Abstract
In familial amyloidotic polyneuropathy, TTR (transthyretin) variants are deposited as amyloid fibrils. It is thought that this process involves TTR tetramer dissociation, which leads to partially unfolded monomers that aggregate and polymerize into amyloid fibrils. This process can be counteracted by stabilization of the tetramer. Several small compounds, such as diclofenac, diflunisal and flufenamic acid, have been reported to bind to TTR in vitro, in the T4 (thyroxine) binding channel that runs through the TTR tetramer, and consequently are considered to stabilize TTR. However, if these agents bind plasma proteins other than TTR, decreased drug availability will occur, compromising their use as therapeutic agents for TTR amyloidosis. In the present work, we compared the action of these compounds and of new derivatives designed to increase both selectivity of binding to TTR and inhibitory potency in relation to TTR amyloid fibril formation. We found two diflunisal derivatives that, in contrast with diclofenac, flufenamic acid and diflunisal, displaced T4 from TTR in plasma preferentially over binding to albumin and thyroxine binding globulin. The same diflunisal derivatives also had a stabilizing effect on TTR tetramers in plasma, as studied by isoelectric focusing of whole plasma under semi-denaturing conditions. In addition, by transmission electron microscopy, we demonstrated that, in contrast with other proposed TTR stabilizers (namely diclofenac, flufenamic acid and diflunisal), one of the diflunisal derivatives tested efficiently inhibited TTR aggregation. Taken together, our ex vivo and in vitro studies present evidence for the selectivity and efficiency of novel diflunisal derivates as TTR stabilizers and as inhibitors of fibril formation.
Keywords: amyloid, diflunisal, neuropathy, stabilizer, thyroxine, transthyretin
Abbreviations: FAP, familial amyloidotic polyneuropathy; IEF, isoelectric focusing; NSAID, non-steroidal anti-inflammatory drug; T4, thyroxine; TBG, thyroxine binding globulin; TEM, transmission electron microscopy; TTR, transthyretin
INTRODUCTION
TTR (transthyretin) is a protein found in the plasma and cerebrospinal fluid that is synthesized mainly by the liver and the choroid plexus of the brain. The protein functions as a transport protein carrying thyroid hormones, namely T4 (thyroxine), as well as retinol through its interaction with retinol binding protein [1]. More than 80 TTR variants have been described to date, most of which are associated with different forms of hereditary amyloidosis [2]. In these diseases, there is a misfolding of the TTR molecule that leads to its aggregation and deposition as amyloid. The deposition occurs systemically, but in many cases there is a predominant involvement of the peripheral nervous system, as occurs in FAP (familial amyloidotic polyneuropathy), the most common form of TTR amyloidosis [3]. The most frequent TTR variant implicated in FAP presents the substitution of valine for methionine at position 30 of the polypeptide chain (TTR V30M). Among the TTR variants described, around 10 are classified as non-pathogenic, such as TTR T119M. This mutation has been detected in heterozygotic carriers and in compound heterozygotic individuals, i.e. carriers of both TTR T119M and TTR V30M. In the latter case, TTR T119M is protective against the effects of TTR V30M in the clinical phenotype [4,5].
The native TTR molecule is a homotetramer, formed by two dimers. TTR possesses a high degree of β-pleated sheet structure [6]. X-ray crystallography reveals that each dimer results from the association of two monomers extending two β-sheets composed of four β-strands, from each monomer, into two β-sheets of eight β-strands. The dimer–dimer interface of the tetramer forms a central hydrophobic channel that has two symmetry-related T4 binding sites presenting negative binding co-operativity [6].
Several investigators have proposed that the amyloidogenic potential of the TTR variants is related to a decrease in the tetrameric stability of the protein [7]. For example, Altland and Winter [8] demonstrated, by a combination of PAGE and IEF (isoelectric focusing) under mild denaturation conditions, that amyloidogenic TTR tetramers had a greater tendency to dissociate compared with their wild-type counterparts. These authors also showed that sulphite exerts a stabilizing effect on the TTR tetramer, increasing the tetramer/monomer ratio.
A recent study, investigating the dynamics of the aggregation of TTR variants into amyloid fibrils, characterized different molecular species in TTR fibrillogenesis that point to a model involving several steps, namely dissociation of the tetramer and polymerization of the resulting modified monomers into non-fibrillar oligomers, leading to protofibrils that elongate further into mature fibrils [9]. According to this proposed mechanism, stabilization of the tetramer would be a fundamental step for the inhibition of amyloid fibril formation. Several small compounds sharing molecular structural similarities with T4 are candidate ligands for the T4 binding sites, and have been proposed as inhibitors of fibril formation in vitro [10,11].
In the present work we investigate, both in vitro and ex vivo: (i) the TTR binding affinity and binding selectivity in plasma of some previously reported fibril inhibitors, namely diflunisal, diclofenac and flufenamic acid, and some new derivatives designed to show increased binding potencies compared with these well known NSAIDs (non-steroidal anti-inflammatory drugs); and (ii) the stabilization effects of the new and reference compounds with regard to the tetrameric structure of TTR.
EXPERIMENTAL
Plasma samples
Whole blood from heterozygotic and homozygotic carriers of TTR V30M, from heterozygotic carriers of TTR T119M, from compound heterozygotic carriers of both TTR V30M and TTR T119M, and from control individuals, was collected in the presence of EDTA and centrifuged. The plasma was separated and kept frozen at −20 °C. The same procedure was used to obtain plasma from TTR knockout mice carrying the human TTR V30M gene [12].
Recombinant proteins
Recombinant wild-type TTR and the TTR variants TTR V30M, TTR T119M and TTR Y78F were produced synthetically in a bacterial expression system using Escherichia coli BL21 [13]. Plasma and recombinant TTRs were isolated according to a previously described protocol [14]. The purity of the proteins was assessed by SDS/PAGE and their concentration determined using the Bio-Rad protein assay (Bio-Rad, Munich, Germany).
Chemical compounds studied for interaction with TTR
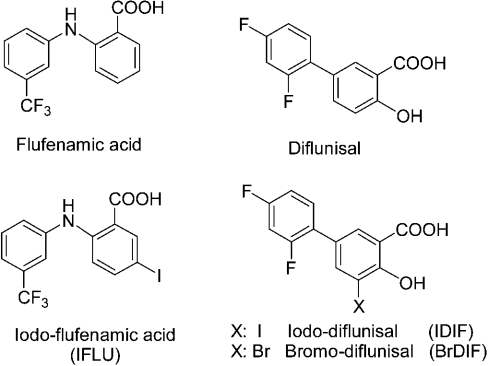
Diflunisal, diclofenac and flufenamic acid were from Sigma. The diflunisal derivatives iododiflunisal (Mr 376.1) and bromodiflunisal (Mr 329.09), and the flufenamic acid derivative iodoflufenamic acid (Mr 407.13) (Figure 1), were synthesized as part of a screening programme for TTR polymerization inhibitors carried out at IIQAB-CSIC, Barcelona, and at the University of Oviedo. After synthesis, the compounds were purified by HPLC (99.9% purity) and characterized fully by NMR, MS and elemental analysis. The synthetic procedure will be reported elsewhere; details are available from G. Valencia on request. The compounds were weighed and dissolved at approx. 10 mg/ml in DMSO.
Figure 1. Chemical structures of the compounds used.
T4 binding assays
Qualitative studies of the displacement of T4 from plasma TTR were carried out by incubation of samples of whole plasma (5 μl) with [125I]T4 (specific radioactivity 1250 μCi/μg; concentration 320 μCi/ml; Perkin Elmer, Boston, MA, U.S.A.) in the presence of different compounds (1 μl of a 10 mM solution), followed by separation of the serum T4 binding proteins by PAGE in a glycine/acetate buffer system as described previously [15]. The gel was dried and subjected to phosphor imaging (Typhoon 8600; Molecular Diagnostics, Amersham Biosciences) and the bands, from four gels, were quantified using the program ImageQuant version 5.1.
Competition of the various compounds with T4 for binding to TTR was assayed quantitatively by a gel filtration procedure as described previously [14,16]. Briefly, a solution of 30 nM TTR in 0.1 M Tris, 0.1 M NaCl and 0.001 M EDTA buffer, pH 8.0, was incubated with a trace amount of [125I]T4 plus increasing concentrations of inhibitor (0–10 μM) overnight at 4 °C. [125I]T4 bound to TTR was separated from unbound T4 by gel filtration chromatography through a column of Bio-Gel P6-DG (Bio-Rad, Hercules, CA, U.S.A.). Non-specific binding was determined using a 100 μM concentration of unlabelled T4. All samples were run in duplicate. The data were analysed using GraphPad Prism 2.0. Percentage binding was plotted against the logarithm of the inhibitor concentration, and the EC50 was determined and compared with the EC50 for T4. The EC50 T4/EC50 competitor ratio reflects the relative inhibition potency.
TTR tetrameric stability assays by IEF under semi-denaturing conditions
To perform this assay, 30 μl of plasma was incubated at 37 °C for 1 h with 5 μl of a 10 mM solution of the compound to be tested. For treatment with iododiflunisal, 10 different plasma samples from TTR V30M carriers and controls were tested. The preparations were then subjected to native PAGE and the gel bands containing TTR were excised and applied to an IEF gel. IEF was carried out in the presence of 4 M urea, containing 5% ampholytes, pH 4–6.5, at 1200 V for 6 h, essentially as described previously [4,17]. These semi-denaturing conditions allow the visualization of bands corresponding to the TTR monomer and tetramer, and also to an oxidized form of the monomer. Proteins were fixed with 20% (v/v) trichloroacetic acid and stained with Coomassie Blue, and the dried gel was scanned (hp Scanjet 4470c; Hewlett Packard) and subjected to densitometry (program UN-SCAN-IT gel version 5.1).
Study of TTR aggregation by TEM (transmission electron microscopy)
Soluble TTR Y78F at 2 mg/ml was dialysed against PBS and incubated at 37 °C in the absence or presence of diflunisal, the diflunisal derivative iododiflunisal or diclofenac at a molar ratio of 1:100. Control samples were incubated with DMSO. The protein concentration was determined by the Lowry method [18]. After 48 h, samples were analysed by TEM.
For visualization by TEM, sample aliquots were diluted (1:50, v/v) in PBS and negatively stained with 1% uranyl acetate. The grids were visualized with a Zeiss microscope (model EM10C), operated at 60 kV.
RESULTS
T4 competition studies
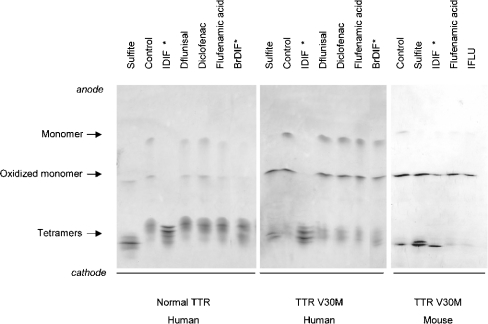
Our first approach to investigating the selectivity of TTR stabilizers was to determine their capacity to compete with T4 for protein binding in plasma. The reference compounds tested were selected on the basis of previous reports of their ability to bind to TTR and inhibit fibril formation, and these were used as parent compounds for the synthesis of new derivatives that may show improved potency of binding to TTR. In particular, we tested diflunisal, flufenamic acid and diclofenac as reference compounds. We also assayed two new iodo derivatives (iododiflunisal and iodoflufenamic acid) and a bromo derivative (bromodiflunisal) (chemical structures are presented in Figure 1). Since sulphite has been described as a stabilizing agent for tetrameric TTR, we also included a sample treated with sulphite. The results obtained for control plasma are shown in Figure 2. In the absence of any drugs, three main proteins bound T4, as shown in the control lane: the main binding protein was TBG (T4 binding globulin), followed by albumin and TTR. Weaker TTR bands were observed in the serum samples incubated with the drugs (except that incubated with sulphite) compared with the TTR band from control serum. This indicates that all compounds tested mediated some inhibition of the binding of T4 to TTR. Iodoflufenamic acid, a derivative of flufenamic acid, particularly inhibited binding of T4 to TBG. Phosphor imaging analysis of the gel demonstrated that the weakest inhibitors of T4 binding to TTR were flufenamic acid and diclofenac, which presented 2–4% inhibition. The most specific compounds to compete with T4 for the binding to TTR were diflunisal and its bromo derivative bromodiflunisal, with inhibition of T4 binding of 70% and 75% respectively; the most potent competitor was found to be the iodo derivative, iododiflunisal, which inhibited 80–100% of T4 binding to TTR. A similar pattern of competition was obtained when plasma samples from carriers of different TTR variants (TTR V30M and TTR T119M) were analysed (results not shown).
Figure 2. T4 binding gel electrophoresis of normal plasma incubated with [125I]T4 and with various compounds as competitors.
The migration of different plasma T4 binding proteins is indicated. The diflunisal derivatives are indicated with an asterisk (*). IDIF, iododiflunisal; BrDIF, bromodiflunisal; IFLU, iododiflufenamic acid. This gel is representative of other gels run in parallel.
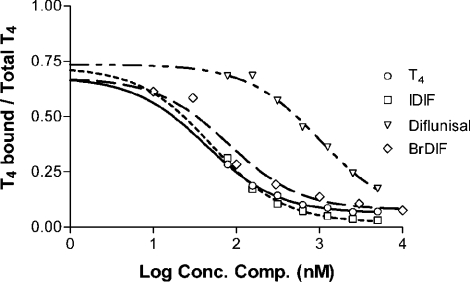
These results allowed us to select compounds to be tested subsequently for their potency in binding to TTR at binding sites for T4. Competition binding studies were then performed for determination of the EC50 for each compound. The relative potency for the inhibition of binding of T4 was defined as the ratio of EC50 T4/EC50 competitor. Figure 3 shows the competition curves obtained for recombinant wild-type TTR (EC50 T4 35.4±8.6 nM). Similar results were obtained for protein isolated from normal serum (EC50 T4 16.8±3.3 nM) (Table 1). The results demonstrate that iododiflunisal, a derivative of diflunisal, was the most potent inhibitor of T4 binding to TTR, with a relative inhibition potency of 0.85; the other diflunisal derivative tested, bromodiflunisal, was also a potent inhibitor of T4 binding, with a relative inhibition potency of 0.53; both derivatives presented a TTR binding affinity similar to that of T4. Flufenamic acid and its derivative displayed similar potencies for binding to TTR, albeit lower than that of iododiflunisal. Diclofenac and diflunisal were the least potent competitors.
Figure 3. Displacement of T4 from wild-type recombinant TTR by diflunisal derivatives.
The curves were obtained using the various compounds indicated as competitive inhibitors. IDIF, iododiflunisal; BrDIF, bromodiflunisal.
Table 1. Relative potency of drug inhibition of T4 binding to wild-type recombinant TTR and to normal TTR isolated from plasma.
| EC50 T4/EC50 competitor | ||
|---|---|---|
| Competitor | Recombinant wild-type TTR | Normal serum TTR |
| T4 | 1 | 1 |
| Iododiflunisal | 0.85 | 0.62 |
| Bromodiflunisal | 0.53 | 0.11 |
| Flufenamic acid | 0.22 | − |
| Iododiflufenamic acid | 0.15 | 0.003 |
| Difluinsal | 0.04 | 0.03 |
| Diclofenac | − | 0.006 |
From the results obtained, we concluded that the diflunisal derivative iododiflunisal was the most interesting and promising compound in terms of the specific displacement of T4 from tetrameric TTR. We next compared this compound with T4 for binding to TTR proteins isolated from the plasma of carriers of different TTR variants, namely heterozygotic TTR V30M, heterozygotic TTR T119M and compound heterozygotic carriers of TTR V30M and TTR T119M. In all cases, the competition curves obtained for iododiflunisal were very similar to the curves obtained for unlabelled T4 (results not shown); the EC50 value for iododiflunisal was lower than that of T4, demonstrating a slightly higher affinity of iododiflunisal for each variant. In addition, like T4, iododiflunisal bound to TTR from TTR V30M carriers with lower affinity than to TTR from control individuals, and with higher affinity to TTR from carriers of TTR T119M. Therefore the TTR binding properties of iododiflunisal and T4 are very similar.
Effects on TTR stability
The effects of the various compounds on TTR stability were investigated by IEF in the presence of 4 M urea. Plasma samples from carriers of different TTR variants (heterozygotic and homozygotic carriers of TTR V30M; heterozygotic carriers of TTR T119M) and from controls were incubated with each of the compounds and, after IEF, the band patterns and tetramer/monomer ratios were compared. The results obtained with plasma from control subjects and from heterozygotic carriers of TTR V30M incubated with selected compounds are shown in Figure 4 (left and middle panels respectively). In parallel, plasma was incubated with sulphite as a reference for the stabilization effect. The incubation with sulphite also results in changes in the oxidation state of the protein, which would explain the small differences in the migration of the tetramer bands. Under the conditions used, TTR presented a characteristic pattern of two main bands, representing monomers (normal and oxidized forms), and several bands of lower pI, representing different forms of tetramers [17,19]. Under the conditions of the assay and in the absence of drugs, the proportion of tetramer was found to be higher in control plasma (75%) than in plasma from the heterozygotic TTR V30M carrier (45%), as determined by densitometry analysis of the gel. The results obtained for normal plasma incubated with the various compounds demonstrated that in all cases there was a slight increase of the percentage of tetramer in the presence of diclofenac (5% increase), whereas sulphite, diflunisal and flufenamic acid had stronger stabilizing effects (15% increase). However, when plasma was incubated with iododiflunisal or bromodiflunisal, only the tetrameric form of the protein was detected. The effect was even more evident with plasma from TTR V30M carriers, where the monomeric band disappeared completely in the plasma incubated with iododiflunisal. A slight increase in the proportion of tetramer (15% increase) was observed for the plasma from the heterozygotic carrier of TTR V30M incubated with sulphite, diflunisal or diclofenac. In contrast, flufenamic acid did not increase the percentage of tetramer in the TTR V30M sample. An effect of bromodiflunisal was not evident for this sample. Therefore the results indicated that the diflunisal derivatives tested (labelled with asterisks in Figure 4), in particular iododiflunisal, had the most pronounced effects on stabilization of the TTR tetramer. We performed a similar analysis with plasma from TTR knockout mice carrying the human gene for TTR V30M [12]. The stabilization effect of iododiflunisal was again evident (Figure 4, right panel), in contrast with flufenamic acid and its derivative, which did not affect tetrameric stability.
Figure 4. Analysis of plasma TTR by IEF in the presence of 4 M urea.
Plasma samples from a control individual, from a heterozygotic carrier of TTR V30M and from knockout mice expressing TTR V30M were incubated with various compounds. TTR was then separated by PAGE and the isolated protein was analysed by IEF. The different molecular species visualized in the IEF gel after Coomassie Blue staining are indicated. Note the stronger bands corresponding to tetramers and the absence of monomer band in samples treated with iododiflunisal (marked with an asterisk), which parallels the effect of sulphite. IDIF, iododiflunisal; BrDIF, bromodiflunisal; IFLU, iododiflufenamic acid. These gels are representative of other gels run in parallel.
Effects on TTR aggregation
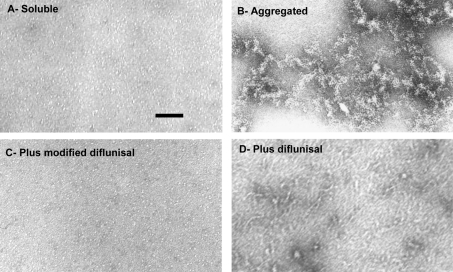
The effects of diflunisal and iododiflunisal on the aggregation of TTR Y78F were compared. This variant is very prone to fibril formation [20] and its aggregation pathway, under physiological conditions (PBS, pH 7.4, 37 °C), has been characterized morphologically by TEM (I. Cardoso and M. J. Saraiva, unpublished work). Incubation of soluble TTR Y78F at 37 °C for 48 h led to substantial aggregate formation (Figure 5B). If the period of incubation was increased to 15 days, fibril formation could be visualized (results not shown). When the protein was pre-incubated with iododiflunisal, no aggregates were visible (Figure 5C), and only round particles resembling native TTR were observed. In contrast, pre-incubation with diflunisal (Figure 5D) did not prevent the formation of very large aggregates after 48 h, indicating that this drug did not inhibit the aggregation of TTR Y78F. Similarly to diflunisal, diclofenac (tested in a parallel experiment; results not shown) did not inhibit TTR aggregation.
Figure 5. Aggregation of TTR Y78F observed by TEM.
(A) The initial preparation contains round particles resembling native TTR WT. (B) After 48 h at 37 °C, the protein aggregates and forms oligomers of different sizes. (C) Co-incubation with iododiflunisal for 48 h at 37 °C inhibits TTR Y78F aggregation and results in structures similar to those observed in (A), whereas (D) co-incubation with diflunisal does not prevent aggregation, and large aggregates are clearly visible. Scale bar=100 nm.
DISCUSSION
Various different proteins are involved in distinct forms of amyloidosis. Despite the structural dissimilarities among the various amyloidogenic precursor proteins, it has been proposed that in each case native soluble proteins undergo partial unfolding, leading to an amyloidogenic structure more prone to amyloid fibril formation [21]. In particular, in the case of TTR amyloidosis, the TTR tetramer dissociates into a non-native monomer (partially unfolded) that has a tendency to form soluble aggregates that can, in turn, seed amyloid fibril formation [22]. The conditions leading to unfolding may be environmental, and thus related to factors such as temperature, ionic strength, pH, oxidation potential or others, or intrinsic to the protein, i.e. due to alterations in its primary structure as a consequence of amino acid substitutions. In TTR amyloidoses, both conditions might apply. While in systemic senile amyloidosis [23] normal TTR is deposited as amyloid, reflecting extrinsic causes of protein fibril formation, in most cases of FAP, mutations are associated with the deposition of TTR as amyloid fibrils [3]. The amino acid substitution in each TTR variant determines structural alterations in the tetramer, as demonstrated by X-ray diffraction analysis of the crystallized mutant proteins. The most striking case is that of TTR L55P, a highly amyloidogenic TTR variant [24]. Analysis of the crystal structure of this variant revealed significant alterations in the regions of dimer–dimer contacts, in accordance with the decreased stability of the tetramer and consequently with its high amylo-idogenicity. Although in most cases the structural alterations induced by amino acid substitutions are not so evident, they still might be sufficient to influence TTR tetramer stability and/or interfere with the binding properties of TTR. On the other hand, there are variants that are non-amyloidogenic and non-pathogenic, such as TTR T119M. In this case, X-ray analysis of the TTR T119M–T4 complex demonstrated that this variant presents alterations in the T4 binding channel, including dimer–dimer contacts. When compared with the wild-type protein or with amyloidogenic TTR V30M, the TTR M119T variant shows novel atomic interactions involving hydrogen bonds occurring within monomers, dimers and the tetramer [25]. These additional interactions explain the greater stability of the variant and its protective effect against the V30M mutation in compound heterozygotic carriers of TTR V30M and TTR T119M [4,5]. A recent study comparing the misfolding energetics of TTR T119M and TTR V30M stressed the importance of stabilization of the TTR tetramer in the process of amyloid fibril formation [26]. Therefore one of the most likely therapeutic approaches to the disease is to stabilize the TTR protein, preventing its partial unfolding that triggers amyloid fibril formation.
Several compounds have been reported to inhibit amyloid fibril formation in vitro, as is the case for some NSAIDs and some analogues [10,11,27,28]. For TTR amyloidoses it has been proposed that these compounds exert their effect through binding to T4 binding sites in TTR, as demonstrated by the crystal structures of some of the complexes, in particular the TTR–diclofenac complex [10,29] and more recently the TTR–diflunisal complex [28]. Other approaches have been used to assess drug–TTR complexes, including MS and isothermal calorimetry [27,30]. Most of these assays have been performed using isolated recombinant TTR in vitro, in the absence of physiological modulating factors, namely other binding proteins. Characterization of the interactions of the different compounds with TTR with regard to specificity and selectivity, to confirm their preferential binding to TTR over other binding proteins in plasma, in particular the highly abundant albumin and the most potent T4 binding protein in human serum, TBG, is of paramount importance. Indeed, more recently, several studies have reported evaluation of the selectivity of inhibitor binding to TTR in plasma by immunoprecipitation of the TTR–inhibitor complexes and determination, by HPLC analysis, of the TTR fraction occupied [27,31]. However, immunoprecipitation assays focus only on the protein precipitated from treated plasma, and not the whole untreated sample. Binding by proteins other than TTR may lead to decreased availability of the drugs in plasma and hence compromise their use as therapeutic agents for TTR amyloidosis. We thus compared the effects of existing drugs and novel derivatives in ex vivo assays employing whole plasma to assess their binding to TTR at T4 binding sites and also their effects on tetrameric stability.
Our qualitative and quantitative studies revealed that the two diflunisal derivatives, iododiflunisal and bromodiflunisal, showed high specificity and high affinity for binding to TTR, as demonstrated by the T4 binding protein profile obtained after electrophoresis of plasma. Both iododiflunisal and bromodiflunisal displaced T4 preferentially from TTR. The affinity of these derivatives for TTR was also determined by in vitro competition with T4 for binding to isolated recombinant TTR, confirming the ex vivo results.
Since we did not find that diclofenac had a high binding affinity for TTR, as compared with that of T4, the formation of a TTR–diclofenac complex might be due to the specific conditions of crystallization, and does not seem to be physiologically relevant. The effect of the conditions of the assay, in particular with regard to crystallization of the complex, has been also raised recently in a study where the binding of small molecules to TTR was characterized by MS [30].
Iododiflunisal and bromodiflunisal not only showed selective, high-affinity binding to TTR, but also efficiently stabilized TTR from dissociating into monomers in plasma from heterozygotic TTR V30M carriers and from control subjects. This was demonstrated by quantifying the resistance of plasma TTR to dissociation, after incubation with the compounds, in IEF experiments [8]. Similar ex vivo results were obtained using plasma from TTR V30M transgenic mice. In this ex vivo assay, diclofenac and flufenamic acid, reported previously to be inhibitors of TTR fibril formation, did not seem to stabilize the TTR tetramer effectively.
When we investigated the potency of our selected stabilizers as inhibitors of amyloid fibril formation by TEM, the inhibitory effect was very evident; in these studies we used TTR Y78F, a mutant protein with a high tendency to form amyloid fibrils. This mutant TTR has revealed epitopes similar to those present in fibrils, as assessed using monoclonal antibodies specific for TTR amyloid fibrils, suggestive of the structure of an early intermediate in the fibrillogenesis pathway [20]. Iododiflunisal inhibited TTR aggregation, as demonstrated by TEM, contrary to what was found for the protein incubated with diflunisal (no inhibition). Under the assay conditions, iododiflunisal prevented the formation of TTR oligomers, considered to be one of the first steps of TTR fibrinillogenesis in vitro [9]. Given that this is a multi-step process, the possibility cannot be excluded that diflunisal or other compounds may act on different stages of fibril formation.
Taken together, our ex vivo and in vitro studies present evidence for the selectivity and efficiency of novel diflunisal derivatives as TTR stabilizers and inhibitors of fibril formation. The criteria of (i) ex vivo selectivity of binding to TTR at T4 binding sites, (ii) ex vivo TTR tetrameric stabilization, and (iii) definition of the inhibitory step of fibrillogenesis, must be taken into consideration in the further testing of drugs with therapeutic interest for the treatment of TTR amyloidosis.
The evaluation of iododiflunisal as a TTR stabilizer will proceed with animal studies. Initial testing of the effect of iododiflunisal on TTR from mouse plasma ex vivo showed a stabilization effect similar to that observed for TTR from human plasma. This is highly encouraging for the pursuit of in vivo studies using selected diflunisal derivatives and for applying the same methodology to screening for stabilizers of tetrameric TTR as potential therapeutics for TTR amyloidoses.
Acknowledgments
We acknowledge excellent technical assistance from Paul Leite Moreira and Isabel Dantas. B.M. and I.C. are supported by fellowships from Fundação para a Ciência e Tecnologia (FCT). The work has been supported by grants from FCT and Fundação Calouste Gulbenkian (Portugal), and a grant from Fundacion LA CAIXA (Spain).
References
- 1.Herbert J., Wilcox J. N., Pham K. T., Fremeau R. T., Jr, Zeviani M., Dwork A., Soprano D. R., Makover A., Goodman D. S., Zimmerman E. A., et al. Transthyretin: a choroid plexus-specific transport protein in human brain. Neurology. 1986;36:900–911. doi: 10.1212/wnl.36.7.900. [DOI] [PubMed] [Google Scholar]
- 2.Saraiva M. J. Sporadic cases of hereditary systemic amyloidosis. N. Engl. J. Med. 2002;346:1818–1819. doi: 10.1056/NEJM200206063462312. [DOI] [PubMed] [Google Scholar]
- 3.Saraiva M. J., Costa P. P., Birken S., Goodman D. S. Presence of an abnormal transthyretin (prealbumin) in Portuguese patients with familial amyloidotic polyneuropathy. Trans. Assoc. Am. Physicians. 1983;96:261–270. [PubMed] [Google Scholar]
- 4.Alves I. L., Hayes M. T., Saraiva M. J. Comparative stability and clearance of [Met30]transthyretin and [Met119]transthyretin. Eur. J. Biochem. 1997;249:662–668. doi: 10.1111/j.1432-1033.1997.00662.x. [DOI] [PubMed] [Google Scholar]
- 5.Coelho T., Carvalho M., Saraiva M. J., Alves I., Almeida M. R., Costa P. P. A strikingly benign evolution of FAP in an individual compound heterozygote for two TTR mutations: TTR Met 30 and TTR Met 119. J. Rheumatol. 1993;20:179. [Google Scholar]
- 6.Blake C. C. F., Geisow M. J., Oatley S. J., Rerat B., Rerat C. Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 Å. J. Mol. Biol. 1978;121:339–356. doi: 10.1016/0022-2836(78)90368-6. [DOI] [PubMed] [Google Scholar]
- 7.Quintas A., Saraiva M. J. M., Brito R. M. M. The amyloidogenic potential of transthyretin variants correlates with their tendency to aggregate in solution. FEBS Lett. 1997;418:297–300. doi: 10.1016/s0014-5793(97)01398-7. [DOI] [PubMed] [Google Scholar]
- 8.Altland K., Winter P. Potential treatment of transthyretin-type amyloidoses by sulfite. Neurogenetics. 1999;2:183–188. doi: 10.1007/s100480050081. [DOI] [PubMed] [Google Scholar]
- 9.Cardoso I., Goldsbury S. A., Muller V., Olivieri S., Wirtz S., Damas A. M., Aebi U., Saraiva M. J. Transthyretin fibrillogenesis entails the assembly of monomers: a molecular model for in vitro assembled transthyretin amyloid-like fibrils. J. Mol. Biol. 2002;317:683–695. doi: 10.1006/jmbi.2002.5441. [DOI] [PubMed] [Google Scholar]
- 10.Baures P. W., Peterson S. A., Kelly J. W. Discovering transthyretin amyloid fibril inhibitors by limited screening. Bioorg. Med. Chem. 1998;6:1389–1401. doi: 10.1016/s0968-0896(98)00130-8. [DOI] [PubMed] [Google Scholar]
- 11.Oza V. B., Smith C., Raman P., Kelly J. Synthesis, structure, and activity of diclofenac analogues as transthyretin amyloid fibril formation inhibitors. J. Med. Chem. 2002;45:321–332. doi: 10.1021/jm010257n. [DOI] [PubMed] [Google Scholar]
- 12.Kohno K., Palha J., Miyakawa K., Saraiva M. J., Ito S., Mabuchi T., Blaner W., Ijima H., Tsukahara S., Episkopou V., et al. Analysis of amyloid deposition in a transgenic mouse model of homozygous familial amyloidotic polyneuropathy. Am. J. Pathol. 1997;150:1497–1508. [PMC free article] [PubMed] [Google Scholar]
- 13.Furuya H., Saraiva M. J. M., Gawinowicz M. A., Alves I. L., Costa P. P., Sasaki H., Goto I., Sakaki Y. Production of recombinant human transthyretin with biological activities toward the understanding of the molecular basis of familial amyloidotic polyneuropathy (FAP) Biochemistry. 1991;30:2415–2421. doi: 10.1021/bi00223a017. [DOI] [PubMed] [Google Scholar]
- 14.Almeida M. R., Damas A. M., Lans M. C., Brouwer A., Saraiva M. J. Thyroxine binding to transthyretin Met 119. Comparative studies of different heterozygotic carriers and structural analysis. Endocrine. 1997;6:309–315. doi: 10.1007/BF02820508. [DOI] [PubMed] [Google Scholar]
- 15.Saraiva M. J. M., Costa P. P., Goodman D. S. Transthyretin (prealbumin) in familial amyloidotic polyneuropathy: genetic and functional aspects. Adv Neurol. 1988;48:189–200. [PubMed] [Google Scholar]
- 16.Lans M. C., Klasson-Wehler E., Willemsen M., Meussen E., Safe S., Brouwer A. Structure-dependent, competitive interaction of hydroxy-polychlorobiphenyls, o-dibenzo-p-dioxins and o-dibenzofurans with human transthyretin. Chem. Biol. Interact. 1993;88:7–21. doi: 10.1016/0009-2797(93)90081-9. [DOI] [PubMed] [Google Scholar]
- 17.Altland K., Rauh S., Hacker R. Demonstration of human prealbumin by double one-dimensional slab gel electrophoresis. Electrophoresis. 1981;2:148–155. [Google Scholar]
- 18.Lowry O. H., Rosebrough N. J., Farr A. L., Randall J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Altland K., Winter P., Sauerborn M. K. Electrically neutral microheterogeneity of human plasma transthyretin (prealbumin) detected by isoelectric focusing in urea gradients. Electrophoresis. 1999;20:1349–1364. doi: 10.1002/(SICI)1522-2683(19990601)20:7<1349::AID-ELPS1349>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Redondo C., Damas A. M., Olofsson A., Lundgren E., Saraiva M. J. Search for intermediate structures in transthyretin fibrillogenesis: soluble tetrameric Tyr78Phe TTR expresses a specific epitope present only in amyloid fibrils. J. Mol. Biol. 2000;304:461–470. doi: 10.1006/jmbi.2000.4220. [DOI] [PubMed] [Google Scholar]
- 21.Kelly J. W., Lansbury P. T. A chemical approach to elucidate the mechanism of transthyretin and protein β-amyloid fibril formation. Amyloid Int. J. Exp. Clin. Invest. 1994;1:186–205. [Google Scholar]
- 22.Quintas A., Saraiva M. J. M., Brito R. M. M. The tetrameric protein transthyretin dissociates to a non-native monomer in solution. A novel model for amyloidogenesis. J. Biol. Chem. 1999;274:32943–32949. doi: 10.1074/jbc.274.46.32943. [DOI] [PubMed] [Google Scholar]
- 23.Westermark P., Sletten K., Johansson B., Cornwell G. G., III Fibril in senile systemic amyloidosis is derived from normal transthyretin. Proc. Natl. Acad. Sci. U.S.A. 1990;87:2843–2845. doi: 10.1073/pnas.87.7.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sebastião M. P., Saraiva M. J. M., Damas A. M. The crystal structure of amyloidogenic Leu55→Pro transthyretin variant reveals a possible pathway for transthyretin polymerization into amyloid fibrils. J. Biol. Chem. 1998;273:24715–24722. doi: 10.1074/jbc.273.38.24715. [DOI] [PubMed] [Google Scholar]
- 25.Sebastião M. P., Lamzim V., Saraiva M. J., Damas A. M. Transthyretin stability as a key factor in amyloidogenesis: X-ray analysis at atomic resolution. J. Mol. Biol. 2001;306:733–744. doi: 10.1006/jmbi.2000.4415. [DOI] [PubMed] [Google Scholar]
- 26.Hammarström P., Wiseman R. L., Powers E. T., Kelly J. W. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science. 2003;299:713–716. doi: 10.1126/science.1079589. [DOI] [PubMed] [Google Scholar]
- 27.Razavi H., Palaninathan S. K., Powers E. T., Wiseman R., Purkey H. E., Mohamedmohaideen N. N., Deechongkit S., Chiang K. P., Dendle M. T. A., Sachettini J. C., Kelly J. W. Benzoaxazoles as transthyretin amyloid fibril inhibitors: synthesis, evaluation and mechanism of action. Angew. Chem. Int. Ed. 2003;42:2758–2761. doi: 10.1002/anie.200351179. [DOI] [PubMed] [Google Scholar]
- 28.Adamski-Werner S. L., Palaninatham S. K., Sacchettini J. C., Kelly J. W. Diflunisal analogues stabilize the native state of transthyretin. Potent inhibition of amyloidogenesis. J. Med. Chem. 2004;47:355–374. doi: 10.1021/jm030347n. [DOI] [PubMed] [Google Scholar]
- 29.Klabunde T., Petrassi H. M., Oza V. B., Raman P., Kelly J. W., Sacchetini J. C. Rational design of potent human transthyretin amyloid disease inhibitors. Nat. Struct. Biol. 2000;7:312–321. doi: 10.1038/74082. [DOI] [PubMed] [Google Scholar]
- 30.McCammon M. G., Scott D. J., Keetch C. A., Greene L. H., Purkey H. E., Petrassi H. M., Kelly J. W., Robinson C. V. Screening transthyretin amyloid fibril inhibitors: characterization of novel multiprotein, multiligand complexes by mass spectrometry. Structure. 2002;10:851–863. doi: 10.1016/s0969-2126(02)00771-2. [DOI] [PubMed] [Google Scholar]
- 31.Purkey H. E., Dorrell M. I., Kelly J. W. Evaluating the binding selectivity of transthyretin amyloid fibril inhibitors. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5566–5571. doi: 10.1073/pnas.091431798. [DOI] [PMC free article] [PubMed] [Google Scholar]