Abstract
RSK2 (p90 ribosomal S6 kinase 2) is activated via the ERK (extracellular-signal-regulated kinase) pathway by phosphorylation on four sites: Ser227 in the activation loop of the N-terminal kinase domain, Ser369 in the linker, Ser386 in the hydrophobic motif and Thr577 in the C-terminal kinase domain of RSK2. In the present study, we demonstrate that RSK2 is associated in vivo with PP2Cδ (protein phosphatase 2Cδ). In epidermal growth factorstimulated cells, RSK2 is partially dephosphorylated on all four sites in an Mn2+-dependent manner, leading to reduced protein kinase activity. Furthermore, PP2Cδ is phosphorylated by ERK on Thr315 and Thr333 in the catalytic domain. Mutation of Thr315 and Thr333 to alanine in a catalytically inactive mutant PP2Cδ(H154D) (His154→Asp) increases the association with RSK2 significantly, whereas mutation to glutamate, mimicking phosphorylation, reduces the binding of RSK2. The domains of interaction are mapped to the N-terminal extension comprising residues 1–71 of PP2Cδ and the N-terminal kinase domain of RSK2. The interaction is specific, since PP2Cδ associates with RSK1–RSK4, MSK1 (mitogen- and stress-activated kinase 1) and MSK2, but not with p70 S6 kinase or phosphoinositide-dependent kinase 1. We conclude that RSK2 is associated with PP2Cδ in vivo and is partially dephosphorylated by it, leading to reduced kinase activity.
Keywords: AGC kinase, extracellular-signal-regulated kinase (ERK), mitogen- and stress-activated kinase (MSK), protein phosphatase 2C (PP2C), p90 ribosomal S6 kinase (RSK)
Abbreviations: CDK, cyclin-dependent kinase; CIP, calf intestinal phosphatase; CTK, C-terminal kinase; EGF, epidermal growth factor; ERK, extracellular-signal-regulated kinase; EST, expressed sequence tag; GST, glutathione S-transferase; HA, haemagglutinin; ILKAP, integrin-linked kinase-associated phosphatase; KAP, CDK-associated protein phosphatase; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase; MSK, mitogen and stress-activated kinase; NTK, N-terminal kinase; p70S6K, p70 S6 kinase; PDK1, phosphoinositide-dependent kinase 1; PP1 (etc.), protein phosphatase 1 (etc.); RSK, p90 ribosomal S6 kinase
INTRODUCTION
Protein phosphorylation, catalysed by protein kinases, is a ubiquitous, intracellular post-translational modification found in eukaryotes as well as prokaryotes. The state of protein phosphorylation is controlled by the relative activity of two families of enzymes with opposing actions, namely protein kinases and protein phosphatases. Reversible protein phosphorylation is involved in the regulation of diverse biological processes such as cell proliferation, differentiation, apoptosis and metabolism [1]. Eukaryotic protein kinases can be classified into two categories based on their target amino acid: protein tyrosine kinases and protein serine/threonine kinases. Modification of serine and threonine residues is much more prevalent compared with tyrosine phosphorylation [2]. RSK (p90 ribosomal S6 kinase) belongs to the large family of serine/threonine kinases. RSK has a unique structure composed of two functional kinase domains connected by a linker [3]. The four RSK isoforms, RSK1–RSK4, are encoded by distinct genes and show the same overall structure, with 80–85% amino acid identity [4–6]. RSKs are implicated in the regulation of a wide range of cellular functions, including transcription [4,7], protein synthesis [8,9], cell survival [10–13] and cell proliferation [3,14]. rsk2 knockout mice are characterized by brain deficits, including poor co-ordination, impaired learning and abnormal cognition [15]. Furthermore, in human, mutations of the rsk2 gene cause Coffin–Lowry syndrome, an X-linked disorder characterized by severe mental retardation and skeletal deformation [16].
RSK is activated in response to growth factors by multi-site phosphorylation of the two kinase domains in a sequential manner. In RSK1, six phosphorylation sites have been identified and these are conserved in all four RSK isoforms [17]. In RSK2, these correspond to Ser227, Thr365, Ser369, Ser386, Thr577 and Ser736. In the basal state, ERK (extracellular-signal-regulated kinase) is associated with RSK2 via a docking site in the C-terminal extension [18]. Activation of ERK leads to phosphorylation of Thr365 and Ser369 in the linker and Thr577 in the activation loop of the CTK (C-terminal kinase) domain [17–20]. Active CTK domain phosphorylates Ser386 located in a hydrophobic motif, which functions as a transient docking site for PDK1 (phosphoinositide-dependent kinase 1) [21]. PDK1 phosphorylates Ser227 in the activation loop of the NTK (N-terminal kinase) domain [22,23]. Finally, NTK domain phosphorylates Ser736 next to the ERK docking site, resulting in the dissociation of ERK [24].
Protein serine/threonine phosphatases can be divided into two distinct families: PPP (polymeric protein phosphatase) and PPM (monomeric protein phosphatase). The PPP family consists of three subtypes: PP1 (protein phosphatase 1), PP2A and PP2B [25]. PPM includes mammalian PP2Cα, PP2Cβ, PP2Cγ and PP2Cδ [26,27]. The catalytic activity of PP2C definitely requires metal ions, Mn2+ or Mg2+. PP2Cs are involved in the regulation of a wide range of physiological functions including cellular stress responses, apoptosis and cell cycle [28–30].
Murine PP2Cδ shows homology with other members of the PP2C subfamily in the catalytic domain, but is distinct from other members in the sequence of its non-catalytic domains. The N-terminal domain of 76 amino acids is unique for PP2Cδ and the C-terminal domain is truncated to three amino acids [26]. Furthermore, the catalytic activity of PP2Cδ is highly dependent on Mn2+, but is inhibited by Mg2+, in contrast with other members of the PP2C subfamily [26,31]. PP2Cδ regulates cell growth negatively by blocking cell cycle progression in early S phase, resulting in inhibition of DNA synthesis and cell death. Human PP2Cδ is 98% similar to murine PP2Cδ and has been identified as an ILKAP (integrin-linked kinase-associated phosphatase) [31].
In the present study, we demonstrate for the first time that RSK2 and PP2Cδ are physically associated in vivo and that RSK2 is partially dephosphorylated by an Mn2+-dependent phosphatase. The decrease in phosphorylation of RSK2 is accompanied by a 50% decrease in kinase activity. The domains of association were mapped to the NTK of RSK2 and the N-terminal domain of PP2Cδ. PP2Cδ is phosphorylated on Thr315 and Thr333 by ERK in vivo and the association with RSK2 is increased by mutation of the ERK phosphorylation sites. Our findings suggest that PP2Cδ forms a complex with RSK2 that is regulated by phosphorylation by ERK and PP2Cδ is involved in the regulation of RSK2 activity.
EXPERIMENTAL
Materials
Human recombinant EGF (epidermal growth factor) was obtained from PreproTech (Rocky Hill, NJ, U.S.A.). S6 peptide (residues 231–239) of human 40 S ribosomal protein 6: RRLSSLRA was from K. J. Ross-Petersen A/S (Copenhagen, Denmark). Phospho-specific antibodies to mouse RSK1 S365 and S380, used to probe for the corresponding S369 and S386 in mouse RSK2, were obtained from Upstate Biotechnology (catalogue nos. 06-824 and 06-826; Lake Placid, NY, U.S.A.). The phospho-specific S227 antibody was from Santa Cruz Biotechnology (catalogue no. sc-12445-R) and the phospho-specific T577 antibody was kindly supplied by K. Merienne (INSERM, Strasbourg, France). Anti-myc and anti-HA antibodies (where HA stands for haemagglutinin) were obtained from Santa Cruz Biotechnology (catalogue nos. sc-789 and Sc-805) and anti-(active MAPK) (mitogen-activated protein kinase) antibody was from Promega (catalogue no. V803A). Anti-PP2Cδ antibody was prepared by immunization of rabbits as described previously [26]. Anti-HA and anti-myc antibodies for immunoprecipitation were from the 12CA5 and 9E10 mouse hybridoma cell lines respectively. [γ-32P]ATP was obtained from Amersham Biosciences. CIP (calf intestinal phosphatase) was purchased from New England Biolabs (Beverly, MA, U.S.A.) and active ERK was from Upstate Biotechnology (cata-logue no. 14-173). Other chemicals were obtained from Sigma.
Mammalian cell line and culture condition
COS-7 and HEK-293 cells were cultured in a humidified incubator at 37 °C and 7.5% CO2 in Dulbecco's modified Eagle's medium with 0.11 g/l sodium pyruvate and pyridoxine (obtained from Life Technologies, Burlington, ON, Canada). To this medium were added 10% (v/v) fetal bovine serum, 5 mM L-glutamine, 100 units/ml penicillin and 0.1 mg/ml streptomycin.
Transfection and precipitation
Monolayers of COS-7 or HEK-293 cells at approx. 75% confluency in 9.6 cm2 dishes were transfected for 4–5 h in serum-free medium with a total of 1.5 μg of DNA construct complexed with 12 μl of LIPOFECTAMINE (Life Technologies) according to the manufacturer's instructions. In double transfections, 0.75 μg of each DNA construct was used. After transfection, cells were cultured for 48 h and then washed twice with serum-free medium. After incubation for 4 h in the absence of serum, the cells were exposed (or not) to 1 ng/ml EGF or 50 nM PMA, washed with PBS and solubilized for 15 min in 500 μl of lysis buffer [0.5% Triton X-100, 150 mM NaCl, 50 mM Tris/HCl (pH 7.4), 1 mM EDTA, 10 μM leupeptin, 10 μM pepstatin, 10 nM calyculin A and 200 kallikrein inhibitor units/ml of aprotinin] on ice. Subsequent manipulations were performed at 0–4 °C. Cell extracts were centrifuged for 10 min at 14000 g, and the supernatant was incubated for 2 h with an anti-HA or anti-myc antibody with the addition of 20 μl of Protein G–agarose beads (Amersham Biosciences) during the final 45 min. Protein G Dynabeads (6 μl; Dynal Biotech, Oslo, Norway) were used in the co-transfection experiments. GST (glutathione S-transferase)–PP2Cδ was incubated with glutathione beads. Finally, beads were precipitated by centrifugation, washed five times with lysis buffer, drained and then dissolved in SDS/PAGE sample buffer. For kinase or phosphatase assays, the final two washes were with 45 mM Tris/HCl, pH 7.4.
Phosphatase and kinase assays
Agarose beads with immunoprecipitated RSK2 from COS-7 cells were drained with a syringe and resuspended in 20 μl of 45 mM Tris/HCl (pH 7.4) and 10 mM dithiothreitol. The phosphatase reaction was initiated by the addition of 10 μl of 30 mM bivalent cations or water. The samples were incubated at 30 °C on a thermoshaker and the immunoprecipitates were then washed three times with 45 mM Tris/HCl (pH 6.8), drained and dissolved in SDS/PAGE sample buffer. The kinase assay of RSK2 was performed as described in [23].
In vitro phosphorylation of GST–PP2Cδ
Overexpressed GST–PP2Cδ was precipitated with glutathione beads from serum-starved COS-7 cells and dephosphorylated with 20 units of CIP for 1 h at 37 °C. CIP was removed by washing, and GST–PP2Cδ was phosphorylated in a kinase buffer for 1 h at 30 °C with 50 ng of active ERK in the presence of 5 μCi of [γ-32P]ATP. The sample was subjected to SDS/PAGE and autoradiography.
Immunoblotting
Samples dissolved in SDS/PAGE sample buffer were fractionated by SDS/PAGE (10% gel) and electroblotted on to a Sequi-Blot™ PVDF membrane (Bio-Rad). Membranes were blocked with 5% (w/v) non-fat dry milk for 40 min. The primary antibody was visualized by incubating with an appropriate secondary antibody coupled with horseradish peroxidase, followed by enhanced chemifluorescence development (Pierce) and image capture with a Fujifilm charge-coupled-device camera.
Plasmid constructs
The coding sequence of rat PP2Cδ was PCR-amplified from the pTR5-PP2Cδ-GFP vector with primers introducing an XhoI site at the 5′-end and a stop codon followed by a KpnI site at the 3′-end of the PP2Cδ coding sequence. The PCR fragment was cut with XhoI and KpnI and cloned into an XhoI- and KpnI-cut pMT2 vector expressing an N-terminal myc epitope in fusion with PP2Cδ. The PP2Cδ point mutations PP2Cδ(H154D) (His154→Asp), PP2Cδ(T315A), PP2Cδ(T315E), PP2Cδ(T333A) and PP2Cδ(T333E) and a combination of these mutations were generated using the QuikChange® (Stratagene) mutagenesis procedure. Deletion mutants PP2Cδ(H154D,T333A)54–393 and PP2Cδ(H154D, T333A)71–393 were generated using PCR to amplify the coding sequence of amino acids 54–393 and 71–393 respectively from the template PP2Cδ(H154D,T333A), with subsequent cloning as mentioned above. Similarly, PP2Cδ1–380 was generated by PCR amplification of the coding sequence of amino acids 1–380 using pTR5-PP2Cδ-GFP as template. N-terminally HA-tagged rat RSK1 and mouse RSK2 in pMT2 were kindly provided by C. Bjørbæk (Beth Israel Hospital, Boston, MA, U.S.A.). HA-tagged human RSK3 was modified due to low expression by replacing RSK3 amino acids 1–20 with RSK2 amino acids 1–30. Modified RSK3 and RSK4 in pMT2 were kindly provided by M. Frödin (Department of Clinical Biochemistry, Glostrup Hospital, Denmark). HA-tagged rat p70S6K (p70 S6 kinase) in pBJ5 was kindly provided by G. Thomas (Friedrich Meischer Institute, Basel, Switzerland). HA-tagged human PDK1 in pMT2 was kindly provided by T. Rasmussen (Novo-Nordisk, Bagsvaerd, Denmark). HA-tagged human MSK1, mouse RSK21–360, mouse RSK21–389 and mouse RSK2375–740 in pMT2 are described in [23]. Human MSK2 was PCR-amplified from I.M.A.G.E. clone 5763859 with primers by introducing an XhoI site and a KpnI site at the 5′- and 3′-ends of the MSK2 coding sequence and cloned into a pMT2 vector expressing an N-terminal HA epitope in fusion with MSK2. All point mutations were confirmed by sequencing. A PP2Cδ splice variant was identified by a BLAST search on human EST (expressed sequence tag) using human PP2Cδ coding sequence as the search parameter. This search identified two EST sequences, GenBank® accession numbers AW602371 and AW602494, which contained skipping of human PP2Cδ exon 10 (corresponding to amino acids 280–318). The corresponding deletion in rat PP2Cδ, PP2Cδ(Δ280–318), was generated using overlap extension [32]. GST-tagged PP2Cδ, PP2Cδ(T315A), PP2Cδ(T333A) and PP2Cδ(T315A,T333A) were prepared by cutting the respective myc-tagged constructs with XhoI and KpnI. The fragments were subsequently cloned into XhoI- and KpnI-cut pMT2 vector expressing an N-terminal GST epitope in fusion with PP2Cδ.
RESULTS
RSK2 association with PP2Cδ
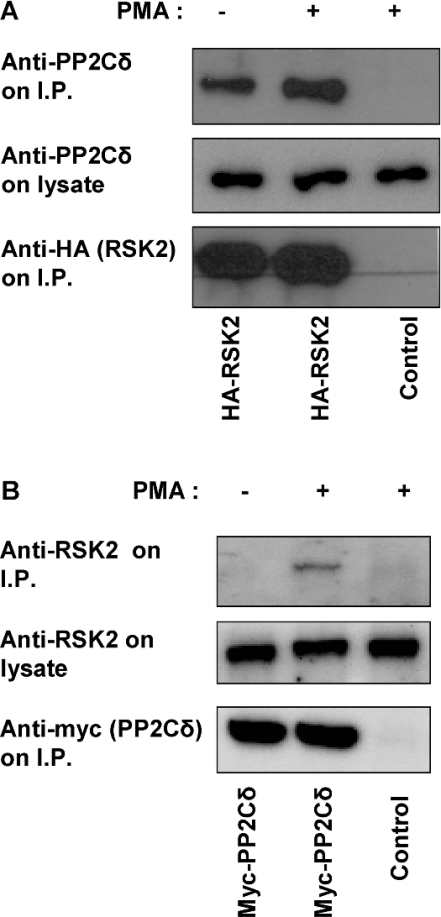
A member of the PP2C family was identified as a binding partner to RSK2 in a yeast two-hybrid screening (M. Frödin, personal communication). To test whether RSK2 is associated with PP2Cδ in mammalian cells, COS-7 cells were transfected with HA–RSK2 and lysed. HA–RSK2 was precipitated and immunoblotted with a specific antibody to PP2Cδ. Figure 1(A) shows that endogenous PP2Cδ is present in the immunoprecipitate associated with RSK2. Furthermore, overexpressed PP2Cδ interacts with endogenous RSK2, as demonstrated by precipitation of myc–PP2Cδ followed by immunoblotting with antibody to RSK2 (Figure 1B). In addition, GST-tagged PP2Cδ could also co-precipitate endogenous RSK2 (results not shown). The interaction between RSK2 and PP2Cδ increased after PMA stimulation.
Figure 1. Co-precipitation of PP2Cδ with RSK2.
COS-7 cells were transfected with an empty vector or a plasmid expressing either HA–RSK2 or myc–PP2Cδ. Cells were stimulated with 50 nM PMA for 20 min and lysed. HA–RSK2 or myc–PP2Cδ was immunoprecipitated using anti-HA or anti-myc antibodies. The samples were washed five times and subjected to SDS/PAGE followed by immunoblotting with either anti-PP2Cδ antibody (A) or anti-RSK2 antibody (B). Overexpressed RSK2 or PP2Cδ was immunoblotted using anti-HA or anti-myc antibodies. I.P., immunoprecipitate.
Dephosphorylation of RSK2
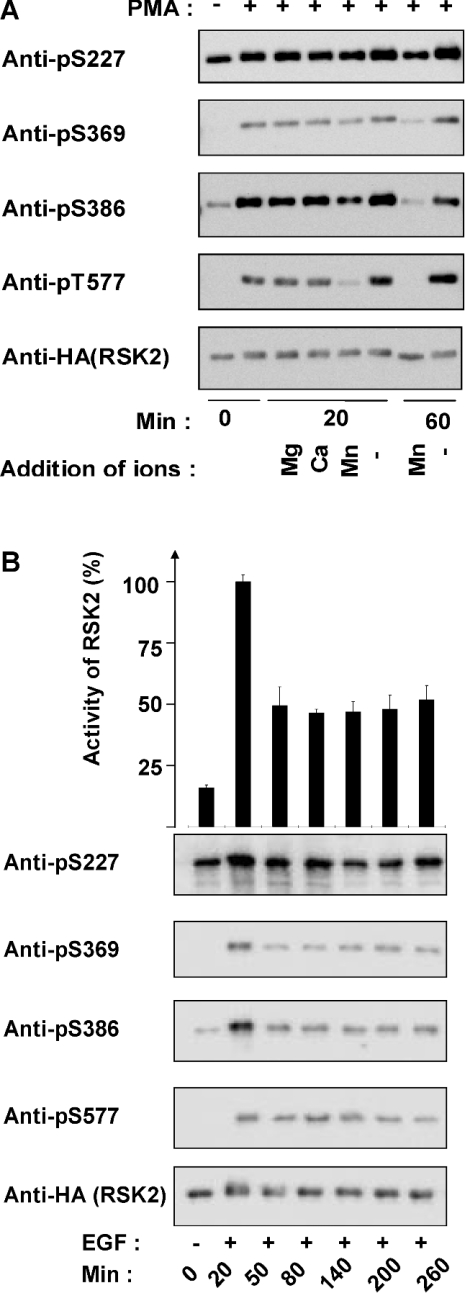
Dephosphorylation of RSK2 was first studied in vitro after transient expression of HA–RSK2 in COS-7 cells and stimulation with PMA before lysis. Immunoprecipitated RSK2 was incubated for 20 or 60 min in the absence or presence of Mg2+, Ca2+ or Mn2+, and analysed by SDS/PAGE and immunoblotting with phosphospecific antibodies against the phosphorylation sites: Ser227, Ser369, Ser386 and Thr577 (Figure 2A). No significant dephosphorylation was observed in the absence of bivalent ions or in the presence of Mg2+ or Ca2+. In contrast, the phosphorylation of all four sites decreased after 20 min in the presence of Mn2+. After 60 min with Mn2+, the phosphorylation was reduced to the level in unstimulated cells. Indeed, PP2Cδ, which is co-immunoprecipitated with overexpressed HA–RSK2 (see Figure 1A), is catalytically active in the presence of Mn2+, but inactive in the presence of Mg2+ [26]. This suggests that RSK2 is dephosphorylated by PP2Cδ in the complex. The catalytic activity of RSK2 could not be quantified due to spontaneous inactivation of the kinase during in vitro incubation at 30 °C.
Figure 2. Dephosphorylation of RSK2.
(A) COS-7 cells were transfected with a plasmid expressing HA-tagged RSK2. After 48 h, the cells were starved overnight. Cells were stimulated with 50 nM PMA for 20 min and lysed. RSK2 was precipitated from the cell lysates with anti-HA antibody. The samples were washed five times. The stimulated samples were pooled and split into eight samples. The samples were frozen (0 min) or incubated for 20 or 60 min at 30 °C in Tris buffer with 10 mM of Mg2+, Ca2+ or Mn2+ or without bivalent cations. After incubation, the samples were washed, analysed by SDS/PAGE and immunoblotted using either phospho-specific antibodies to RSK2 phosphorylated on Ser227, Ser369, Ser386 or Thr577 or anti-HA antibody. (B) COS-7 cells were transfected with a plasmid expressing HA–RSK2 for 48 h, followed by overnight serum starvation. The cells were treated with 1 ng/ml EGF for 20–260 min and lysed. HA–RSK2 was immunoprecipitated from the cell lysates using anti-HA antibody and washed five times. The immunoprecipitates were analysed by protein kinase assay (top panel) or SDS/PAGE and immunoblotting with the indicated phospho-specific antibodies or anti-HA antibody (bottom panel). The RSK2 activity is expressed as a percentage of the maximal value obtained. Results are expressed as the means±S.D. for three independent experiments performed in triplicate.
Next, the dephosphorylation of RSK2 was studied in vivo in COS-7 cells expressing HA–RSK2. After EGF stimulation, the cells were lysed and the phosphorylation of four residues in immunoprecipitated RSK2 was determined by Western blotting with phospho-specific antibodies. Figure 2(B) shows that the phosphorylation of Ser227, Ser369, Ser386 and Thr577 in RSK2 was significantly increased after 20 min of stimulation with EGF. The phosphorylation of all four sites was decreased after 50 min and thereafter the level of phosphorylation was constant up to 260 min. In parallel, the kinase activity of immunoprecipitated RSK2 was determined. Figure 2(B) shows that the kinase activity was increased 6-fold after 20 min of EGF stimulation. After 50 min, the kinase activity was reduced to approx. 50% of its maximal activity and this level was maintained for an additional 210 min. These results show that RSK2 is partially dephosphorylated in vivo, followed by reduced catalytic activity.
ERK-mediated phosphorylation of PP2Cδ
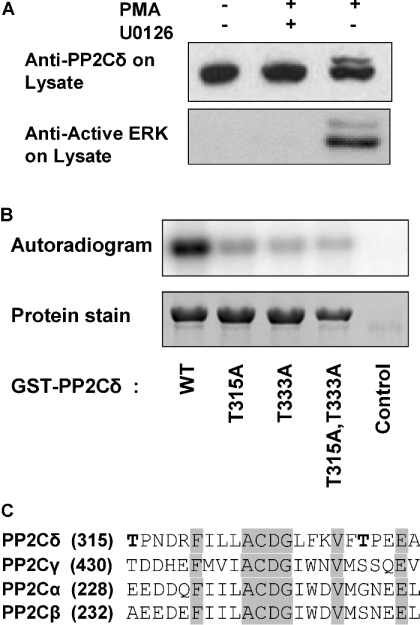
To determine whether PP2Cδ is phosphorylated by ERK, HEK-293 cells were stimulated with PMA in the absence or presence of the MEK (MAPK/ERK kinase) inhibitor U0126. The cells were lysed and analysed by Western blotting using anti-PP2Cδ antibody (Figure 3A). The immunoblot showed a single band of PP2Cδ in unstimulated cells, whereas an additional band of slower mobility appeared in cells stimulated with PMA. Pretreatment of PMA-stimulated cells with the MEK inhibitor U0126 blocked the appearance of modified PP2Cδ. Furthermore, pretreatment of EGF-stimulated cells with the AGC kinase inhibitor RO318220 did not inhibit the appearance of the slow-migrating PP2Cδ band (results not shown). PP2Cδ was phosphorylated in vitro by ERK as shown by incubation of GST–PP2Cδ with active ERK (Figure 3B). PP2Cδ contains two putative ERK phosphorylation sites with the consensus sequence Thr-Pro located at Thr315 and Thr333 in the catalytic domain (Figure 3C). Mutation of these two sites to alanine residues decreased the phosphorylation of PP2Cδ by ERK significantly (Figure 3B). This suggests that PP2Cδ is phosphorylated by ERK on Thr315 and Thr333.
Figure 3. Phosphorylation of PP2Cδ.
(A) HEK-293 cells were serum-starved for 4 h and incubated in the absence or presence of 50 nM PMA for 20 min. PMA-treated cells were preincubated with 10 μM U0126 for 40 min. The cells were lysed, subjected to SDS/PAGE and immunoblotted with anti-PP2Cδ antibody or anti-(active MAPK) antibody. (B) COS-7 cells were transfected with an empty vector or a plasmid expressing GST–PP2Cδ. Wild-type PP2Cδ, mutant PP2Cδ(T315A), PP2Cδ(T333A) and PP2Cδ(T315A,T333A) were incubated with activated ERK in the presence of [γ-32P]ATP and analysed by SDS/PAGE and autoradiography. (C) Four members of the PP2C family were aligned using the Vector NTI. Identical residues are shaded. The two putative ERK phosphorylation sites in PP2Cδ are shown in bold.
Increased association of RSK2 with PP2Cδ mutants
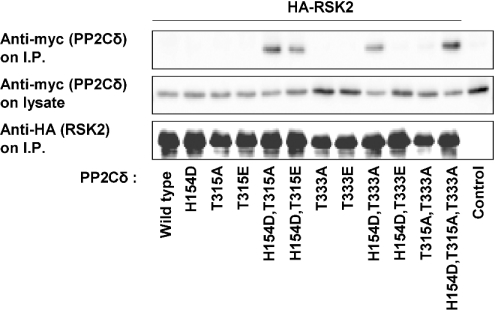
The association between RSK2 and PP2Cδ was investigated in more detail using three PP2Cδ mutants: one phosphatase-inactive PP2Cδ mutant with mutation of His154 to aspartate in the catalytic site [31] and two PP2Cδ mutants with replacement of the two ERK phosphorylation sites, Thr315 and Thr333, with alanine. The three mutations were combined to give double and triple PP2Cδ mutants. HA-tagged RSK2 and myc-tagged PP2Cδ were co-expressed in COS-7 cells, followed by immunoprecipitation of HA–RSK2 and Western blotting of myc–PP2Cδ. Figure 4 shows that neither overexpressed wild-type PP2Cδ nor the three single mutants, PP2Cδ(H154D), PP2Cδ(T315A) and PP2Cδ(T333A), were associated with overexpressed RSK2. In contrast, the double and triple mutants PP2Cδ(H154D,T315A), PP2Cδ(H154D,T333A) and PP2Cδ(H154D,T315A,T333A) were associated with RSK2. Furthermore, ERK phosphorylation of PP2Cδ was mimicked by replacement of Thr315 or Thr333 with glutamate in PP2Cδ(H154D). The association of RSK2 with two mutants, PP2Cδ(H154D,T315E) and PP2Cδ(H154D,T333E), was significantly decreased. This may indicate that the complex between RSK2 and PP2Cδ dissociates after phosphorylation of PP2Cδ by ERK.
Figure 4. Association of PP2Cδ mutants with RSK2.
COS-7 cells were co-transfected with plasmids expressing wild-type or mutant myc–PP2Cδ and HA–RSK2. Transfection of myc–PP2Cδ(H154D,T333A) without HA–RSK was used as the control. Cells were lysed and HA–RSK2 was precipitated from the cell lysates with anti-HA antibody and washed five times. The samples were subjected to SDS/PAGE followed by immunoblotting with anti-myc or anti-HA antibody. The cellular expression level of PP2Cδ was determined by immunoblotting of lysates with anti-myc antibody.
PP2Cδ interacts with the NTK of RSK2
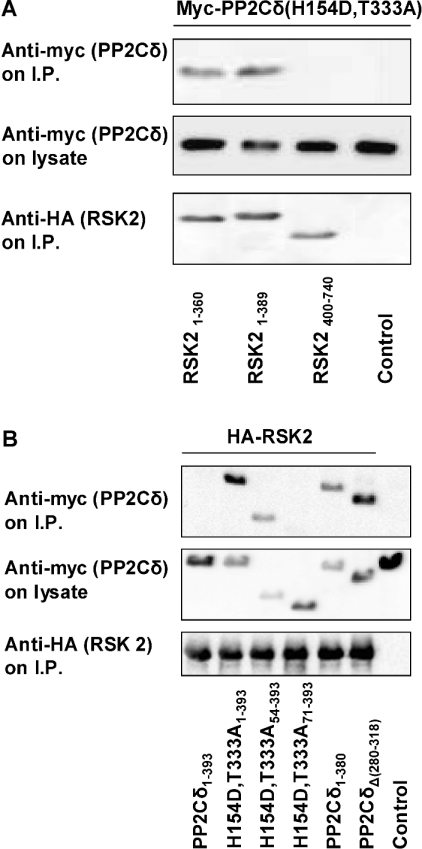
The region of RSK2 involved in association with PP2Cδ was investigated by co-expression and immunoprecipitation of RSK2 deletion mutants and PP2Cδ(H154D,T333A). The RSK2 mutants were composed of either NTK with increasing portions of the linker region or CTK without the linker. The HA-tagged RSK2 mutants were co-expressed with myc-tagged PP2Cδ mutant in COS-7 cells, immunoprecipitated and analysed by Western blotting. Figure 5(A) shows that the RSK2 deletion mutants containing NTK associated with PP2Cδ. In contrast, the RSK2 deletion mutant composed of CTK alone did not interact with PP2Cδ. In conclusion, these findings suggest that PP2Cδ interacts with the NTK of RSK2. The interaction was not mapped in detail due to instability and low expression of RSK2 NTK after further deletions in the kinase domain.
Figure 5. Association of RSK2 mutants with PP2Cδ.
(A) COS-7 cells were co-transfected with plasmids expressing myc–PP2Cδ(H154D,T333A) and truncated HA–RSK2 mutants. Transfection of myc–PP2Cδ(H154D,T333A) without HA–RSK was used as a control. HA–RSK2 was precipitated from the cell lysates with anti-HA antibody, washed five times and subjected to SDS/PAGE followed by immunoblotting with anti-myc or anti-HA antibody. The cellular expression level of PP2Cδ(H154D,T333A) was determined by immunoblotting of lysates with anti-myc antibody. (B) COS-7 cells were co-transfected with plasmids expressing truncated myc–PP2Cδ(H154D,T333A) and HA–RSK2. Transfection of myc–PP2Cδ(H154D,T333A) without HA–RSK2 was used as a control. HA–RSK2 was precipitated from the cell lysates with anti-HA antibody. The precipitates were washed five times and the samples were subjected to SDS/PAGE followed by immunoblotting with anti-myc or anti-HA antibody. The expression level of PP2Cδ in the cells was determined by immunoblotting of lysates with anti-myc antibody.
The N-terminal region of PP2Cδ is required for binding to RSK2
The region of PP2Cδ involved in the association with RSK2 was investigated using deletion mutants of PP2Cδ. Myc-tagged PP2Cδ mutants were co-expressed with HA–RSK2 in COS-7 cells, immunoprecipitated and immunoblotted. Figure 5(B) shows that deletion of 54 amino acids of the N-terminal region of the PP2Cδ(H154D,T333A) mutant decreased the association with RSK, whereas deletion of the entire N-terminal extension of 71 amino acids abolished the interaction with RSK. This suggested that the N-terminal residues 1–71 of PP2Cδ are involved in the association with RSK2. Furthermore, deletion of the last 13 amino acids in PP2Cδ increased the association with RSK2 almost to the same extent as PP2Cδ(H154D,T333A). Finally, an alternative splice variant of PP2Cδ, where exon 10 is missing within the catalytic domain, was discovered by database search. This splice variant PP2Cδ(Δ280–318) showed increased association with RSK2 similar to PP2Cδ(H154D,T333). These findings suggest that the association between RSK2 and PP2Cδ is complex and is influenced positively and negatively by several domains of PP2Cδ. These include the N-terminal residues 1–71, which promote the interaction with RSK2. The alternatively spliced domain 280–318 and the C-terminal 13 residues seem to inhibit the interaction with RSK.
PP2Cδ associates with kinases of the AGC family
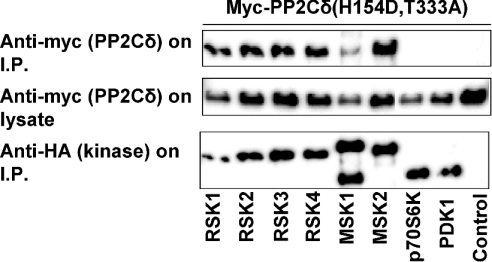
The specificity of the interaction between PP2Cδ and RSK2 was investigated by measuring the association of PP2Cδ with other members of the RSK subfamily of kinases, two RSK-related kinases, mitogen- and stress-activated kinases MSK1 and MSK2, and two other AGC kinases, p70S6K(αII) and PDK1. In these experiments, the double-mutant PP2Cδ(H154D,T333A) was used because of its strong association with RSK2. Myc-tagged PP2Cδ was co-expressed with HA-tagged protein kinases, immunoprecipitated and immunoblotted. Figure 6 shows that PP2Cδ is associated with all four members of the RSK subfamily and two members of the MSK subfamily, MSK1 and MSK2. In contrast, PP2Cδ was not associated with p70S6K(αII) and PDK1. These findings indicate that the association of PP2Cδ is specific for related kinases belonging to the AGC family of kinases.
Figure 6. Association between PP2Cδ and members of the RSK and MSK family of kinases.
COS-7 cells were co-transfected with plasmids expressing myc–tagged PP2Cδ (H154D,T333A) and HA-tagged protein kinases. Transfection of myc–PP2Cδ(H154D,T333A) without HA-tagged protein kinase was used as a control. After 48 h, the cells were lysed and the protein kinases were precipitated using anti-HA antibody. The samples were washed five times and subjected to SDS/PAGE followed by immunoblotting with anti-myc or anti-HA antibody. The expression level of PP2Cδ in the cells was determined by immunoblotting of lysates with anti-myc antibody.
DISCUSSION
RSK2 is a member of the superfamily of AGC kinases, which are characterized by a high sequence homology in their catalytic domain and mechanism of activation. However, the AGC kinases are distinct in their regulatory and interaction domains. RSK2 is activated by ERK and phosphorylated on several sites including a docking site in the linker domain for PDK1 that phosphorylates the activation loop of NTK [22,23,33]. In the present study, the dephosphorylation and deactivation of RSK2 is analysed for the first time. We show that PP2Cδ forms a complex with RSK2 in vivo and suggest that the complex formation may be regulated by the phosphorylation of PP2Cδ by ERK. Furthermore, PP2Cδ is probably involved in dephosphorylation of RSK2 on four sites, leading to decreased kinase activity.
The interaction between RSK2 and PP2Cδ is specific, since all four isoforms of RSK are capable of associating with PP2Cδ as well as the related MSK1 and MSK2, whereas P70S6K and PDK1 are not capable of forming a complex. Association between kinases and phosphatases has been described in several studies. These include the complexes between ERK and MKP-1 (MAPK phosphatase-1) [34], CDK1 (cyclin-dependent kinase 1), CDK2 and KAP (CDK-associated protein phosphatase) [35], ILK1 (integrin-linked kinase 1) and ILKAP, i.e. human PP2Cδ [31], and receptor serine/threonine kinase RLK5 (receptor-like kinase 5) and KAPP (kinase-associated protein phosphatase), i.e. PP2C from Arabidopsis thaliana [36].
The interaction of PP2Cδ with RSK2 involves several domains in PP2Cδ, including the N-terminal extension. The N-terminal tail is composed of 76 residues and is different from the other members of the PP2C family and probably confers specificity to the binding of substrates to PP2Cδ. This is analogous to PP2Cβ where a unique C-terminal tail is involved in the determination of substrate specificity [37].
The alternatively spliced domain 280–318 in the catalytic domain of PP2Cδ is also involved in the binding of RSK2. This is based on the observation that the association of RSK2 with the splice variant PP2Cδ(Δ280–318) is higher compared with full-length PP2Cδ. The crystal structure of PP2Cα has been solved [38] and we assume that the catalytic domain of PP2Cδ, which shows 32.3% identity with PP2Cα, has a similar structure. The alternatively spliced domain of PP2Cδ consists of a long loop and a β-sheet (β9) in the structure of the homologous PP2Cα. The splice variant of PP2Cδ(Δ280–318) was found in the human EST database. PP2Cδ(Δ280–318) has no phosphatase activity (results not shown) and we speculate that it may be functionally significant as a competitive inhibitor of the dephosphorylation of RSK2. The C-terminal portion 380–392 of the catalytic domain of PP2Cδ may also be involved in the binding of RSK2, since removal of this domain increases the association with RSK2. In PPC2α, this region corresponds to a β-sheet (β11) [38].
Furthermore, we have shown that RSK2 and PP2Cδ form a complex after PMA stimulation (Figures 1A and 1B). The interaction between RSK2 and PP2Cδ depends on two putative ERK phosphorylation sites in the catalytic domain of PP2Cδ (Figure 4). Mutation of Thr315 and Thr333 to alanine increases the interaction of PP2Cδ with RSK2. This may suggest a regulatory mechanism whereby PP2Cδ is recruited to the activated RSK2. The binding of RSK2 to PP2Cδ then induces a conformational change in the latter, allowing ERK to phosphorylate PP2Cδ, leading to dissociation of the RSK2–PP2Cδ complex. Indeed, mutation of Thr315 and Thr333 to glutamate reduced the binding of RSK2. Thr315 and Thr333 are located in two loops connecting β9 and β10 and α4 and α5 respectively in the structure of PP2Cα. These loops surround one of the invariant aspartic residues, Asp226, involved in the binding of Mn2+ to site 1 in the catalytic centre. It is presumed that phosphorylation of these two threonine residues may interfere with the binding of the substrate and the metal-catalysed dephosphorylation.
Alignment of the two ERK phosphorylation sites, Thr315 and Thr333, in PP2Cδ with other members of the PP2C family shows conservation of a phosphor-acceptor residue or an acidic residue, suggesting that the site is functionally significant regarding phosphorylation (Figure 3C). On the other hand, the adjacent proline residue is only found in PP2Cδ, suggesting that only proline-directed ERK phosphorylation plays a role in PP2Cδ. This stresses the functional relevance of PP2Cδ in the dephosphorylation of RSK and MSK, both of which are activated by ERK.
PP2Cδ forms a complex with the NTK of RSK2, but the sites in NTK involved in recognition of PP2Cδ have not been mapped in further detail. The crystal structure of a complex between kinase and phosphatase has been resolved for CDK2 and KAP [39]. The structure showed that the interaction involved two sites of CDK2: phospho-Thr160 in the activation loop, which projects into the catalytic site of KAP, and a second site comprising CDK2 residues 205–210 (GDSEID of the G α-helix) and 235–237 (DYK of the L14 loop).
Finally, dephosphorylation of RSK2 in vivo in EGF-stimulated cells correlates with decreased catalytic activity. We suggest that RSK2 is dephosphorylated by PP2Cδ in vivo, but we have not been able to demonstrate this. Unfortunately, we could not assay the kinase activity of RSK2 in vitro after dephosphorylation by PP2Cδ due to deactivation of RSK2 after 20–60 min of incubation at 30 °C. However, the marked dependence on Mn2+ when RSK2 is dephosphorylated in vitro indicates that PP2Cδ could be involved. It is characteristic of the PP2C family that the catalytic activity is dependent on either Mn2+ or Mg2+ and that PP2Cδ is stimulated only by Mn2+ and not by Mg2+ [26].
We did not attempt to address the functional role of dephosphorylation of RSK2 by PP2Cδ in vivo in cells. However, previous studies have suggested that PP2Cδ may be involved in the inhibition of cell growth. Indeed, cell cycle progression and DNA synthesis are blocked in cells overexpressing PP2Cδ [26]. RSK2 is required for growth factor-stimulated expression of c-Fos and transcriptional activation of Elk-1 and the serum response factor [40]. It is probable that the effect of PP2Cδ on cell growth is mediated by dephosphorylation and inactivation of RSK2. So far, PP2Cδ has only been shown to dephosphorylate ILK1 in mammals and RLK5 in A. thaliana [31,36].
Acknowledgments
We are indebted to M. Frödin for discussions and comments on this paper. This work was supported by grants from the Danish Medical Research Council, the Danish Research Ministry and the Danish Cancer Society.
References
- 1.Cohen P. The structure and regulation of protein phosphatases. Annu. Rev. Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 2.Hanks S. K., Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- 3.Frödin M., Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol. Cell. Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 4.Schouten G. J., Vertegaal A. C., Whiteside S. T., Israel A., Toebes M., Dorsman J. C., van der Eb A. J., Zantema A. κBα is a target for the mitogen-activated 90 kDa ribosomal S6 kinase. EMBO J. 1997;16:3133–3144. doi: 10.1093/emboj/16.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yntema H. G., van den H. B., Kissing J., van Duijnhoven G., Poppelaars F., Chelly J., Moraine C., Fryns J. P., Hamel B. C., Heilbronner H., et al. A novel ribosomal S6-kinase (RSK4; RPS6KA6) is commonly deleted in patients with complex X-linked mental retardation. Genomics. 1999;62:332–343. doi: 10.1006/geno.1999.6004. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y., Bjorbaek C., Weremowicz S., Morton C. C., Moller D. E. RSK3 encodes a novel pp90rsk isoform with a unique N-terminal sequence: growth factor-stimulated kinase function and nuclear translocation. Mol. Cell. Biol. 1995;15:4353–4363. doi: 10.1128/mcb.15.8.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xing J., Ginty D. D., Greenberg M. E. Coupling of the RAS–MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 8.Wang X., Li W., Williams M., Terada N., Alessi D. R., Proud C. G. Regulation of elongation factor 2 kinase by p90RSK1 and p70 S6 kinase. EMBO J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welsh G. I., Miller C. M., Loughlin A. J., Price N. T., Proud C. G. Regulation of eukaryotic initiation factor eIF2B: glycogen synthase kinase-3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Lett. 1998;421:125–130. doi: 10.1016/s0014-5793(97)01548-2. [DOI] [PubMed] [Google Scholar]
- 10.Bonni A., Brunet A., West A. E., Datta S. R., Takasu M. A., Greenberg M. E. Cell survival promoted by the Ras–MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 11.Shimamura A., Ballif B. A., Richards S. A., Blenis J. Rsk1 mediates a MEK-MAP kinase cell survival signal. Curr. Biol. 2000;10:127–135. doi: 10.1016/s0960-9822(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 12.Lizcano J. M., Morrice N., Cohen P. Regulation of BAD by cAMP-dependent protein kinase is mediated via phosphorylation of a novel site, Ser155. Biochem. J. 2000;349:547–557. doi: 10.1042/0264-6021:3490547. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Tan Y., Ruan H., Demeter M. R., Comb M. J. p90RSK blocks bad-mediated cell death via a protein kinase C-dependent pathway. J. Biol. Chem. 1999;274:34859–34867. doi: 10.1074/jbc.274.49.34859. [DOI] [PubMed] [Google Scholar]
- 14.Palmer A., Gavin A. C., Nebreda A. R. A link between MAP kinase and p34cdc2/cyclin B during oocyte maturation: p90rsk phosphorylates and inactivates the p34cdc2 inhibitory kinase Myt1. EMBO J. 1998;17:5037–5047. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dufresne S. D., Bjorbaek C., El Haschimi K., Zhao Y., Aschenbach W. G., Moller D. E., Goodyear L. J. Altered extracellular signal-regulated kinase signaling and glycogen metabolism in skeletal muscle from p90 ribosomal S6 kinase 2 knockout mice. Mol. Cell. Biol. 2001;21:81–87. doi: 10.1128/MCB.21.1.81-87.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trivier E., De Cesare D., Jacquot S., Pannetier S., Zackai E., Young I., Mandel J. L., Sassone-Corsi P., Hanauer A. Mutations in the kinase Rsk-2 associated with Coffin–Lowry syndrome. Nature (London) 1996;384:567–570. doi: 10.1038/384567a0. [DOI] [PubMed] [Google Scholar]
- 17.Dalby K. N., Morrice N., Caudwell F. B., Avruch J., Cohen P. Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J. Biol. Chem. 1998;273:1496–1505. doi: 10.1074/jbc.273.3.1496. [DOI] [PubMed] [Google Scholar]
- 18.Smith J. A., Poteet-Smith C. E., Malarkey K., Sturgill T. W. Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J. Biol. Chem. 1999;274:2893–2898. doi: 10.1074/jbc.274.5.2893. [DOI] [PubMed] [Google Scholar]
- 19.Gavin A. C., Nebreda A. R. A MAP kinase docking site is required for phosphorylation and activation of p90rsk/MAPKAP kinase-1. Curr. Biol. 1999;9:281–284. doi: 10.1016/s0960-9822(99)80120-1. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y., Bjorbaek C., Moller D. E. Regulation and interaction of pp90(rsk) isoforms with mitogen-activated protein kinases. J. Biol. Chem. 1996;271:29773–29779. doi: 10.1074/jbc.271.47.29773. [DOI] [PubMed] [Google Scholar]
- 21.Vik T. A., Ryder J. W. Identification of serine 380 as the major site of autophosphorylation of Xenopus pp90rsk. Biochem. Biophys. Res. Commun. 1997;235:398–402. doi: 10.1006/bbrc.1997.6794. [DOI] [PubMed] [Google Scholar]
- 22.Frödin M., Jensen C. J., Merienne K., Gammeltoft S. A phosphoserine-regulated docking site in the protein kinase RSK2 that recruits and activates PDK1. EMBO J. 2000;19:2924–2934. doi: 10.1093/emboj/19.12.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen C. J., Buch M. B., Krag T. O., Hemmings B. A., Gammeltoft S., Frödin M. 90-kDa ribosomal S6 kinase is phosphorylated and activated by 3-phosphoinositide-dependent protein kinase-1. J. Biol. Chem. 1999;274:27168–27176. doi: 10.1074/jbc.274.38.27168. [DOI] [PubMed] [Google Scholar]
- 24.Roux P. P., Richards S. A., Blenis J. Phosphorylation of p90 ribosomal S6 kinase (RSK) regulates extracellular signal-regulated kinase docking and RSK activity. Mol. Cell. Biol. 2003;23:4796–4804. doi: 10.1128/MCB.23.14.4796-4804.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barford D. Molecular mechanisms of the protein serine/threonine phosphatases. Trends Biochem. Sci. 1996;21:407–412. doi: 10.1016/s0968-0004(96)10060-8. [DOI] [PubMed] [Google Scholar]
- 26.Tong Y., Quirion R., Shen S. H. Cloning and characterization of a novel mammalian PP2C isozyme. J. Biol. Chem. 1998;273:35282–35290. doi: 10.1074/jbc.273.52.35282. [DOI] [PubMed] [Google Scholar]
- 27.Cohen P. T., Brewis N. D., Hughes V., Mann D. J. Protein serine/threonine phosphatases; an expanding family. FEBS Lett. 1990;268:355–359. doi: 10.1016/0014-5793(90)81285-v. [DOI] [PubMed] [Google Scholar]
- 28.Klumpp S., Selke D., Krieglstein J. Protein phosphatase type 2C dephosphorylates BAD. Neurochem. Int. 2003;42:555–560. doi: 10.1016/s0197-0186(02)00174-2. [DOI] [PubMed] [Google Scholar]
- 29.Ofek P., Ben Meir D., Kariv-Inbal Z., Oren M., Lavi S. Cell cycle regulation and p53 activation by protein phosphatase 2Cα. J. Biol. Chem. 2003;278:14299–14305. doi: 10.1074/jbc.M211699200. [DOI] [PubMed] [Google Scholar]
- 30.Hanada M., Kobayashi T., Ohnishi M., Ikeda S., Wang H., Katsura K., Yanagawa Y., Hiraga A., Kanamaru R., Tamura S. Selective suppression of stress-activated protein kinase pathway by protein phosphatase 2C in mammalian cells. FEBS Lett. 1998;437:172–176. doi: 10.1016/s0014-5793(98)01229-0. [DOI] [PubMed] [Google Scholar]
- 31.Leung-Hagesteijn C., Mahendra A., Naruszewicz I., Hannigan G. E. Modulation of integrin signal transduction by ILKAP, a protein phosphatase 2C associating with the integrin-linked kinase, ILK1. EMBO J. 2001;20:2160–2170. doi: 10.1093/emboj/20.9.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 33.Richards S. A., Dreisbach V. C., Murphy L. O., Blenis J. Characterization of regulatory events associated with membrane targeting of p90 ribosomal S6 kinase 1. Mol. Cell. Biol. 2001;21:7470–7480. doi: 10.1128/MCB.21.21.7470-7480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun H., Charles C. H., Lau L. F., Tonks N. K. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell (Cambridge, Mass.) 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 35.Hannon G. J., Casso D., Beach D. KAP: a dual specificity phosphatase that interacts with cyclin-dependent kinases. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1731–1735. doi: 10.1073/pnas.91.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stone J. M., Collinge M. A., Smith R. D., Horn M. A., Walker J. C. Interaction of a protein phosphatase with an Arabidopsis serine-threonine receptor kinase. Science. 1994;266:793–795. doi: 10.1126/science.7973632. [DOI] [PubMed] [Google Scholar]
- 37.Kusuda K., Kobayashi T., Ikeda S., Ohnishi M., Chida N., Yanagawa Y., Shineha R., Nishihira T., Satomi S., Hiraga A., et al. Mutational analysis of the domain structure of mouse protein phosphatase 2Cβ. Biochem. J. 1998;332:243–250. doi: 10.1042/bj3320243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das A. K., Helps N. R., Cohen P. T., Barford D. Crystal structure of the protein serine/threonine phosphatase 2C at 2.0 Å resolution. EMBO J. 1996;15:6798–6809. [PMC free article] [PubMed] [Google Scholar]
- 39.Song H., Hanlon N., Brown N. R., Noble M. E., Johnson L. N., Barford D. Phosphoprotein–protein interactions revealed by the crystal structure of kinase-associated phosphatase in complex with phosphoCDK2. Mol. Cell. 2001;7:615–626. doi: 10.1016/s1097-2765(01)00208-8. [DOI] [PubMed] [Google Scholar]
- 40.Bruning J. C., Gillette J. A., Zhao Y., Bjorbaek C., Kotzka J., Knebel B., Avci H., Hanstein B., Lingohr P., Moller D. E., et al. Ribosomal subunit kinase-2 is required for growth factor-stimulated transcription of the c-Fos gene. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2462–2467. doi: 10.1073/pnas.97.6.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]