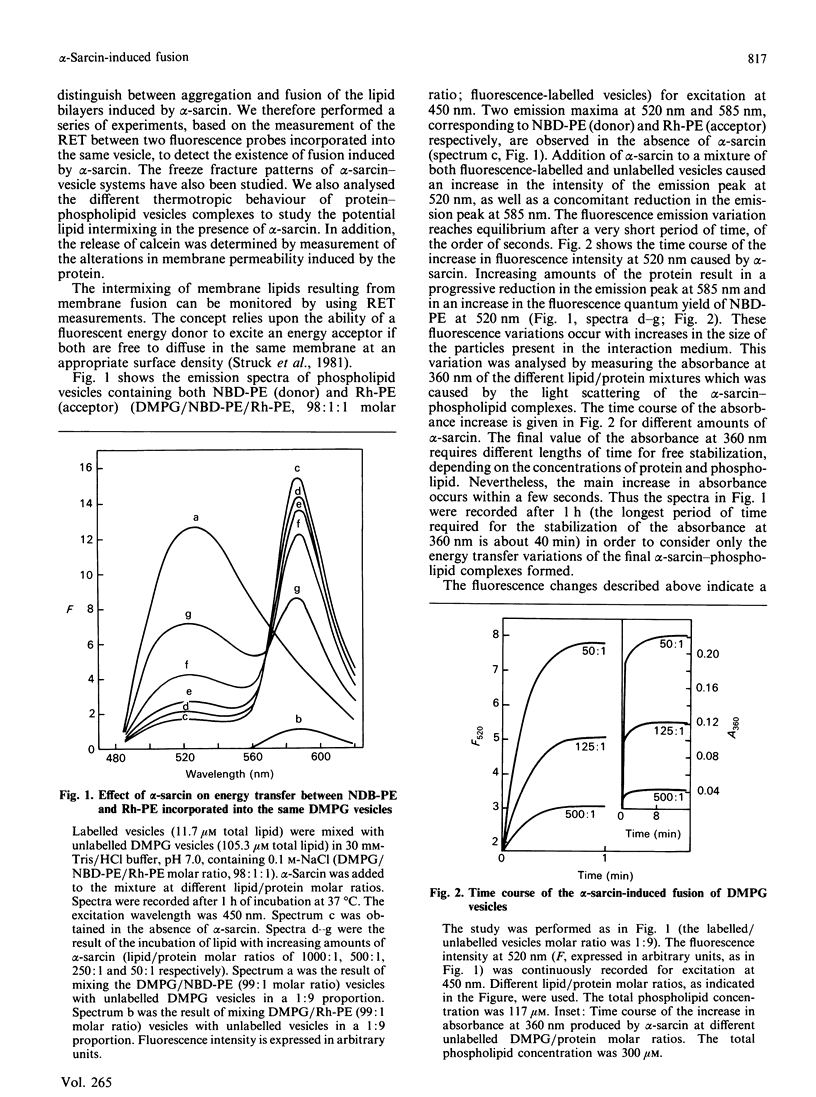
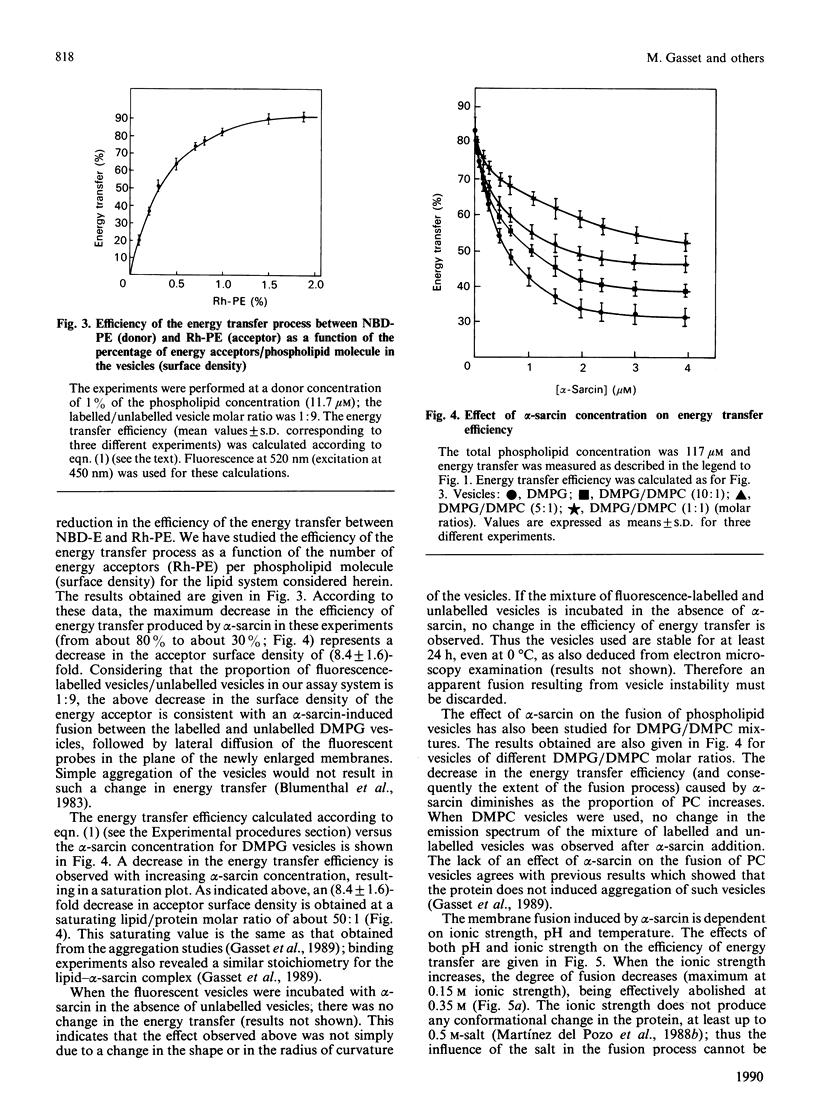
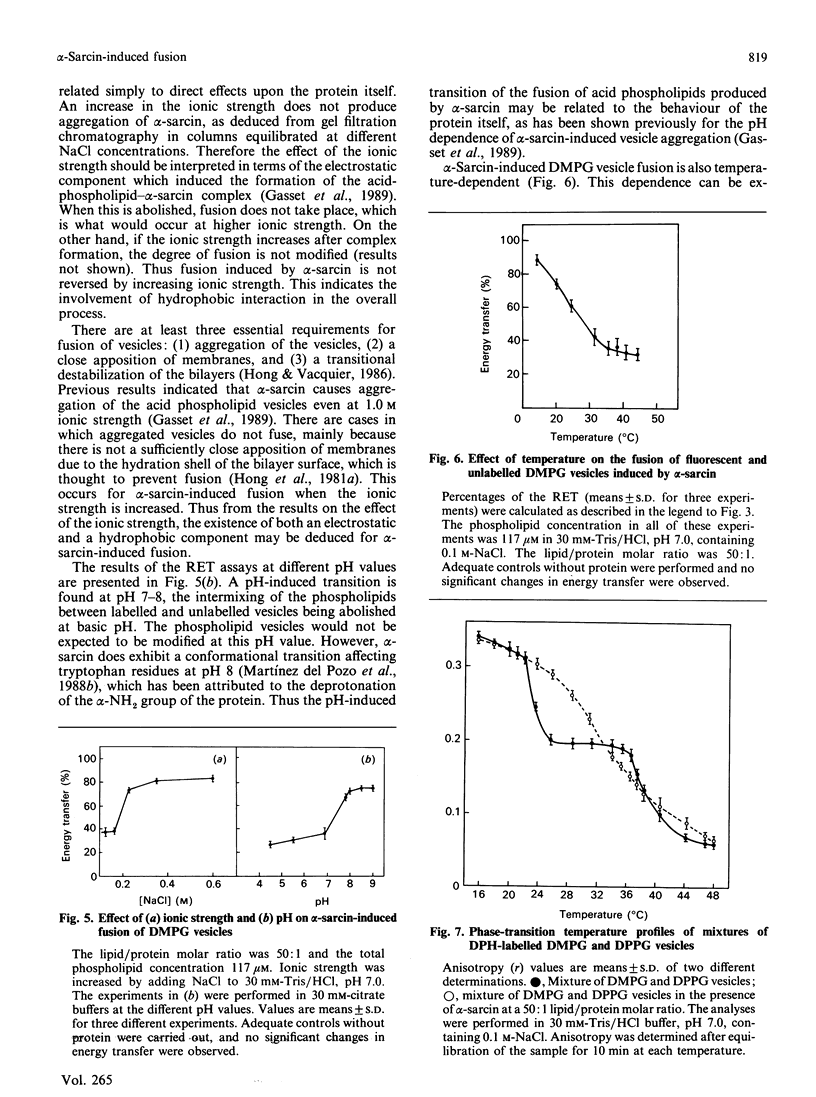
Abstract
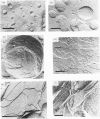
The anti-tumour protein alpha-sarcin causes fusion of bilayers of phospholipid vesicles at neutral pH. This is demonstrated by measuring the decrease in the efficiency of the fluorescence energy transfer between N-(7-nitro-2-1,3-benzoxadiazol-4-yl)-dimyristoylphosphatidylethano lamine (NDB-PE) (donor) and N-(lissamine rhodamine B sulphonyl)-diacylphosphatidylethanolamine (Rh-PE) (acceptor) incorporated in dimyristoylphosphatidylcholine (DMPG) vesicles. The effect of alpha-sarcin is a maximum at 0.15 M ionic strength and is abolished at basic pH. alpha-Sarcin promotes fusion between 1,6-diphenylhexa-1,3,5-triene (DPH)-labelled DMPG and dipalmitoyl-PG (DPPG) vesicles, resulting in a single thermotropic transition for the population of fused phospholipid vesicles. Bilayers composed of DMPC and DMPG, at different molar ratios in the range 1:1 to 1:10 PC/PG, are also fused by alpha-sarcin. Freeze-fracture electron micrographs corroborate the occurrence of fusion induced by the protein. alpha-Sarcin also modifies the permeability of the bilayers, causing the leakage of calcein in dye-trapped PG vesicles. All of the observed effects reach saturation at a 50:1 phospholipid/protein molar ratio, which is coincident with the binding stoichiometry previously described.
Full text
PDF

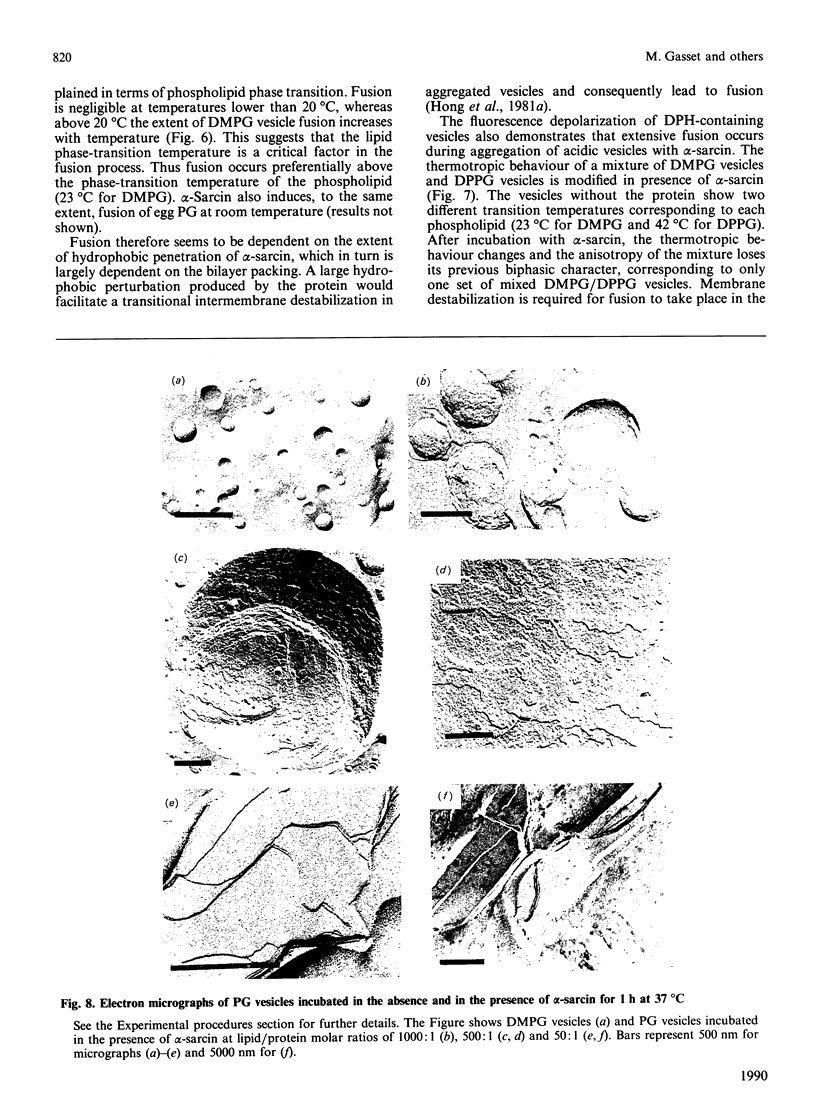
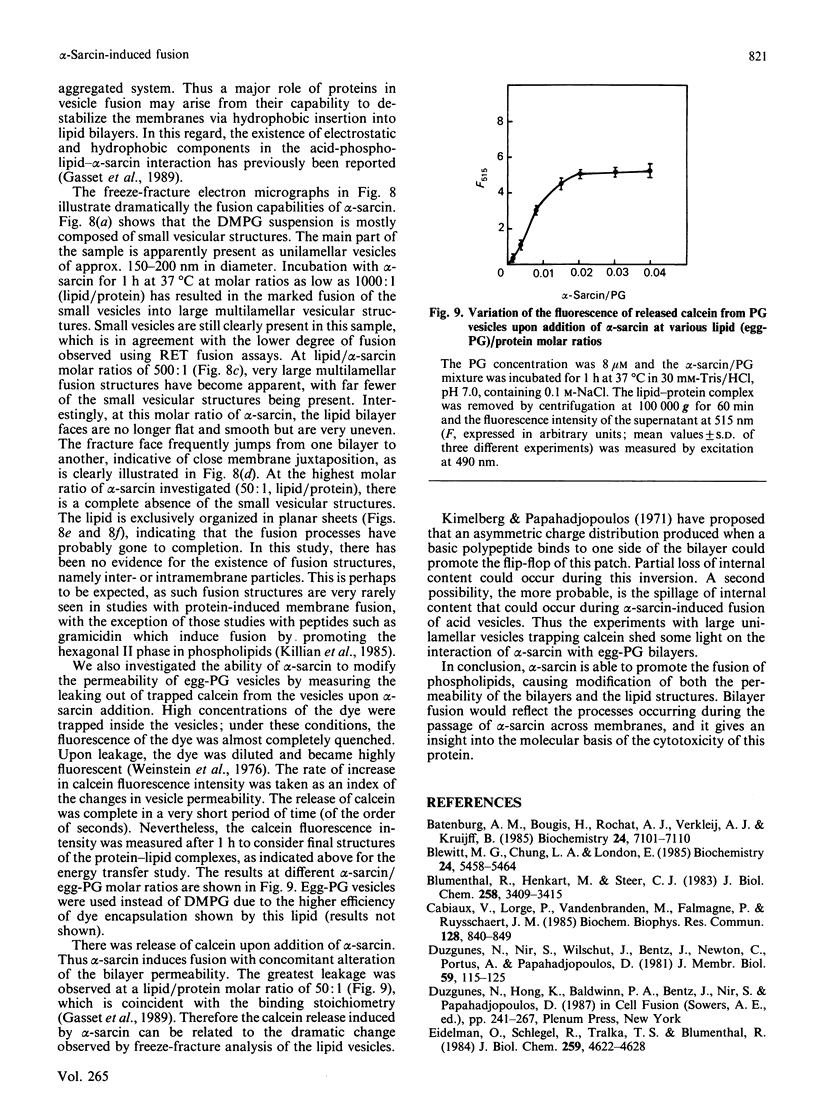

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batenburg A. M., Bougis P. E., Rochat H., Verkleij A. J., de Kruijff B. Penetration of a cardiotoxin into cardiolipin model membranes and its implications on lipid organization. Biochemistry. 1985 Dec 3;24(25):7101–7110. doi: 10.1021/bi00346a013. [DOI] [PubMed] [Google Scholar]
- Blewitt M. G., Chung L. A., London E. Effect of pH on the conformation of diphtheria toxin and its implications for membrane penetration. Biochemistry. 1985 Sep 24;24(20):5458–5464. doi: 10.1021/bi00341a027. [DOI] [PubMed] [Google Scholar]
- Blumenthal R., Henkart M., Steer C. J. Clathrin-induced pH-dependent fusion of phosphatidylcholine vesicles. J Biol Chem. 1983 Mar 10;258(5):3409–3415. [PubMed] [Google Scholar]
- Cabiaux V., Lorge P., Vandenbranden M., Falmagne P., Ruysschaert J. M. Tetanus toxin induces fusion and aggregation of lipid vesicles containing phosphatidylinositol at low pH. Biochem Biophys Res Commun. 1985 Apr 30;128(2):840–849. doi: 10.1016/0006-291x(85)90123-8. [DOI] [PubMed] [Google Scholar]
- Düzgünes N., Nir S., Wilschut J., Bentz J., Newton C., Portis A., Papahadjopoulos D. Calcium- and magnesium-induced fusion of mixed phosphatidylserine/phosphatidylcholine vesicles: effect of ion binding. J Membr Biol. 1981 Apr 15;59(2):115–125. doi: 10.1007/BF01875709. [DOI] [PubMed] [Google Scholar]
- Eidelman O., Schlegel R., Tralka T. S., Blumenthal R. pH-dependent fusion induced by vesicular stomatitis virus glycoprotein reconstituted into phospholipid vesicles. J Biol Chem. 1984 Apr 10;259(7):4622–4628. [PubMed] [Google Scholar]
- Gad A. E. Cationic polypeptide-induced fusion of acidic liposomes. Biochim Biophys Acta. 1983 Mar 9;728(3):377–382. doi: 10.1016/0005-2736(83)90509-6. [DOI] [PubMed] [Google Scholar]
- Gasset M., Martinez del Pozo A., Oñaderra M., Gavilanes J. G. Study of the interaction between the antitumour protein alpha-sarcin and phospholipid vesicles. Biochem J. 1989 Mar 1;258(2):569–575. doi: 10.1042/bj2580569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavilanes J. G., Lizarbe M. A., Municio A. M., Oñaderra M. Fluorescence studies on the lipoprotein complex of the fatty acid synthetase from the insect Ceratitis capitata. Biochemistry. 1981 Sep 29;20(20):5689–5694. doi: 10.1021/bi00523a008. [DOI] [PubMed] [Google Scholar]
- Gavilanes J. G., Lizarbe M. A., Munico A. M., Oñaderra M. Interaction of dipalmitoyl-phosphatidylcholine with calf thymus histone H1. Int J Pept Protein Res. 1985 Aug;26(2):187–194. doi: 10.1111/j.1399-3011.1985.tb03196.x. [DOI] [PubMed] [Google Scholar]
- Glabe C. G. Interaction of the sperm adhesive protein, bindin, with phospholipid vesicles. I. Specific association of bindin with gel-phase phospholipid vesicles. J Cell Biol. 1985 Mar;100(3):794–799. doi: 10.1083/jcb.100.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanspal M., Ralston G. B. Purification of a trypsin-insensitive fragment of spectrin from human erythrocyte membranes. Biochim Biophys Acta. 1981 Jul 28;669(2):133–139. doi: 10.1016/0005-2795(81)90234-8. [DOI] [PubMed] [Google Scholar]
- Hong K., Düzgüneş N., Papahadjopoulos D. Role of synexin in membrane fusion. Enhancement of calcium-dependent fusion of phospholipid vesicles. J Biol Chem. 1981 Apr 25;256(8):3641–3644. [PubMed] [Google Scholar]
- Hong K., Vacquier V. D. Fusion of liposomes induced by a cationic protein from the acrosome granule of abalone spermatozoa. Biochemistry. 1986 Feb 11;25(3):543–549. doi: 10.1021/bi00351a004. [DOI] [PubMed] [Google Scholar]
- JENNINGS J. C., OLSON B. H., ROGA V., JUNEK A. J., SCHUURMANS D. M. ALPHA SARCIN, A NEW ANTITUMOR AGENT. II. FERMENTATION AND ANTITUMOR SPECTRUM. Appl Microbiol. 1965 May;13:322–326. doi: 10.1128/am.13.3.322-326.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977 Oct 25;252(20):7384–7390. [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Interactions of basic proteins with phospholipid membranes. Binding and changes in the sodium permeability of phosphatidylserine vesicles. J Biol Chem. 1971 Feb 25;246(4):1142–1148. [PubMed] [Google Scholar]
- Martinez del Pozo A., Oñaderra M., Laynez J., Gavilanes J. G. Interaction of type I collagen with phosphatidylcholine vesicles. Coll Relat Res. 1988 Mar;8(2):133–144. doi: 10.1016/s0174-173x(88)80025-6. [DOI] [PubMed] [Google Scholar]
- Martínez del Pozo A., Gasset M., Oñaderra M., Gavilanes J. G. Conformational study of the antitumor protein alpha-sarcin. Biochim Biophys Acta. 1988 Apr 14;953(3):280–288. doi: 10.1016/0167-4838(88)90036-2. [DOI] [PubMed] [Google Scholar]
- Ohki S. A mechanism of divalent ion-induced phosphatidylserine membrane fusion. Biochim Biophys Acta. 1982 Jul 14;689(1):1–11. doi: 10.1016/0005-2736(82)90182-1. [DOI] [PubMed] [Google Scholar]
- Oku N., Yamaguchi N., Yamaguchi N., Shibamoto S., Ito F., Nango M. The fusogenic effect of synthetic polycations on negatively charged lipid bilayers. J Biochem. 1986 Oct;100(4):935–944. doi: 10.1093/oxfordjournals.jbchem.a121806. [DOI] [PubMed] [Google Scholar]
- Rodriguez R., Lopez-Otin C., Barber D., Fernandez-Luna J. L., Gonzalez G., Mendez E. Amino acid sequence homologies in alfa-sarcin, restrictocin and mitogillin. Biochem Biophys Res Commun. 1982 Sep 16;108(1):315–321. doi: 10.1016/0006-291x(82)91868-x. [DOI] [PubMed] [Google Scholar]
- Roga V., Hedeman L. P., Olson B. H. Evaluation of mitogillin (NSC-69529) in the treatment of naturally occurring canine neoplasms. Cancer Chemother Rep. 1971 Apr;55(2):101–113. [PubMed] [Google Scholar]
- Steer C. J., Klausner R. D., Blumenthal R. Interaction of liver clathrin coat protein with lipid model membranes. J Biol Chem. 1982 Jul 25;257(14):8533–8540. [PubMed] [Google Scholar]
- Struck D. K., Hoekstra D., Pagano R. E. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 1981 Jul 7;20(14):4093–4099. doi: 10.1021/bi00517a023. [DOI] [PubMed] [Google Scholar]
- Walter A., Steer C. J., Blumenthal R. Polylysine induces pH-dependent fusion of acidic phospholipid vesicles: a model for polycation-induced fusion. Biochim Biophys Acta. 1986 Oct 9;861(2):319–330. doi: 10.1016/0005-2736(86)90434-7. [DOI] [PubMed] [Google Scholar]
- Weinstein J. N., Yoshikami S., Henkart P., Blumenthal R., Hagins W. A. Liposome-cell interaction: transfer and intracellular release of a trapped fluorescent marker. Science. 1977 Feb 4;195(4277):489–492. doi: 10.1126/science.835007. [DOI] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]