Abstract
Cellular and organism survival depends upon the regulation of pH, which is regulated by highly specialized cell membrane transporters, the solute carriers (SLC)1. The SLC4 family of bicarbonate (HCO3−) transporters consists of ten members, sorted by their coupling to either sodium (NBCe1, NBCe2, NBCn1, NBCn2, NDCBE), chloride (AE1, AE2, AE3), or borate (BTR1). The ionic coupling of SLC4A9 (AE4) remains controversial. These SLC4 bicarbonate transporters may be controlled by cellular ionic gradients, cellular membrane voltage, and signaling molecules to maintain critical cellular and systemic pH (acid-base) balance.
There are profound consequences when blood pH deviates even a small amount outside the normal range (7.35–7.45). Chiefly, Na+ coupled bicarbonate transporters (NCBT) control intracellular pH in nearly every living cell, maintaining the biological pH required for life. Additionally, NCBTs have important roles to regulate cell volume and maintain salt balance as well as absorption and secretion of acid-base equivalents. Due to their varied tissue expression, NCBTs have roles in pathophysiology, which become apparent in physiologic responses when their expression is reduced or genetically deleted. Variations in physiological pH are seen in a wide variety of conditions, from canonically acid-base related conditions to pathologies not necessarily associated with acid-base dysfunction such as cancer, glaucoma, or various neurological diseases. The membranous location of the SLC4 transporters as well as recent advances in discovering their structural biology makes them accessible and attractive as a druggable target in a disease context. The role of sodium-coupled bicarbonate transporters in such a large array of conditions illustrates the potential of treating a wide range of disease states by modifying function of these transporters, whether that be through inhibition or enhancement.
Introduction
The SLC4 family of bicarbonate (HCO3−) transporters can initially be separated into three groups [139]: Na+ independent, Na+ dependent, and variable. Na+ independent HCO3− transporters, i.e., the anion exchangers (AEs: AE1, AE2, AE3), exchange chloride ions for HCO3− ions. The AEs are encoded by the SLC4A1–3 genes. The second group is Na+ dependent (NBCe1, NBCe2, NBCn1, NBCn2, NDCBE). The Na+ dependent transporters are encoded by the SLC4A4, 5, 7, 8, and 10 genes. The controversial category includes SLC4A9 and SLC4A11. AE4, the gene product of SLC4A9, has not been fully characterized and tends to remain controversial, however it has been recently shown to affect acid-base sensing in intercalated cells of the distal nephron [195]. SLC4A11 (BTR1) function has been the subject of debate with early studies indicating electrogenic Na+ borate cotransport (mouse and human) [138], while more detailed analysis of mammalian Slc4a11 revealed neither borate nor HCO3− coupling, but rather an EIPA-sensitive pH regulator [134] NH3 transporter [213,131]. Later studies in the euryhaline teleost Takafugu obscurus showed that one of the orthologs of Slc4a11 in this fish functions as a cation-independent boric acid channel or uniporter [92]. The primary focus of this review will be the SLC4 family members that are Na+ coupled HCO3− transporters, including NDCBE which is a hybrid Na+ and Cl−-dependent HCO3− transporter.
Nomenclature and rationale for discussion
Many biological anions and cations, and therefore involving a variety of SLC transporters, are pH buffers and used in animal cells and tissues (for review see [158]). As biological fluids exist in an obligate steady state with CO2, for most tissues HCO3− and HCO3− transporters are more critical for pH-homeostasis than H+ and other organic buffer systems. Moreover, HCO3− transporting proteins are also found in the Slc26 gene family [4,176] and the CFTR Cl− channel is known to conduct HCO3− [149,175,83,113]. For this review, we will confine the discussion to Na+ coupled members of the SLC4 family.
Na+ coupled HCO3− transporters are colloquially referred to as NBCs or NCBTs (Na+ coupled HCO3− transporter [123]), and this abbreviation will be used throughout. The nomenclature of the SLC4 family members has been inconsistent and changed with the discovery that NBCe1 (the first Na+ coupled HCO3− transporter) was part of the SLC4 family along with anion exchangers (AE1, AE2, AE3) [157]. This inconsistent nomenclature necessitates an explanation of the terms that will be used in this review. We will be covering the electrogenic NCBTs and the electroneutral NCBTs separately and will briefly discuss NCBTs that do not fit into a category- SLC4A9 and SLC4A11. For a summary of these transporters’ naming conventions and properties, including which SLC4 gene names correspond to which protein names see Table 1.
Table 1.
NCBT genes, protein names, coupling, localization, and further information on the transporters covered in this review.
| Gene | Protein name | Gene locus | Na+ coupling | Net charge | Human tissue expression | Monogenic disease and other associated conditions |
|---|---|---|---|---|---|---|
| SLC4A4 | NBCe1 | 4q21 | 1 Na+: 2 or 3 HCO3−; 1 Na+: 3 HCO3− (HCO3− & CO32−) |
−1 / −2 | Kidney, pancreas, heart, eye, teeth Basolateral (BL) membrane in epithelia |
Monogenic: Proximal renal tubular acidosis [81,82], glaucoma and cataracts [82], problems with dentition [82] Cancer [28,38,65,69,111,145,205,214], diabetes mellitus [24], cystic fibrosis [162], cognition [82], mental health [133], ischemia-reperfusion injury [94] |
| SLC4A5 | NBCe2 | 2p13 | 1 Na+: 2 or 3 HCO3−; 1 Na+: 3 HCO3− (HCO3− & CO32−) |
−1 / −2 | Liver, spleen, testis, heart, placenta, stomach, choroid plexus epithelium Apical membrane in epithelia |
Salt sensitivity [29], hypertension [72,183,29] brain and neurologic impairment [127,91], distal renal tubular acidosis [203], retinal pathologies [42] |
| SLC4A7 | NBCn1 | 3p22 | 1 Na+:1 HCO3− | 0 | Muscle (skeletal and smooth), heart, breast, brain, bone | Breast cancer [37,14,104,13], rheumatoid arthritis [87], osteopetrosis [21], kidney metabolism of ammonia [105] |
| SLC4A10 | NBCn2 | 2q24 | 1 Na+:1 HCO3− | 0 | CNS, kidney, reproductive system, small intestine Apical membrane in epithelia |
Neuronal excitability [85,173] |
| SLC4A8 | NDCBE | 12q13 | 1 Na+ & 2HCO3− (CO32−) for 1 Cl− |
0 | CNS, testis, ovary, kidney Apical membrane in epithelia |
pH regulation of the CNS [35,36,102], hypokalemia and volume depletion associated with thiazide diuretics [109,174] |
| SLC4A9 | AE4 | 5q31 | (A) | Kidney, fetal brain, testis BL membrane in epithelia |
||
| SLC4A11 | BTR1 | 20p12 | (B) | (C) | Ubiquitous |
Monogenic: FUCHS dystrophy [194] Corneal issues [143] |
Function of SLC4A9 is disputed. Slc4a9 was originally defined as a Na+ independent Cl−−HCO3− exchanger [95]. Parker and colleagues found that Slc4a9 functions as a Cl− IN-Dependent, electroneutral Na+ bicarbonate cotransporter (NBCn) [140]. More recently, Vitzthum and coworkers have suggested that Slc4a9 may be a HCO3− sensing protein [195].
Function of human SLC4A11 and non-human versions of Slc4a11 has likely the highest diversity of functions attributed to the single cDNA ranging from Na+ coupled borate cotransporter [138], EIPA-sensitive transporter [134], NH3 transporter [213], OH− transporter [131], and a boric acid channel (uniporter) [92].
Tissue expression, role in biology and diseases
NCBTs have varying functions, but these are apparently crucial for physiological processes due to modifying intracellular pH (pHi) and extracellular pH (pHo) especially in the nervous system. A few examples of NCBT function include regulation of cell volume, NaCl resorption in various epithelia including the ilium or proximal colon, both secretion and resorption of acid-base equivalents in many organs and organ systems, and regulation of pHi in almost every cell in the body [154]. NCBTs are also expressed in many tissues: they can be found in the epithelia of kidney, pancreas, eye, heart, lung, brain, testis, and gastrointestinal tract [139], the central and peripheral nervous systems [123], and in a variety of tumors [28,111,125,145,205,214].
Due to their broad distribution, NCBT dysfunction can be involved in many different diseases and syndromes, either due to genetic mutations that change function based on structural abnormalities or acid-base dysregulation in diseases that result in perturbed NCBT function. Some of these conditions can include systemic acid-base related conditions such as proximal renal tubular acidosis or any conditions involving metabolic or respiratory acidosis or alkalosis, in addition to conditions not generally thought of as being associated with primary acid-base dysregulation including tumor formation and cancer [37,28,70,111,38,125,145,205,214], glaucoma [51], cystic fibrosis [162,129,211], neurologic diseases [85], heart conditions [207,196,189,136,72,61,48,3] or rheumatoid arthritis [86].
Potential for pharmacology and need for further research
All NCBTs have been shown to be sensitive to 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS) [6], except for SLC4A4 whose DIDS sensitivity seems to be variable depending on tissue expression and experimental conditions [150,15]. S0859, an N-cyanosulphonamide compound also acts as a generic inhibitor of sodium-coupled HCO3− transport [56] and appears to inhibit both electrogenic and electroneutral transport [166,208,31]. Conversely, diphenylamine-2-carboxylate (DPC), and 5-nitro-2-(3-phenylpropyl-amino) benzoic acid (NPPB) both seem to inhibit a variety of Cl− transporters and channels without affecting HCO3− transporters. A notable exception is the Na+ driven Cl−-HCO3− exchanger, NDAE1, in Drosophila [139]. However, these compounds have not been thoroughly explored for therapeutic use in treating conditions associated with NCBT dysfunction.
Though NCBTs are posited to be a suitable drug target for several conditions, their broad distribution in the body does present a challenge for targeted treatment. If one were to broadly inhibit an NCBT in the heart, for example, it would ultimately present challenges in acid-base compensation elsewhere. It may be possible to use a tissue-specific delivery method to reduce off-target effects or toxicity [215,48,49], but this treatment avenue for Na+-HCO3− transport is under-investigated. Additionally, sequence variation of NCBTs and other associated genes in conditions that have been discovered to have NCBT involvement or dysfunction represent many novel avenues of pharmacological treatment, including immunotherapy for pancreatic cancer [28]. Cappellesso and colleagues demonstrated that Slc4a4-knockdown in combination with anti-PD1 (programmed death ligand 1) and anti-CTLA-4 (cytotoxic T-lymphocyte–associated antigen 4) can resolve pancreatic tumors [28]2. NCBTs as drivers of disease have been under-investigated, indicating a need for further exploration into sodium-dependent HCO3− transporters as well as the compounds that could therapeutically inhibit or augment their function.
The electrogenic NCBTs: NBCe1, NBCe2
NBCe1/SLC4A4
Overview
NBCe1, also known as the electrogenic HCO3− cotransporter, is the most studied and characterized of the NCBTs. It is encoded by the SLC4A4 gene and located on human chromosome 4q21 [2]. It was cloned in 1997 by expression cloning from salamander kidney [157].
Function of SLC4A4 (NBCe1)
NBCe1 transports Na+ and HCO3− at a ratio of 1 Na+ to either 2 or 3 HCO3−, (functional and structural studies indicate CO32− may substitute for HCO3− [130,204,103] (Table 1, Fig. 1). There have been three isoforms of NBCe1 that have been extensively characterized: NBCe1-A as expressed primarily in the proximal renal tubule, NBCe1-B having a wider tissue expression distribution in the pancreas, heart, and eye, and NBCe1-C which is primarily expressed in the central nervous system (CNS), referring to several parts of the brain, optic nerve, and spinal column [157,43]. NBCe1 D and E isoforms have also been discovered in the reproductive tract of mice [117], however they have not yet been extensively characterized in either human or murine tissue. NBCe1 has 14 transmembrane domains (TMs) which are identical among isoforms [220]. NBCe1-B and NBCe1-C have alternative and longer N-termini than NBCe1-A. This longer N-terminus in the B and C isoforms encodes an autoinhibitory domain (AID) [80,171] that is a binding site for inositol 1,4,5-triphosphate (IP3) receptor-binding protein (IRBIT). IRBIT binding to the AID of NBCe1 augments the function of NBCe1-B and C to NBCe1-A levels [80,171]. Thus, isoform, membrane ion and voltage gradients, and interacting protein(s) control NBCe1 transport direction and overall activity.
Figure 1.
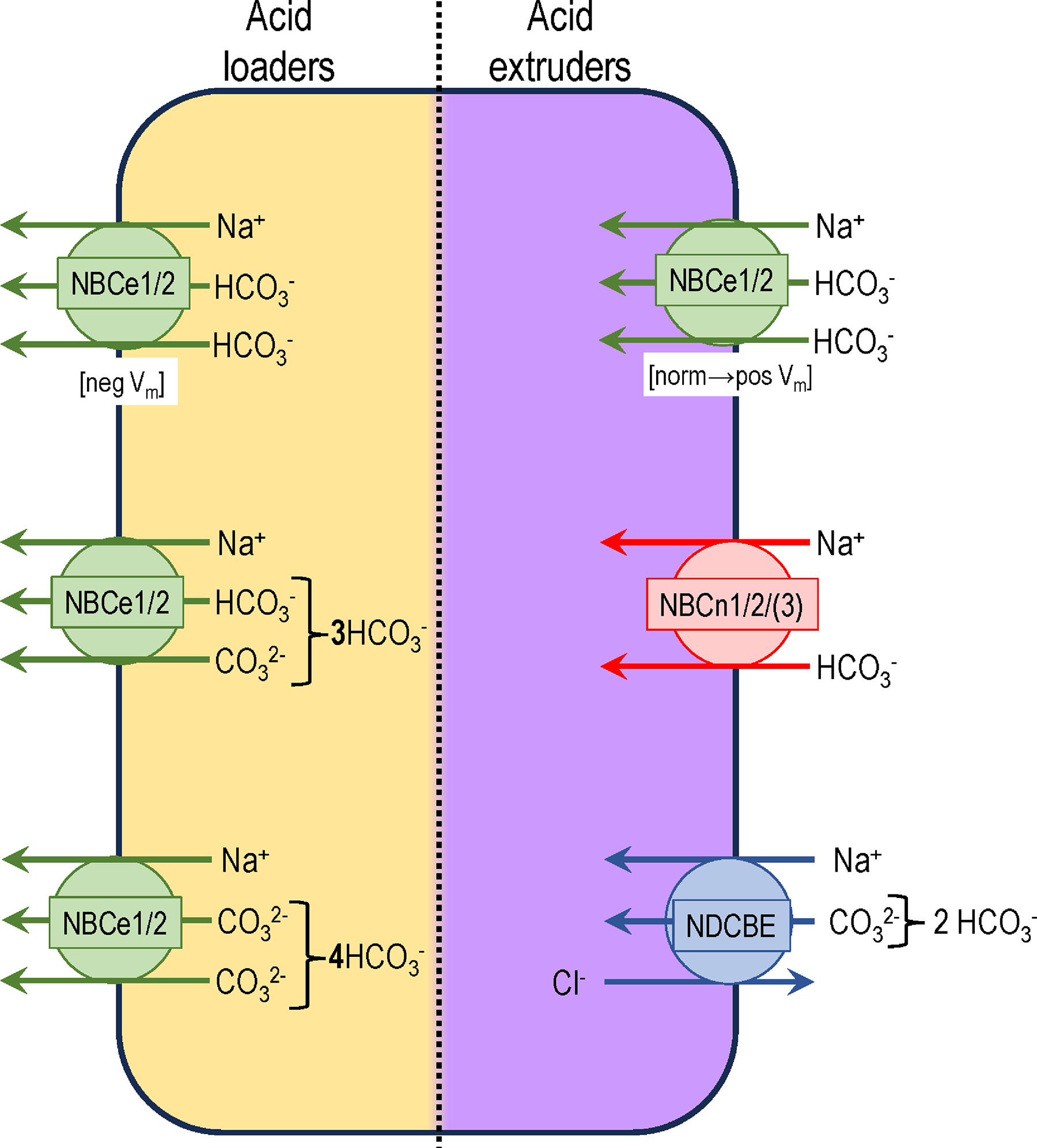
Schematic of ion transport of NCBTs.
A schematic showing the direction of ion movement for NBCe1/2 (green text), NBCn1/2/3 (red text), and NDCBE (blue text). Note: AE9, a relatively uncharacterized SLC4 family member, is referred to here as NBCn3.
Primary disease manifestations of SLC4A4 (NBCe1) pathological variants
Proximal renal tubular acidosis (pRTA)
The A isoform of NBCe1 is predominantly expressed in the kidney at the basolateral membrane of the proximal tubule (PT) [165]. Using isoform specific antibodies and isoform specific nbce1-knockout, NBCe1-B protein has been found in proximal straight tubules of the outer medullary collecting duct [60,23]. PTs are one of the most metabolically active tissues in the body and contain an abundance of mitochondria, being second in O2 consumption only to the heart due to the immense volume of solute exchange [76]. The kidneys are responsible for controlling how much acid or base goes into the blood by modulating HCO3− transport across the plasma membrane; 80% of the filtered load of HCO3− is reabsorbed by the proximal tubule [77], i.e., 1 lb baking soda per day in an average 70 kg adult. While other acid-base transporters are found in the proximal tubule, e.g., NHEs, H+ pumps, mutation or deletion of Slc4a4 (NBCe1) is the only one that causes a severe acid-base disturbance. Proximal renal tubular acidosis (pRTA, OMIM #604278) occurs when there is defective ability to reabsorb HCO3− in the PT, which ultimately leads to a decrease in the amount of HCO3− absorbed into the blood [84,51,178,75,137]. pRTA can be inherited in either an autosomal dominant or autosomal recessive pattern, in addition to sporadic de-novo mutations and one instance of compound heterozygosity [132]. The pathogenic variants (aka, disease mutations) associated with pRTA are further outlined in Table 2.
Table 2.
Mutations in SLC4A4 associated with pRTA.
| Mutation | Mutation Type | Inheritance Mode | Phenotype | Biochemical consequences | References |
|---|---|---|---|---|---|
| Q29X | Non-sense | Autosomal Recessive |
(A) developmental delay |
Truncation of NBCe1-A by 1007 amino acids, full-length protein not expressed | [82,9] |
| R298S | Missense | Autosomal Recessive | (A) | Disruption of HCO3 “tunnel,” impaired delivery of bicarbonate to the ion permeation pathway | [81,78,110] |
| S427L | Missense | Autosomal Recessive | (A) | Serine hydrophobicity lost, leading to perturbation of TM1 helix packing and change in configuration of ion permeation pathway | [51,110,218,220] |
| T485S | Missense | Autosomal Recessive | (A) | Loss of electrogenicity due to conformational changes occurring in an aqueous accessible portion | [78,180,220] |
| G486R | Missense | Autosomal Recessive | (A) | Blocking of the ion coordination site with large arginine residue | [180,181,220] |
| R510H | Missense | Autosomal Recessive | (A) | Loss of positive arginine charge and loss of interaction between TMs that is required for proper protein folding, retention of transporter in ER | [81,78,110,181,219] |
| W516X | Non-sense | Autosomal Recessive |
(A) developmental delay renal K+ wasting |
Nonsense-mediated decay surveillance mechanisms preventing full translation of mutant transcripts | [120] |
| L522P | Missense | Autosomal Recessive |
(A) growth retardation, developmental delay |
Proline residue causing helix disruption and significant misfolding, retention of transporter in ER | [47,180,181,219,209] |
| A799V | Missense | Autosomal Recessive |
(A) hypokalemia, muscle weakness |
Cation leak in TM10 | [46,78,181,220,144] |
| R881C | Missense | Autosomal Recessive | (A) | Impairment of TM12 helix packing and protein plasma membrane trafficking, ER retention (does not affect basal transport function) | [78,187,181,219] |
| Q931R + R510H |
Missense | Compound heterozygosity |
(A) developmental delay |
Loss of positive arginine charge and loss of interaction between TMs that is required for proper protein (R510H), anion leak (Q913) | [132] |
pRTA with ocular abnormalities. These are common to all the known SLC4A4 recessive mutations.
pRTA is characterized clinically primarily by severe metabolic acidosis (i.e., blood pH <7.2) but additionally can have features of growth retardation, cognitive defects, ocular pathologies (specifically cataracts and glaucoma, see below section), and hypokalemia [52,220,100]. There have been great efforts to elucidate the mechanics behind pRTA as relating to functional mutations in SLC4A4. Many of these mutations disturb the structure of the transporter and impair its function. The biochemical consequences of these mutations that disrupt function are further discussed in Table 2. Interestingly, some of the NBCe1 variants that cause pRTA do not modify basic transporter function but rather have mislocalized NBCe1 to the endoplasmic reticulum instead of the basolateral membrane of the PT [187].
Another clinical feature seen in pRTA is impaired growth and development: patients with pRTA tend to be short in stature and can experience developmental delay [51,47,84]. With low blood [HCO3−] some buffering of the blood occurs by scavenging phosphate from bone or preventing phosphate incorporation. The phenotype of impeded growth is posited to be due to the growth hormone insensitivity caused by chronic metabolic acidosis [26]. NBCe1 is expressed in the brain [177] and the phenotype of developmental delay seen in pRTA patients is potentially caused by effects in nervous transmission due to altered pH-dependent action potential propagation due to either changes in pHi or pHo [99].
Most cases of pRTA show an autosomal recessive pattern of inheritance, however autosomal dominant and sporadic methods of inheritance have also been documented [99]. A novel NBCe1 variant has been discovered in compound-heterozygous inheritance with mutations from both parents in Q931R and R510H [132]. The Q931R mutation by itself does not seem to have a pathologic effect, but in combination with R510H halts function of NBCe1 [132]. For further information on these mutations as well as their biochemical consequences, see Table 2.
Glaucoma / cataracts
NBCe1-B has high expression in ocular tissue and there are several mutations in NBCe1 that have been canonically associated with pRTA with ocular pathology, specifically glaucoma and cataracts [157,47]. The etiology of the glaucoma is not clear, as even the one patient with just NBCe1-A affected (Q29X), is clinically diagnosed with glaucoma [82].
NBCe1-B is located in the basolateral membrane of the corneal endothelium [16]. Slc4a4-null mice have been characterized as having corneal edema [120]. This fluid buildup upon perturbation is likely due to NBCe1-B contributing to ion movements that would normally draw fluid out of the corneal stroma [22]. Interestingly, corneal edema has not been reported in humans with NBCe1 mutations, potentially due to other eye pathologies such as glaucoma or cataracts having a higher prevalence compared to corneal edema [22].
Other health phenotypes associated with SLC4A4 (NBCe1)
Diabetes mellitus
Pancreatic β cells are responsible for making and secreting insulin [53]. In type 1 diabetes mellitus (T1DM), β cells are destroyed due to autoimmunity. Though type 2 diabetes mellitus (T2DM) is a disease of many etiologies, β cells are still dysfunctional: not through autoimmunity, but potentially due to metabolic stress of an obesogenic environment [63].
SLC4A4 has been identified through single-cell transcriptomics as being enriched in immature and dedifferentiated T2DM β cells and downregulated in nondiabetic (ND) adult β cells [200]. Some of the genetic changes seen in β cells to promote survival resemble those seen in cancer, specifically the “reverse pH gradient,” which consists of an acidic tumor microenvironment and an alkaline pHi [201]. Regardless of the exact etiology, T2DM causes inappropriate NBCe1 expression in both mouse and human pancreatic β cells [24]. If it is impossible for mouse β cells to make NBCe1, then mice do not show a T2DM phenotype [25].
The increased pHi in cancer cells allows them to undergo specific metabolic adaptions (further discussed in below cancer section), shifting from mostly relying on oxidative phosphorylation to almost exclusively relying on anaerobic glycolysis, which results in an increase in the amount of lactic acid produced, mirroring the Warburg effect as seen in various forms of cancer. HCO3− transporters which alkalize pHi have been shown to increase in several disease states, making this an interesting avenue for further studies in broad acid-base transport biology. It should also be noted that electrogenic transport represents an additional area of investigation into the mechanosensory and signaling processes of β cells. If charge movement across the plasma membrane of these sensitive cells is altered, it could present an additional dysfunction that is not solely pH-based or worsened upon pH dysregulation. The potential of NBCe1 to be a therapeutic target in T2DM is exciting, though it presents a challenge. First, NBCe1’s wide tissue expression would require targeted pharmacology. Second, there is more NBCe1 mRNA and protein expression in the pancreas (ductal epithelia and acinar cells) than even the kidney. Thus, even targeting the pancreas would be too broad. In further studies examining possible drug design to treat T2DM, it would be necessary to consider a tissue-specific method of NBCe1 inhibition such as using a glucagon-like peptide 1 attached to an NBCe1 inhibitor [215].
Lung Physiology & Cystic Fibrosis
In cystic fibrosis (CF, OMIM #219700), one of the most common autosomal recessive diseases (~1:3500 Caucasian births), impaired Cl− and HCO3− secretion has been characterized to decrease the volume of fluid in the airway, ultimately leading to ciliary dysfunction mucus stasis, and airway obstruction [211]. The role of HCO3− transport in lung epithelia has been less extensively investigated in CF. However, studies have shown that by impairing HCO3− secretion via NBCe1 inhibition decreases the pH of airway surface liquid (ASL), that in turn compromises post-secretory mucin maturation and clearance and impairs antimicrobial properties in the epithelia. Impaired HCO3− secretion also increases fluid absorption in the airway, further decreasing airway fluid hydration [129]. According to further analysis of RNA-seq data [161,162], the B isoform of NBCe1 is more highly expressed in the lung compared to the A isoform. Inhibiting NBCe1 can ultimately induce airway acidification and reduce the ability of the lungs to recover from an acid load due to a disruption of HCO3− entering the airway lumen. Though the mechanism behind basolateral HCO3− transport in native pulmonary tissue is not fully characterized, it has been posited that adding HCO3− could increase the height of human airway epithelial cells, thus restoring normal mucus properties in CF patients. This represents an area in which more research into how HCO3− transport works in lung and airway epithelia could inform drug treatments or even gene therapy in CF patients.
At the level of systemic acid-base homeostasis, as a first approximation, blood pH is a balancing of PCO2 (controlled by minute volume and respiratory rate) and the kidneys (secreting and absorbing both HCO3− and H+). Based on several human recessive SLC4A4 variants (disease mutations) with pRTA, some patients seem to have a complicating respiratory acidosis [51]. In addition to HCO3− secretion to maintain proper ASL pH, impairment of PCO2 control would indicate an NBCe1 role in the respiratory responses to acidosis and alkalosis. Recently, Brady and coworkers showed that mice lacking NBCe1-B/C have a depressed respiratory response to metabolic acidosis [23].That is, rather than the expected increase in minute volume thereby decreasing PCO2, the B/C-knockout animals rely on the kidney alone to maintain blood pH.
Cognition and Mental Health
The C isoform of NBCe1 is primarily expressed in the CNS. Interestingly, NBCe1-C localizes to astrocytes, glia, and even neurons throughout the CNS [12,54,123,124]. Its involvement has been identified in the acute stimulation of neuronal glycolysis, specifically in astrocytes [159,184,185]. Mouse studies have shown that Slc4a4 null mutants respond to an increase in extracellular K+ with increasing glucose levels, ultimately inhibiting glycolysis. The dysfunction of NBCe1 would ultimately lead to pH imbalances and many of the steps in the glycolytic pathway are sensitive to pH showing that pH is a glycolytic regulator [159].
SLC4A4 mutations have also been associated with familial migraine in patients with pRTA and interestingly, heterozygous relatives have a phenotype of mild to moderate migraine symptoms [181]. A genome-wide association study (GWAS) of patients with several psychiatric disorders including bipolar disorder indicates that NBCe1 expression is strongly associated with a suicide “phenotype” [133]. Interestingly, lithium carbonate (Li2CO3) is commonly used to treat bipolar disorder; and the Li+ cation is poorly or not transported by species orthologs of NBCe1 [7,168,106]. Though this is currently speculative, this may influence brain chemistry or signal propagation and alter the efficacy of Li2CO3 treatment, leading to worsening of psychiatric disorder symptoms. Further investigation is necessary to ascertain whether SLC4A4 represents a biomarker for pharmacologic treatment of psychiatric disorders, e.g., as a potential individualized therapeutic.
Protective role in ischemia-reperfusion injury
SLC4A4, SLC4A5, and SLC4A7 (NBCn1, see below) are both expressed in cardiac tissue, however the relative mRNA expression level of SLC4A4 in the heart is approximately two-fold higher than that of SLC4A7 [196]. Acute myocardial infarctions (AMI), colloquially known as heart attacks are a serious and often fatal cardiac disease. The oxygen deprivation caused by AMI can lead to the development of ischemia-reperfusion injury (IRI), which can be worsened by excess sodium influx [121,189]. Lactic acid accumulation in cardiomyocytes resulting from IRI can lead to intracellular metabolic acidosis, ultimately causing additional, irreversible injury to the myocardium- including congestive heart failure [94]. It has been shown that by inhibiting Slc4a4 in a conditional knockout mouse model causes a decreased AMI size during initial reperfusion [189]. Additionally, this same knockout model with simulated IRI had no effect on contractility or relaxation of the heart muscle [121]. Fantinelli and colleagues developed antibodies against extracellular loops 3 and 4 of NBCe1 (aL3 and aL4, respectively) [61]. In a rat model of regional IRI, the aL3 antibody administered at the start of reperfusion was found to improve both infarct size and myocardial function where S0859 only improved infarct size [61] This aL3 also had benefits on post-ischemic myocardial and mitochondrial effects, likely through calcineurin-mediated p38MAPK activation [41]. While targeted NBCe1-inhibition, specifically with specific antibodies may be a viable, individualized preventative treatment for some IRI, the utility may be limited to rodents as the extracellular loops vary in sequence between rodents and human.
Role in cardiac physiology
NBCe1 mRNA and protein are found in the heart [156,157] and later NBCe2 (see NBCe2), initially called NBC4, from human heart [152,193]. The main buffering systems in the heart are NHE1 (SLC9A1), NBCe1 (SLC4A4), and NBCn1 (SLC4A7) as well as several Cl−-HCO3− exchangers (SLC4A3, SLC26A3, SLC26A6) and monocarboxylate transporters (MCT, SLC19 family) [191]. Nevertheless, the HCO3− transporters’ roles in cardiac cell buffering seem to account for ~20% of the overall acid-base flux (see Fig 1c in [191]), while buffering in most other tissues, e.g., epithelia, rely mostly on CO2/HCO3− transporters [30,17] (see also Ch 28 in [18]).
There are two forms of cardiac hypertrophy (CH): physiologic and pathologic. Following prolonged volume or pressure overload, the heart responds by increasing cardiomyocyte growth [163]. Physiologic CH develops in circumstances of enhanced cardiovascular training or pregnancy and involves a maintained cardiac structure with sustained or improved contractility. Pathologic CH is the more severe leading to heart failure and often irreversible due to contractility loss[163]. NHE1 in CH has been extensively characterized, and NHE1 inhibition prevents CH. The roles of NBCe1, NBCe2, and NBCn1 are more complex and less thoroughly investigated.
In a mouse model of exercise-induced physiologic CH brought on by voluntary wheel running, NBCe1 was found to be significantly upregulated while NHE1 and NBCn1 remained unchanged [126]. These authors reason that NBCe1 works to move Na+ out of the cell and thus decreased levels of intracellular Na+ from upregulated NBCe1 improves the recovery of intracellular acidosis [126]. This hypothesis seems contrary to the electrochemical gradient observed in the kidney proximal tubule where the transmembrane voltage is sufficiently negative to allow Na+ and 2 or 3 HCO3− equivalents (2 HCO3− are chemically similar to 1x CO32−, see section “SLC4 Structures” for details). Nevertheless, NBCe1 appears upregulated in pathologic CH [207,136,49]; however, in a rat heart CH model, NBCe1inhibition led to increases in NHE1 and NBCn1, potentially as a compensatory mechanism [48]. The renin-angiotensin system (RAAS) is activated in CH development via angiotensin II (Ang II) stimulating NCBT activity as found in spontaneously hypertensive rats (SHR) [136]. NBCe1 trafficking is changed such that NBCe1 appears showing nuclear rather than sarcolemmal and T-tubules membranes [136]. NBCe1’s varying role in both pathologic and physiologic CH necessitates further investigation to exploit its properties as a therapeutic target. CH seems to invoke a variety of NCBT responses that require more detailed molecular characterization to elucidate individual roles; nonetheless, Na+, HCO3−, Ca2+, and Vm gradients and control are likely all invoked.
NBCe2 (SLC4A5)
Overview
NBCe2, another electrogenic NCBT, is also involved in regulating acid-base balance and control of pHi. The SLC4A5 gene has been mapped to chromosome 2p13 and encodes NBCe2, a polypeptide consisting of 1074 residues [1]. Notably, it has been less characterized compared to NBCe1. It was first isolated from human testis and heart [152] and has since been found to be expressed in the liver, spleen, eyes, heart, kidney, placenta, and stomach. [193,152]. NBCe2 has high expression specifically in choroid plexus epithelial cells, which differs from other members of the SLC4 family, which tend to have wider expression throughout the CNS [91]. It is posited to function similarly to NBCe1 in buffering the cell and controlling pHi in the context of metabolic acidosis, but predominantly in the liver and connecting tubule and collecting duct in mouse [10]. No monogenic diseases have been associated with mutations in SLC4A5, although SNPs are correlated with hypertension [68,183,29]. Ithas been characterized as playing a role in various conditions including salt sensitivity and various neurologic and retinal pathologies [42,29,67,68,91].
Function of SLC4A5 (NBCe2)
NBCe2 transports Na+:HCO3− at a ratio of 1:2 and 1:3 [193] (Table 1, Fig. 1). NBCe2 has six total splice variants (A-F), though only the A and C isoform have intact transmembrane domains and are relevant in the context of Na+-HCO3− transport [19]. NBCe2-A has not been characterized as having electrogenic activity; this is potentially due to a 16 amino acid sequence near the end of the C terminus. Therefore NBCe2-C is the only variant with electrogenic activity [19]. NBCe2 shares 56% similarity to NBCe1 and 40% similarity with NBCn1 [164,152] (see below).
Primary disease manifestations of SLC4A5 (NBCe2) mutation
Retina
NBCe2 localizes to the Golgi apparatus as well as the apical and basolateral membranes of retinal pigment epithelial (RPE) cells [42]. Recently, Collin and coworkers demonstrated that NBCe2 is involved in RPE development [42]. Perturbations in the murine Slc4a5 gene- specifically a disrupted splice donor site in the 5’ region- have been associated with a loss of retinal function in addition to retinal detachment [42,91]. The specific mechanism behind this phenomenon is not yet fully understood. Additional studies of human gene profiling for retinal detachment or defects in RPE development could indicate whether SLC4A5 variants or SNPs are associated with these conditions.
Salt sensitivity and hypertension
Salt sensitivity is a condition observed in both hypertensive and normotensive populations. This condition has similar cardiovascular and renal consequences as hypertension [122]. Through chromosomal mapping of patients with salt sensitivity and elevated blood pressure, several SNPs within SLC4A5 have been identified as being associated with both salt sensitivity and hypertension in various populations [68,183,29]. Increased Na+ intake increases NBCe2 protein and migration from the sub-apical compartment to microvilli [62]. Gildea and coworkers propose that NBCe1 decreases at the PT basolateral membrane and NBCe2 expression is increased in the luminal membrane [68]. If true in humans, differential control of NBCe1 and NBCe2 in the kidney could help control salt sensitivity.
Brain and neurologic impairment
SLC4A5 mRNA specifically localizes to the choroid plexus, which is responsible for the production of cerebrospinal fluid (CSF) [91]. Choroid plexus epithelial cells (CPEs) have a variety of transport proteins that allow for necessary ions and nutrients to flow into the CSF and for toxins to flow out. NBCe2 protein is localized to the apical membrane of the choroid plexus epithelia (i.e., opposite membrane localization in epithelia than NBCe1) [40]. Studies using Slc4a5-null mice have shown differences in CSF ion chemistry in addition to cranial pressure changes and additionally are more resistant to seizures when treated with convulsant drugs [91] and control CSF pH [40]. For decades it has been well-documented that both intracellular and extracellular pH control neuronal activity directly and indirectly. While there is not necessarily a therapeutic avenue to control NBCe2, these data make it clear that controlling [HCO3−] and CSF-pH could be a generalized mode to alter CNS activity in a variety of neurologic conditions.
Distal tubule renal acidosis
NBCe2 is expressed in the kidney, though it differs from NBCe1 in its renal expression. NBCe2 has higher levels of expression in the renal distal tubule (DT) than the renal proximal tubule [164]. This distribution means that removal of NBCe2 in PT is unlikely to elicit a systemic acid-base response whereas loos of NBCe2 in the DT could result in a distal renal tubular acidosis (dRTA). Using mice lacking Slc4a5, Wen and colleagues showed that indeed NBCe2 loss manifests as dRTA although only after acid loading [203]. Accordingly, SLC4A5 polymorphisms may be associated with unresolved (genetically) human dRTA.
The electroneutral NCBTs (NBCn1, NBCn2, NDCBE)
NBCn1 (SLC4A7)
Overview
Originally named NBC3, the SLC4A7 cDNA was cloned from a human skeletal muscle library and characterized as an EIPA-sensitive Na+ and HCO3− cotransporter by Pushkin et al. [150]. While this was consistent with electroneutral Na+ HCO3− cotransport of muscle, it was Choi and coworkers who characterized a rat Slc4a7 isoform as NBCn1, an electroneutral Na+ HCO3− cotransporter, however, this NBCn1 also contained a discrete and separate Na+ conductance [39]. NBCn1 was the first electroneutral NCBT. SLC4A7 is located on human chromosome 3p22 [151]. Upon first cloning, NBC3-mRNA was found in skeletal muscle and cardiac tissue [150], and proposed to be the major HCO3− and intracellular pH (pHi) regulator previously described in skeletal, smooth, and cardiac muscle. Subsequently, NBCn1 mRNA isoforms have been found in other tissues including breast, brain, and osteoclasts [13,21,39]. One or more NBCn1 isoforms are associated with cellular migration in both cancers (its role in breast cancer has been most extensively studied)3 and rheumatoid arthritis (RA) [86].
Function of SLC4A7 (NBCn1)
As an electroneutral transporter, by definition this means that NBCn1 cotransports Na+:HCO3− at a ratio of 1:1 without changing Vm [39] (Table 1, Fig. 1). NBCn1 has been characterized as having 16 splice variants (A-P) with varying N and C terms and 12 TMs [116,186]. The Slc4a7 mRNA variants have differential expression based upon tissue type and all seem to be functional cotransporters [116]. DIDS sensitivity of NBCn1 is variable, i.e. DIDS-insensitive [150] to ~90% sensitive [15], depending on Slc4a7-isoform expressed. Choi et al. attributed the variable DIDS sensitivity to a lack of a DIDS-binding motif in some isoforms [39].
Primary disease manifestations of SLC4A7 (NBCn1) mutation
Rheumatoid arthritis
The long-term progression of RA involves the movement of synovial fibroblasts from affected joints to unaffected joints [108]. Ion channels and transporters are involved in cell migration and motility. Ji and associates found NBCn1 mRNA in primary cultures of RA-fibroblasts and that S0859 decreased fibroblast migration after cytokine challenge [87,86]. Histological characterization of RA shows hyperplasia of fibroblast-like synoviocytes (FLSs). The growth environment, like many cancers, is characterized with higher anaerobic metabolism and glycolysis, causing an acidic extracellular environment which, in turn, recruits NCBTs to correct the acid load. Growth of these FLS cells as measured by TNF-α (an inflammatory marker) levels is halted when NBCn1 is inhibited by S0859 or DIDS [86]. Interestingly, dexmedetomidine, an α2-adrenergic receptor agonist has been shown to both halt NCBT activity (not specifically a NBCn1 assay) as well as diminishing the severity of RA symptoms [107]. These data suggest that final actor in α2-adrenergic, NBCn1, could be a RA drug target to halt RA progression. Given the primary localization of NBCn1 and role in RA, it would be interesting to determine if inflammatory conditions such as atherosclerosis might have features of disrupted NBCn1 expression, protein distribution or activity.
Osteopetrosis
Osteoclasts are bone cells that normally dissolve bone to be remodeled by osteoblasts. Osteopetrosis (OMIM #16660, yet other forms exist) is most commonly an autosomal dominant condition associated with disrupted osteoclast expression leading to a phenotype of dense, brittle bones and previously linked to anion exchanger function [88]. The low (4.5–4.8) pH of the bone lacunae (bone absorption) [90] is maintained by membrane proteins, including H+ ATPases and chloride channels [90]. SLC4A7 mRNA expression is observed after osteoclast formation [153]. When NBCn1 is disturbed, H+ efflux and low lacunae pH is lost leading to the pathologic buildup of hydroxyapatite [153]. The mechanism behind this involves colony-stimulating factor 1, which can induce alkalinization, depending on the amount of CO2 and HCO3− in the environment [21]. This alkalinization is posited to be associated with inhibition of caspase-8 and −3, both of which are associated with cell death [21]. Thus, targeted NBCn1 expression represents a potential therapeutic target for both osteopetrosis and osteoporosis.
Kidney metabolism of ammonia
The normal kidney response to systemic acidosis is initiation of ammoniagenesis. This process is a function of the proximal tubule initiated by glutamine uptake (produced by liver as part of ureagenesis), and subsequent metabolism to NH4+ (secreted apically into the forming urine) and mitochondrial HCO3− generation (absorbed to blood by NBCe1). This urinary NH4+ excretion helps to increase the amounts of urinary acid that is excreted [202]. Conversely, NBCn1 is an acid extruder (into blood) by neutralizing intracellular H+ which is released during NH4+ absorption (Fig. 1). Mouse knockout studies have demonstrated that Slc4a7 null mice have upregulated basolateral Na+/HCO3− cotransport, compromised NH4+ reabsorption in the medullary thick ascending limb (mTAL) [101] and ultimately have acidotic arterial blood for longer amounts of time [135]. Interestingly, these knockout mice also demonstrate upregulated basolateral Na+/HCO3− cotransport in mTALs during metabolic acidosis, so NBCn1 is probably not related to this form of transport [135]. Investigating the NBCn1’s role in renal ammonia metabolism will give insight into whether polymorphisms of this gene could contribute to forms of dRTA.
NBCn2 (SLC4A10)
Overview
NBCn2, encoded by the SLC4A10 gene, is another electroneutral NCBT located on chromosome 2q23 [210]. It has been less extensively characterized compared to NBCn1. SLC4A10 mRNA is found in the brain, specifically in the choroid plexus, hippocampus, the molecular layer of the cerebellum, and specific brainstem regions [118,44]. The mRNA is also expressed in kidney [73], reproductive system [116], and small intestine [198]. Before documentation of NBCe2’s role in generating CSF, NBCn2 was hypothesized to contribute to electrolyte transport within CSF due to localization in the choroid plexus, basolateral membrane [66]. This has not been reexamined since NBCe2’s role was elucidated.
Function of SLC4A10 (NBCn2)
NBCn2 transports Na+ and HCO3− 1:1 and thus is electroneutral [142] (Table 1, Fig. 1). Slc4a10 was initially characterized as Cl− and Na+ coupled and called “NCBE” [66], implicating the Na+ dependent Cl−-HCO3− exchanger described in squid axon [20,160]. However, detailed electrophysiology experiments revealed a futile Cl− self-exchange activity [142,71]. The only Cl−-HCO3− exchange activity occurs with complete absence of extracellular chloride, which is an entirely nonphysiological condition [142]. Human tissues reveal four NBCn2 splice variants (A, B, C, and D) with differential brain tissue expression [118]. Additional NBCn2 splice variants have been found in mice [198] although human homologous are unclear [116]. As with many SLC4 members, an N-terminal splicing doubles the total number of protein isoforms.
In the mouse intestine these splicing events seems yet more complex as the main transcript indicates internal and N-terminal splicing, but isoform specific antibodies detect only one of these isoforms [197]. The functional significance of particularly a luminally located NBCn2 in the small intestine is unclear. The protein distribution mirrors NHE3 with highest expression in the jejunum and decrements with NaCl dietary supplement. These investigators propose that electroneutral Na+ and HCO3− uptake with the known apical Slc26 proteins [93] would create a futile cycle for HCO3− and a parallel path for jejunal NaCl absorption [197]. While this mechanism is plausible, more explicit tissue or cellular experiments are needed to test this hypothesis.
Primary disease manifestations of SLC4A10 (NBCn2) mutation
Neuronal excitability
NBCn2 mediates acid extrusion (base absorption) in all tissues because [Na+] and [HCO3−] are almost always higher in extracellular fluids. That said, pH changes in the CNS can have significant effects on basal cellular function via alteration of both the excitability and synaptic activity of neurons. In humans, NBCn2 mRNA is found within the cerebrum, hippocampus, and choroid plexus [44] in both inhibitory and excitatory neurons [173,85]. In Slc4a10 knockout mice, Jacobs and colleagues found that knockout mice were more resistance to induced seizures (via proconvulsant drugs) compared to wild-type animals [85]. Similarly, patients with SLC4A10 disruption experience complex partial epilepsy in addition to lowered cognitive abilities and moderate intellectual disability [74].
NDCBE (SLC4A8)
Overview
The SLC4A8 gene (chromosome 12q13) encodes a Na+ dependent Cl−-HCO3− exchanger (NDCBE) with properties of both anion exchangers and Na+ cotransporters[71]. NDCBE shows high expression in both brain and testis with lesser mRNA in kidney and ovary [71]. Within the CNS, SLC4A8 mRNA localizes to the cerebral cortex, cerebellum, medulla, thalamus, and hippocampus as well as the spinal cord [71]. Thus, NDCBE should play a significant role in CNS pH regulation. Mutations (SNPs) in SLC4A8 are not currently known to be associated with any human diseases, although it has important roles in neuronal function and signaling [35,27].
Function of SLC4A8 (NDCBE)
NDCBE is a complex transporter with one Cl− being exchanged for one CO32− and one Na+ [142] mirroring the functional SLC4 paralog NDAE1 characterized in Drosophila [155,167] (Table 1, Fig. 1). The SLC4A8 gene has four splice variants that are controlled by separate promoters [141]. The splice variants have differentially influenced transcription based on various factors such as cell type, developmental stage, and response to external stimuli, e.g., local pH changes in metabolic acidosis [141]. The structure of NDCBE has been solved x-ray crystallography [5] and cryoEM [199].
Primary disease manifestations of SLC4A8 (NDCBE) mutation
pH regulation the CNS
As discussed in the NBCn2 CNS section, extracellular pH changes in the brain can affect neuronal excitability [57,85] and synaptic transmission [50]. This is illustrated in C. elegans in which the NDAE1-homolog controls whether GABA(A) Cl− channels are excitatory or inhibitory by controlling intracellular [Cl−] [11]. NDCBE is similarly involved in neurotransmitter release [27]. Burette and associates postulate that NDCBE plays a prominent role in regulation of neurotransmitter release, due to its activity coupling Cl− transport to many different physiological processes (pH balance, metabolic activity, and basal synaptic activity) [27].
Hypokalemia and volume depletion
Thiazide diuretics are often the first line of treatment for both hypertension and hypercalciuria. When they were first introduced, at higher doses than currently used therapeutically, severe hypokalemia was a common side effect [58]. Thiazide diuretics’ canonical target is the sodium chloride transporter (NCC), although higher doses have been demonstrated to additionally block NDCBE in the β-intercalated cells of the cortical collecting duct [109].
Cancer involves multiple NCBTs
pH dysregulation is seen in many cancers, especially those involving solid tumors, and this “pH dysregulation” has been hypothesized to be both a direct and indirect driver of oncogenesis since the 1980s. The Warburg effect explains the tendency of tumor microenvironments to have a lower extracellular pH (pHo) and either a normal or slightly elevated pHi [112]. This phenomenon results from the shift from primarily oxidative phosphorylation (aerobic respiration) to glycolytic activity (anaerobic respiration). This glycolysis increase causes lactic acid accumulation extracellularly (decreased pHo) while pHi is normal or slightly elevated. The process of cellular carcinogenesis is complex and can include epigenetic changes which can alter the expression of genes, including those which influence epithelial-to-mesenchymal transition, cell proliferation, apoptosis, and differentiation. SLC4A7, (NBCn1 gene) and SLC4A4 (NBCe1 gene), have both been identified as having various roles in many forms of cancer.
NBCe1 (SLC4A4)
SLC4A4 has been implicated in several types of cancers, including prostate cancer [111,119,169], colorectal cancer [38,217], various types of thyroid cancers [69], renal carcinoma [205], various leukemias [65], and pancreatic cancer [28]. The elucidation of specific genes associated with the development of cancer represents a major area of investigation as many cancers have genetic risk factors.
Prostate cancer is one of the most common cancers in males and is the second-most common cause of cancer-related mortality in males in Western countries [169]. Despite advances in early detection and treatment, the specific genetic mechanism contributing to the development of prostate cancer has not yet been fully elucidated. Prostate cancer is also a highly heritable cancer, necessitating the study of potential causative genes. It has been demonstrated that genetically or pharmacologically inhibiting NBCe1 in prostate cancer cell lines decreases the level of cancer cell proliferation and associated cellular markers. It has been posited that this is due to changes in the AKT phosphorylation pathway as well as HIF1α involvement following NBCe1’s correcting of an acid load [111,119].
Pancreatic cancer has a great disease burden with a very poor prognosis upon diagnosis (10–20% 5-year survival rate depending on staging) [172]. Unfortunately, due to vague symptom presentation [128], it is frequently diagnosed in later stages when metastasis is more likely. Reliable pancreatic diagnostic tools, as seen with routine mammograms or prostate exams, do not exist [128]. Data from several RNA-seq studies performed by Cappellesso and colleagues has identified SLC4A4 as being upregulated to a clinically relevant degree in pancreatic ductal adenocarcinoma (PDAC), specifically in the ductal epithelial cells [28]. Additionally, in a mouse PDAC model inhibiting NBCe1, either genetically or pharmacologically, improved immune response that is often associated with aggressive cancers. NBCe1 inhibition in combination with immune checkpoint blockade also allowed the mice to overcome resistance to immunotherapy as seen in certain varieties of PDAC and ultimately survive for a longer amount of time [28]. Inhibiting NBCe1’s function likely allows for HCO3− to accumulate in the extracellular space (increasing pHo), and potentially slowing oncogenic metabolism.
Nevertheless, SLC4A4’s broad role in cancer and tumorigenesis appears to be unclear. In certain cases, SLC4A4 appears upregulated- specifically in prostate cancer, colorectal cancer, renal carcinoma, and leukemias [38,64,65,70,119,205] while it is reliably downregulated in thyroid cancer (diagnostic marker) [69]. Despite this important discovery, the role of SLC4A4 in cancer development, progression, and treatment remains under-investigated.
NBCn1 (SLC4A7)
Solid cancers, including breast carcinomas are characterized as having similar properties, including changes in both pHi and pHo [70]. Some of these pH changes are due to elevated metabolic rates, prominent glycolytic activity, and a preference towards anaerobic metabolism, both of which lead to a hypoxic and acidic tumor microenvironment [45,182]. This hypoxia and lower pH are due to intracellular acid loading and the subsequent compensation leads eventually to an increase in cellular net acid extrusion, accomplished by NBCn1.
A GWAS examining genes involved in breast cancer development identified SLC4A7 as being upregulated twofold during the process of human breast carcinogenesis and identified a SLC4A7 variant (rs4973768) associated with an increased risk [37]. Importantly, NBCn1 localizes to breast tissue in both cancerous and normal tissue, although NBCn1 is 2.5-fold higher in mouse breast cancer organoids [104]. Interestingly, NBCn1 expression is upregulated during the process of breast carcinogenesis and not significantly upregulated or otherwise changed in later stages of breast cancer [13]. This implies a role of NBCn1 in solely the cellular migration of cancer, mirroring its role in conditions such as rheumatoid arthritis.
Disputed & variable ion transport: AE4 (SLC4A9) and BTR1 (SLC4A11)
AE4 (SLC4A9)
Slc4a9 was first cloned in 2001 by Tsuganezawa and coworkers [188] with Lipovich et al. reporting the human chromosomal location (5q31) [115]. SLC4A9 has 14 different splice variants [115]. Through various experimental means, it has been characterized as being expressed in the kidney, testis, and fetal brain [115]. SLC4A9 variants have not been associated with monogenic diseases or syndrome. However, SLC4A9 protein colocalizes and interacts with SLC26A4 (Pendrin), e.g., intercalated cells of the kidney collecting duct [195].
Function of SLC4A9 is disputed. Slc4a9 was originally reported as a Na+ IN-dependent Cl--HCO3− exchanger [188,95,206] and apparently confirmed [146]. Using pH-electrode experiments Parker and Boron showed that at least one Slc4a9 isoform is an electroneutral Na+ HCO3− cotransporter, i.e., NBCn3 [140] (Fig. 1). Pena-Munzenmayer and coworkers have reported that the mouse SLC4A9 and human SLC4A9 are a unique electroneutral monovalent cation-dependent Cl−-HCO3− exchanger capable of using any of the Group I alkali-metals [147]. More recently, Vitzthum and coworkers have suggested that Slc4a9 may be a HCO3− sensing protein of the kidney distal nephron [195]. Further detailed studies will be needed to determine the correct and perhaps variable role of the Slc4a9 isoforms.
BTR1 (SLC4A11)
SLC4A11 was identified by Parker and Tanner as a sequence with unknown function (i.e., bicarbonate transporter 1, BTR1) [143]. Function of SLC4A11 has been heavily debated with early studies indicating electrogenic Na+ borate cotransport (mouse and human) [138]. More detailed analysis of mammalian Slc4a11 revealed neither borate nor HCO3− coupling, but rather an EIPA-sensitive pH regulator [134], NH3 transporter [213,131], and OH− transport [131]. Later studies in the euryhaline teleost Takafugu obscurus showed that this fish Slc4a11 functions as a cation-independent boric acid channel or uniporter [92]. SLC4A11 is at 20p12 [143]. Many different SLC4A11 variants cause a recessive congenital hereditary endothelial dystrophy (CHED2, OMIM #613268, 217400, 21770), a condition involving corneal dystrophy and opacification [194,96] and FUCHS dystrophy (FECD1, OMIM#136800; FECD4, OMIM#613268). Clearly understanding the possible functions of human SLC4A11 isoforms will be critical to treat or possibly prevent these corneal issues.
SLC4 Structures
The protein structures of several SLC4 family members (AE1, NBCe1, and NDCBE) have been solved with cryo electron microscopy (cryoEM) [79,193,199] (see Fig. 2A) and X-ray crystallography [5,8,212]:
Figure 2. Structural comparisons of NDCBE, NBCe1, and AE1.
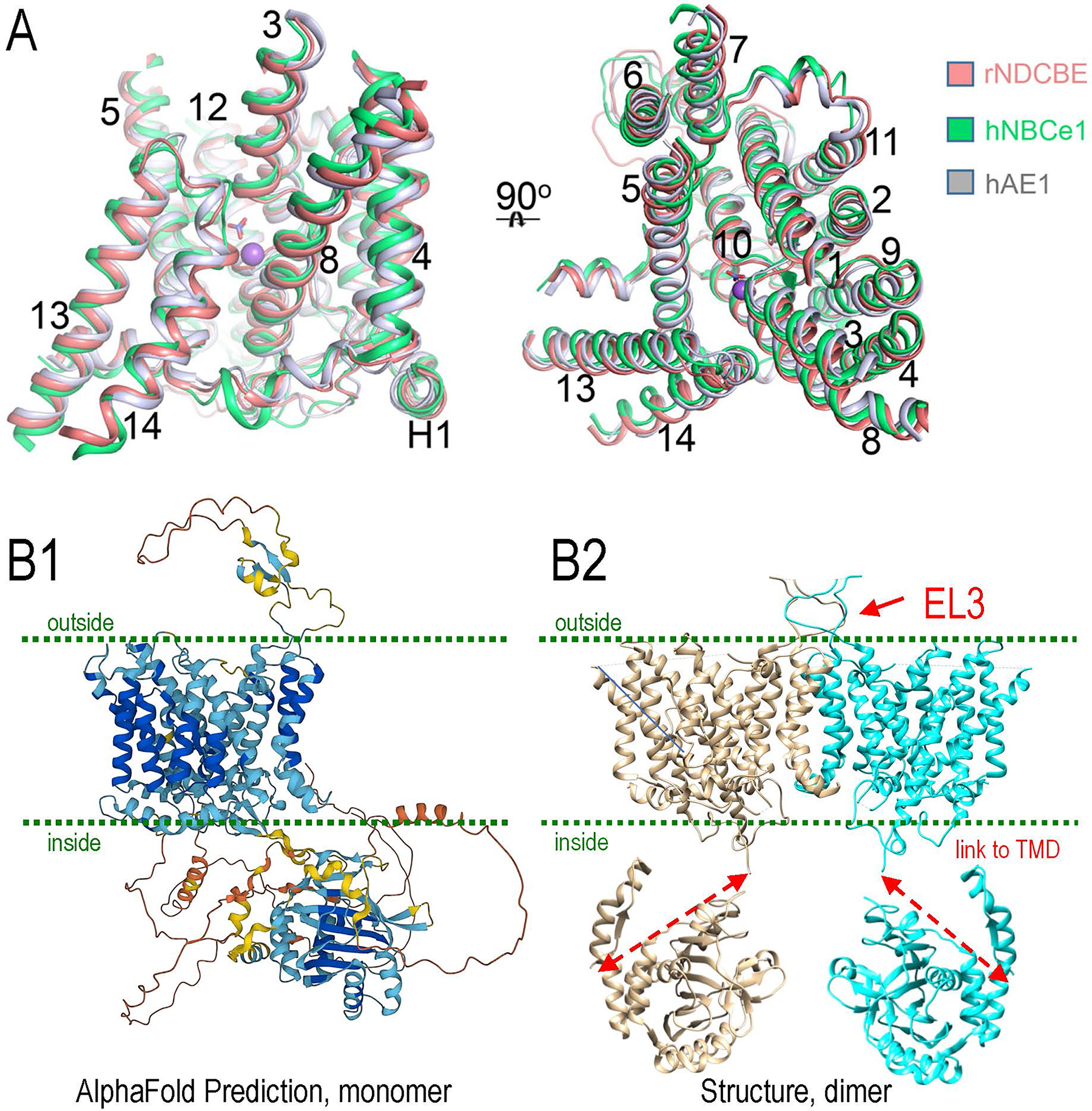
A. The structural superimposition of NDCBE (salmon), AE1 (gray) and NBCe1 (green) in the TMD. For clarity, all loops including EL3 are not shown.
B1. AlphaFold prediction of monomeric NBCe1 (SLC4A4) 3D-structure, based on UniProt “Q9Y6R1.” The color coding indicates levels of similarity of known X-ray or cryoEN structures (ground truth: dark blue = >90; light blue = 70–90%; yellow = 50–70%; orange = <50%). Areas of known structure [1HYN (AE1 globular domain, Xray) [212], 6CAA (NBCe1, cryoEM) [79]] show >70% similarity, but still have areas of divergence in these structures as well as linker regions.
B2. Dimer structure of NBCe1 [6CAA (cryoEM) [79]] connected to the globular domain of SLC4A1 (1HYN, Xray) [212] shown experimentally to be similar in human NBCe1 [33]. Dotted red lines indicate where linkers are which are not defined in any of the ground truth structures. EL3 is the major extracellular loop in the SLC4 family members, but is not defined in structures but rather only models.
8D9N (AE1 whole protein, cryoEM) [8], 1HYN (AE1 globular domain, Xray) [212]
6CAA (NBCe1, cryoEM) [79]
7RTM (NDCBE, cryoEM) [199]
Structures of the other SLC4 family members have been partially elucidated using functional mutagenesis. Of the structures that have been extensively validated, there are some conserved features, even between anion exchangers (AE1, AE2, AE3) and NCBTs: exist as dimers (except AE1 tetramer [8]); similar spanning transmembrane segments (TMs) [79,193,199,55]; and have variable C- and N-termini ends. Recently available tools such as AlphaFold [89,190] allow for a visualization of the predicted protein structures of all NCBTs in the SLC4 family.
While AlphaFold can be a good predictor of unknown structures, when compared to “ground truth” structures from X-ray crystallography or cryoEM, there are very obvious differences vs PDB structures (compare Fig 2B1 to 2B2), the agreement is overall in the 70–90+ % agreement for the transmembrane domains. The Xray structure defining the globular intracellular domain of AE1 (pdb = 1HYN) is also >70% conserved. However, the extreme N- and C-termini as well as the connections between the membrane and globular domain tend to all be below 50%. While neither result is surprising, the reviewer is correct that we should comment on this as even in the membrane spans, where AlphaFold should be the best predictor, is NOT >90% accurate for the identical sequence. This of course means that the machine learning datasets for training algorithms and the resulting AI are data deficient. To drive this point home a bit more, we have added a paragraph in the structural section as well as adding and additional panel to Figure 2. This panel addition illustrates the AlphaFold AI-prediction against the “ground truth” of the NBCe1 cryoEM (6CAA) coupled with the AE1-cytoplasmic, globular domain (1HYN) that we have previously shown is an extremely good predictive model of NBCe1 [33].
The electrogenic NCBTs: NBCe1, NBCe2
NBCe1 (SLC4A4)
A 3.9 Å resolution structure of human NBCe1 has been solved via cryoEM, giving insight into several functionally important areas within the protein, including the ion coordination site and ion accessibility pathway [79]. The NBCe1 N- and C- termini are intracellular and truncation of either causes dysfunction of the transporter [59]. Mutations have identified the ion coordination site helped to characterize pathogenic NBCe1 variants [79]. NBCe1 appears to exist as a homodimer in solution and membranes [32]. Recent modeling and functional expression reveal only two anion coordination sites that may hold HCO3− or CO32− [204] (Fig. 3).
Figure 3. Ion coordination in NBCe1.
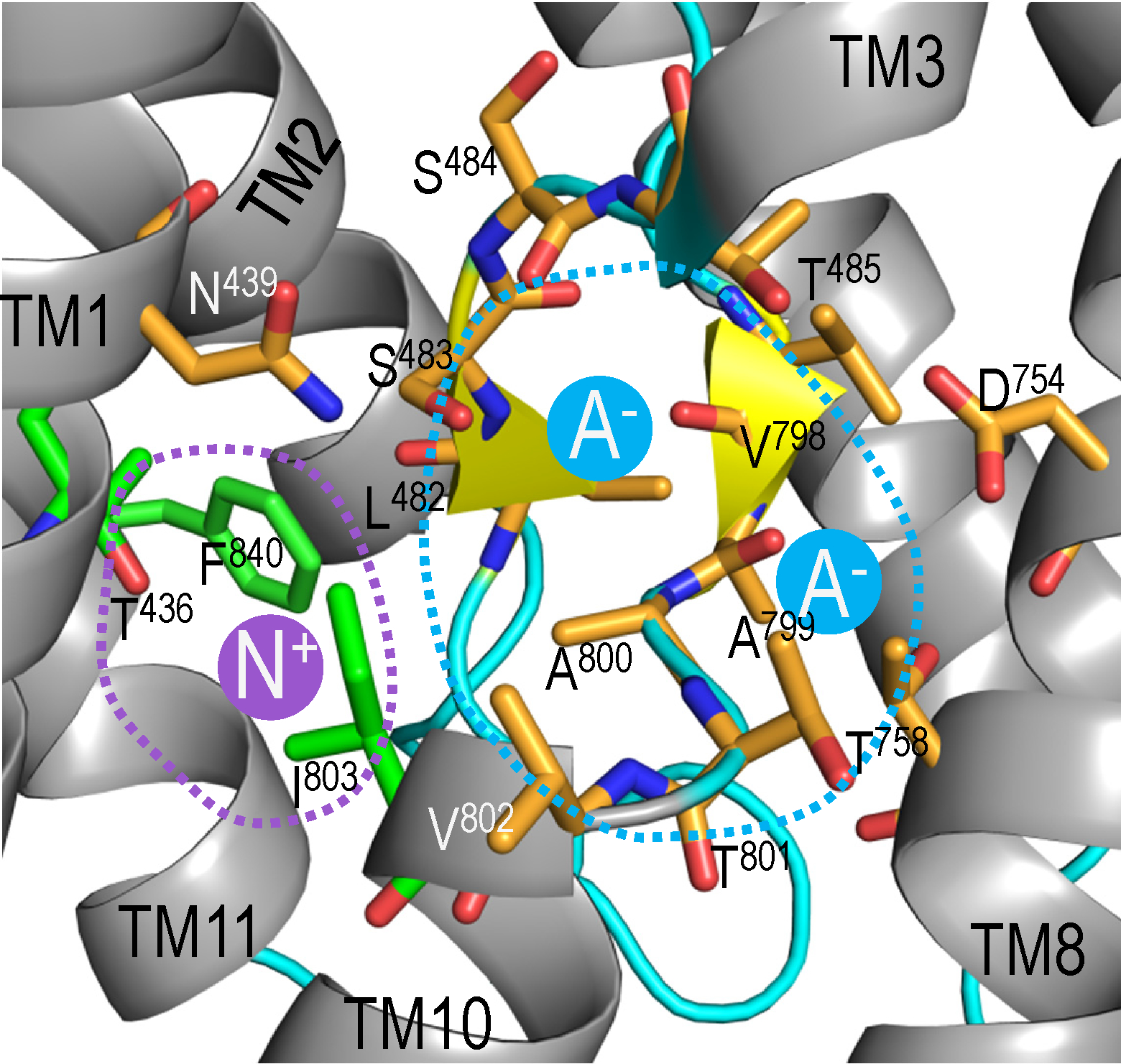
A structural model showing the region for coordination of Na+ (indicated by dashed purple line) and the region for HCO3− (indicated by blue dashed line) in the substrate pocket of NBCe1. NBCe1 has the capacity to bind two anions in the substrate pocket [204].
Material in panel A adapted from: ‘Wang et al., Cryo-EM structure of the sodium-driven chloride/bicarbonate exchanger NDCBE, Nature Communications, published 2021, Nature Portfolio’ [199].
Creative commons licence can be found at https://creativecommons.org/licenses/by/4.0/
Material in panel B adapted from: ‘Wu et al., Molecular insight into coordination sites for substrates and their coupling kinetics in Na+/HCO3− cotransporter NBCe1, The Journal of Physiology, published 2022, The Physiological Society’ [204]. Licence number for reuse: 5687740758558.
NBCe1 shares structural similarities to AE1 [8], and NDCBE [5,199] (Fig. 2A) with the caveat that AE1 forms homotetramers in red blood cells [8]. Additionally, there are NCBT structural similarities between the SLC4 and SLC26 families theorized to perform ion and solute transport with an “elevator alternating-access mode” [55].
The electroneutral NCBTs: NBCn1, NBCn2, NDCBE
3D structures for neither NBCn1 nor NBCn2 have been determined. However, mutagenesis studies and tools such as AlphaFold [89,190] have provided some structural insights controlling function.
NDCBE (SLC4A8)
The NDCBE structure was solved by cryoEM at 3.4 Å [199], and x-ray crystallography was used to elucidate its regulatory domain [5] (Fig. 2A). As hypothesized or determined for most SLC4 members, NDCBE is a homodimer [199]. Likewise, NBDCE’s structure contains regions resembling AE1 [8] and NBCe1 [79]. For example, its N-terminus is cytoplasmic and smaller the large C-terminal transmembrane domain. The binding pocket architecture is particularly interesting. Mutation of 5 key residues in the ion coordination area of NBCe1 adds Cl−-HCO3− exchange activity [79]. The NDCBE structure seems to illustrate a bridge between AE1 and NCBTs of SLC4, having properties similar to both, illustrated by close helix overlap (Fig. 2A). Table 3 provides ion coordination sites for Na+, HCO3−, CO32−, and Cl− based on these structures. As illustrated in Fig 1 and Fig 3, these NCBT protein seem to coordinate at most two anions (HCO3−, CO32−, or Cl−) indicating that within the protein one CO32− is physiologically equivalent to two HCO3− ions.
Table 3.
Critical NCBT amino acids: residues involved in Na+, HCO3− (CO32−), and Cl− transport
| Property | NBCe1 | NDCBE | AE1 |
|---|---|---|---|
| Na+ binding | Thr436 | Asp800 | Ala |
| Ile803 | Leu849 | Arg | |
| Phe840 | Ala | ||
| HCO3− binding |
Asn439 | Gly536 | Pro |
| Leu482 | Ser537 | Val1,3/Ile2* | |
| Ser483 | Thr538 | Gly | |
| Ser484 | Asp800 | Phe | |
| Thr485 | Asp780 | Ser | |
| Asp754 | Val844 | Glu | |
| Thr758 | Ala846 | Thr | |
| Val798 | Leu849 | Ser1 Ala2 Thr3 | |
| Ala800 | Thr1/Ala2,3 | ||
| Thr801 | Thr | ||
| Val802 | Val | ||
| Changes required for Cl− transport | Gly483, Phe484, Ser485 | Thr847 | Gly463, Phe484, Ser485 |
| Glu754 | Val848 | Glu684 | |
| Ser798 | Ser725 | ||
| Thr800 | Thr727 | ||
| Arg803 | Arg730 | ||
Evolution of NCBT family in chordates (including vertebrates).
Human SLC4A4 (NBCe1), SLC4A5 (NBCe2), SLC4A7 (NBCn1), SLC4A8 (NDCBE), SLC4A9 (AE4), and SLC4A10 (NBCn2) form a subfamily and are closely related evolutionarily [139,154]. The recent increase in the number of animal genome data make it possible to determine the composition, number, and phylogenetic relationships of NCBT genes in various animal species by using homology searches and molecular phylogenetic analysis (Fig. 4). Only a few of these genes have been given names based on actual measurements of the activity of their protein products, and many others have been given names simply on the basis of homology with related genes, so whether they actually have the activity as named is a matter of guesswork.
Figure 4. NCBTs in animals.
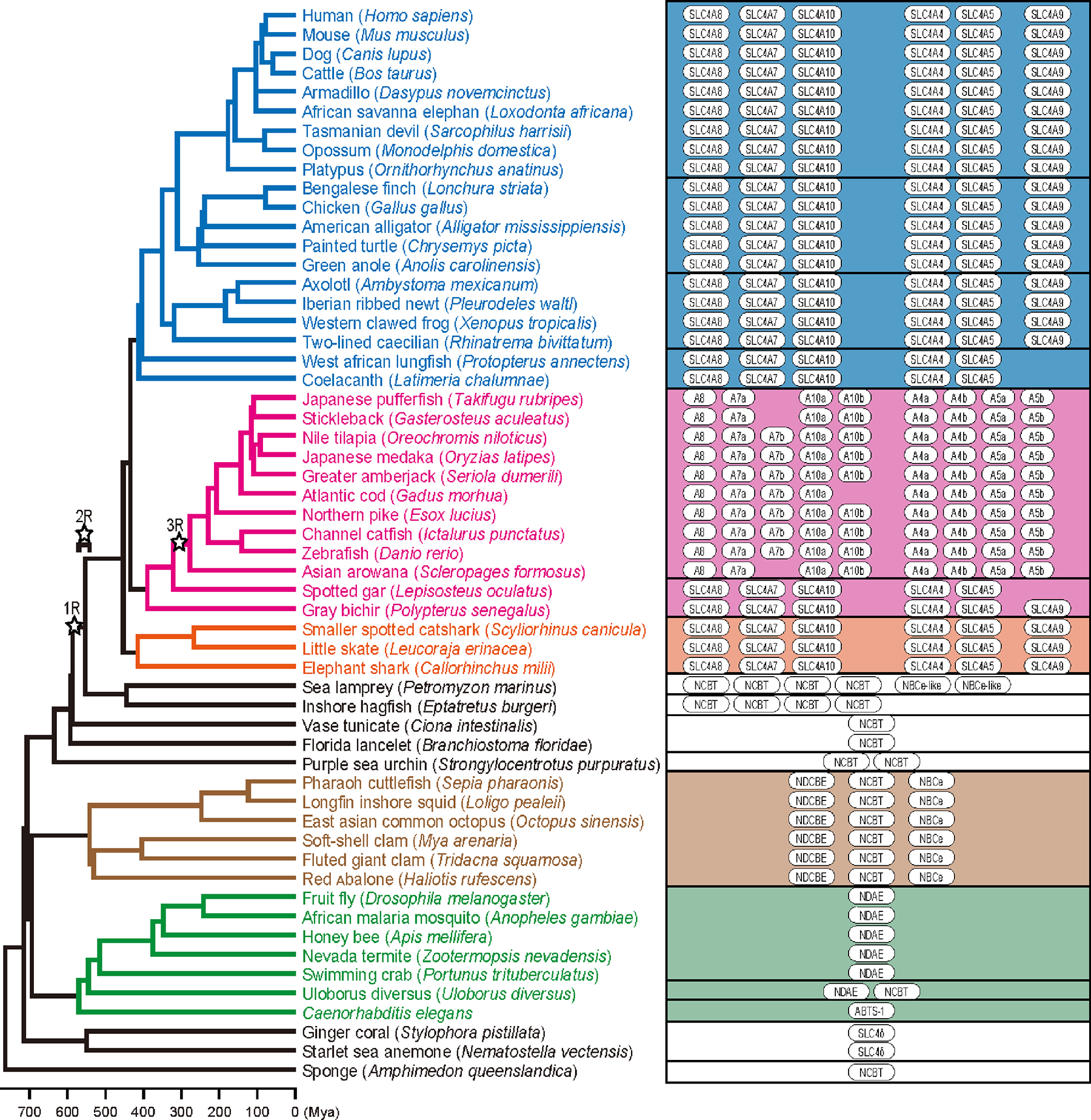
An overview of the distribution of NCBTs in the genome databases of various animal species are shown with the phylogeny of various vertebrate species based on the TimeTree database (http://www.timetree.org/) [97]. Tetrapods shown in blue section, teleosts shown in pink and orange, primitive fish shown in middle white section, mollusks shown in brown, insects, anilids and arthropods shown in green, and cnidaria shown in bottom white section. Open stars indicate the timing of three rounds of whole-genome duplication occurred in vertebrate evolution.
Mammals, birds/reptiles, and amphibians have one Slc4a4, 5, 7, 8, 9, and 10 gene each without exception, indicating that the genetic composition of the NCBT family is well conserved among tetrapods (Fig. 4, blue section). Lobe-finned fishes (lungfish and coelacanth) have single Slc4a4, 5, 7, and 8 genes, but lack Slc4a9. Chondrichthyans possess one each of the Slc4a4, 5, 7, 8, 9, and 10 genes, indicating that these gene sets were already present in the common ancestor of jawed vertebrates (Fig. 4, pink section). Polypterus and gar belong to “ancient” ray-finned fishes. Polypterus possess one each of the Slc4a4, 5, 7, 8, and 10 genes, whereas gar lacks Slc4a9 (Fig. 4, middle white section). Teleosts have two Slc4a4, two Slc4a5, one or two Slc4a7, one Slc4a8, and one or two Slc4a10, suggesting that teleosts maintained paralogs caused by teleost specific whole genome duplication according to species [216]. Teleost species lack the Slc4a9 gene. These indicate that Slc4a9 was lost secondarily in lobe-finned fishes, gars, and teleosts. Zebrafish and pufferfish orthologs of Slc4a4/NBCe1 were cloned and functionally confirmed to be NBCe’s [179,98,34] (Fig. 4, pink and orange sections).
The distribution of NCBTs in the genome databases of jawless vertebrates shows a different pattern between hagfish and lamprey. Hagfish has four NCBT genes related to NDCBE/NBCn, but no genes close to NBCe. On the other hand, lamprey has four NCBT genes related to NDCBE/NBCn but two genes similar to NBCe. Lower Chordates, e.g., Ciona and lancelet, have only one NCBT gene related to vertebrate NCBTs (Fig. 4 middle white section).
Evolution of the NCBT family in various invertebrates.
The genome database of one of the most primitive animal species, the sponge (Amphimedon queenslandica), shows the presence of one NCBT gene. Corals, which also belong to the primitive Cnidarians, have one NCBT gene (SLC4δ) [221] (Fig. 4, bottom white section). Caenorhabditis elegans has one NCBT gene known as ABTS-1 [170]. Dipterans, e.g., Drosophila and Anopheles, have one gene belonging to NCBT, and activity measurements have been reported as NDAE [155,114]. Other insects and arthropods, including crabs, have a NCBT gene closely related to Drosophila NDAE. Beyond NDAE, spiders have an additional NCBT gene (Fig. 4, green section). A NBCe and a NDCBE have been cloned from squid Loligo pealei, and functionally analyzed [192,148]. Squids, octopi, and shellfish have one ortholog each of the L. pealei-like NBCe and NDCBE as well as a third NCBT (Fig. 4, brown section). Sea urchin (Echinodermata closely related to Chordata) has two NCBTs that are close to the chordate NCBTs (Fig. 4, middle white section).
Conclusion and further discussion
NCBTs and their isoforms are extensively expressed in a variety of tissues, and active in both normal and pathophysiologic states. Every cell in the body requires a mechanism for balancing pHi, as deviations of even 0.1 pH units can lead to deleterious effects, including protein misfolding and enzyme dysfunction. This also means that acid-base imbalance is implicated in a variety of acute and chronic conditions, e.g., respiratory and metabolic alkalosis and acidosis, the cancer metabolism, excitability of neurons, osteopetrosis, diabetes, and even migratory cell growth (rheumatoid arthritis). This variety of pathologic states that could be controlled by NCBT activity control implies that these NCBT-transporters should likely be used in small molecule screens, or even gene therapies.
The elucidation of these transporters’ biophysical properties with the use of new structure-solving tools and further advancements in experimental methods represents a critical area of investigation for many different scientific disciplines. Further insight into these transporters’ biophysical properties could lead to the discovery of compounds that could interact with important areas, yielding a therapeutic effect based upon inhibition or augmentation, depending on the transporter’s role in health and disease.
The most exciting outlook regarding NCBTs is that multiple scientific disciplines and organisms reveals that NCBT-proteins are used for many aspects of animal life. These widespread NCBT niches mean that nature and evolution have already performed many experiments to generate the variety of functions. This also means that is much we can learn about “other-normals” and activity control from many species beyond mammals which could be useful in treating a wide range of human diseases or enhancing normal physiology.
Acknowledgements:
This review in part helped fulfill degree requirements (SRH) for Mayo Clinic Graduate School of Biomedical Sciences, Biochemistry and Molecular Biology Track, Mayo Clinic, Rochester, MN. We thank Drs. Peter C. Harris, Aleksey Matveyenko, and Lisa Schimmenti for guidance and comments on the manuscript.
Funding
This work was supported by DK057061, DK101405, DK128844, DK129897, and the Mayo Foundation. The Kato laboratory work was supported by Japan Society for the Promotion of Science (grant number 21H02281) and Laboratory for Design of Social Innovation in Global Networks (DLab), Tokyo Institute of Technology, Japan.
Footnotes
For a comprehensive list of the solute carrier family members, see: https://www.bioparadigms.org/slc/
For further information on NBCe1’s role in cancer, see below section on cancer.
As multiple NBCTs are implicated in various cancers, these are discussed together in the Cancer section.
References
- 1.Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I (1998) Molecular Cloning, Chromosomal Localization, Tissue Distribution, and Functional Expression of the Human Pancreatic Sodium Bicarbonate Cotransporter. J Biol Chem 273:17689–17695 [DOI] [PubMed] [Google Scholar]
- 2.Abuladze N, Song M, Pushkin A, Newman D, Lee I, Nicholas S, Kurtz I (2000) Structural organization of the human NBC1 gene: kNBC1 is transcribed from an alternative promoter in intron 3. Gene 251:109–122 [DOI] [PubMed] [Google Scholar]
- 3.Allen DG, Xiao XH (2003) Role of the cardiac Na+/H+ exchanger during ischemia and reperfusion. Cardiovasc Res 57:934–941. doi:S0008636302008362 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Alper SL, Sharma AK (2013) The SLC26 gene family of anion transporters and channels. Mol Aspects Med 34:494–515. doi: 10.1016/j.mam.2012.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvadia CM, Sommer T, Bjerregaard-Andersen K, Damkier HH, Montrasio M, Aalkjaer C, Morth JP (2017) The crystal structure of the regulatory domain of the human sodium-driven chloride/bicarbonate exchanger. Sci Rep 7:12131. doi: 10.1038/s41598-017-12409-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amlal H, Burnham CE, Soleimani M (1999) Characterization of Na+/HCO3- cotransporter isoform NBC-3. Am J Physiol 276:F903–913 [DOI] [PubMed] [Google Scholar]
- 7.Amlal H, Wang Z, Burnham C, Soleimani M (1998) Functional characterization of a cloned human kidney Na+:HCO3- cotransporter. J Biol Chem 273:16810–16815 [DOI] [PubMed] [Google Scholar]
- 8.Arakawa T, Kobayashi-Yurugi T, Alguel Y, Iwanari H, Hatae H, Iwata M, Abe Y, Hino T, Ikeda-Suno C, Kuma H, Kang D, Murata T, Hamakubo T, Cameron AD, Kobayashi T, Hamasaki N, Iwata S (2015) Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science 350:680–684. doi: 10.1126/science.aaa4335 [DOI] [PubMed] [Google Scholar]
- 9.Azimov R, Abuladze N, Sassani P, Newman D, Kao L, Liu W, Orozco N, Ruchala P, Pushkin A, Kurtz I (2008) G418-mediated ribosomal read-through of a nonsense mutation causing autosomal recessive proximal renal tubular acidosis. Am J Physiol Renal Physiol 295:F633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbuskaite D, Pedersen FD, Christensen HL, Johnsen LØ, Praetorius J, Damkier HH (2020) NBCe2 (Slc4a5) Is Expressed in the Renal Connecting Tubules and Cortical Collecting Ducts and Mediates Base Extrusion. Frontiers in Physiology 11. doi: 10.3389/fphys.2020.00560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellemer A, Hirata T, Romero MF, Koelle MR (2011) Two types of chloride-extruding transporters are required for GABAA receptor-mediated inhibition in C. elegans. The EMBO journal 30:1852–1863. doi: 10.1038/emboj.2011.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF (2000) An electrogenic Na/HCO3 cotransporter (NBC) with a novel C terminus, cloned from rat brain. Am J Physiol Cell Physiol 278:C1200–C1211 [DOI] [PubMed] [Google Scholar]
- 13.Boedtkjer E (2019) Na(+),HCO(3)(−) cotransporter NBCn1 accelerates breast carcinogenesis. Cancer Metastasis Rev 38:165–178. doi: 10.1007/s10555-019-09784-7 [DOI] [PubMed] [Google Scholar]
- 14.Boedtkjer E, Moreira JM, Mele M, Vahl P, Wielenga VT, Christiansen PM, Jensen VE, Pedersen SF, Aalkjaer C (2013) Contribution of Na+,HCO3(−)-cotransport to cellular pH control in human breast cancer: a role for the breast cancer susceptibility locus NBCn1 (SLC4A7). Int J Cancer 132:1288–1299. doi: 10.1002/ijc.27782 [DOI] [PubMed] [Google Scholar]
- 15.Boedtkjer E, Praetorius J, Aalkjaer C (2006) NBCn1 (slc4a7) mediates the Na+-dependent bicarbonate transport important for regulation of intracellular pH in mouse vascular smooth muscle cells. Circulation research 98:515–523. doi: 10.1161/01.RES.0000204750.04971.76 [DOI] [PubMed] [Google Scholar]
- 16.Bok D, Schibler MJ, Pushkin A, Sassani P, Abuladze N, Naser Z, Kurtz I (2001) Immunolocalization of electrogenic sodium-bicarbonate cotransporters pNBC1 and kNBC1 in the rat eye. Am J Physiol Renal Physiol 281:F920–935 [DOI] [PubMed] [Google Scholar]
- 17.Boron WF (1986) Intracellular pH Regulation in Epithelial Cells. Annual Review of Physiology 48:377–388. doi: 10.1146/annurev.ph.48.030186.002113 [DOI] [PubMed] [Google Scholar]
- 18.Boron WF, Boulpaep EL (2017) Medical Physiology. 3rd edn. Elsevier, Philadelphia, PA [Google Scholar]
- 19.Boron WF, Chen L, Parker MD (2009) Modular structure of sodium-coupled bicarbonate transporters. J Exp Biol 212:1697–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boron WF, Sackin H (1983) Measurement of intracellular ionic composition and activities in renal tubules. Annu Rev Physiol 45:483–496 [DOI] [PubMed] [Google Scholar]
- 21.Bouyer P, Sakai H, Itokawa T, Kawano T, Fulton CM, Boron WF, Insogna KL (2007) Colony-stimulating factor-1 increases osteoclast intracellular pH and promotes survival via the electroneutral Na/HCO3 cotransporter NBCn1. Endocrinology 148:831–840. doi: 10.1210/en.2006-0547 [DOI] [PubMed] [Google Scholar]
- 22.Brady CT, Dugandžić A, Parker M, Romero MF (2021) NBCe1, an electrogenic Na+ bicarbonate (carbonate) cotransporter, in epithelia. In: Hamilton KL, Devor DC(eds) Ion Channels and Transporters of Epithelia in Health and Disease, 2nd edition. Springer (Am Physiol Soc), New York, pp 93–124. doi: 10.1007/978-3-030-55454-5_4 [DOI] [Google Scholar]
- 23.Brady CT, Marshall A, Zhang C, Parker MD (2023) NBCe1-B/C-knockout mice exhibit an impaired respiratory response and an enhanced renal response to metabolic acidosis. Front Physiol 14:1201034. doi: 10.3389/fphys.2023.1201034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown MR, Holmes H, Rakshit K, Javeed N, Her T, Stiller A, Shull GE, Prakash YS, Romero MF, Matveyenko AV (2021) Electrogenic Na+-nHCO3- cotransporter NBCe1 is a novel regulator of pancreatic β cell function in Type 2 diabetes. J Clin Invest 131:e142365. doi: 10.1172/JCI142365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown MR, Holmes H, Rakshit K, Javeed N, Her TK, Stiller AA, Sen S, Shull GE, Prakash YS, Romero MF, Matveyenko AV (2021) Electrogenic sodium bicarbonate cotransporter NBCe1 regulates pancreatic β cell function in type 2 diabetes. The Journal of Clinical Investigation 131:e142365. doi: 10.1172/JCI142365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brungger M, Hulter HN, Krapf R (1997) Effect of chronic metabolic acidosis on the growth hormone/IGF-1 endocrine axis: new cause of growth hormone insensitivity in humans. Kidney Int 51:216–221. doi: 10.1038/ki.1997.26 [DOI] [PubMed] [Google Scholar]
- 27.Burette AC, Weinberg RJ, Sassani P, Abuladze N, Kao L, Kurtz I (2012) The sodium-driven chloride/bicarbonate exchanger in presynaptic terminals. J Comp Neurol 520:1481–1492. doi: 10.1002/cne.22806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cappellesso F, Orban MP, Shirgaonkar N, Berardi E, Serneels J, Neveu MA, Di Molfetta D, Piccapane F, Caroppo R, Debellis L, Ostyn T, Joudiou N, Mignion L, Richiardone E, Jordan BF, Gallez B, Corbet C, Roskams T, DasGupta R, Tejpar S, Di Matteo M, Taverna D, Reshkin SJ, Topal B, Virga F, Mazzone M (2022) Targeting the bicarbonate transporter SLC4A4 overcomes immunosuppression and immunotherapy resistance in pancreatic cancer. Nat Cancer 3:1464–1483. doi: 10.1038/s43018-022-00470-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carey RM, Schoeffel CD, Gildea JJ, Jones JE, McGrath HE, Gordon LN, Park MJ, Sobota RS, Underwood PC, Williams J, Sun B, Raby B, Lasky-Su J, Hopkins PN, Adler GK, Williams SM, Jose PA, Felder RA (2012) Salt sensitivity of blood pressure is associated with polymorphisms in the sodium-bicarbonate cotransporter. Hypertension 60:1359–1366. doi: 10.1161/HYPERTENSIONAHA.112.196071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casey JR, Grinstein S, Orlowski J (2010) Sensors and regulators of intracellular pH. Nature Reviews Molecular Cell Biology 11:50–61. doi: 10.1038/nrm2820 [DOI] [PubMed] [Google Scholar]
- 31.Ch’en FF, Villafuerte FC, Swietach P, Cobden PM, Vaughan-Jones RD (2008) S0859, an N-cyanosulphonamide inhibitor of sodium-bicarbonate cotransport in the heart. British journal of pharmacology 153:972–982. doi: 10.1038/sj.bjp.0707667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang M-H, Chen A-P, Romero MF (2014) NBCe1A dimer assemble visualized by bimolecular fluorescence complementation (BiFC). Am J Physiol Renal Physiol 306:F672–680. doi: 10.1152/ajprenal.00284.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang M-H, DiPiero JM, Sönnichsen FD, Romero MF (2008) Entry to “HCO3 tunnel” revealed by human mutation and structural model. J Biol Chem 283:18402–18410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang M-H, Plata C, Kurita Y, Kato A, Hirose S, Romero MF (2012) Euryhaline Pufferfish NBCe1 differs from non-marine species NBCe1 physiology. Am J Physiol Cell Physiol 302:C1083–1095. doi:doi: 10.1152/ajpcell.00233.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen LM, Haddad GG, Boron WF (2008) Effects of chronic continuous hypoxia on the expression of SLC4A8 (NDCBE) in neonatal versus adult mouse brain. Brain Res 1238:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen LM, Kelly ML, Parker MD, Bouyer P, Gill HS, Felie JM, Davis BA, Boron WF (2008) Expression and localization of Na-driven Cl-HCO(3)(−) exchanger (SLC4A8) in rodent CNS. Neuroscience 153:162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen W, Zhong R, Ming J, Zou L, Zhu B, Lu X, Ke J, Zhang Y, Liu L, Miao X, Huang T (2012) The SLC4A7 variant rs4973768 is associated with breast cancer risk: evidence from a case-control study and a meta-analysis. Breast Cancer Res Treat 136:847–857. doi: 10.1007/s10549-012-2309-9 [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Chen J, Feng Y, Guan W (2020) Prognostic Value of SLC4A4 and its Correlation with Immune Infiltration in Colon Adenocarcinoma. Med Sci Monit 26:e925016. doi: 10.12659/MSM.925016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi I, Aalkjaer C, Boulpaep EL, Boron WF (2000) An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 405:571–575 [DOI] [PubMed] [Google Scholar]
- 40.Christensen HL, Barbuskaite D, Rojek A, Malte H, Christensen IB, Fuchtbauer AC, Fuchtbauer EM, Wang T, Praetorius J, Damkier HH (2018) The choroid plexus sodium-bicarbonate cotransporter NBCe2 regulates mouse cerebrospinal fluid pH. J Physiol 596:4709–4728. doi: 10.1113/JP275489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciocci Pardo A, Gonzalez Arbelaez LF, Fantinelli JC, Aiello EA, Mosca SM (2019) Calcineurin/P38MAPK/HSP27-dependent pathways are involved in the attenuation of postischemic mitochondrial injury afforded by sodium bicarbonate co-transporter (NBCe1) inhibition. Biochem Pharmacol 161:26–36. doi: 10.1016/j.bcp.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 42.Collin GB, Shi L, Yu M, Akturk N, Charette JR, Hyde LF, Weatherly SM, Pera MF, Naggert JK, Peachey NS, Nishina PM, Krebs MP (2022) A Splicing Mutation in Slc4a5 Results in Retinal Detachment and Retinal Pigment Epithelium Dysfunction. Int J Mol Sci 23. doi: 10.3390/ijms23042220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colmenares-Aguilar M-G, Mazzone A, Eisenman S, Strege P, Bernard C, Holmes H, Romero M, Farrugia G, Gibbons S (2021) Expression of the Regulated Isoform of the Electrogenic Na+/HCO3- Cotransporter, NBCe1, is Enriched in Pacemaker Interstitial Cells of Cajal. Am J Physiol-GI & Liver Physiology 320:G93–G107. doi: 10.1152/ajpgi.00255.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Damkier HH, Nielsen S, Praetorius J (2007) Molecular expression of SLC4 derived Na+ dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol [DOI] [PubMed] [Google Scholar]
- 45.Danielsen AA, Parker MD, Lee S, Boron WF, Aalkjaer C, Boedtkjer E (2013) Splice cassette II of Na+,HCO3(−) cotransporter NBCn1 (slc4a7) interacts with calcineurin A: implications for transporter activity and intracellular pH control during rat artery contractions. The Journal of biological chemistry 288:8146–8155. doi: 10.1074/jbc.M113.455386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deda G, Ekim M, Guven A, Karagol U, Tumer N (2001) Hypopotassemic paralysis: a rare presentation of proximal renal tubular acidosis. J Child Neurol 16:770–771 [DOI] [PubMed] [Google Scholar]
- 47.Demirci FY, Chang M-H, Mah TS, Romero MF, Gorin MB (2006) Proximal renal tubular acidosis and ocular pathology: L522P, a novel missense mutation in the gene (SLC4A4) for Sodium Bicarbonate Cotransporter protein (NBCe1). Molecular Vision 12:324–330 [PubMed] [Google Scholar]
- 48.Di Mattia RA, Diaz-Zegarra LA, Blanco PG, Valverde CA, Gonano LA, Jaquenod De Giusti C, Portiansky EL, Vila-Petroff MG, Aiello EA, Orlowski A (2023) The specific inhibition of the cardiac electrogenic sodium/bicarbonate cotransporter leads to cardiac hypertrophy. Life Sci 312:121219. doi: 10.1016/j.lfs.2022.121219 [DOI] [PubMed] [Google Scholar]
- 49.Di Mattia RA, Diaz Zegarra LA, Valverde CA, Blanco PG, Jaquenod De Giusti C, Portiansky EL, Aiello EA, Orlowski A (2022) In vivo Overexpression of Electrogenic Sodium/Bicarbonate Cotransporter (NBCe1) by AAV9 Modifies the Cardiac Action Potential and the QT Interval in Mice. Front Cardiovasc Med 9:862118. doi: 10.3389/fcvm.2022.862118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dietrich CJ, Morad M (2010) Synaptic acidification enhances GABAA signaling. J Neurosci 30:16044–16052. doi: 10.1523/JNEUROSCI.6364-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dinour D, Chang M-H, Satoh J-I, Smith BL, Angle N, Knecht A, Serban I, Holtzman EJ, Romero MF (2004) A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J Biol Chem 279:52238–52246 [DOI] [PubMed] [Google Scholar]
- 52.Dinour D, Mini S, Polak-Charcon S, Lotan D, Holtzman EJ (2004) Progressive nephropathy associated with mitochondrial tRNA gene mutation. Clinical nephrology 62:149–154 [DOI] [PubMed] [Google Scholar]
- 53.Dolensek J, Rupnik MS, Stozer A (2015) Structural similarities and differences between the human and the mouse pancreas. Islets 7:e1024405. doi: 10.1080/19382014.2015.1024405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Douglas RM, Schmitt BM, Xia Y, Bevensee MO, Biemesderfer D, Boron WF, Haddad GG (2001) Sodium-hydrogen exchangers and sodium-bicarbonate co-transporters: ontogeny of protein expression in the rat brain. Neuroscience 102:217–228. [DOI] [PubMed] [Google Scholar]
- 55.Drew D, Boudker O (2016) Shared Molecular Mechanisms of Membrane Transporters. Annu Rev Biochem 85:543–572. doi: 10.1146/annurev-biochem-060815-014520 [DOI] [PubMed] [Google Scholar]
- 56.Ducoudret O, Diakov A, Muller-Berger S, Romero MF, Fromter E (2001) The renal Na-HCO3-cotransporter expressed in Xenopus laevis oocytes: inhibition by tenidap and benzamil and effect of temperature on transport rate and stoichiometry. Pflugers Arch 442:709–717. doi: 10.1007/s004240100594 [DOI] [PubMed] [Google Scholar]
- 57.Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ, Masino SA (2005) Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron 48:1011–1023. doi: 10.1016/j.neuron.2005.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ellison DH, Loffing J (2009) Thiazide effects and adverse effects: insights from molecular genetics. Hypertension 54:196–202. doi: 10.1161/HYPERTENSIONAHA.109.129171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Espiritu DJ, Bernardo AA, Arruda JA (2006) The role of the NH2 and the COOH termini in targeting, stability and activity of sodium bicarbonate cotransporter 1 (NBC1). Am J Physiol Renal Physiol [DOI] [PubMed] [Google Scholar]
- 60.Fang L, Lee HW, Chen C, Harris AN, Romero MF, Verlander JW, Weiner ID (2018) Expression of the B splice variant of NBCe1 (SLC4A4) in the mouse kidney. Am J Physiol Renal Physiol 315:F417–F428. doi: 10.1152/ajprenal.00515.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fantinelli JC, Orlowski A, Aiello EA, Mosca SM (2014) The electrogenic cardiac sodium bicarbonate co-transporter (NBCe1) contributes to the reperfusion injury. Cardiovasc Pathol 23:224–230. doi: 10.1016/j.carpath.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 62.Felder RA, Jose PA, Xu P, Gildea JJ (2016) The Renal Sodium Bicarbonate Cotransporter NBCe2: Is It a Major Contributor to Sodium and pH Homeostasis? Current hypertension reports 18:71. doi: 10.1007/s11906-016-0679-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fu Z R Gilbert E, Liu D (2013) Regulation of Insulin Synthesis and Secretion and Pancreatic Beta-Cell Dysfunction in Diabetes. Current Diabetes Reviews 9:25–53. doi: 10.2174/157339913804143225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao X, Yang J (2020) Identification of Genes Related to Clinicopathological Characteristics and Prognosis of Patients with Colorectal Cancer. DNA Cell Biol 39:690–699. doi: 10.1089/dna.2019.5088 [DOI] [PubMed] [Google Scholar]
- 65.Gerber JM, Gucwa JL, Esopi D, Gurel M, Haffner MC, Vala M, Nelson WG, Jones RJ, Yegnasubramanian S (2013) Genome-wide comparison of the transcriptomes of highly enriched normal and chronic myeloid leukemia stem and progenitor cell populations. Oncotarget 4:715–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giffard RG, Lee YS, Ouyang YB, Murphy SL, Monyer H (2003) Two variants of the rat brain sodium-driven chloride bicarbonate exchanger (NCBE): developmental expression and addition of a PDZ motif. Eur J Neurosci 18:2935–2945 [DOI] [PubMed] [Google Scholar]
- 67.Gildea JJ, Xu P, Carlson JM, Gaglione RT, Bigler Wang D, Kemp BA, Reyes CM, McGrath HE, Carey RM, Jose PA, Felder RA (2015) The sodium-bicarbonate cotransporter NBCe2 (slc4a5) expressed in human renal proximal tubules shows increased apical expression under high-salt conditions. American journal of physiology Regulatory, integrative and comparative physiology 309:R1447–1459. doi: 10.1152/ajpregu.00150.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gildea JJ, Xu P, Kemp BA, Carlson JM, Tran HT, Bigler Wang D, Langouet-Astrie CJ, McGrath HE, Carey RM, Jose PA, Felder RA (2018) Sodium bicarbonate cotransporter NBCe2 gene variants increase sodium and bicarbonate transport in human renal proximal tubule cells. PLoS One 13:e0189464. doi: 10.1371/journal.pone.0189464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gomez-Rueda H, Palacios-Corona R, Gutierrez-Hermosillo H, Trevino V (2016) A robust biomarker of differential correlations improves the diagnosis of cytologically indeterminate thyroid cancers. International journal of molecular medicine 37:1355–1362. doi: 10.3892/ijmm.2016.2534 [DOI] [PubMed] [Google Scholar]
- 70.Gorbatenko A, Olesen CW, Boedtkjer E, Pedersen SF (2014) Regulation and roles of bicarbonate transporters in cancer. Front Physiol 5:130. doi: 10.3389/fphys.2014.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grichtchenko II, II, Choi II, Zhong X, Bray-Ward P, Russell JM, Boron WF (2001) Cloning, characterization and chromosomal mapping of a human electroneutral Na+- driven Cl-HCO3 exchanger. J Biol Chem 276:8358–8363 [DOI] [PubMed] [Google Scholar]
- 72.Groger N, Vitzthum H, Frohlich H, Kruger M, Ehmke H, Braun T, Boettger T (2012) Targeted mutation of SLC4A5 induces arterial hypertension and renal metabolic acidosis. Hum Mol Genet 21:1025–1036. doi:ddr533 [pii], 10.1093/hmg/ddr533 [DOI] [PubMed] [Google Scholar]
- 73.Guo YM, Liu Y, Liu M, Wang JL, Xie ZD, Chen KJ, Wang DK, Occhipinti R, Boron WF, Chen LM (2017) Na(+)/HCO3(−) Cotransporter NBCn2 Mediates HCO3(−) Reclamation in the Apical Membrane of Renal Proximal Tubules. Journal of the American Society of Nephrology : JASN 28:2409–2419. doi: 10.1681/ASN.2016080930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gurnett CA, Veile R, Zempel J, Blackburn L, Lovett M, Bowcock A (2008) Disruption of sodium bicarbonate transporter SLC4A10 in a patient with complex partial epilepsy and mental retardation. Archives of neurology 65:550–553. doi: 10.1001/archneur.65.4.550 [DOI] [PubMed] [Google Scholar]
- 75.Hamm LL, Nakhoul N, Hering-Smith KS (2015) Acid-Base Homeostasis. Clin J Am Soc Nephrol 10:2232–2242. doi: 10.2215/CJN.07400715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hansell P, Welch WJ, Blantz RC, Palm F (2013) Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin Exp Pharmacol Physiol 40:123–137. doi: 10.1111/1440-1681.12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haque SK, Ariceta G, Batlle D (2012) Proximal renal tubular acidosis: a not so rare disorder of multiple etiologies. Nephrol Dial Transplant 27:4273–4287. doi: 10.1093/ndt/gfs493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horita S, Yamada H, Inatomi J, Moriyama N, Sekine T, Igarashi T, Endo Y, Dasouki M, Ekim M, Al-Gazali L, Shimadzu M, Seki G, Fujita T (2005) Functional Analysis of NBC1 Mutants Associated with Proximal Renal Tubular Acidosis and Ocular Abnormalities. J Am Soc Nephrol 16:2270–2278 [DOI] [PubMed] [Google Scholar]
- 79.Huynh KW, Jiang J, Abuladze N, Tsirulnikov K, Kao L, Shao X, Newman D, Azimov R, Pushkin A, Zhou ZH, Kurtz I (2018) CryoEM structure of the human SLC4A4 sodium-coupled acid-base transporter NBCe1. Nature communications 9:900. doi: 10.1038/s41467-018-03271-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hwang S, Shin DM, Hong JH (2020) Protective Role of IRBIT on Sodium Bicarbonate Cotransporter-n1 for Migratory Cancer Cells. Pharmaceutics 12. doi: 10.3390/pharmaceutics12090816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, Tsukamoto K, Satoh H, Shimadzu M, Tozawa F, Mori T, Shiobara M, Seki G, Endou H (1999) Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet 23:264–266 [DOI] [PubMed] [Google Scholar]
- 82.Igarashi T, Inatomi J, Sekine T, Seki G, Shimadzu M, Tozawa F, Takeshima Y, Takumi T, Takahashi T, Yoshikawa N, Nakamura H, Endou H (2001) Novel nonsense mutation in the Na+/HCO3- cotransporter gene (SLC4A4) in a patient with permanent isolated proximal renal tubular acidosis and bilateral glaucoma. J Am Soc Nephrol 12:713–718 [DOI] [PubMed] [Google Scholar]
- 83.Illek B, Yankaskas JR, Machen TE (1997) cAMP and genistein stimulate HCO3- conductance through CFTR in human airway epithelia. American Journal of Physiology-Lung Cellular and Molecular Physiology 272:L752–L761. doi: 10.1152/ajplung.1997.272.4.L752 [DOI] [PubMed] [Google Scholar]
- 84.Inatomi J, Horita S, Braverman N, Sekine T, Yamada H, Suzuki Y, Kawahara K, Moriyama N, Kudo A, Kawakami H, Shimadzu M, Endou H, Fujita T, Seki G, Igarashi T (2004) Mutational and functional analysis of SLC4A4 in a patient with proximal renal tubular acidosis. Pflugers Arch 448:438–444 [DOI] [PubMed] [Google Scholar]
- 85.Jacobs S, Ruusuvuori E, Sipila ST, Haapanen A, Damkier HH, Kurth I, Hentschke M, Schweizer M, Rudhard Y, Laatikainen LM, Tyynela J, Praetorius J, Voipio J, Hubner CA (2008) Mice with targeted Slc4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proc Natl Acad Sci U S A 105:311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ji M, Ryu HJ, Baek HM, Shin DM, Hong JH (2022) Dynamic synovial fibroblasts are modulated by NBCn1 as a potential target in rheumatoid arthritis. Exp Mol Med 54:503–517. doi: 10.1038/s12276-022-00756-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ji M, Ryu HJ, Hong JH (2021) Signalling and putative therapeutic molecules on the regulation of synoviocyte signalling in rheumatoid arthritis. Bone Joint Res 10:285–297. doi: 10.1302/2046-3758.104.BJR-2020-0331.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Josephsen K, Praetorius J, Frische S, Gawenis LR, Kwon TH, Agre P, Nielsen S, Fejerskov O (2009) Targeted disruption of the Cl-/HCO3- exchanger Ae2 results in osteopetrosis in mice. Proc Natl Acad Sci U S A 106:1638–1641. doi: 10.1073/pnas.0811682106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Zidek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D (2021) Highly accurate protein structure prediction with AlphaFold. Nature 596:583–589. doi: 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kalervo Väänänen H, Salo J, Lehenkari P (1996) Mechanism of osteoclast-mediated bone resorption. Journal of Bone and Mineral Metabolism 14:187–192. doi: 10.1007/bf01763818 [DOI] [Google Scholar]
- 91.Kao L, Kurtz LM, Shao X, Papadopoulos MC, Liu L, Bok D, Nusinowitz S, Chen B, Stella SL, Andre M, Weinreb J, Luong SS, Piri N, Kwong JM, Newman D, Kurtz I (2011) Severe Neurologic Impairment in Mice with Targeted Disruption of the Electrogenic Sodium Bicarbonate Cotransporter NBCe2 (Slc4a5 Gene). J Biol Chem 286:32563–32574. doi:M111.249961 [pii] 10.1074/jbc.M111.249961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kato A, Kimura Y, Kurita Y, Chang M-H, Kasai K, Fujiwara T, Hirata T, Doi H, Hirose S, Romero MF (2023) Seawater fish use an electrogenic boric acid transporter, Slc4a11A, for boric acid excretion by the kidney J Biol Chem 299:102740. doi: 10.1016/j.jbc.2022.102740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kato A, Romero MF (2011) Regulation of Electroneutral NaCl Absorption by the Small Intestine. Annual Review of Physiology 73:261–281. doi:doi: 10.1146/annurev-physiol-012110-142244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khandoudi N, Albadine J, Robert P, Krief S, Berrebi-Bertrand I, Martin X, Bevensee MO, Boron WF, Bril A (2001) Inhibition of the cardiac electrogenic sodium bicarbonate cotransporter reduces ischemic injury. Cardiovasc Res 52:387–396. [DOI] [PubMed] [Google Scholar]
- 95.Ko SB, Luo X, Hager H, Rojek A, Choi JY, Licht C, Suzuki M, Muallem S, Nielsen S, Ishibashi K (2002) AE4 is a DIDS-sensitive Cl−/HCO3- exchanger in the basolateral membrane of the renal CCD and the SMG duct. Am J Physiol Cell Physiol 283:C1206–1218 [DOI] [PubMed] [Google Scholar]
- 96.Kumar A, Bhattacharjee S, Prakash DR, Sadanand CS (2007) Genetic analysis of two Indian families affected with congenital hereditary endothelial dystrophy: two novel mutations in SLC4A11. Mol Vis 13:39–46 [PMC free article] [PubMed] [Google Scholar]
- 97.Kumar S, Stecher G, Suleski M, Hedges SB (2017) TimeTree: A Resource for Timelines, Timetrees, and Divergence Times. Mol Biol Evol 34:1812–1819. doi: 10.1093/molbev/msx116 [DOI] [PubMed] [Google Scholar]
- 98.Kurita Y, Nakada T, Kato A, Mistry AC, Chang M-H, Romero MF, Hirose S (2008) Identification of intestinal bicarbonate transporters involved in formation of carbonate precipitates to stimulate water absorption in marine teleost fish. Am J Physiol - Comp & Reg Physiol 284:R1402–1412 [DOI] [PubMed] [Google Scholar]
- 99.Kurtz I, Zhu Q (2013) Proximal renal tubular acidosis mediated by mutations in NBCe1-A: unraveling the transporter’s structure-functional properties. Frontiers in physiology 4:350. doi: 10.3389/fphys.2013.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kurtz I, Zhu Q (2013) Structure, function, and regulation of the SLC4 NBCe1 transporter and its role in causing proximal renal tubular acidosis. Current opinion in nephrology and hypertension 22:572–583. doi: 10.1097/MNH.0b013e328363ff43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kwon TH, Fulton C, Wang W, Kurtz I, Frokiaer J, Aalkjaer C, Nielsen S (2002) Chronic metabolic acidosis upregulates rat kidney Na-HCO3 cotransporters NBCn1 and NBC3 but not NBC1. Am J Physiol Renal Physiol 282:F341–351. [DOI] [PubMed] [Google Scholar]
- 102.Lee HJ, Park HJ, Lee S, Kim YH, Choi I (2011) The sodium-driven chloride/bicarbonate exchanger NDCBE in rat brain is upregulated by chronic metabolic acidosis. Brain research 1377:13–20. doi: 10.1016/j.brainres.2010.12.062 [DOI] [PubMed] [Google Scholar]
- 103.Lee S-K, Occhipinti R, Moss FJ, Parker MD, Grichtchenko II, Boron WF (2023) Distinguishing among HCO3−, CO3=, and H+ as Substrates of Proteins That Appear To Be “Bicarbonate” Transporters. Journal of the American Society of Nephrology 34:40–54. doi: 10.1681/asn.2022030289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee S, Axelsen TV, Andersen AP, Vahl P, Pedersen SF, Boedtkjer E (2016) Disrupting Na(+), HCO(3)(−)-cotransporter NBCn1 (Slc4a7) delays murine breast cancer development. Oncogene 35:2112–2122. doi: 10.1038/onc.2015.273 [DOI] [PubMed] [Google Scholar]
- 105.Lee S, Lee HJ, Yang HS, Thornell IM, Bevensee MO, Choi I (2010) Sodium-bicarbonate cotransporter NBCn1 in the kidney medullary thick ascending limb cell line is upregulated under acidic conditions and enhances ammonium transport. Experimental physiology 95:926–937. doi: 10.1113/expphysiol.2010.053967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee SK, Boron WF, Parker MD (2013) Substrate specificity of the electrogenic sodium/bicarbonate cotransporter NBCe1-A (SLC4A4, variant A) from humans and rabbits. American journal of physiology Renal physiology 304:F883–899. doi: 10.1152/ajprenal.00612.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee SK, Occhipinti R, Moss FJ, Parker MD, Grichtchenko II, Boron WF (2023) Distinguishing among HCO 3-, CO 3=, and H + as Substrates of Proteins That Appear To Be “Bicarbonate” Transporters. J Am Soc Nephrol 34:40–54. doi: 10.1681/ASN.2022030289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lefevre S, Knedla A, Tennie C, Kampmann A, Wunrau C, Dinser R, Korb A, Schnaker EM, Tarner IH, Robbins PD, Evans CH, Sturz H, Steinmeyer J, Gay S, Scholmerich J, Pap T, Muller-Ladner U, Neumann E (2009) Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat Med 15:1414–1420. doi: 10.1038/nm.2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D (2010) The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. The Journal of clinical investigation 120:1627–1635. doi: 10.1172/JCI40145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li HC, Szigligeti P, Worrell RT, Matthews JB, Conforti L, Soleimani M (2005) Missense mutations in Na+:HCO3- cotransporter NBC1 show abnormal trafficking in polarized kidney cells: A basis of proximal renal tubular acidosis. Am J Physiol Renal Physiol 289:F61–71 [DOI] [PubMed] [Google Scholar]
- 111.Li JM, Lee S, Zafar R, Shin E, Choi I (2021) Sodium bicarbonate transporter NBCe1 regulates proliferation and viability of human prostate cancer cells LNCaP and PC3. Oncol Rep 46. doi: 10.3892/or.2021.8080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liberti MV, Locasale JW (2016) The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci 41:211–218. doi: 10.1016/j.tibs.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Linsdell P, Tabcharani JA, Rommens JM, Hou Y-X, Chang X-B, Tsui L-C, Riordan JR, Hanrahan JW (1997) Permeability of Wild-Type and Mutant Cystic Fibrosis Transmembrane Conductance Regulator Chloride Channels to Polyatomic Anions. Journal of General Physiology 110:355–364. doi: 10.1085/jgp.110.4.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Linser PJ, Neira Oviedo M, Hirata T, Seron TJ, Smith KE, Piermarini PM, Romero MF (2012) Slc4-like anion transporters of the larval mosquito alimentary canal. J Insect Physiol 58:551–562. doi: 10.1016/j.jinsphys.2012.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lipovich L, Lynch ED, Lee MK, King MC (2001) A novel sodium bicarbonate cotransporter-like gene in an ancient duplicated region: SLC4A9 at 5q31. Genome Biol 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Y, Qin X, Wang DK, Guo YM, Gill HS, Morris N, Parker MD, Chen LM, Boron WF (2013) Effects of optional structural elements, including two alternative amino termini and a new splicing cassette IV, on the function of the sodium-bicarbonate cotransporter NBCn1 (SLC4A7). The Journal of physiology 591:4983–5004. doi: 10.1113/jphysiol.2013.258673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Y, Xu JY, Wang DK, Wang L, Chen LM (2011) Cloning and identification of two novel NBCe1 splice variants from mouse reproductive tract tissues: a comparative study of NCBT genes. Genomics 98:112–119. doi: 10.1016/j.ygeno.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 118.Liu Y, Xu K, Chen LM, Sun X, Parker MD, Kelly ML, LaManna JC, Boron WF (2010) Distribution of NBCn2 (SLC4A10) splice variants in mouse brain. Neuroscience 169:951–964. doi: 10.1016/j.neuroscience.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Z, Wang Q, Zhai G, Ke S, Yu X, Guo J (2022) SLC4A4 promotes prostate cancer progression in vivo and in vitro via AKT-mediated signalling pathway. Cancer Cell International 22. doi: 10.1186/s12935-022-02546-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lo YF, Yang SS, Seki G, Yamada H, Horita S, Yamazaki O, Fujita T, Usui T, Tsai JD, Yu IS, Lin SW, Lin SH (2011) Severe metabolic acidosis causes early lethality in NBC1 W516X knock-in mice as a model of human isolated proximal renal tubular acidosis. Kidney international 79:730–741. doi: 10.1038/ki.2010.523 [DOI] [PubMed] [Google Scholar]
- 121.Lu M, Jia M, Wang Q, Guo Y, Li C, Ren B, Qian F, Wu J (2021) The electrogenic sodium bicarbonate cotransporter and its roles in the myocardial ischemia-reperfusion induced cardiac diseases. Life Sci 270:119153. doi: 10.1016/j.lfs.2021.119153 [DOI] [PubMed] [Google Scholar]
- 122.Majid DS, Prieto MC, Navar LG (2015) Salt-Sensitive Hypertension: Perspectives on Intrarenal Mechanisms. Curr Hypertens Rev 11:38–48. doi: 10.2174/1573402111666150530203858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Majumdar D, Bevensee MO (2010) Na-coupled bicarbonate transporters of the solute carrier 4 family in the nervous system: function, localization, and relevance to neurologic function. Neuroscience 171:951–972. doi: 10.1016/j.neuroscience.2010.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Majumdar D, Maunsbach AB, Shacka JJ, Williams JB, Berger UV, Schultz KP, Harkins LE, Boron WF, Roth KA, Bevensee MO (2008) Localization of electrogenic Na/bicarbonate cotransporter NBCe1 variants in rat brain. Neuroscience 155:818–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McIntyre A, Hulikova A, Ledaki I, Snell C, Singleton D, Steers G, Seden P, Jones D, Bridges E, Wigfield S, Li JL, Russell A, Swietach P, Harris AL (2016) Disrupting Hypoxia-Induced Bicarbonate Transport Acidifies Tumor Cells and Suppresses Tumor Growth. Cancer research 76:3744–3755. doi: 10.1158/0008-5472.CAN-15-1862 [DOI] [PubMed] [Google Scholar]
- 126.Medina AJ, Ibanez AM, Diaz-Zegarra LA, Portiansky EL, Blanco PG, Pereyra EV, de Giusti VC, Aiello EA, Yeves AM, Ennis IL (2020) Cardiac up-regulation of NBCe1 emerges as a beneficial consequence of voluntary wheel running in mice. Arch Biochem Biophys 694:108600. doi: 10.1016/j.abb.2020.108600 [DOI] [PubMed] [Google Scholar]
- 127.Millar ID, Brown PD (2008) NBCe2 exhibits a 3 HCO3(−):1 Na+ stoichiometry in mouse choroid plexus epithelial cells. Biochem Biophys Res Commun 373:550–554. doi: 10.1016/j.bbrc.2008.06.053 [DOI] [PubMed] [Google Scholar]
- 128.Mizrahi JD, Surana R, Valle JW, Shroff RT (2020) Pancreatic cancer. Lancet 395:2008–2020. doi: 10.1016/S0140-6736(20)30974-0 [DOI] [PubMed] [Google Scholar]
- 129.Morrison CB, Shaffer KM, Araba KC, Markovetz MR, Wykoff JA, Quinney NL, Hao S, Delion MF, Flen AL, Morton LC, Liao J, Hill DB, Drumm ML, O’Neal WK, Kesimer M, Gentzsch M, Ehre C (2022) Treatment of cystic fibrosis airway cells with CFTR modulators reverses aberrant mucus properties via hydration. Eur Respir J 59. doi: 10.1183/13993003.00185-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moss FJ, Boron WF (2020) Carbonic anhydrases enhance activity of endogenous Na-H exchangers and not the electrogenic Na/HCO3 cotransporter NBCe1-A, expressed in Xenopus oocytes. J Physiol 598:5821–5856. doi: 10.1113/JP280143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Myers EJ, Marshall A, Jennings ML, Parker MD (2016) Mouse Slc4a11 expressed in Xenopus oocytes is an ideally selective H+/OH- conductance pathway that is stimulated by rises in intracellular and extracellular pH. American journal of physiology Cell physiology 311:C945–C959. doi: 10.1152/ajpcell.00259.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Myers EJ, Yuan L, Felmlee MA, Lin YY, Jiang Y, Pei Y, Wang O, Li M, Xing XP, Marshall A, Xia WB, Parker MD (2016) A novel mutant Na+ /HCO3- cotransporter NBCe1 in a case of compound-heterozygous inheritance of proximal renal tubular acidosis. The Journal of physiology 594:6267–6286. doi: 10.1113/JP272252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Niculescu AB, Le-Niculescu H, Levey DF, Phalen PL, Dainton HL, Roseberry K, Niculescu EM, Niezer JO, Williams A, Graham DL, Jones TJ, Venugopal V, Ballew A, Yard M, Gelbart T, Kurian SM, Shekhar A, Schork NJ, Sandusky GE, Salomon DR (2017) Precision medicine for suicidality: from universality to subtypes and personalization. Molecular psychiatry 22:1250–1273. doi: 10.1038/mp.2017.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ogando DG, Jalimarada SS, Zhang W, Vithana EN, Bonanno JA (2013) SLC4A11 is an EIPA-sensitive Na(+) permeable pHi regulator. American journal of physiology Cell physiology 305:C716–727. doi: 10.1152/ajpcell.00056.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Olsen JSM, Svendsen S, Berg P, Dam VS, Sorensen MV, Matchkov VV, Leipziger J, Boedtkjer E (2021) NBCn1 Increases NH(4) (+) Reabsorption Across Thick Ascending Limbs, the Capacity for Urinary NH(4) (+) Excretion, and Early Recovery from Metabolic Acidosis. J Am Soc Nephrol 32:852–865. doi: 10.1681/ASN.2019060613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Orlowski A, Ciancio MC, Caldiz CI, De Giusti VC, Aiello EA (2014) Reduced sarcolemmal expression and function of the NBCe1 isoform of the Na(+)-HCO(3)(−) cotransporter in hypertrophied cardiomyocytes of spontaneously hypertensive rats: role of the renin-angiotensin system. Cardiovascular research 101:211–219. doi: 10.1093/cvr/cvt255 [DOI] [PubMed] [Google Scholar]
- 137.Palmer BF, Kelepouris E, Clegg DJ (2021) Renal Tubular Acidosis and Management Strategies: A Narrative Review. Advances in Therapy 38:949–968. doi: 10.1007/s12325-020-01587-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Park M, Li Q, Shcheynikov N, Zeng W, Muallem S (2004) NaBC1 Is a Ubiquitous Electrogenic Na(+)-Coupled Borate Transporter Essential for Cellular Boron Homeostasis and Cell Growth and Proliferation. Mol Cell 16:331–341 [DOI] [PubMed] [Google Scholar]
- 139.Parker MD, Boron WF (2013) The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiological reviews 93:803–959. doi: 10.1152/physrev.00023.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Parker MD, Boron WF, Tanner MJ (2002) Characterization of human ‘AE4’ as an electroneutral, sodium-dependent bicarbonate transporter. FASEB J 16:A796 [Google Scholar]
- 141.Parker MD, Bouyer P, Daly CM, Boron WF (2008) Cloning and characterization of novel human SLC4A8 gene products encoding Na+-driven Cl-/HCO3(−) exchanger variants NDCBE-A, -C, and -D. Physiol Genomics 34:265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Parker MD, Musa-Aziz R, Rojas JD, Choi I, Daly CM, Boron WF (2008) Characterization of human SLC4A10 as an electroneutral Na/HCO3 cotransporter (NBCn2) with Cl- self-exchange activity. J Biol Chem 283:12777–12788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Parker MD, Ourmozdi EP, Tanner MJ (2001) Human BTR1, a new bicarbonate transporter superfamily member and human AE4 from kidney. Biochem Biophys Res Commun 282:1103–1109 [DOI] [PubMed] [Google Scholar]
- 144.Parker MD, Qin X, Williamson RC, Toye AM, Boron WF (2012) HCO(3)(−)-independent conductance with a mutant Na(+)/HCO(3)(−) cotransporter (SLC4A4) in a case of proximal renal tubular acidosis with hypokalaemic paralysis. The Journal of physiology 590:2009–2034. doi: 10.1113/jphysiol.2011.224733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Parks SK, Pouyssegur J (2015) The Na(+)/HCO3(−) Co-Transporter SLC4A4 Plays a Role in Growth and Migration of Colon and Breast Cancer Cells. J Cell Physiol 230:1954–1963. doi: 10.1002/jcp.24930 [DOI] [PubMed] [Google Scholar]
- 146.Pena-Munzenmayer G, Catalan MA, Kondo Y, Jaramillo Y, Liu F, Shull GE, Melvin JE (2015) Ae4 (Slc4a9) Anion Exchanger Drives Cl- Uptake-dependent Fluid Secretion by Mouse Submandibular Gland Acinar Cells. The Journal of biological chemistry 290:10677–10688. doi: 10.1074/jbc.M114.612895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pena-Munzenmayer G, George AT, Shull GE, Melvin JE, Catalan MA (2016) Ae4 (Slc4a9) is an electroneutral monovalent cation-dependent Cl-/HCO3- exchanger. J Gen Physiol 147:423–436. doi: 10.1085/jgp.201611571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Piermarini PM, Choi I, Boron WF (2007) Cloning and characterization of an electrogenic Na/HCO3- cotransporter from the squid giant fiber lobe. Am J Physiol Cell Physiol 292:C2032–2045 [DOI] [PubMed] [Google Scholar]
- 149.Poulsen JH, Fischer H, Illek B, Machen TE (1994) Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A 91:5340–5344. doi: 10.1073/pnas.91.12.5340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I (1999) Cloning, Tissue Distribution, Genomic Organization, and Functional Characterization of NBC3, a New Member of the Sodium Bicarbonate Cotransporter Family. J Biol Chem 274:16569–16575 [DOI] [PubMed] [Google Scholar]
- 151.Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I (1999) Mapping of the human NBC3 (SLC4A7) gene to chromosome 3p22. Genomics 57:321–322 [DOI] [PubMed] [Google Scholar]
- 152.Pushkin A, Abuladze N, Newman D, Lee I, Xu G, Kurtz I (2000) Cloning, characterization and chromosomal assignment of NBC4, a new member of the sodium bicarbonate cotransporter family. Biochim Biophys Acta 1493:215–218 [DOI] [PubMed] [Google Scholar]
- 153.Riihonen R, Nielsen S, Vaananen HK, Laitala-Leinonen T, Kwon TH (2010) Degradation of hydroxyapatite in vivo and in vitro requires osteoclastic sodium-bicarbonate co-transporter NBCn1. Matrix biology : journal of the International Society for Matrix Biology 29:287–294. doi: 10.1016/j.matbio.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 154.Romero MF, Chen AP, Parker MD, Boron WF (2013) The SLC4 Family of Bicarbonate (HCO3−) Transporters. Molecular Aspects of Medicine 34:159–182. doi: 10.1016/j.mam.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Romero MF, Dillon AK, Harte PJ, Sciortino CM (2000) The Drosophila ndae1 gene encodes a Functional Na+ Dependent Cl-HCO3 Exchanger. FASEB J 14:A356 [Google Scholar]
- 156.Romero MF, Fong P, Berger UV, Hediger MA, Boron WF (1998) Cloning and functional expression of rNBC, an electrogenic Na+-HCO3- cotransporter from rat kidney. Am J Physiol 274:F425–432 [DOI] [PubMed] [Google Scholar]
- 157.Romero MF, Hediger MA, Boulpaep EL, Boron WF (1997) Expression cloning and characterization of a renal electrogenic Na+/HCO3- cotransporter. Nature 387:409–413. doi: 10.1038/387409a0 [DOI] [PubMed] [Google Scholar]
- 158.Romero MF, Rossano AJ (2019) Acid-Base Basics. Semin Nephrol 39:316–327. doi: 10.1016/j.semnephrol.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ruminot I, Gutierrez R, Pena-Munzenmayer G, Anazco C, Sotelo-Hitschfeld T, Lerchundi R, Niemeyer MI, Shull GE, Barros LF (2011) NBCe1 mediates the acute stimulation of astrocytic glycolysis by extracellular K+. J Neurosci 31:14264–14271. doi:31/40/14264 [pii] 10.1523/JNEUROSCI.2310-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Russell JM, Boron WF (1976) Role of choloride transport in regulation of intracellular pH. Nature 264:73–74. doi: 10.1038/264073a0 [DOI] [PubMed] [Google Scholar]
- 161.Saint-Criq V, Delpiano L, Casement J, Onuora JC, Lin J, Gray MA (2020) Choice of Differentiation Media Significantly Impacts Cell Lineage and Response to CFTR Modulators in Fully Differentiated Primary Cultures of Cystic Fibrosis Human Airway Epithelial Cells. Cells 9. doi: 10.3390/cells9092137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Saint-Criq V, Guequen A, Philp AR, Villanueva S, Apablaza T, Fernandez-Moncada I, Mansilla A, Delpiano L, Ruminot I, Carrasco C, Gray MA, Flores CA (2022) Inhibition of the sodium-dependent HCO(3)(−) transporter SLC4A4, produces a cystic fibrosis-like airway disease phenotype. Elife 11. doi: 10.7554/eLife.75871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Samak M, Fatullayev J, Sabashnikov A, Zeriouh M, Schmack B, Farag M, Popov AF, Dohmen PM, Choi YH, Wahlers T, Weymann A (2016) Cardiac Hypertrophy: An Introduction to Molecular and Cellular Basis. Med Sci Monit Basic Res 22:75–79. doi: 10.12659/MSMBR.900437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sassani P, Pushkin A, Gross E, Gomer A, Abuladze N, Dukkipati R, Carpenito G, Kurtz I (2002) Functional characterization of NBC4: a new electrogenic sodium- bicarbonate cotransporter. Am J Physiol Cell Physiol 282:C408–416. [DOI] [PubMed] [Google Scholar]
- 165.Schmitt BM, Biemesderfer D, Boulpaep EL, Romero MF, Boron WF (1999) Immunolocalization of the electrogenic Na+/ HCO3- cotransporter in mammalian and amphibian kidney. Am J Physiol 276:F27–36 [DOI] [PubMed] [Google Scholar]
- 166.Schwab A, Rossmann H, Klein M, Dieterich P, Gassner B, Neff C, Stock C, Seidler U (2005) Functional role of Na+-HCO3- cotransport in migration of transformed renal epithelial cells. J Physiol 568:445–458. doi: 10.1113/jphysiol.2005.092957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sciortino CM, Fletcher BR, Shrode LD, Harte PJ, Romero MF (2001) NDAE1, Na+-driven anion exchanger from Drosophila, is a plasma membrane protein expressed in the nervous system and alimentary tract. FASEB J 15:A502 [Google Scholar]
- 168.Sciortino CM, Romero MF (1999) Cation and voltage dependence of rat kidney, electrogenic Na+/HCO3- cotransporter, rkNBC, expressed in oocytes. Am J Physiol 277:F611–623 [DOI] [PubMed] [Google Scholar]
- 169.Shang Z, Yu J, Sun L, Tian J, Zhu S, Zhang B, Dong Q, Jiang N, Flores-Morales A, Chang C, Niu Y (2019) LncRNA PCAT1 activates AKT and NF-kappaB signaling in castration-resistant prostate cancer by regulating the PHLPP/FKBP51/IKKalpha complex. Nucleic Acids Res 47:4211–4225. doi: 10.1093/nar/gkz108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sherman T, Chernova MN, Clark JS, Jiang L, Alper SL, Nehrke K (2005) The abts and sulp families of anion transporters from Caenorhabditis elegans. Am J Physiol Cell Physiol 289:C341–351 [DOI] [PubMed] [Google Scholar]
- 171.Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K (2006) IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3- cotransporter 1 (pNBC1). Proc Natl Acad Sci U S A 103:9542–9547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 173.Sinning A, Liebmann L, Hubner CA (2015) Disruption of Slc4a10 augments neuronal excitability and modulates synaptic short-term plasticity. Front Cell Neurosci 9:223. doi: 10.3389/fncel.2015.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sinning A, Radionov N, Trepiccione F, Lopez-Cayuqueo KI, Jayat M, Baron S, Corniere N, Alexander RT, Hadchouel J, Eladari D, Hubner CA, Chambrey R (2017) Double Knockout of the Na+-Driven Cl-/HCO3- Exchanger and Na+/Cl- Cotransporter Induces Hypokalemia and Volume Depletion. J Am Soc Nephrol 28:130–139. doi: 10.1681/ASN.2015070734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Smith JJ, Welsh MJ (1992) cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest 89:1148–1153. doi: 10.1172/jci115696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Soleimani M (2013) SLC26 Cl-/HCO3- exchangers in the kidney: roles in health and disease. Kidney international 84:657–666. doi: 10.1038/ki.2013.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Soleimani M, Burnham CE (2000) Physiologic and molecular aspects of the Na+:HCO3- cotransporter in health and disease processes. Kidney Int 57:371–384 [DOI] [PubMed] [Google Scholar]
- 178.Soleimani M, Rastegar A (2016) Pathophysiology of Renal Tubular Acidosis: Core Curriculum 2016. American Journal of Kidney Diseases 68:488–498. doi: 10.1053/j.ajkd.2016.03.422 [DOI] [PubMed] [Google Scholar]
- 179.Sussman CR, Zhao J, Plata C, Daly JLC, Angle N, DiPiero J, Drummond IA, Liang JO, Boron WF, Romero MF, Chang M-H (2009) Cloning, localization and functional expression of the electrogenic Na+ bicarbonate cotransporter (NBCe1) from zebrafish. Am J Physiol - Cell Physiol 297:C865 – C875. doi:doi: 10.1152/ajpcell.00679.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Suzuki M, Vaisbich MH, Yamada H, Horita S, Li Y, Sekine T, Moriyama N, Igarashi T, Endo Y, Cardoso TP, de Sa LC, Koch VH, Seki G, Fujita T (2008) Functional analysis of a novel missense NBC1 mutation and of other mutations causing proximal renal tubular acidosis. Pflugers Arch 455:583–593 [DOI] [PubMed] [Google Scholar]
- 181.Suzuki M, Van Paesschen W, Stalmans I, Horita S, Yamada H, Bergmans BA, Legius E, Riant F, De Jonghe P, Li Y, Sekine T, Igarashi T, Fujimoto I, Mikoshiba K, Shimadzu M, Shiohara M, Braverman N, Al-Gazali L, Fujita T, Seki G (2010) Defective membrane expression of the Na(+)-HCO(3)(−) cotransporter NBCe1 is associated with familial migraine. Proceedings of the National Academy of Sciences of the United States of America 107:15963–15968. doi: 10.1073/pnas.1008705107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Swietach P, Boedtkjer E, Pedersen SF (2023) How protons pave the way to aggressive cancers. Nat Rev Cancer 23:825–841. doi: 10.1038/s41568-023-00628-9 [DOI] [PubMed] [Google Scholar]
- 183.Taylor JY, Wu CY, Darling D, Sun YV, Kardia SLR, Jackson JS (2012) Gene-Environment Effects of SLC4A5 and Skin Color on Blood Pressure among African American Women.pdf. Ethnicity and Disease [PMC free article] [PubMed] [Google Scholar]
- 184.Theparambil SM, Hosford PS, Ruminot I, Kopach O, Reynolds JR, Sandoval PY, Rusakov DA, Barros LF, Gourine AV (2020) Astrocytes regulate brain extracellular pH via a neuronal activity-dependent bicarbonate shuttle. Nat Commun 11:5073. doi: 10.1038/s41467-020-18756-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Theparambil SM, Ruminot I, Schneider HP, Shull GE, Deitmer JW (2014) The electrogenic sodium bicarbonate cotransporter NBCe1 is a high-affinity bicarbonate carrier in cortical astrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience 34:1148–1157. doi: 10.1523/JNEUROSCI.2377-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Thornell IM, Bevensee MO (2015) Regulators of Slc4 bicarbonate transporter activity. Frontiers in Physiology 6. doi: 10.3389/fphys.2015.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Toye AM, Parker MD, Daly CM, Lu J, Virkki LV, Pelletier MF, Boron WF (2006) The Human NBCe1-A Mutant R881C, Associated With Proximal Renal Tubular Acidosis, Retains Function But Is Mistargeted In Polarized Renal Epithelia. Am J Physiol Cell Physiol 291:C788–801 [DOI] [PubMed] [Google Scholar]
- 188.Tsuganezawa H, Kobayashi K, Iyori M, Araki T, Koizumi A, Watanabe S-I, Kaneko A, Fukao T, Monkawa T, Yoshida T, Kim DK, Kanai Y, Endou H, Hayashi M, Saruta T (2001) A new member of the HCO3- transporter superfamily is an apical anion exchanger of b -intercalated cells in the kidney. Journal of Biological Chemistry 276:8180–8189 [DOI] [PubMed] [Google Scholar]
- 189.Vairamani K, Prasad V, Wang Y, Huang W, Chen Y, Medvedovic M, Lorenz JN, Shull GE (2018) NBCe1 Na(+)-HCO3(−) cotransporter ablation causes reduced apoptosis following cardiac ischemia-reperfusion injury in vivo. World J Cardiol 10:97–109. doi: 10.4330/wjc.v10.i9.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, Yuan D, Stroe O, Wood G, Laydon A, Zidek A, Green T, Tunyasuvunakool K, Petersen S, Jumper J, Clancy E, Green R, Vora A, Lutfi M, Figurnov M, Cowie A, Hobbs N, Kohli P, Kleywegt G, Birney E, Hassabis D, Velankar S (2022) AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res 50:D439–D444. doi: 10.1093/nar/gkab1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vaughan-Jones RD, Spitzer KW, Swietach P (2009) Intracellular pH regulation in heart. J Mol Cell Cardiol 46:318–331. doi: 10.1016/j.yjmcc.2008.10.024 [DOI] [PubMed] [Google Scholar]
- 192.Virkki LV, Choi I, Davis BA, Boron WF (2003) Cloning of a Na+-driven Cl/HCO3 exchanger from squid giant fiber lobe. Am J Physiol Cell Physiol 285:C771–780 [DOI] [PubMed] [Google Scholar]
- 193.Virkki LV, Wilson DA, Vaughan-Jones RD, Boron WF (2002) Functional characterization of human NBC4 as an electrogenic Na/HCO3 cotransporter (NBCe2). Am J Physiol Cell Physiol 282:C1278–1289 [DOI] [PubMed] [Google Scholar]
- 194.Vithana EN, Morgan P, Sundaresan P, Ebenezer ND, Tan DT, Mohamed MD, Anand S, Khine KO, Venkataraman D, Yong VH, Salto-Tellez M, Venkatraman A, Guo K, Hemadevi B, Srinivasan M, Prajna V, Khine M, Casey JR, Inglehearn CF, Aung T (2006) Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2). Nat Genet 38:755–757 [DOI] [PubMed] [Google Scholar]
- 195.Vitzthum H, Koch M, Eckermann L, Svendsen SL, Berg P, Hubner CA, Wagner CA, Leipziger J, Meyer-Schwesinger C, Ehmke H (2023) The AE4 transporter mediates kidney acid-base sensing. Nat Commun 14:3051. doi: 10.1038/s41467-023-38562-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang HS, Chen Y, Vairamani K, Shull GE (2014) Critical role of bicarbonate and bicarbonate transporters in cardiac function. World journal of biological chemistry 5:334–345. doi: 10.4331/wjbc.v5.i3.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang JL, Zhao L, Zhu J, Wang DK, Ren MJ, Wang M, Liu Y, Boron WF, Chen LM (2019) Expression, Localization, and Effect of High Salt Intake on Electroneutral Na(+)/HCO3 (−) Cotransporter NBCn2 in Rat Small Intestine: Implication in Intestinal NaCl Absorption. Front Physiol 10:1334. doi: 10.3389/fphys.2019.01334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang P, Song Y, Zhong H, Lin S, Zhang X, Li J, Che L, Feng B, Lin Y, Xu S, Zhuo Y, Wu D, Burrin DG, Fang Z (2019) Transcriptome Profiling of Placenta through Pregnancy Reveals Dysregulation of Bile Acids Transport and Detoxification Function. International Journal of Molecular Sciences 20:4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang W, Tsirulnikov K, Zhekova HR, Kayik G, Khan HM, Azimov R, Abuladze N, Kao L, Newman D, Noskov SY, Zhou ZH, Pushkin A, Kurtz I (2021) Cryo-EM structure of the sodium-driven chloride/bicarbonate exchanger NDCBE. Nat Commun 12:5690. doi: 10.1038/s41467-021-25998-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang YJ, Schug J, Won KJ, Liu C, Naji A, Avrahami D, Golson ML, Kaestner KH (2016) Single-Cell Transcriptomics of the Human Endocrine Pancreas. Diabetes 65:3028–3038. doi: 10.2337/db16-0405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Webb BA, Chimenti M, Jacobson MP, Barber DL (2011) Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 11:671–677. doi: 10.1038/nrc3110 [DOI] [PubMed] [Google Scholar]
- 202.Weiner ID, Verlander JW (2013) Renal ammonia metabolism and transport. Compr Physiol 3:201–220. doi: 10.1002/cphy.c120010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wen D, Yuan Y, Cornelius RJ, Li H, Warner PC, Wang B, Wang-France J, Boettger T, Sansom SC (2015) Deficient acid handling with distal RTA in the NBCe2 knockout mouse. Am J Physiol Renal Physiol 309:F523–530. doi: 10.1152/ajprenal.00163.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wu H, Liu S, Su P, Xie ZD, Gui TX, Zhao L, Liu Y, Chen LM (2022) Molecular insight into coordination sites for substrates and their coupling kinetics in Na(+) /HCO3 (−) cotransporter NBCe1. J Physiol:doi: 10.1113/JP282034. doi: 10.1113/JP282034 [DOI] [PubMed] [Google Scholar]
- 205.Xiao W, Wang X, Wang T, Xing J (2019) MiR-223–3p promotes cell proliferation and metastasis by downregulating SLC4A4 in clear cell renal cell carcinoma. Aging (Albany NY) 11:615–633. doi: 10.18632/aging.101763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Xu J, Barone S, Petrovic S, Wang Z, Seidler U, Riederer B, Ramaswamy K, Dudeja PK, Shull GE, Soleimani M (2003) Identification of an apical Cl−/HCO3- exchanger in gastric surface mucous and duodenal villus cells. Am J Physiol Gastrointest Liver Physiol 285:G1225–1234 [DOI] [PubMed] [Google Scholar]
- 207.Yamamoto T, Shirayama T, Sakatani T, Takahashi T, Tanaka H, Takamatsu T, Spitzer KW, Matsubara H (2007) Enhanced activity of ventricular Na+-HCO3- cotransport in pressure overload hypertrophy. Am J Physiol Heart Circ Physiol 293:H1254–1264 [DOI] [PubMed] [Google Scholar]
- 208.Yamamoto T, Swietach P, Rossini A, Loh SH, Vaughan-Jones RD, Spitzer KW (2005) Functional diversity of electrogenic Na+-HCO3- cotransport in ventricular myocytes from rat, rabbit and guinea pig. J Physiol 562:455–475. doi: 10.1113/jphysiol.2004.071068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yamazaki O, Yamada H, Suzuki M, Horita S, Shirai A, Nakamura M, Satoh N, Fujita T, Seki G (2013) Identification of dominant negative effect of L522P mutation in the electrogenic Na(+)-HCO(3)(−) cotransporter NBCe1. Pflugers Archiv : European journal of physiology 465:1281–1291. doi: 10.1007/s00424-013-1277-1 [DOI] [PubMed] [Google Scholar]
- 210.Yano H, Wang C, Yamashita S, Yokoyama Y, Yokoi N, Seino S (2000) Assignment(1) of the human solute carrier family 4, sodium bicarbonate cotransporter-like, member 10 gene (SLC4A10) to 2q23-->q24 by in situ hybridization and radiation hybrid mapping [In Process Citation]. Cytogenet Cell Genet 89:276–277 [DOI] [PubMed] [Google Scholar]
- 211.Zajac M, Dreano E, Edwards A, Planelles G, Sermet-Gaudelus I (2021) Airway Surface Liquid pH Regulation in Airway Epithelium Current Understandings and Gaps in Knowledge. Int J Mol Sci 22. doi: 10.3390/ijms22073384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang D, Kiyatkin A, Bolin JT, Low PS (2000) Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood 96:2925–2933 [PubMed] [Google Scholar]
- 213.Zhang W, Ogando DG, Bonanno JA, Obukhov AG (2015) Human SLC4A11 Is a Novel NH3/H+ Co-transporter. The Journal of biological chemistry 290:16894–16905. doi: 10.1074/jbc.M114.627455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhang X, Tan P, Zhuang Y, Du L (2020) hsa_circRNA_001587 upregulates SLC4A4 expression to inhibit migration, invasion, and angiogenesis of pancreatic cancer cells via binding to microRNA-223. Am J Physiol Gastrointest Liver Physiol 319:G703–G717. doi: 10.1152/ajpgi.00118.2020 [DOI] [PubMed] [Google Scholar]
- 215.Zhao Z, Ukidve A, Kim J, Mitragotri S (2020) Targeting Strategies for Tissue-Specific Drug Delivery. Cell 181:151–167. doi: 10.1016/j.cell.2020.02.001 [DOI] [PubMed] [Google Scholar]
- 216.Zhou C, Hu B, Tang Y, Chen X, Ma Z, Ding Q, Nie G (2022) Genome-wide characterization of the Triplophysa dalaica slc4 gene family and expression profiles in response to salinity changes. BMC Genomics 23:824. doi: 10.1186/s12864-022-09057-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhou J, Xie Z, Cui P, Su Q, Zhang Y, Luo L, Li Z, Ye L, Liang H, Huang J (2020) SLC1A1, SLC16A9, and CNTN3 Are Potential Biomarkers for the Occurrence of Colorectal Cancer. Biomed Res Int 2020:1204605. doi: 10.1155/2020/1204605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhu Q, Azimov R, Kao L, Newman D, Liu W, Abuladze N, Pushkin A, Kurtz I (2009) NBCe1-A transmembrane segment 1 lines the ion translocation pathway. J Biol Chem 284:8918–8929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhu Q, Kao L, Azimov R, Abuladze N, Newman D, Pushkin A, Liu W, Chang C, Kurtz I (2010) Structural and functional characterization of the C-terminal transmembrane region of NBCe1-A. J Biol Chem [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhu Q, Kao L, Azimov R, Newman D, Liu W, Pushkin A, Abuladze N, Kurtz I (2010) Topological location and structural importance of the NBCe1-A residues mutated in proximal renal tubular acidosis. J Biol Chem 285:13416–13426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zoccola D, Ganot P, Bertucci A, Caminiti-Segonds N, Techer N, Voolstra CR, Aranda M, Tambutte E, Allemand D, Casey JR, Tambutte S (2015) Bicarbonate transporters in corals point towards a key step in the evolution of cnidarian calcification. Sci Rep 5:9983. doi: 10.1038/srep09983 [DOI] [PMC free article] [PubMed] [Google Scholar]


