Abstract
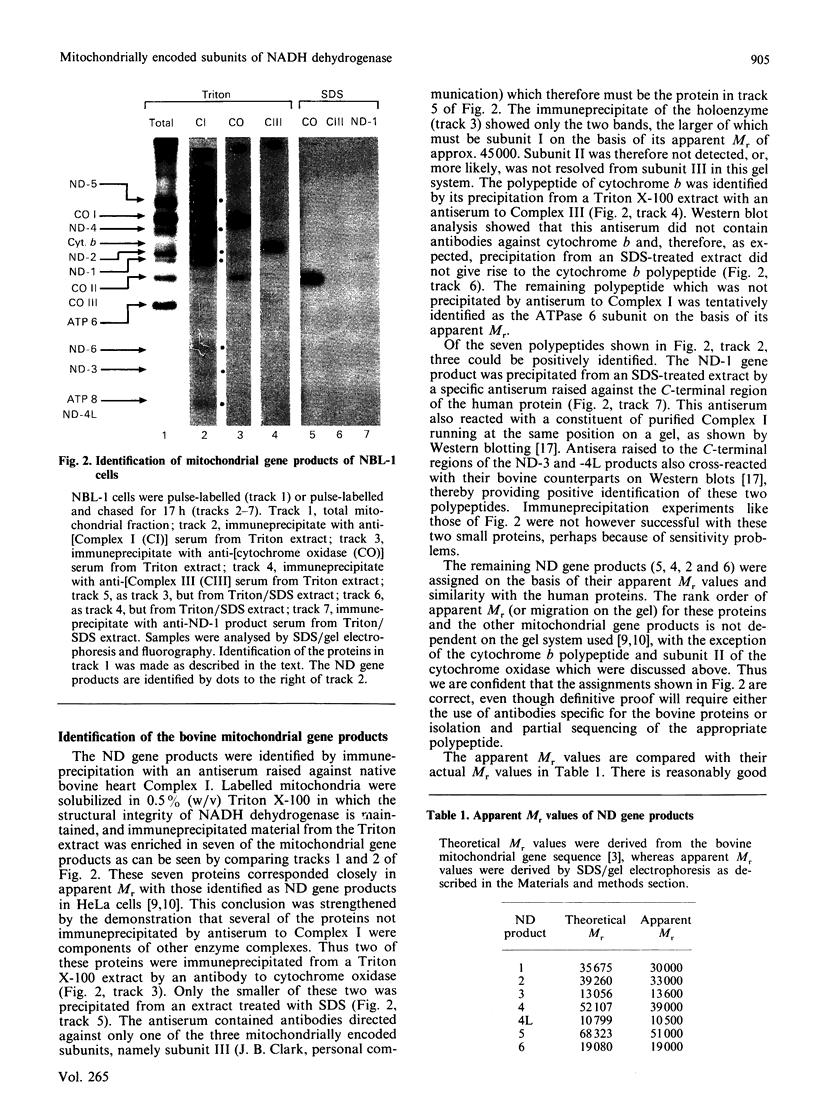
Products of the mitochondrial genome were identified in the bovine kidney cell line NBL-1 by labelling with [35S]methionine in the presence of cycloheximide. Seven proteins were precipitated by an antiserum to bovine heart NADH dehydrogenase, corresponding to the seven mitochondrial gene products identified in the human HeLa cell line. Comparison of these mitochondrial gene products with purified bovine NADH dehydrogenase by SDS/gel electrophoresis revealed that the ND-5 product is probably a previously unidentified protein of apparent Mr 51,000, and the ND-4 product is the protein of apparent Mr 39,000.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Cleeter M. W., Ragan C. I., Riley M., Doolittle R. F., Attardi G. URF6, last unidentified reading frame of human mtDNA, codes for an NADH dehydrogenase subunit. Science. 1986 Oct 31;234(4776):614–618. doi: 10.1126/science.3764430. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Mariottini P., Cleeter M. W., Ragan C. I., Matsuno-Yagi A., Hatefi Y., Doolittle R. F., Attardi G. Six unidentified reading frames of human mitochondrial DNA encode components of the respiratory-chain NADH dehydrogenase. Nature. 1985 Apr 18;314(6012):592–597. doi: 10.1038/314592a0. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Patel S. D., Cleeter M. W., Ragan C. I., Attardi G. The site of synthesis of the iron-sulfur subunits of the flavoprotein and iron-protein fractions of human NADH dehydrogenase. J Biol Chem. 1988 Nov 5;263(31):16395–16400. [PubMed] [Google Scholar]
- Cleeter M. W., Ragan C. I. The polypeptide composition of the mitochondrial NADH: ubiquinone reductase complex from several mammalian species. Biochem J. 1985 Sep 15;230(3):739–746. doi: 10.1042/bj2300739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley F. G., Patel S. D., Ragan I., Attardi G. Photolabelling of a mitochondrially encoded subunit of NADH dehydrogenase with [3H]dihydrorotenone. FEBS Lett. 1987 Jul 13;219(1):108–112. doi: 10.1016/0014-5793(87)81200-0. [DOI] [PubMed] [Google Scholar]
- Gibb G. M., Ragan C. I. Biosynthetic studies of several of the nuclear-encoded subunits of mammalian NADH dehydrogenase. Eur J Biochem. 1989 Jun 15;182(2):367–372. doi: 10.1111/j.1432-1033.1989.tb14840.x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mariottini P., Chomyn A., Attardi G., Trovato D., Strong D. D., Doolittle R. F. Antibodies against synthetic peptides reveal that the unidentified reading frame A6L, overlapping the ATPase 6 gene, is expressed in human mitochondria. Cell. 1983 Apr;32(4):1269–1277. doi: 10.1016/0092-8674(83)90308-2. [DOI] [PubMed] [Google Scholar]
- Mori M., Morita T., Miura S., Tatibana M. Uptake and processing of the precursor for rat liver ornithine transcarbamylase by isolated mitochondria. Inhibition by uncouplers. J Biol Chem. 1981 Aug 25;256(16):8263–8266. [PubMed] [Google Scholar]
- Patel S. D., Ragan C. I. Structural studies on mitochondrial NADH dehydrogenase using chemical cross-linking. Biochem J. 1988 Dec 1;256(2):521–528. doi: 10.1042/bj2560521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe G., Holtrop M., Gadaleta G., Kroon A. M., Cantatore P., Gallerani R., De Benedetto C., Quagliariello C., Sbisà E., Saccone C. Non-random patterns of nucleotide substitutions and codon strategy in the mammalian mitochondrial genes coding for identified and unidentified reading frames. Biochem Int. 1983 Apr;6(4):553–563. [PubMed] [Google Scholar]
- Yagi T., Hatefi Y. Identification of the dicyclohexylcarbodiimide-binding subunit of NADH-ubiquinone oxidoreductase (Complex I). J Biol Chem. 1988 Nov 5;263(31):16150–16155. [PubMed] [Google Scholar]




