Abstract
Malaria, a tropical disease caused by Plasmodium sp., has been haunting mankind for ages. Unsuccessful attempts to develop a vaccine, the emergence of resistance against the existing drugs and the increasing mortality rate all call for immediate strategies to treat it. Intense attempts are underway to develop potent analogues of the current antimalarials, as well as a search for novel drug targets in the parasite. The indispensability of apicoplast (plastid) to the survival of the parasite has attracted a lot of attention in the recent past. The present review describes the origin and the essentiality of this relict organelle to the parasite. We also show that among the apicoplast specific pathways, the fatty acid biosynthesis system is an attractive target, because its inhibition decimates the parasite swiftly unlike the ‘delayed death’ phenotype exhibited by the inhibition of the other apicoplast processes. As the enzymes of the fatty acid biosynthesis system are present as discrete entities, unlike those of the host, they are amenable to inhibition without impairing the operation of the host-specific pathway. The present review describes the role of these enzymes, the status of their molecular characterization and the current advancements in the area of developing inhibitors against each of the enzymes of the pathway.
Keywords: antimalarial, apicoplast, fatty acid biosynthesis pathway, malaria, Plasmodium falciparum, triclosan
Abbreviations: ACAT, acyl-CoA:ACP transacylase; ACC, acetyl-CoA carboxylase; ACP, acyl carrier protein; CER, cerulenin; FAS, fatty acid synthase; INH, isoniazid; InhA, enoyl-ACP reductase of Mycobacterium tuberculosis; KAS, β-oxoacyl-ACP synthase (β-ketoacyl-ACP synthase); MCAT, malonyl-CoA:ACP transacylase; ORF, open reading frame; PDH, pyruvate dehydrogenase; PEP, phosphoenolpyruvate; Pf, Plasmodium falciparum; TLM, thiolactomycin
“With rings on her fingers and (death) bells on her toes…”
Banbury Cross nursery rhyme
Be it in the marshy areas of age-old Rome or in the thickets of Africa and South America, there has always been a battle between malaria and mankind. Afflictions sum up to half a billion, of which a million succumb to death every year [1]. Although man has tried his best to eliminate malaria for some centuries by targeted vector control and changing lifestyles, the disease caused by the apicomplexan protozoan parasite, Plasmodium, is seeing a resurgence, and malaria is identified among the top ten killers today [2].
A quick look at the life cycle of the most virulent species of this parasite, Plasmodium falciparum, in its two hosts, humans and mosquitoes [3], draws our attention to three facts. The complexity of the life cycle, the short-lived extracellular appearance of the merozoites and the intracellular nature of the other asexual stages in an immune-privileged site makes it extremely difficult for the host to mount an effective immune response. Natural acquired immunity against the parasite is short-lived, requires consistent exposure to infection and is effective only in adults [4]. Additionally, antigenic variation, which results in an evasion of the immune response, and inhibition of T-cell stimulation by the parasite present hurdles in the development of a vaccine.
There are no raging successes today when it comes to currently prescribed antimalarial drugs either. Aminoquinolines and quinines, although they target specifically the disease-causing parasite, P. falciparum, act in an as yet unknown manner [5]. Antifolates, artemisinins, and sulphones have known targets, but affect the host biosynthesis machinery too [6–8]. These limitations of current antimalarials, as well as the upsurge of drug-resistant P. falciparum strains have fuelled the quest for new antimalarials.
The recent discovery of the apicoplast, the plastid-like organelle in Plasmodium, Toxoplasma and other apicomplexans offers promise in this regard. In the present review, we focus on the indispensability of the apicoplast to the parasite, particularly with regard to the fatty acid biosynthesis pathway operating within it. We believe that the exploration of this pathway presents us with unique opportunities to tackle P. falciparum because of its distinctive organization when compared with that of the host.
FATTY ACID BIOSYNTHESIS
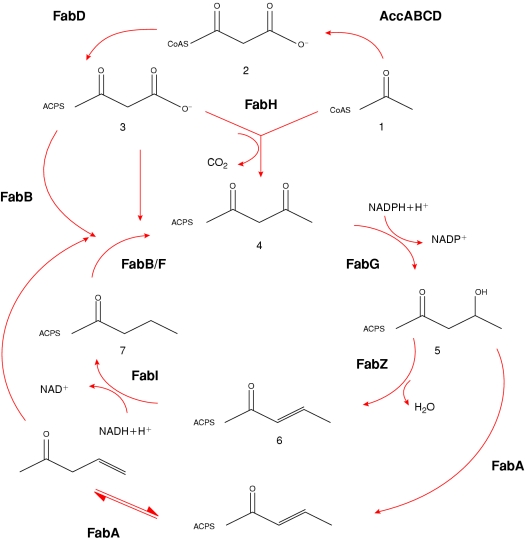
Fatty acid biosynthesis is fundamental to cell growth, differentiation and homoeostasis. All living organisms synthesize fatty acids, except for the mycoplasmas, which import them from their surroundings. The production of malonyl-CoA by ACC (acetyl-CoA carboxylase) and the transfer of malonyl group by MCAT [malonyl-CoA:ACP (acyl carrier protein) transacylase] (also known as FabD) to ACP to form malonyl-ACP, which condenses in a reaction catalysed by β-oxoacyl-ACP synthase (β-ketoacyl-ACP synthase; KAS) III (FabH) with an acetyl group from acetyl-CoA or acetyl-ACP set the stage for fatty acid biosynthesis. Subsequently, repeated cycles of elongation each comprising a condensation, a reduction, a dehydration and a reduction step by KAS I and II (FabB/F), β-oxoacyl-ACP reductase (FabG), β-hydroxyacyl-ACP dehydratases (FabZ/FabA) and enoyl-ACP reductase (FabI) respectively yield fatty acids (Scheme 1). The four chemical reactions required to complete successive cycles of fatty acid elongation are catalysed by distinct enzymes encoded by unique genes in bacteria and plants in what is called the Type II or the ‘dissociative’ pathway. This is in contrast with the Type I or associative pathway in mammals and fungi, where a multifunctional enzyme catalyses all the steps of the pathway.
Scheme 1. Type II fatty acid biosynthesis system.
Acetyl-CoA (1) is converted into malonyl-CoA (2) by ACC and then to malonyl-ACP (3) by FabD. The resulting malonyl-ACP condenses with another molecule of acetyl-CoA to form β-oxoacyl-ACP (4) catalysed by β-oxoacyl ACP synthase III (FabH). This is then converted into β-hydroxyacyl-ACP (5) by β-oxoacyl ACP reductase (FabG) and then dehydrated by β-hydroxyacyl ACP dehydratases (FabZ/FabA). The synthesis of unsaturated fatty acids branches out at this step catalysed first by FabA and then by FabB. The dehydrated product, enoyl-ACP (6) is then reduced by enoyl-ACP reductase (FabI) to form butyryl-ACP (7). This product re-enters the FAS cycle and the growing chain is elongated by two carbon units per cycle. The condensing enzymes involved in elongation are FabB and FabF.
Fatty acid biosynthesis in P. falciparum
The identification of nuclear-encoded apicoplast-targeted genes for three enzymes of the fatty acid biosynthesis pathway: ACP, KAS III and β-hydroxyacyl-ACP dehydratase [9] in P. falciparum constituted indirect evidence for the existence of a fatty acid biosynthesis pathway in the relict plastid of the parasite. Later, incorporation of 14C-labelled acetate into 10-, 12-, and 14-carbon-long fatty acid chains by P. falciparum in culture and that of [14C]malonyl-CoA by the cell-free extracts of the parasite confirmed that P. falciparum indeed has a de novo fatty acid synthesis pathway [10]. This was reinforced by the demonstration of enoyl-ACP reductase activity in the enzyme isolated from P. falciparum cultures, demonstration of enzyme activity in purified, recombinant P. falciparum FabZ, FabG and FabI proteins expressed in Escherichia coli [10–12] and inhibition studies. Triclosan, a broad-spectrum biocide, acts on the Type II fatty acid biosynthesis system [13]. The incorporation of [14C]acetate was inhibited in the presence of triclosan both in the in vitro culture of P. falciparum and in the cell-free assay of fatty acid synthesis, thus confirming that P. falciparum has a functional Type II fatty acid biosynthesis pathway. The cardinal importance of the fatty acid biosynthesis system and the inherent difference between the fatty acid biosynthesis pathways of the parasite (Type II) and the human host (Type I) make it an appealing target for the development of antimalarials.
The two steps involved in synthesizing fatty acids are, broadly, initiation and elongation. The enzymes involved and their inhibitors are described briefly below. Some of their characteristics are also listed in Table 1.
Table 1. P. falciparum FAS enzymes.
| Enzyme | Molecular mass (kDa) | Subunit composition | Kinetic constants | Inhibitor | Inhibition parameters | Reference |
|---|---|---|---|---|---|---|
| ACC | 391* | – | – | |||
| FabH | 36† | – | Km 17.9 μM (acetyl-CoA) | 1,2-Dithiole-3-one compounds | IC50 0.53–10.4 μM | [22] |
| kcat 230 min−1 | ||||||
| Km 35.7 μM (butyryl-CoA) | ||||||
| Km 64.9 μM (malonyl-ACP) | Thiolactomycin | IC50>330 μM | – | |||
| kcat 200 min−1 (butyryl-CoA and malonyl-ACP) | ||||||
| FabD | 34.5† | – | – | |||
| FabG | 28 | – | Km 75 μM (acetoacetyl-CoA) | [12] | ||
| Vmax 0.0054 μmol/ml per min | ||||||
| kcat 0.014 s−1 | ||||||
| FabZ | 17 | 2 | Km 199 μM (β-hydroxybutyryl-CoA) | NAS-21 | Ki 1.3, IC50 100 μM | [11] |
| kcat 15.99 s−1 | NAS-91 | Ki 1.5, IC50 7.4 μM | ||||
| Km 86 μM (crotonoyl-CoA) | ||||||
| kcat 18.9 s−1 | ||||||
| FabI | 41 | 4 | Km 165 μM (crotonoyl-CoA) | Triclosan | Ki 0.4 mM (with respect to NADH) | [37] |
| Km 33 μM (NADH) | Ki 0.03 mM (with respect to NAD+) | |||||
| Ki (ternary complex) 96 pM | ||||||
| IC50 700 nM | ||||||
| 2,2-Dihydroxyphenyl ether | Ki 0.28 μM (with respect to NADH) | |||||
| Ki 18 nM (with respect to NAD+) | ||||||
| FabB/F | 45† | 2‡ |
* Inferred from genomic sequence reported in Sanger Centre, PlasmoDB (http://plasmodb.org/).
† Molecular mass from S. Sharma, S. K. Sharma, A. Misra and A. Surolia, unpublished work.
‡ Subunit composition from S. Sharma, S. K. Sharma, A. Misra and A. Surolia, unpublished work.
ACC
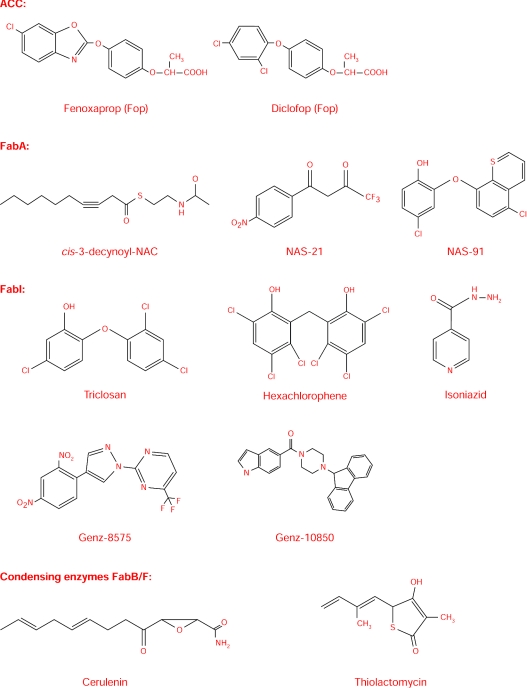
Fatty acid synthesis is initiated by the carboxylation of acetyl-CoA to malonyl-CoA using bicarbonate as the source of the carboxy group. This step is catalysed by the biotin-containing enzyme, ACC. As this enzyme is responsible for initiating the FAS (fatty acid synthase) pathway and thus regulating the metabolic flux through the pathway, its inhibition should significantly affect the fatty acid production and thereby affect the growth of the organism. Indeed, the apicoplast ACC of Toxoplasma gondii, being a multidomain protein like the ACC of grasses, is sensitive to fops, a class of aryloxyphenoxypropionate herbicides [14] (Figure 1). Fops such as fenoxaprop and diclofop are also known to inhibit P. falciparum, at comparable concentrations [15]. Unlike the enzymes involved in fatty acid elongation, the ACC is eukaryotic in nature. However, human ACC is a part of a multifunctional enzyme complex, whereas the ACC of P. falciparum is a discrete multidomain enzyme. This feature confers the selectivity of the herbicides to the apicoplast ACC, thus making it a promising target for the development of antimalarials.
Figure 1. Structures of the inhibitors of different enzymes of the Type II fatty acid biosynthetic pathway.
MCAT (FabD)
This enzyme transfers the malonyl moiety from malonyl-CoA to ACP through a Ping Pong mechanism of catalysis. Mutation of the FabD enzyme has been shown to be lethal in many pathogenic organisms [16–18]. The sequence is known [19,20] and the crystal structure of E. coli FabD has been solved [21]. Recently, Prigge et al. [22] reported the purification and expression of MCAT of P. falciparum. Although the enzyme has been implicated in the survival of the organism, it is hitherto unexploited as a target for antimalarials.
KAS III (FabH)
This is the first enzyme of the elongation pathway, which condenses acetyl-CoA and malonyl-ACP to form acetoacetyl-ACP. KAS III enzymes can have two activities. They can transfer the acetyl moiety from CoA to ACP [ACAT (acyl-CoA:ACP transacylase)] or condense malonyl-ACP and acetyl-CoA [23]. To compare these two activities of KAS III in P. falciparum, 14C-labelled acetyl-CoA was used along with PfACP (P. falciparum ACP) and malonyl-PfACP, and it was shown that KAS activity dominates well over the ACAT activity. The specificity for butyryl-CoA as a substrate over isobutyryl-CoA points to the fact that P. falciparum may not synthesize branched chain lipids.
TLM (thiolactomycin) (Figure 1) proved to be a poor inhibitor of KAS III with an IC50 of >330 μM. Since the IC50 of TLM is <50 μM in cultures of P. falciparum in human red blood cells, it probably inhibits the other condensing enzymes of the pathway such as KAS I/II. However, three compounds, 1,2-dithiole-3-ones, structurally related to TLM were shown to inhibit KAS III with IC50 values below 10 μM. These compounds also inhibit P. falciparum cultures, both sensitive and resistant to chloroquine [22]. CER (cerulenin) (Figure 1), an inhibitor of the other condensing enzymes, FabB and FabF, does not inhibit FabH.
β-Oxoacyl-ACP reductase (FabG)
The resulting condensed product, acetoacetyl-ACP, is reduced by β-oxoacyl-ACP reductase (FabG) in a NADPH-dependent manner to produce β-hydroxyacyl-ACP. Following the characterization of the enzyme in E. coli and Brassica napus, it has been characterized in P. falciparum [12,24,25]. The distinctive feature of this enzyme is that, thus far, only one isoform of FabG is identified. This target remained unexploited until Zhang and Rock [26] assessed the inhibitory activity of plant polyphenols against FabG and FabH, which, although less potent than TLM, inhibit more than one enzyme in the pathway, definitely a preferred feature for an inhibitor.
β-Hydroxyacyl-ACP dehydratases (FabZ/FabA)
The β-hydroxyacyl-ACP so produced is dehydrated in the next step to form enoyl-ACP. In E. coli, there are two dehydratases that can perform this function: FabZ and FabA. Although both have dehydratase activity, FabA is a bifunctional enzyme which also catalyses the isomerization of trans-2-decenoyl-ACP to cis-3-decenoyl-ACP in addition to dehydration. This cis-3-decenoyl-ACP is then used by FabB, and thus the flux is diverted towards the production of unsaturated fatty acids. However, overexpression of FabA does not result in overproduction of unsaturated fatty acids [27], because FabA cannot dehydrate cis-unsaturated β-hydroxyacyl-ACP since its active site is too small to accommodate the bent unsaturated fatty acid. Thus, in further cycles of elongation of unsaturated fatty acids, FabZ plays an important role. Also, FabZ is found to be more active than FabA on both long-chain and short-chain saturated acyl-ACPs, crediting FabZ to be the primary dehydratase [28].
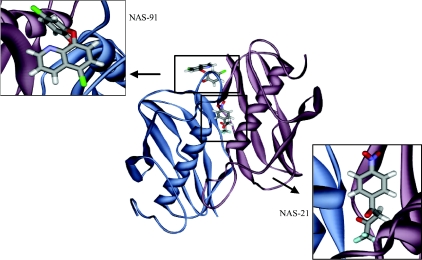
E. coli FabA is shown to be inhibited by 3-decynoyl-N-acetylcysteamine (Figure 1), an analogue of cis-3-decenoyl-ACP [29]. This is remarkable in that this is the first suicide inhibitor or mechanism-based inhibitor described. This is also supported by the recently solved crystal structure of FabA with the inhibitor [30]. Despite the importance of FabZ in fatty acid synthesis, it has not yet been exploited in the development of drugs. Only recently has the FabZ of P. falciparum been cloned, expressed and characterized, and inhibitors identified which happen to be the first of their kind [11]. Homology modelling of PfFabZ was performed with E. coli FabA because of the 70% amino acid similarity. The major contributing factor is the identity of residues between 60 and 90, which accounts for 21% identity. Based on this, docking studies were carried out, and the rational synthesis of the two inhibitors, NAS91 and NAS21 (Figure 1) accomplished. Both inhibitors were competitive for crotonoyl-CoA and β-hydroxybutyryl-CoA. Also, both of them inhibited fatty acid synthesis in the cell-free extracts. Moreover, the mode of binding of these inhibitors was also investigated which should stimulate further research in this area. Figure 2 shows NAS-91 and NAS-21 docked with homology-modelled PfFabZ. Also, recently, PfFabZ has been crystallized [31]. Solution of its structure should augment the process of designing rational inhibitors.
Figure 2. NAS-91 and NAS-21 docked with homology-modelled FabZ from P. falciparum.
Pfal FabZ being a dimer, has two active sites, which, hence, can house either two molecules of NAS-21 or NAS-91, or one of each, simultaneously. In this Figure, however, for clarity, only one of the active sites has been demonstrated. While NAS-21 occludes the entry to the active site, NAS-91 sits in the active site and inhibits FabZ.
Enoyl-ACP reductase (FabI)
Enoyl-ACP reductase, which catalyses the reduction of the double bond in the dehydrated product, enoyl-ACP, catalyses the rate-determining step in fatty acid synthesis, and so is an appealing target not only for antimalarials, but also for antibacterials. Its quintessential nature is well evident from the fact that mutant FabI (Ts) temperature-sensitive strain is also sensitive to antibiotics, and leakage of cytoplasmic contents occurs due to membrane perturbations at non-permissive temperatures [32]. Research on the mechanism of action of diazoborines led to the discovery of the gene encoding the NADH-dependent enoyl-ACP reductase [33]. This enzyme appears to be the target of many broad-spectrum antimicrobial biocides [34], one of which is triclosan, possessing broad-spectrum antibacterial action, and it is used in many consumer products (Figure 1).
Contradicting the earlier views that triclosan inhibits growth by disrupting the cell membrane, McMurry et al. [13] showed that triclosan inhibited the lipid synthesis in bacteria. Heath et al. [35] reported that triclosan and other 2-hydroxydiphenyl ethers inhibit FabI activity. Triclosan has IC50 values varying from 0.2 to 1.2 μM for different P. falciparum strains, and shows differential inhibition for the different stages of the parasite, with the trophozoite and ring stage showing the greatest inhibition [10,15].
A homology model of Plasmodium FabI complexed with its cofactor NAD+ and triclosan built by Suguna et al. [36] showed that FabI of P. falciparum prefers NAD+ to NADP+. It also accounted for the 1000-fold increased affinity of Plasmodium FabI for triclosan when compared with 2,2′-dihydroxydiphenyl ether, because the chlorine at the 2′ position of the former makes favourable contacts with PfFabI and NAD+.
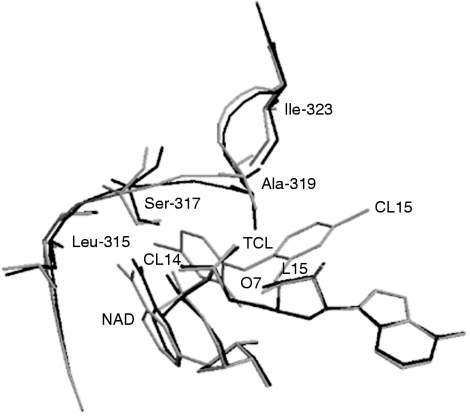
The characterization of Plasmodium FabI in E. coli and inhibition studies carried out by Kapoor et al. [37] showed that triclosan is competitive with respect to NADH and shows uncompetitive inhibition with NAD+. In fact, the binding of NAD+ to the enzyme promotes the binding of triclosan. Kinetic studies on the binding of triclosan with FabI show that the inhibition is faster when NAD+, the product of the reaction, is added. Pre-incubation of the enzyme with NAD+ increases the degree of inhibition further. This accounts for the slow-binding nature of triclosan. Although the binding is reversible, it is exceptionally tight given the very low dissociation rate of triclosan from its ternary complex [38]. In continuation with these efforts, time-dependent inhibition of FabI by triclosan was investigated using steady-state kinetics, and it was shown that triclosan is a slow tight inhibitor of FabI [39]. Surface plasmon resonance studies conducted with immobilized FabI reveal that, while NAD+ binding to FabI is not detectable, in the presence of triclosan, the binding constant is 6.5×104 M−1. Similarly, triclosan binding to FabI is increased 300-fold in the presence of NAD+ [40,41]. The crystal structure of P. falciparum enoyl-ACP reductase (FabI) as a binary complex with NADPH and as a ternary complex with triclosan and NAD+ have been solved, and it was demonstrated that stacking interactions, hydrogen bonds and van der Waal's interactions aid in stabilizing the ternary complex. This was also similar to the mode of binding of diazoborine and E. coli FabI, suggesting that all 2-hydroxydiphenyl ethers plausibly have similar mode of binding to FabI. It has also been shown that the outward movement of the loop (residues 318–324) plays a major role in inhibitor binding and substrate recognition [40,41]. This outward movement of the loop increases the affinity and rapidity of the interaction for binding of both triclosan and NAD+, and thus promotes the formation of the ternary complex (Figure 3). These results contribute to our understanding of the inhibition of FabI by triclosan, and should help in synthesizing more potent analogues.
Figure 3. Superposition of the binary complex of FabI–NADH and the ternary complex of FabI–NAD+–triclosan.
Superposition of the binary complex of FabI–NADH (black) and the ternary complex of FabI–NAD+–triclosan (grey). Movement of residues 318–324 of the substrate-binding loop and the nicotinamide ring of NAD+ result in increased van der Waal's contacts, explaining the increased affinity of NAD+ and triclosan for FabI in the presence of triclosan and NAD+ respectively. TCL, triclosan.
Studies of Suguna et al. [36] had averred that the replacement of a methionine by alanine in the malarial enzyme leaves enough room for introducing bulkier substituents in the B-ring of triclosan. Indeed, attempts have already been made to test some derivatives of triclosan, such as naphthalene derivatives, with this intent. Hexachlorophene, an antimicrobial compound used in scrubs and detergents was also tested against E. coli FabI (Figure 1). But it was found to be less effective compared with triclosan due to its inability to form ternary complexes [42]. 2,9-Disubstituted 1,2,3,4-tetrahydropyrido[3,4-b]indoles [43], aminopyridines [44], indole naphthyridinones [45] and 1,4-disubstituted imidazoles [46] were tested against FabI of E. coli. Although these are found to be less effective than triclosan, such studies help to underscore the importance of groups responsible for the stabilization of triclosan thereby helping us retain those characteristics in further syntheses of efficacious analogues.
The other important inhibitor of enoyl-ACP reductase is INH (isoniazid), a frontline drug used for the treatment of tuberculosis (Figure 1). It has been shown that triclosan is an inhibitor of InhA (enoyl-ACP reductase of Mycobacterium tuberculosis) [47]. This was confirmed by the crystal structure of InhA with triclosan. It provides additional evidence that triclosan binds to a site different from INH [48]. Two other novel inhibitors were found, namely Genz-8575 and Genz-10850 (Figure 1), but these showed IC50s of 32 μM and 18 μM respectively, far above that of triclosan. It is surprising that, although triclosan, which has been shown to be an antimalarial, has been found to inhibit InhA, the activity of the celebrated antitubercular drug, INH, on P. falciparum enoyl-ACP reductase has not yet been investigated. Nevertheless, this would require activating the drug INH into its acyl radical in the same way as it is activated by an enzyme, KatG, for exhibiting its inhibitory action on M. tuberculosis. Nonetheless, a combination of INH with rifampicin and chloroquine has already been shown to constitute an effective antidote for malaria in mice [49]. However, the ease with which INH resistance develops in M. tuberculosis, and the fact that many individuals in the developing world, and now even in the developed world, are already hosting this organism, call for caution in using INH in any formulation for treating malaria.
KAS I and II (FabB/F)
The fatty acids synthesized in the previous step are either transferred to glycerol phosphate by the acyl transferase system to be incorporated into phospholipids or elongated by two enzymes KAS I (FabB) or II (FabF). FabB is required in a critical step for the elongation of unsaturated fatty acids [50,51], and FabF is required for the thermal regulation of fatty acid composition. Both contain the His-His-Cys catalytic triad in their active site [52]. To date, two inhibitors of these enzymes are known. CER, synthesized by the fungus Cephalosporium ceruleans irreversibly inhibits FabB and FabF by binding to the active-site cysteine residue [53–56]. But, as β-oxoacyl ACP synthase III (FabH) has an aspartate instead of a histidine residue in its active site, CER is found to be inactive against it [57]. These residues are found to be conserved in P. falciparum FabB and FabF. CER was also found to act synergistically with triclosan on P. falciparum culture [10]. However, the disadvantage is that CER can be accommodated in the Type I FAS active site too, thereby limiting its use as an antimalarial. However, analogues of CER can be synthesized which would be specific only to the Type II FAS system.
While TLM specifically targets FabB [58], it is also an inhibitor of the other condensing enzymes, FabF and FabH [59,60]. Unlike CER, which mimics the transition state of the condensation reaction, TLM mimics malonyl-ACP. The sensitivity of TLM to the condensing enzymes is in the order FabF>FabB≫FabH. The isoprenoid moiety fits into the hydrophobic pocket of FabB/F and is stabilized by stacking interactions [57]. These studies also showed that the hydrophobic pocket is not completely filled with the side chain of TLM, leaving space for further substitutions on it for a better fitting molecule. These are confirmed by analysing TLM analogues, wherein increased side-chain length and increased unsaturation enhanced their potencies. TLM has been shown to inhibit the growth of P. falciparum [61]. Furthermore, P. falciparum FabB/F exhibits significant homology with E. coli FabB. This draws our attention to their potential as an antimalarial target.
Stage-specific expression of FAS enzymes
High-density oligonucleotide arrays have been used to generate expression profiles of the human and mosquito stages of the malarial parasite's life cycle [62,63]. The transcriptome of the enzymes involved in the fatty acid biosynthesis pathway reveals that FabZ mRNA is expressed in all stages of the malarial parasite to almost similar levels. However, while FabB/F and FabH are expressed in the late trophozoite and schizont stages, and FabG in the trophozoite stage, expression profiles of these enzymes, as well as those of FabD and FabI, are associated with low confidence levels. Triclosan and TLM inhibition data demonstrate that the stages most affected by inhibition of fatty acid biosynthesis are the late ring and the trophozoite stages [9,10], suggesting perhaps that the fatty acid biosynthesis enzymes are expressed maximally at these stages. More rigorous data on the expression profiles of these enzymes would be required to resolve this issue, which will help in deciding a stratagem for the therapeutic use of inhibitors towards various enzymes.
Apicoplast
The multifunctional enzyme of the type I pathway has the advantage that substrates for consecutive reactions are channelled from one domain of the enzyme to another without diffusion of substrates limiting the rate of the reaction. Plasmodium, which has the Type II fatty acid biosynthesis pathway, has perhaps got around this problem by confining all the reactions of fatty acid biosynthesis to a small organelle: the apicoplast. This unique, apically positioned, plastid-like organelle, which has been implicated in several other biological processes, such as haem biosynthesis, protein synthesis and developmental processes [10,64], owes its discovery to its 35 kb genome. First identified in the avian malarial parasite, Plasmodium lophurae and attributed to the mitochondria [65], this extrachromosomal DNA was suggested to be plastid in origin after analysis of the rRNA genes which are arranged in an inverted repeat form, like in chloroplasts. Immunogold labelling and in situ hybridization experiments confirmed the presence of the relict plastid both in T. gondii [66] and in Plasmodium sp.
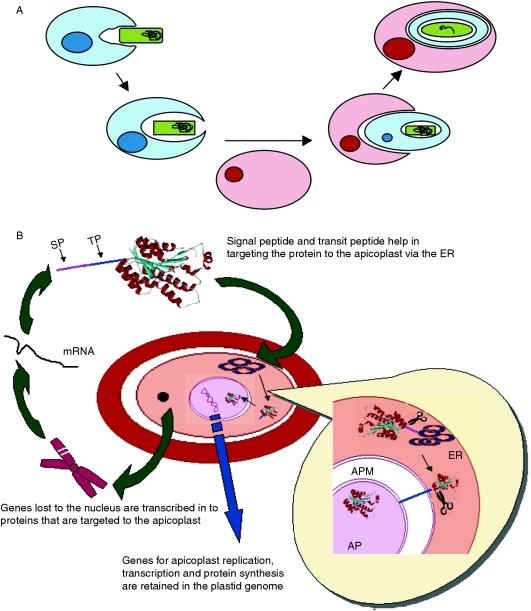
Evidence such as the presence of four membranes around the organelle, susceptibility of malarial parasites to rifampicin, a drug that inhibits prokaryotic RNA polymerase, but not eukaryotic RNA polymerase, and comprehensive analysis of genes suggests that, like plant plastids, the apicoplast arose by secondary endosymbiosis probably of a red alga [67] (Figure 4A). It is assumed that biochemical exchanges during this process resulted in the endosymbionts (cyanobacterial-like prokaryote) losing genes that were no longer required in its new environment, and transferring other genes that are essential for endosymbiont functions to the host cell nucleus [68–70] (Figure 4A). Although a few proteins involved in apicoplast function, such as protein synthesis, are encoded by the reduced apicoplast genome itself, most are nuclear encoded and targeted to the plastid with the help of a transit peptide via the endomembrane system [64,71] (Figure 4B). An apicoplast-encoded chaperone, ClpC, renders assistance in their entry into the organelle [72].
Figure 4. Pictorial representation of the origin of the apicoplast.
(A) The photosynthetic cyanobacterium (green) is engulfed by a primary eukaryote (light blue) and some of the genes from the primary endosymbiont are transferred to the host nucleus (dark blue). Subsequently, this eukaryote containing the endosymbiont is engulfed further by a secondary eukaryote (buff). Here again, some genes are transferred to the host nucleus (red) reducing the apicoplast genome to the bare minimum. (B) During evolution, most of the genes were transferred from the plastid to the nucleus. Some of these genes code for proteins destined to the apicoplast (APM, apicoplast membrane). These proteins carry a signal peptide (SP; shown in pink) followed by a transit peptide (TP; shown in blue). The SP directs the protein into the endoplasmic reticulum where it is cleaved by a signal peptidase I. The resultant protein carries the TP, which directs its entry into the apicoplast. The TP is then cleaved by the plastid peptidase. Thus the nuclear-encoded proteins enter the apicoplast by a bipartite targeting signal.
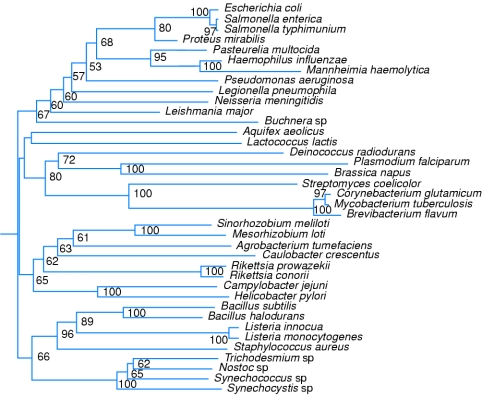
The phylogenetic relationship of FabI, a nucleus-encoded, plastid-targeted protein with those of other organisms is depicted as a representative example of the proteins of FAS II pathway of the parasite in Figure 5. Consistent with the fact that apicoplasts have many characteristics of plant plastids, the enoyl-ACP reductase was similar to FabI found in the plastid of the plant, B. napus.
Figure 5. Phylogenetic relationship of FabI of P. falciparum with those of other organisms.
The values shown represent those with confidence levels above 50%.
Apicoplast and fatty acid synthesis
The apicoplast membrane is impermeable to acetyl-CoA, the substrate of the FAS system, akin to the mitochondrial membrane. In mitochondria, where acetyl-CoA is the precursor of the TCA (tricarboxylic acid) cycle, the glycolytic end product, pyruvate, is transported into the mitochondria by a pyruvate–H+ symport and is converted into acetyl-CoA inside the organelle. It is possible that in the apicoplast too, a similar mechanism exists wherein pyruvate is formed from PEP (phosphoenolpyruvate), which is transported from the cytosol into the apicoplast [73]. This is corroborated by the findings of Kubis and Rawsthorne [74] that PEP transporters play an important role in providing resources for the fatty acid synthesis system in plant plastids. Assuming that pyruvate is transported across the apicoplast membrane, a PDH (pyruvate dehydrogenase) complex would be required to convert the pyruvate, inside the apicoplast, into acetyl-CoA. To explore this, the genome sequence of P. falciparum at PlasmoDB (http://plasmodb.org/) and at the Sanger Institute P. falciparum Genome Project (http://www.sanger.ac.uk) were searched for the genes coding for the subunits of PDH complex (E1α, E1β, E2 and E3). Indeed, four ORFs (open reading frames) corresponding to the four subunits of the PDH complex are found in the P. falciparum genome (accession codes PF11_0256, PF14_0441, PF10_0407 and PF08_0066), implicating the possibility of a PDH in the apicoplast. We find it compelling to hypothesize that pyruvate, either transported directly or generated from PEP, acts as a source of the substrate, acetyl-CoA, for fatty acid synthesis in the apicoplast. Nevertheless, one cannot rule out the other possibility that acetyl-CoA can be derived from acetate by the action of acetyl-CoA synthetase. But, although there are three annotated ORFs (accession codes PFF1350c, MAL6P1.150 and PF14_0357) in the P. falciparum genome sequence, which could have acetyl-CoA synthetase activity, neither is there an apicoplast-targeting signal in any of these ORFs, nor has the enzyme activity been demonstrated in their expressed protein products. Whether it is the PDH complex or the acetyl-CoA synthetase that generates acetyl-CoA in the apicoplast has yet to be experimentally demonstrated.
Apicoplast and delayed death
Its indispensability for the survival of the parasite and the presence of parasite-specific metabolic pathways of the prokaryotic type make the apicoplast a suitable drug target. The fatty acid biosynthesis system appears to be appealing as a potential target due to the dependence of the parasite on fatty acids for survival and infection. What makes FAS score over the other pathways that are present in the apicoplast and are thus unique to the parasite? It has been shown that antibiotics such as ciprofloxacin and clindamycin target apicoplast replication by inhibiting DNA replication and protein synthesis respectively [75,76]. But the kinetics of inhibition by these drugs is peculiar in the sense that, although they are parasiticidal, there is a delay in the death after the administration of the drug. For instance, in the case of T. gondii, when DNA replication in the apicoplast is affected by clindamycin, the drug has its effect only on the second replication cycle when the tachyzoites re-infect the host [77].
We observed that clindamycin and chloramphenicol invoked delayed death in the cultures of P. falciparum. The result was the same when the cultures were incubated for the entire duration of the experiment or only for the first 48 h, asserting that delayed death is the cause of the anti-parasitic activity of these drugs (Figures 6 and 7). In an attempt to understand the rationale behind the delayed-death phenomenon, it was hypothesized that, in the case of T. gondii, the apicoplast was required for the establishment of the parasitophorous vacuole, inside which the parasite grows and divides, in the second host [78]. One of the hypotheses suggests that the specific fatty acids and lipids synthesized in the apicoplast are required for the successful establishment of the parasitophorous vacuole [77,79]. If this is true, then the drugs inhibiting the fatty acid synthesis pathway should lead to a delayed-death phenotype. But, on the other hand, if the fatty acid biosynthesis pathway was essential to the survival of the parasite in the first cell itself, then the drugs targeting it, such as triclosan, should effect immediate death, unlike the inhibition of other processes of the apicoplast-like DNA replication, which cause only a delayed death. This is of paramount importance in treating cases of cerebral malaria, where delay in the action of drug is synonymous with death of the individual.
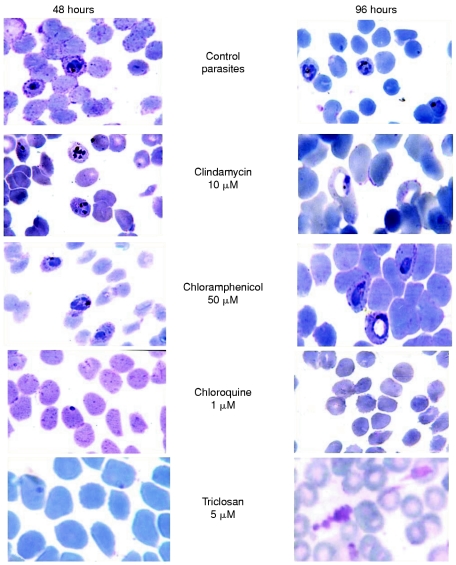
Figure 6. Giemsa-stained blood smears of the Plasmodium culture.
Cultures were treated with various reagents as indicated and were incubated for 48 or 96 hours.
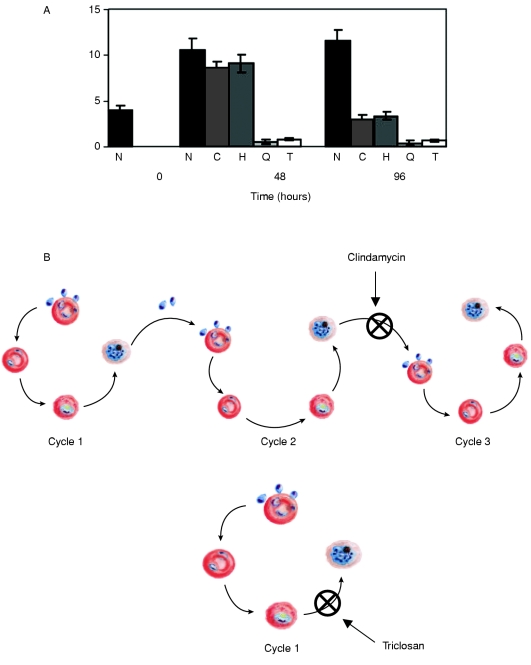
Figure 7. Death kinetics invoked by apicoplast-targeting drugs: triclosan and clindamycin.
(A) Chloroquine (Q), triclosan (T) and CER (results not shown) ablate the parasites within the first cycle of asexual reproduction, unlike clindamycin (C) and chloramphenicol (H) which invoke parasite death only after 84 h (towards the end of the second cycle). Parasitaemia remained high in the untreated culture (N, no drug control). Parasite growth was monitored by making Giemsa-stained smears and counting number of infected cells per total number of cells, as well as by hypoxanthine incorporation (results not shown). Results are means±S.E.M. The statistical significance of changes in parasitaemia was confirmed using the two-tailed Student's t test (P<0.05). (B) Cartoon depicting the effect of the action of clindamycin and triclosan on the culture of P. falciparum. In the case of clindamycin, the effect is felt only in the second asexual cycle leading to the delayed-death phenotype, whereas triclosan effects immediate death by inhibiting the first asexual cycle itself.
To investigate the parasiticidal effect of triclosan and compare with the other drugs that invoke delayed death, P. falciparum cultures were incubated with clindamycin or triclosan, and assessed using standard smear staining and hypoxanthine-incorporation assay. We noted that, in the case of clindamycin, the parasitaemia decreases sharply only after 84 h, corresponding to the end of the second asexual cycle, and the surviving trophozoites showed enlarged vacuoles in the cytoplasm characteristic of dying parasites (Figure 6). On the other hand, triclosan showed a rapid parasiticidal effect ablating young trophozoites within 24 h of incubation (Figure 7a). To confirm that the death is not of delayed type, quantitative hybridization analysis with probes specific for single-copy genes in nucleus (FabI) and plastid [EF-Tu (elongation factor Tu)] genomes were performed. In a normal parasite, the apicoplast genome is present at approx. 1.75 copies per haploid nuclear genome. We observed a specific reduction in the plastid genome copy number to approx. 1.2 copies after 48 h and then approx. 0.7 copies after 96 h on treatment with clindamycin. On the contrary, in the case of triclosan, the nuclear and plastid genome copies decreased in parallel as indicated by their constant ratio (T. N. C. Ramya, N. Surolia and A. Surolia, unpublished work). Thus triclosan does not cause delayed death, but rather leads to a rapid inhibition (depicted in Figure 7b), underpinning the fact that fatty acid synthesis in P. falciparum is of prime importance for the survival of the parasite.
Future drug targets
The complete genome of P. falciparum sequenced by Gardner et al. [71] has provided a plethora of information on the genes encoded by the parasite, particularly those which are apicoplast-encoded or -targeted [71]. Taking the leads, many enzymes of the fatty acid biosynthetic pathway have been cloned, characterized and purified. High-throughput assays devised to test the inhibitory activity of many putative drug candidates and in vitro testing of the same against the cell culture P. falciparum with [3H]hypoxanthine-uptake studies has helped identify a few putative drug molecules, which are described earlier in the present review. However, inhibition of many of the enzymes, including regulatory enzymes, is unexplored, opening new and rich avenues for research. Also interesting are the condensing enzymes. A common inhibitor of the condensing enzymes would affect more than one step of the FAS pathway. The development of resistance in such cases is difficult, making the drug more effective.
The elucidation of mechanism of inhibition by crystallizing the enzymes with the inhibitors would help us to synthesize more potent analogues of such molecules while retaining the basic interactions that are important for the inhibition. This is also aided by computational tools such as modelling and docking studies of the enzymes with the potential drug candidates. QSAR (quantitative structure–activity relationship) studies have been employed recently where model pharmacophores are built using the structure and activity of a set of lead compounds. These identify the interacting sites of the compounds in the training set and develop a model pharmacophore, which can be used as a paradigm for design and synthesis of drugs. One such pharamacophore model has been built recently based on the structure–activity relationship of trypanthrin (indolo[2,1-b]quinazoline-6,12-dione) [80]. The pharmacophore was found to map well with existing potent antimalarials, although the target enzyme is yet to be found. Such studies can also be extended to develop pharamocophores against key enzymes in the FAS pathway.
Apart from the distinctive nature of FAS enzymes of the parasite from those of its host, what makes it all the more appealing is that inhibiting fatty acid synthesis rapidly compromises the growth of the parasite, unlike apicoplast DNA replication, transcription and translation. Biochemical approaches, together with computational approaches, should provide us with the required thrust to identify many compounds, which may become potent drugs in the future and help us put an end to the sound of the ringing death bells.
Acknowledgments
The work outlined in the present review is supported by a grant from the Department of Biotechnology, Government of India and partially from the Council of Scientific and Industrial Research (CSIR), India, to N.S. and A.S. T.N.C.R. is a CSIR senior research fellow. We thank I. Surolia for editorial assistance, and G. Kumar and P.L. Mukhi for Figures 2 and 3.
References
- 1.World Health Organization. The World Health Report 2002: reducing risks, promoting healthy life. 2002 doi: 10.1080/1357628031000116808. [DOI] [PubMed]
- 2.World Health Organization. The World Health Report 1995. 1995
- 3.Ramya T. N. C., Surolia N., Surolia A. Survival strategies of the malarial parasite Plasmodium falciparum. Curr. Sci. 2002;83:818–825. [Google Scholar]
- 4.Baird J. K. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol. Today. 1995;11:105–111. doi: 10.1016/0169-4758(95)80167-7. [DOI] [PubMed] [Google Scholar]
- 5.Robert A., Benoit-Vical F., Dechy-Cabaret O., Meunier B. From classical antimalarial drugs to new compounds based on the mechanism of action of artemisinin. Pure Appl. Chem. 2001;73:1173–1188. [Google Scholar]
- 6.Eckstein-Ludwig U., Webb R. J., van Goethem I. D. A., East J. M., Lee A. G., Kimura M., O'Neill P. M., Bray P. G., Ward S. A., Krishna S. Artemisinins target the SERCA of Plasmodium falciparum. Nature (London) 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 7.Nontprasert A., Nosten-Bertrand M., Pukrittayakamee S., Vanijanonta S., Angus B. J., White N. J. Assessment of the neurotoxicity of parenteral artemisinin derivatives in mice. Am. J. Trop. Med. Hyg. 1998;59:519–522. doi: 10.4269/ajtmh.1998.59.519. [DOI] [PubMed] [Google Scholar]
- 8.Nontprasert A., Pukrittayakamee S., Nosten-Bertrand M., Vanijanonta S., White N. J. Studies of the neurotoxicity of oral artemisinin derivatives in mice. Am. J. Trop. Med. Hyg. 2000;62:409–412. doi: 10.4269/ajtmh.2000.62.409. [DOI] [PubMed] [Google Scholar]
- 9.Waller R. F., Keeling P. J., Donald R. G. K., Striepen B., Handman E., Lang-Unnasch N., Cowman A. F., Besra G. S., Roos D. S., McFadden G. I. Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc. Natl. Acad. Sci. U.S.A. 1998;95:12352–12357. doi: 10.1073/pnas.95.21.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surolia N., Surolia A. Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nat. Med. 2001;7:167–173. doi: 10.1038/84612. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S. K., Kapoor M., Ramya T. N. C., Kumar S., Kumar G., Modak R., Sharma S., Surolia N., Surolia A. Identification, characterization and inhibition of Plasmodium falciparum β-hydroxyacyl-acyl carrier protein dehydratase (FabZ) J. Biol. Chem. 2003;278:45661–45671. doi: 10.1074/jbc.M304283200. [DOI] [PubMed] [Google Scholar]
- 12.Pillai S., Rajagopal C., Kapoor M., Kumar G., Gupta A., Surolia N. Functional characterization of β-ketoacyl-ACP reductase (FabG) from Plasmodium falciparum. Biochem. Biophys. Res. Commun. 2003;303:387–392. doi: 10.1016/s0006-291x(03)00321-8. [DOI] [PubMed] [Google Scholar]
- 13.McMurry L. M., Oethinger M., Levy S. B. Triclosan targets lipid synthesis. Nature (London) 1998;394:531–532. doi: 10.1038/28970. [DOI] [PubMed] [Google Scholar]
- 14.Zuther E., Johnson J. J., Haselkorn R., McLeod R., Gornicki P. Growth of Toxoplasma gondii is inhibited by aryloxyphenoxypropionate herbicides targeting acetyl-CoA carboxylase. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13387–13392. doi: 10.1073/pnas.96.23.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waller R. F., Ralph S. A., Reed M. B., Su V., Douglas J. D., Minnikin D. E., Cowman A. F., Besra G. S., McFadden G. I. A type II pathway for fatty acid biosynthesis presents drug targets in Plasmodium falciparum. Antimicrob. Agents Chemother. 2003;47:297–301. doi: 10.1128/AAC.47.1.297-301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verwoert I. I., Verhagen E. F., van der Linden K. H., Verbree E. C., Nijkamp H. J., Stuitje A. R. Molecular characterization of an Escherichia coli mutant with a temperature-sensitive malonyl coenzyme A-acyl carrier protein transacylase. FEBS Lett. 1994;348:311–316. doi: 10.1016/0014-5793(94)00630-x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., Cronan J. E., Jr Transcriptional analysis of essential genes of the Escherichia coli fatty acid biosynthesis gene cluster by functional replacement with the analogous Salmonella typhimurium gene cluster. J. Bacteriol. 1998;180:3295–3303. doi: 10.1128/jb.180.13.3295-3303.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutchma A. J., Hoang T. T., Schweizer H. P. Characterization of a Pseudomonas aeruginosa fatty acid biosynthetic gene cluster: purification of acyl carrier protein (ACP) and malonyl-coenzyme A:ACP transacylase (FabD) J. Bacteriol. 1999;181:5498–5504. doi: 10.1128/jb.181.17.5498-5504.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verwoert I. I., Verbree E. C., van der Linden K. H., Nijkamp H. J., Stuitje A. R. Cloning, nucleotide sequence, and expression of the Escherichia coli fabD gene, encoding malonyl coenzyme A-acyl carrier protein transacylase. J. Bacteriol. 1992;174:2851–2857. doi: 10.1128/jb.174.9.2851-2857.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magnuson K., Oh W., Larson T. J., Cronan J. E., Jr Cloning and nucleotide sequence of the fabD gene encoding malonyl coenzyme A-acyl carrier protein transacylase of Escherichia coli. FEBS Lett. 1992;299:262–266. doi: 10.1016/0014-5793(92)80128-4. [DOI] [PubMed] [Google Scholar]
- 21.Serre L., Verbree E. C., Dauter Z., Stuitje A. R., Derewenda Z. S. The Escherichia coli malonyl-CoA:acyl carrier protein transacylase at 1.5-Å resolution: crystal structure of a fatty acid synthase component. J. Biol. Chem. 1995;270:12961–12964. doi: 10.1074/jbc.270.22.12961. [DOI] [PubMed] [Google Scholar]
- 22.Prigge S. T., He X., Gerena L., Waters N. C., Reynolds K. A. The initiating steps of a type II fatty acid synthase in Plasmodium falciparum are catalyzed by PfACP, PfMCAT and PfKASIII. Biochemistry. 2003;42:1160–1169. doi: 10.1021/bi026847k. [DOI] [PubMed] [Google Scholar]
- 23.Waters N. C., Kopydlowski K. M., Guszczynski T., Wei L., Sellers P., Ferlan J. T., Lee P. J., Li Z., Woodard C. L., Shallom S., et al. Functional characterization of the acyl carrier protein (PfACP) and β-ketoacyl ACP synthase III (PfKASIII) from Plasmodium falciparum. Mol. Biochem. Parasitol. 2002;123:85–94. doi: 10.1016/s0166-6851(02)00140-8. [DOI] [PubMed] [Google Scholar]
- 24.Tsay J. T., Oh W., Larson T. J., Jackowski S., Rock C. O. Isolation and characterization of the β-ketoacyl-acyl carrier protein synthase III gene (fabH) from Escherichia coli K-12. J. Biol. Chem. 1992;267:6807–6814. [PubMed] [Google Scholar]
- 25.Fisher M., Kroon J. T. M., Martindale W., Stuitje A. R., Slabas A. R., Rafferty J. B. The X-ray structure of Brassica napus β-ketoacyl-acyl carrier protein reductase and its implications for substrate binding and catalysis. Structure. 2000;8:339–347. doi: 10.1016/s0969-2126(00)00115-5. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y.-M., Rock C. O. Evaluation of epigallocatechin gallate and related plant polyphenols as inhibitors of the FabG and FabI reductases of bacterial type II fatty acid synthase. J. Biol. Chem. 2004;279:30994–31001. doi: 10.1074/jbc.M403697200. [DOI] [PubMed] [Google Scholar]
- 27.Clark D. P., de Mendoza D., Polacco M. L., Cronan J. E., Jr β-Hydroxydecanoyl thio ester dehydrase does not catalyze a rate-limiting step in Escherichia coli unsaturated fatty acid synthesis. Biochemistry. 1983;22:5897–5902. doi: 10.1021/bi00294a032. [DOI] [PubMed] [Google Scholar]
- 28.Heath R. J., Rock C. O. Roles of the FabA and FabZ β-hydroxyacyl-acyl carrier protein dehydratases in Escherichia coli fatty acid biosynthesis. J. Biol. Chem. 1996;271:27795–27801. doi: 10.1074/jbc.271.44.27795. [DOI] [PubMed] [Google Scholar]
- 29.Helmkamp G. M., Jr, Brock D. J. H., Bloch K. β-Hydroxydecanoyl thioester dehydrase: specificity of substrates and acetylenic inhibitors. J. Biol. Chem. 1968;243:3229–3232. [PubMed] [Google Scholar]
- 30.Leesong M., Henderson B. S., Gillig J. R., Schwab J. M., Smith J. L. Structure of a dehydratase-isomerase from the bacterial pathway for biosynthesis of unsaturated fatty acids: two catalytic activities in one active site. Structure. 1996;4:253–264. doi: 10.1016/s0969-2126(96)00030-5. [DOI] [PubMed] [Google Scholar]
- 31.Swarna Mukhi P. L., Sharma S. K., Kapoor M., Surolia N., Surolia A., Suguna K. Crystallization and preliminary crystallographic analysis of β-hydroxyacyl ACP dehydratase (FabZ) from Plasmodium falciparum. Acta Crystallogr. D Biol. Crystallogr. 2004;60:120–121. doi: 10.1107/s0907444903022327. [DOI] [PubMed] [Google Scholar]
- 32.Egan A. F., Russell R. R. B. Conditional mutants affecting the cell envelope of Escherichia coli. Genet. Res. 1973;21:3603–3611. doi: 10.1017/s001667230001332x. [DOI] [PubMed] [Google Scholar]
- 33.Heath R. J., Rock C. O. Enoyl-acyl carrier protein reductase (fabI) plays a determinant role in completing cycles of fatty acid elongation in Escherichia coli. J. Biol. Chem. 1995;270:26538–26542. doi: 10.1074/jbc.270.44.26538. [DOI] [PubMed] [Google Scholar]
- 34.Heath R. J., Yu Y.-T., Shapiro M. A., Olson E., Rock C. O. Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis. J. Biol. Chem. 1998;273:30316–30321. doi: 10.1074/jbc.273.46.30316. [DOI] [PubMed] [Google Scholar]
- 35.Heath R. J., Rubin J. R., Holland D. R., Zhang E., Snow M. E., Rock C. O. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J. Biol. Chem. 1999;274:11110–11114. doi: 10.1074/jbc.274.16.11110. [DOI] [PubMed] [Google Scholar]
- 36.Suguna K., Surolia A., Surolia N. Structural basis for triclosan and NAD binding to enoyl-ACP reductase of Plasmodium falciparum. Biochem. Biophys. Res. Commun. 2001;283:224–228. doi: 10.1006/bbrc.2001.4747. [DOI] [PubMed] [Google Scholar]
- 37.Kapoor M., Dar M. J., Surolia A., Surolia N. Kinetic determinants of the interaction of enoyl-ACP reductase from Plasmodium falciparum with its substrates and inhibitors. Biochem. Biophys. Res. Commun. 2001;289:832–837. doi: 10.1006/bbrc.2001.6061. [DOI] [PubMed] [Google Scholar]
- 38.Ward W. H. J., Holdgate G. A., Rowsell S., McLean E. G., Pauptit R. A., Clayton E., Nichols W. W., Minshull C. A., Jude D. A., Mistry A., et al. Kinetic and structural characteristics of the inhibition of enoyl (acyl carrier protein) reductase by triclosan. Biochemistry. 1999;38:12514–12525. doi: 10.1021/bi9907779. [DOI] [PubMed] [Google Scholar]
- 39.Kapoor M., Reddy C. C., Krishnasastry M. V., Surolia N., Surolia A. Slow-tight binding inhibition of enoyl-acyl carrier protein reductase from Plasmodium falciparum by triclosan. Biochem. J. 2004;381:735–741. doi: 10.1042/BJ20031821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kapoor M. Bangalore, India: Indian Institute of Science; 2003. Kinetic studies on enoyl-ACP reductase from Plasmodium falciparum: a potent target of antimalarials. Ph.D. Thesis. [Google Scholar]
- 41.Kapoor M., Mukhi P. L., Surolia N., Suguna K., Surolia A. Kinetic and structural analysis of the increased affinity of enoyl-ACP reductase for triclosan in the presence of NAD+ Biochem. J. 2004;381:725–733. doi: 10.1042/BJ20040228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heath R. J., Li J., Ronald G. E., Rock C. O. Inhibition of the Staphylococcus aureus NADPH-dependent enoyl-acyl carrier protein reductase by triclosan and hexachlorophene. J. Biol. Chem. 2000;275:4654–4659. doi: 10.1074/jbc.275.7.4654. [DOI] [PubMed] [Google Scholar]
- 43.Seefeld M. A., Miller W. H., Newlander K. A., Burgess W. J., Payne D. J., Rittenhouse S. F., Moore T. D., DeWolf W. E., Jr, Keller P. M., Qiu X., et al. Inhibitors of bacterial enoyl acyl carrier protein reductase (FabI): 2,9-disubstituted 1,2,3,4-tetrahydropyrido[3,4-b]indoles as potential antibacterial agents. Bioorg. Med. Chem. Lett. 2001;11:2241–2244. doi: 10.1016/s0960-894x(01)00405-x. [DOI] [PubMed] [Google Scholar]
- 44.Miller W. H., Seefeld M. A., Newlander K. A., Uzinskas I. N., Burgess W. J., Heerding D. A., Yuan C. C. K., Head M. S., Payne D. J., Rittenhouse S. F., et al. Discovery of aminopyridine-based inhibitors of bacterial enoyl-ACP reductase (FabI) J. Med. Chem. 2002;45:3246–3256. doi: 10.1021/jm020050+. [DOI] [PubMed] [Google Scholar]
- 45.Seefeld M. A., Miller W. H., Newlander K. A., Burgess W. J., DeWolf W. E., Jr, Elkins P. A., Head M. S., Jakas D. R., Janson C. A., Keller P. M., et al. Indole naphthyridinones as inhibitors of bacterial enoyl-ACP reductases FabI and FabK. J. Med. Chem. 2003;46:1627–1635. doi: 10.1021/jm0204035. [DOI] [PubMed] [Google Scholar]
- 46.Heerding D. A., Chan G., DeWolf W. E., Jr, Fosberry A. P., Janson C. A., Jaworski D. D., McManus E., Miller W. H., Moore T. D., Payne D. J., et al. 1,4-Disubstituted imidazoles are potential antibacterial agents functioning as inhibitors of enoyl acyl carrier protein reductase (FabI) Bioorg. Med. Chem. Lett. 2001;11:2061–2065. doi: 10.1016/s0960-894x(01)00404-8. [DOI] [PubMed] [Google Scholar]
- 47.Parikh S. L., Xiao G., Tonge P. J. Inhibition of InhA, the enoyl reductase from Mycobacterium tuberculosis, by triclosan and isoniazid. Biochemistry. 2000;39:7645–7650. doi: 10.1021/bi0008940. [DOI] [PubMed] [Google Scholar]
- 48.Kuo M. R., Morbidoni H. R., Alland D., Sneddon S. F., Gourlie B. B., Staveski M. M., Leonard M., Gregory J. S., Janjigian A. D., Yee C., et al. Targeting tuberculosis and malaria through inhibition of enoyl reductase. J. Biol. Chem. 2003;278:20851–20859. doi: 10.1074/jbc.M211968200. [DOI] [PubMed] [Google Scholar]
- 49.Goerg H., Ochola S. A., Goerg R. Treatment of malaria tropica with a fixed combination of rifampicin, co-trimoxazole and isoniazid: a clinical study. Chemotherapy. 1999;45:68–76. doi: 10.1159/000007167. [DOI] [PubMed] [Google Scholar]
- 50.Rosenfeld I. S., D'Agnolo G., Vagelos P. R. Synthesis of unsaturated fatty acids and the lesion in fabB mutants. J. Biol. Chem. 1973;248:2452–2460. [PubMed] [Google Scholar]
- 51.D'Agnolo G., Rosenfeld I. S., Vagelos P. R. Multiple forms of β-ketoacyl-acyl carrier protein synthetase in Escherichia coli. J. Biol. Chem. 1975;250:5289–5294. [PubMed] [Google Scholar]
- 52.Garwin J. L., Klages A. L., Cronan J. E., Jr β-Ketoacyl-acyl carrier protein synthase II of Escherichia coli: evidence for function in the thermal regulation of fatty acid synthesis. J. Biol. Chem. 1980;255:3263–3265. [PubMed] [Google Scholar]
- 53.Vance D. E., Goldberg I., Mitsuhashi O., Bloch K., Omura S., Nomura S. Inhibition of fatty acid synthetases by the antibiotic cerulenin. Biochem. Biophys. Res. Commun. 1972;48:649–656. doi: 10.1016/0006-291x(72)90397-x. [DOI] [PubMed] [Google Scholar]
- 54.Kawaguchi A., Tomoda H., Nozoe S., Omura S., Okuda S. Mechanism of action of cerulenin on fatty acid synthetase: effect of cerulenin on iodoacetamide-induced malonyl-CoA decarboxylase activity. J. Biochem. (Tokyo) 1982;92:7–12. doi: 10.1093/oxfordjournals.jbchem.a133933. [DOI] [PubMed] [Google Scholar]
- 55.Kauppinen S., Siggaard-Anderson M., van Wettstein-Knowles P. β-Ketoacyl-ACP synthase I of Escherichia coli: nucleotide sequence of the fabB gene and identification of the cerulenin binding residue. Carlsberg Res. Commun. 1988;53:357–370. doi: 10.1007/BF02983311. [DOI] [PubMed] [Google Scholar]
- 56.Heath R. J., White S. W., Rock C. O. Inhibitors of fatty acid synthesis as antimicrobial chemotherapeutics. Appl. Microbiol. Biotechnol. 2002;58:695–703. doi: 10.1007/s00253-001-0918-z. [DOI] [PubMed] [Google Scholar]
- 57.Price A. C., Choi K.-H., Heath R. J., Li Z., White S. W., Rock C. O. Inhibition of β-ketoacyl-acyl carrier protein synthases by thiolactomycin and cerulenin. J. Biol. Chem. 2001;276:6551–6559. doi: 10.1074/jbc.M007101200. [DOI] [PubMed] [Google Scholar]
- 58.Tsay J.-T., Rock C. O., Jackowski S. Overproduction of β-ketoacyl-acyl carrier protein synthase I imparts thiolactomycin resistance to Escherichia coli K-12. J. Bacteriol. 1992;174:508–513. doi: 10.1128/jb.174.2.508-513.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jackowski S., Murphy C. M., Cronan J. E., Jr, Rock C. O. Acetoacetyl-acyl carrier protein synthase: a target for the antibiotic thiolactomycin. J. Biol. Chem. 1989;264:7624–7629. [PubMed] [Google Scholar]
- 60.Hayashi T., Yamamoto O., Sasaki H., Kawaguchi A., Okazaki H. Mechanism of action of the antibiotic thiolactomycin inhibition of fatty acid synthesis of Escherichia coli. Biochem. Biophys. Res. Commun. 1983;115:1108–1113. doi: 10.1016/s0006-291x(83)80050-3. [DOI] [PubMed] [Google Scholar]
- 61.Waller R. F., Reed M. B., Cowman A. F., McFadden G. I. Protein trafficking to the plastid of Plasmodium falciparum is via the secretory pathway. EMBO J. 2000;19:1794–1802. doi: 10.1093/emboj/19.8.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bozdech Z., Llinas M., Pulliam B. L., Wong E. D., Zhu J., DeRisi J. L. The transcriptome of the Intraerythrocytic developmental cycle of Plasmodium. PLoS Biol. 2003;1:E5. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.LeRoch K. G., Zhou Y., Blair P. L., Grainger M., Moch J. K., Haynes J. D., De La Vega P., Holder A. A., Batalov S., Carucci D. J., Winzeler E. A. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301:1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 64.Maréchal E., Cesbron-Delauw M.-F. The apicoplast: a new member of the plastid family. Trends Plant Sci. 2001;6:200–205. doi: 10.1016/s1360-1385(01)01921-5. [DOI] [PubMed] [Google Scholar]
- 65.Gleeson M. T. The plastid in Apicomplexa: what use is it? Int. J. Parasitol. 2000;30:1053–1070. doi: 10.1016/s0020-7519(00)00100-4. [DOI] [PubMed] [Google Scholar]
- 66.McFadden G. I., Reith M., Munholland J., Lang-Unnasch N. Plastid in human parasites. Nature (London) 1996;381:482. doi: 10.1038/381482a0. [DOI] [PubMed] [Google Scholar]
- 67.Wilson R. J. M. Plastids better red than dead. Nature (London) 1993;366:638. doi: 10.1038/366638a0. [DOI] [PubMed] [Google Scholar]
- 68.McFadden G. I., Gilson P. Something borrowed, something green: lateral transfer of chloroplasts by secondary endosymbiosis. Trends Ecol. Evol. 1995;10:12–17. doi: 10.1016/s0169-5347(00)88954-5. [DOI] [PubMed] [Google Scholar]
- 69.Martin W., Stoebe B., Goremykin V., Hapsmann S., Hasegawa M., Kowallik K. V. Gene transfer to the nucleus and the evolution of chloroplasts. Nature (London) 1998;14:162–165. doi: 10.1038/30234. [DOI] [PubMed] [Google Scholar]
- 70.McFadden G. I. Chloroplast origin and integration. Plant Physiol. 2001;125:50–53. doi: 10.1104/pp.125.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gardner M. J., Shallom S. J., Carlton J. M., Salzberg S. L., Nene V., Shoaibi A., Ciecko A., Lynn J., Rizzo M., Weaver B., et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature (London) 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sato S., Tews I., Wilson R. Impact of a plastid-bearing endocytobiont on apicomplexan genomes. Int. J. Parasitol. 2000;30:427–439. doi: 10.1016/s0020-7519(99)00185-x. [DOI] [PubMed] [Google Scholar]
- 73.Ralph S. A., van Dooren G. G., Waller R. F., Crawford M. J., Fraunholz M. J., Foth B. J., Tonkin C. J., Roos D. S., McFadden G. I. Metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat. Rev. Microbiol. 2004;2:203–216. doi: 10.1038/nrmicro843. [DOI] [PubMed] [Google Scholar]
- 74.Kubis S. E., Rawsthorne S. The role of plastidial transporters in developing embryos of oilseed rape (Brassica napus L.) for fatty acid synthesis. Biochem. Soc. Trans. 2000;28:665–666. [PubMed] [Google Scholar]
- 75.Furet Y. X., Pechere J. C. Newly documented antimicrobial activity of quinolines. Eur. J. Clin. Microbiol. Infect. Dis. 1991;10:249–254. doi: 10.1007/BF01966997. [DOI] [PubMed] [Google Scholar]
- 76.Cundliffe E. Recognition sites for antibiotics within rRNA. In: Hill W. H., et al., editors. The Ribosome: Structure, Function and Evolution. Washington: American Society of Microbiology; 1990. pp. 479–490. [Google Scholar]
- 77.Fichera M. E., Bhopale M. K., Roos D. S. In vitro assays elucidate peculiar kinetics of clindamycin action against Toxoplasma gondii. Antimicrob. Agents Chemother. 1995;39:1530–1537. doi: 10.1128/aac.39.7.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He C. Y., Shaw M. K., Pletcher C. H., Striepen B., Tilney L. G., Roos D. S. A plastid segregation defect in the protozoan parasite Toxoplasma gondii. EMBO J. 2001;20:330–339. doi: 10.1093/emboj/20.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McFadden G. I., Roos D. S. Apicomplexan plastids as drug targets. Trends Microbiol. 1999;7:328–333. doi: 10.1016/s0966-842x(99)01547-4. [DOI] [PubMed] [Google Scholar]
- 80.Bhattacharjee A. K., Hartell M. G., Nichols D. A., Hicks R. P., Stanton B., van Hamont J. E., Milhous W. K. Structure–activity relationship study of antimalarial indolo[2,1-b]quinazoline-6,12-diones (tryptanthrins): three dimensional pharmacophore modeling and identification of new antimalarial candidates. Eur. J. Med. Chem. 2004;39:59–67. doi: 10.1016/j.ejmech.2003.10.004. [DOI] [PubMed] [Google Scholar]