 3 in the pathogenesis of solid organ injury. Cell Death Dis. 2021;12(8):734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Szigety KM, Liu F, Yuan CY, et al. HDAC3 ensures stepwise epidermal stratification via NCoR/SMRT-reliant mechanisms independent of its histone deacetylase activity. Genes Dev. 2020;34(13–14):973‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ng I, Luk IY, Nightingale R, et al. Intestinal-specific hdac3 deletion increases susceptibility to colitis and small intestinal tumor development in mice fed a high-fat diet. Am J Physiol Gastrointest Liver Physiol. 2023;325(6):G508‐G517. [DOI] [PubMed] [Google Scholar]
- 48. Chen S, Lu W, Yueh MF, et al. Intestinal NCoR1, a regulator of epithelial cell maturation, controls neonatal hyperbilirubinemia. Proc Natl Acad Sci USA. 2017;114(8):E1432‐E1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Ritter M. 2024. Data from: “Nuclear Receptor Corepressors NCOR1 and SMRT Regulate Metabolism via Intestinal Regulation of Carbohydrate Transport”. Mendeley Data. https://data.mendeley.com/datasets/rry567r92p/1. Date of deposit 26 July 2024. [DOI] [PMC free article] [PubMed]
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in “References.”