Abstract
Fru-2,6-P2 (fructose 2,6-bisphosphate) is a signal molecule that controls glycolysis. Since its discovery more than 20 years ago, inroads have been made towards the understanding of the structure–function relationships in PFK-2 (6-phosphofructo-2-kinase)/FBPase-2 (fructose-2,6-bisphosphatase), the homodimeric bifunctional enzyme that catalyses the synthesis and degradation of Fru-2,6-P2. The FBPase-2 domain of the enzyme subunit bears sequence, mechanistic and structural similarity to the histidine phosphatase family of enzymes. The PFK-2 domain was originally thought to resemble bacterial PFK-1 (6-phosphofructo-1-kinase), but this proved not to be correct. Molecular modelling of the PFK-2 domain revealed that, instead, it has the same fold as adenylate kinase. This was confirmed by X-ray crystallography. A PFK-2/FBPase-2 sequence in the genome of one prokaryote, the proteobacterium Desulfovibrio desulfuricans, could be the result of horizontal gene transfer from a eukaryote distantly related to all other organisms, possibly a protist. This, together with the presence of PFK-2/FBPase-2 genes in trypanosomatids (albeit with possibly only one of the domains active), indicates that fusion of genes initially coding for separate PFK-2 and FBPase-2 domains might have occurred early in evolution. In the enzyme homodimer, the PFK-2 domains come together in a head-to-head like fashion, whereas the FBPase-2 domains can function as monomers. There are four PFK-2/FBPase-2 isoenzymes in mammals, each coded by a different gene that expresses several isoforms of each isoenzyme. In these genes, regulatory sequences have been identified which account for their long-term control by hormones and tissue-specific transcription factors. One of these, HNF-6 (hepatocyte nuclear factor-6), was discovered in this way. As to short-term control, the liver isoenzyme is phosphorylated at the N-terminus, adjacent to the PFK-2 domain, by PKA (cAMP-dependent protein kinase), leading to PFK-2 inactivation and FBPase-2 activation. In contrast, the heart isoenzyme is phosphorylated at the C-terminus by several protein kinases in different signalling pathways, resulting in PFK-2 activation.
Keywords: catalysis; evolution; fructose 2,6-bisphosphate; gene; glycolysis; phosphorylation
Abbreviations: ACC, acetyl-CoA carboxylase; AK, adenylate kinase; AMPK, AMP-activated protein kinase; Ca/CAMK, Ca2+/calmodulin-activated protein kinase; C/EBP, CCAAT/enhancer-binding protein; EGF, epidermal growth factor; FBPase-1, fructose-1,6-bisphosphatase; FBPase-2, fructose-2,6-bisphosphatase; Fru-6-P, fructose 6-phosphate; Fru-2,6-P2, fructose 2,6-bisphosphate; GAP, GTPase-activating protein; GLUT4, glucose transporter 4; GR, glucocorticoid receptor; GRU, glucocorticoid-response unit; HNF, hepatocyte nuclear factor; MAPK, mitogen-activated protein kinase; NF-1, nuclear factor 1; OC, Onecut; PDK, phosphoinositide-dependent protein kinase; PEPCK, phosphoenolpyruvate carboxykinase; PFK-1, 6-phosphofructo-1-kinase; PFK-2, 6-phosphofructo-2-kinase; iPFK-2, inducible PFK-2; PI3K, phosphoinositide 3-kinase; PKA, cAMP-dependent protein kinase; PKB, protein kinase B; PKC, protein kinase C; p70S6k, p70 ribosomal protein S6 kinase; 3D, three-dimensional; WISK, wortmannin-sensitive, insulin-stimulated protein kinase
INTRODUCTION
Fru-2,6-P2 (fructose 2,6-bisphosphate) is found in all mammalian tissues, throughout the animal and plant kingdoms, and in fungi and certain unicellular eukaryotes, but not in bacteria (however, see the section on evolution below) (for earlier reviews see [1–7]). In most of these organisms, this molecule is a potent positive allosteric effector of PFK-1 (6-phosphofructo-1-kinase) (except in some protists in which it is an allosteric stimulator of pyruvate kinase – see below) and thus stimulates glycolysis. In liver, Fru-2,6-P2 is an inhibitor of FBPase-1 (fructose-1,6-bisphosphatase), a regulatory enzyme of gluconeogenesis. Glucagon decreases the concentration of hepatic Fru-2,6-P2, thereby relieving the inhibition of FBPase-1 and allowing gluconeogenesis to prevail. Therefore Fru-2,6-P2 plays a unique role in the control of glucose homoeostasis by allowing the liver to switch from glycolysis to gluconeogenesis. In most mammalian tissues, which do not contain FBPase-1, Fru-2,6-P2 acts as a glucose signal to stimulate glycolysis when glucose is available. In heart, insulin and anoxia increase Fru-2,6-P2 concentrations, which contributes to the stimulation of glycolysis under these conditions [8,9].
The synthesis of Fru-2,6-P2 from Fru-6-P (fructose 6-phosphate) and MgATP is catalysed by PFK-2 (6-phosphofructo-2-kinase), whereas its hydrolysis to Fru-6-P and Pi is catalysed by FBPase-2 (fructose-2,6-bisphosphatase) [1–7]. The PFK-2 and FBPase-2 reactions are catalysed on the same polypeptide of a homodimeric protein. The PFK-2 reaction is catalysed in the N-terminal half of the enzyme subunit, whereas the FBPase-2 reaction is catalysed in the C-terminal half [6,7]. PFK-2/FBPase-2 isoenzymes were first described in mammalian tissues and one distinguishing feature is their PFK-2/FBPase-2 activity ratio. The skeletal muscle isoenzyme has a Vmax of FBPase-2 5–10 times greater than that of PFK-2, and in this respect resembles the liver isoenzyme after phosphorylation by PKA (cAMP-dependent protein kinase) [2]. When assayed with physiological concentrations of substrates, the heart [10] and testis [11] isoenzymes possess a higher PFK-2/FBPase-2 activity ratio than the liver isoenzyme [2,4] and a molecular basis for this difference was recently proposed [12] (see below).
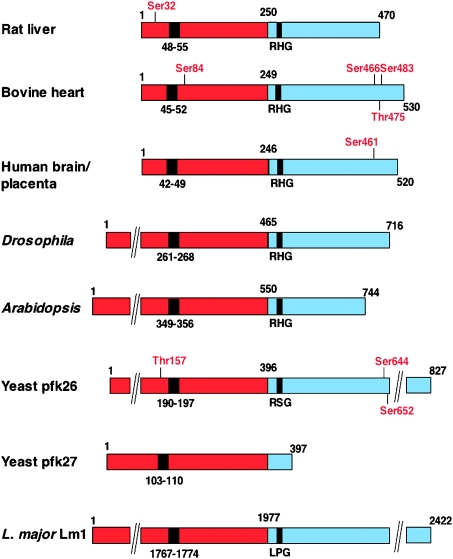
In the present review, we have clarified the classification of the mammalian PFK-2/FBPase-2 isoenzymes/isoforms, which is now based on their gene origin rather than on tissue distribution. PFK-2/FBPase-2 isoenzymes have also been identified in birds, amphibians, worms, fish, insects, plants, fungi and trypanosomatids. Figure 1 shows the organization of domains in some of the PFK-2/FBPase-2 isoenzymes, particularly those with unusual extensions or truncations and those which are controlled by phosphorylation. In the present review, we discuss the evolution of the bifunctional enzyme, which seems to have arisen by gene fusion. However, in trypanosomatids and yeasts, monofunctional isoenzymes have evolved in which one or the other catalytic domain has become inactive.
Figure 1. Domain organization of the subunit sequences of some of the PFK-2/FBPase-2 isoenzymes.
The N-terminal PFK-2 domain is shown in red and the C-terminal FBPase-2 domain is in blue. Numbering refers to the individual isoenzyme sequences. The black box in the PFK-2 domain indicates the position of the glycine-rich loop in the nucleotide-binding fold with the signature G(X)4GKT/S. The black box in the FBPase-2 domain contains the active site histidine in the RHG motif which has become mutated in some of the monofunctional isoforms. Phosphorylation sites for protein kinases are indicated in red (see text).
FBPase-2 is a classical Ping Pong reaction with formation of a phosphoryl-enzyme intermediate on a histidine residue [6,7]. This histidine residue is located in an RHG (Arg-His-Gly) motif, which is characteristic of the so-called ‘histidine phosphatase’ family (histidine is indicated in bold) of enzymes, including the acid phosphatases and phosphoglycerate mutases. Indeed, the homology with the histidine phosphatases helped in our understanding of the structure and catalysis in the FBPase-2 domain, which has been extensively reviewed elsewhere [6,7] and is not covered in the present review. On the other hand, the PFK-2 mechanism has proved more elusive and the structure–activity relationships in the PFK-2 domain is one of the subjects of the present review. Lastly, we consider how phosphorylation controls the activity of the bifunctional enzyme. The heart isoenzyme is particularly interesting as it is multisite-phosphorylated, integrating signalling from many pathways via protein kinase cascades to a single molecule, Fru-2,6-P2, to stimulate glycolysis.
THE PFK-2/FBPase-2 GENES IN MAMMALS
In mammals, and in keeping with the phylogenetic analysis (see below), there are four PFK-2/FBPase-2 isoenzymes, namely the liver, heart, brain (or placenta) and testis isoenzymes, which differ by the sequence of their bifunctional catalytic core. They have been named after the tissue from which they were first purified, but they are expressed in other tissues as well, except for the testis isoenzyme. A number of isoforms have been described for each of these isoenzymes. The isoforms share the catalytic core of the parent isoenzyme, but differ by flanking sequences, which often contain sites for phosphorylation by protein kinases. As these additional sequences also influence the catalytic properties and PFK-2/FBPases-2 ratio, some isoforms, e.g. liver and muscle, have been considered as distinct isoenzymes.
A prerequisite for understanding the origin of the PFK-2/FBPase-2 isoenzymes and isoforms, their tissue-specific expression, and their long-term control by hormones and growth factors was to clone the corresponding gene(s). Four genes have been characterized in mammals, one for each isoenzyme (Table 1). They are called PFKFB1 (also called gene A, located on human chromosome X) [13], PFKFB2 (gene B, on human chromosome 1) [14], PFKFB3 (on human chromosome 10) [15] and PFKFB4 (on human chromosome 3) [16]. These genes all have a similar organization, which explains how each one codes for one isoenzyme, for several isoforms by differential splicing, and for even more mRNAs because of the presence of several promoters and 5′ non-coding exons.
Table 1. Mammalian FPK-2/FBPase-2 gene products.
All the mRNAs listed, except M and F, differ in terms of coding sequence. The M isoform is sometimes called the muscle isoenzyme. Only the mRNAs from gene 1 (formerly called gene A) are referred to in the literature as indicated. We propose here an ad hoc nomenclature for the other mRNAs. As gene 2 was called gene B, its transcripts were referred to earlier as B1 to B4. Some of the isoforms are coded by more mRNAs than indicated, due to transcription from several promoters and due to differential splicing with non-coding exons. See text for explanations and references.
| Gene | Chromosome locus | Isoenzyme | mRNA | Isoform |
|---|---|---|---|---|
| PFKFB1 | Human Xp11.21 | Liver | L | L |
| Rat Xq22-q31 | M | M | ||
| F | M | |||
| PFKFB2 | Human 1q31 | Heart | H1, H2, H4 | Long (58 kDa) |
| Rat 13 | H3 | Short (54 kDa) | ||
| PFKFB3 | Human 10p14-p15 | Brain/placenta | U | Ubiquitous |
| Rat 17q12.3 | I | Inducible | ||
| PFKFB4 | Human 3p21-p22 | Testis | T | T |
| Rat 8q32 |
The PFKFB1 gene
This gene (60 kb, 17 exons), which codes for the liver isoenzyme, gives rise, from distinct promoters (called L, M and F from 3′ to 5′), to the L, M and F mRNAs respectively [13,17,18]. These three mRNAs differ by their 5′ end and share 12 consecutive exons (exons 2–13) corresponding to the catalytic core, six coding for the PFK-2 domain, followed by six for the FBPase-2 domain. An additional 5′ coding exon (exon1L) is present in the L mRNA, which codes for the L isoform, and another one (exon1M) in the M mRNA, which codes for the M isoform. The two F mRNAs, which contain two non-coding exons upstream from, and spliced with part of, exon1M also code for the M isoform. Thus the M isoform has the same sequence as the L isoform except for the N-terminus, where the first 32 residues, including the PKA-phosphorylated Ser32, are replaced by an unrelated nonapeptide devoid of phosphorylation sites. The L isoform, expressed from the L promoter, is the main PFK-2/FBPase-2 in liver; it is also found in white adipose tissue [19]. The M isoform is expressed from the M promoter in skeletal muscle and white adipose tissue, and from the F promoter in fibroblasts, foetal tissues and proliferating cells.
The PFKFB2 gene
This gene codes for the heart isoenzyme and its two isoforms. The rat gene (22 kb, 20 exons) [14,20] contains 12 consecutive exons (3–14), which are very similar to those of the PFKFB1 gene that code for the core catalytic domain. Exon 15 contains several phosphorylation sites for protein kinases. In bovine heart, alternative splicing with deletion of exon 15 gives rise to an additional, shorter (54 kDa instead of 58 kDa) isoform that lacks the sequence coded by this regulatory exon [21,22]. The PFKFB2 gene gives rise, from distinct promoters, to at least four mRNAs that differ by non-coding sequences at the 5′ end [20,23]. How these distinct 5′ ends relate to the three mRNAs (H1, H2 and H4) that give rise to the 58 kDa isoform and the mRNA (H3) for the 54 kDa isoform, is unknown. Moreover, none of these mRNAs are strictly heart-specific [20]. For instance, the heart isoenzyme is sometimes called the kidney isoenzyme.
The PFKFB3 gene
This gene, which contains at least 16 exons, codes for an isoenzyme originally cloned from bovine brain [24] and from human placenta [25]. Alternative splicing of exon 15 and possibly differential promoter usage yields two main isoforms that differ by a short C-terminal sequence [15]. These are the so-called ubiquitous (or constitutive) isoform [26] and the inducible isoform [27], the expression of the latter being very low in adult tissues, high in tumour cell lines and increased by pro-inflammatory stimuli. Additional splice variants of this gene have been described: six in rat brain [28] and four in human brain [29].
The PFKFB4 gene
The testis isoenzyme [16,30] is encoded by the PFKFB4 gene, which has been characterized in humans [16]. The expression of this isoenzyme, possibly translated from two mRNAs that share the same coding sequence, is reportedly testis-specific [16].
THE PFK-2 REACTION: CATALYSIS, SITE-DIRECTED MUTAGENESIS AND MODELLING
Kinetic studies of product inhibition [31,32] and stereochemical investigations [33] showed that the PFK-2 reaction involves ternary complex formation with direct in-line transfer and inversion of configuration of the γ-phosphate of ATP. Based on a sequence alignment, Pilkis and co-workers speculated that the PFK-2 domain was analogous to bacterial PFK-1 [34,35]. Accordingly, Cys160 of the PFK-2 domain (numbering refers to the rat liver isoenzyme unless specified otherwise), corresponding to Asp127 in bacterial PFK-1, would act as a base catalyst. This would explain why the kcat of PFK-2 is approx. 1000-times less than that of PFK-1, since a cysteine residue is expected to be a much poorer base catalyst than an aspartate residue. However, our site-directed mutagenesis experiments indicated that mutation of Cys160 to aspartic acid or serine barely affected the kcat [36], arguing against the PFK-1 analogy. A closer inspection of the PFK-2 sequence revealed two regions containing the so-called ‘Walker A’ and ‘Walker B’ motifs or nucleotide-binding fold [37,38]. These motifs are found in the AK (adenylate kinase), Ras and EF-Tu family of nucleotide binding proteins [39,40]. Indeed, mutagenesis of Lys54 and Thr55 [37] in the Walker A motif, and Asp130 [38] in the Walker B motif drastically decreased PFK-2 activity. Multiple sequence alignments of the PFK-2 domains of the PFK-2/FBPase-2 isoenzymes using the ‘Matchbox’ algorithm revealed conserved boxes of residues and mutagenesis of residues within these boxes allowed us to pinpoint residues that are important for substrate binding and catalysis [41–44]. An important residue for catalysis was identified as Lys174 [43]. In the yeast fbp26 isoenzyme, which lacks PFK-2 activity, this amino acid is replaced by a glycine residue. Indeed, mutation of Lys174 to glycine in the PFK-2 domain of the liver isoenzyme drastically reduced the kcat [43], and it is likely that this residue, together with Thr55 of the Walker A motif, stabilizes the transition state of the PFK-2 reaction during phosphate transfer (see below). A summary of the residues that are important for the PFK-2 reaction that we, and others, have identified as being important for substrate binding and catalysis, is shown in Figure 2(A).
Figure 2. Residues involved in catalysis and substrate binding in the PFK-2 domain (A), stereo view of the location of critical residues for ATP-binding and catalysis (B) and Fru-6-P binding (C) in the 3D structure of the testis isoenzyme.
The numbering of residues refers to the liver isoenzyme sequence. In (A), residues involved in substrate binding and catalysis in the PFK-2 domain are shown. In (B) and (C), the X-ray co-ordinates were retrieved from the PDB database (accession code 1BIF) corresponding to the testis isoenzyme containing ATPγS in the PFK-2 domain. The position of Fru-6-P was modelled as described [44]. The trace of the backbone is shown in green. Substrates and side-chains of critical residues are represented with carbon atoms in white, oxygen in red, nitrogen in blue and phosphate in yellow. Distances indicated by dotted lines are in Å.
Sequence similarities were found between the PFK-2 domain and other nucleotide-binding proteins, the most similar being AK [41]. Sequence alignments and secondary-structure prediction allowed us to propose a structure model of the PFK-2 domain, based on the 3D (three-dimensional) structure of AK [41]. The model was consistent with the roles of amino acid residues proposed from site-directed mutagenesis studies and was confirmed in the 3D structure of the testis isoenzyme, solved by Uyeda and co-workers [45]. The crystal structure of this isoenzyme contained an ATP analogue, but the other substrate, Fru-6-P was not present. Initial mutagenesis experiments suggested that Arg195 [46] and Arg104 [47] are important for Fru-6-P binding. Using the X-ray co-ordinates of the testis isoenzyme, we were able to identify residues in the Fru-6-P-binding pocket by computer docking (Figure 2C) and the role of these residues was confirmed by site-directed mutagenesis [44]. These studies indicate that five arginine residues are involved in Fru-6-P binding (Figure 2A).
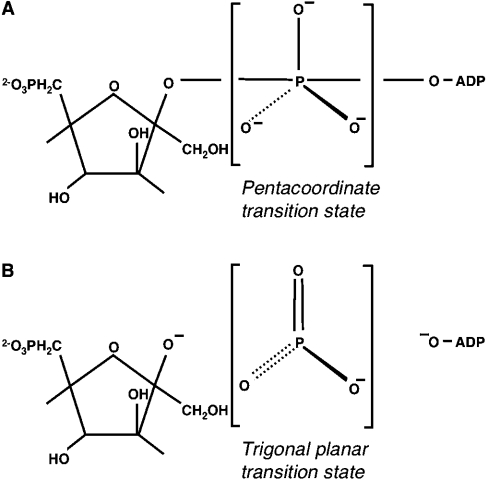
Although the PFK-2 domain displays structural similarity with AK, the kcat of PFK-2 (at best, approx. 0.1 s−1) is more than three orders of magnitude less than that of AK [48]. Another family member, the small G-protein Ras, has evolved to maintain a low catalytic activity [49] (kcat 1000 times lower than that of PFK-2), due to a highly flexible and mobile active site. However, in the presence of the GTPase-activating protein GAP, the kcat is increased 105-fold to about 20 s−1 [50]. The catalytic mechanism of Ras was originally proposed to involve proton abstraction from water by a general base, Gln61. This would help to explain the low kcat of Ras, since a glutamine residue would be expected to be a poor base catalyst. Indeed, replacement of Gln61 by glutamic acid increased the rate of GTP hydrolysis 20-fold [49]. However, an alternative mechanism for Ras-catalysed GTP hydrolysis has been proposed (substrate-assisted catalysis) [51]. In this mechanism, it is the γ-phosphoryl group of GTP, the substrate, that acts as a base catalyst. A central question in the understanding of phosphotransferase reaction mechanisms is whether they operate principally via an associative (SN2-like) or dissociative (SN1-like) transition state [52–54]. For the PFK-2 reaction, the two mechanisms are shown in Figure 3. If a negative charge develops on the γ-phosphate oxygens, the reaction is associative and occurs via a pentaco-ordinated phosphoryl intermediate. In a dissociative mechanism, a negative charge arises on the leaving group, which for the PFK-2 reaction is the β–γ-bridging oxygen atom of ADP. The Ras-catalysed hydrolysis of GTP was suggested to be associative, with Gln61 stabilizing the transition state by correctly positioning the attacking water molecule [55]. However, others have made arguments in favour of a dissociative transition state [54]. GAP accelerates the GTPase reaction by introducing the so-called ‘arginine finger’ (Arg789) to stabilize further the β–γ-bridge oxygen [56]. For the PFK-2 domain, all attempts to identify a residue that could fulfil the role of a base catalyst by site-directed mutagenesis have failed. It is noteworthy that catalysis in AK and related kinases does not seem to involve a base catalyst. The position of Lys174 in the PFK-2 domain structure [45] is such that it could stabilize a negative charge on the β–γ-bridge oxygen of ATP, favouring a dissociative transition state, in a manner similar to the arginine finger of GAP in Ras-catalysed GTP hydrolysis [54]. The low kcat of PFK-2 could be due to either a ‘loose’ transition state or the two substrates being far away from each other.
Figure 3. Associative and dissociative transition states for phosphotransferase catalysis in PFK-2.
In the single displacement reaction, both substrates must be bound to the enzyme forming a ternary complex. Phosphoryl transfer can then take place either via (A) an associative transition state (SN2-like) or (B) a dissociative transition state (SN1-like).
STRUCTURE OF PFK-2/FBPase-2
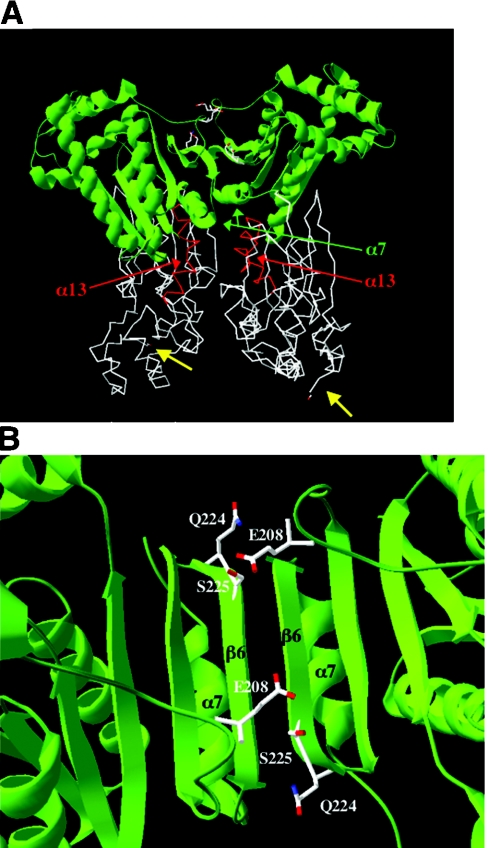
The crystal structure of the rat testis isoenzyme [45] confirmed that the PFK-2 domain is related structurally to the AK/Ras family, whereas the FBPase-2 domain is related to the histidine phosphatases. In the homodimeric structure [45], the two PFK-2 domains come together in a head-to-head fashion, whereas the FBPase-2 domains are basically independent (Figure 4A), with only one connecting salt bridge. The structure contained two bound phosphates, indicating the position of the FBPase-2 active site, and a molecule of ATPγS in the PFK-2 active site ([45], and see Figure 5). The PFK-2 domain is a six-stranded β-sheet surrounded by seven α-helices (Figure 5). The Walker A motif is located at the C-terminal end of the first β-strand and the phosphates of ATPγS are bound in the anion hole of the phosphate-binding loop. Lys54 of the Walker A motif interacts with both the β- and γ-phosphates of ATP (Figure 2B), while Thr55 and Asp130 (Walker B motif) provide two ligands for the octahedral Mg2+ ion, another two being provided by the β- and γ-phosphates of ATP [45]. ATP is also bound by the stacking of the adenine ring against non-polar side chains. Although the crystal structure was devoid of Fru-6-P, its binding site was predicted to be analogous to the AMP site in AK [44], with the 6-phosphate group of Fru-6-P bound by Arg104, Arg138 and Arg195 (Figure 2C).
Figure 4. 3D structure of the testis PFK-2/FBPase-2 homodimer (A) and close-up view of the head-to-head dimer interface (B).
(A) The co-ordinates were retrieved from the PDB database (accession code 1BIF). In this Figure, the PFK-2 domains are in green and come together in a head-to-head like fashion. The FBPase-2 domains are below in white. The α7 helices in each subunit are indicated by green arrows, and the α13 helices are in red (see text). The C-terminal ends in each subunit are indicated by yellow arrows. The side chains of residues in the PFK-2 domain shown in (B) are also shown in (A) and are coloured white in the upper domain. (B) Top view of the head-to-head interaction seen after turning the structure through 90 ° towards the reader along the plane of the β-sheet. Part of the interacting continuous 12-stranded β-sheet is shown together with the positions of some of the residues that differ between the testis and liver isoenzymes, namely Ser225 (replaced by arginine in the liver isoenzyme), Gln224 (replaced by threonine in the liver isoenzyme), along with Glu208, which is conserved (see text).
Figure 5. 3D structure of one enzyme subunit of the testis PFK-2/FBPase-2 isoenzyme.
The co-ordinates were retrieved from the PDB database (accession code 1BIF) containing ATPγS in the PFK-2 domain. The position of Fru-6-P was modelled as described in [44]. In the upper right hand PFK-2 domain, ATP is on the left and Fru-6-P is on the right. In the lower left hand FBPase-2 domain, two inorganic phosphates indicate the position of the Fru-2,6-P2-binding site. The PFK-2 domain is composed of a β-sheet surrounded by α-helices. Two subdomains, composed of α-helices (above) form a flexible cover and are involved in Fru-6-P binding and catalysis (induced fit, see text).
The two central six-stranded β-sheets of the PFK-2 domains interact to form a continuous twelve-stranded inter-monomer β-sheet [45], part of which is shown in Figure 4(B). Therefore PFK-2 functions as a homodimer, in contrast with AK, which, in mammals, is a 22 kDa monomer. Substrate-induced conformational changes have been proposed for kinases such as hexokinase [57] and AK [58]. AK contains a cleft at the active site. ATP and AMP bind at opposite ends of this cleft with their phosphates pointing towards each other and lying in the centre of the cleft. Substrate binding causes a switch to the structure in which the cleft closes [58]. By analogy with AK, the PFK-2 domain is predicted to contain two mobile segments which trap Fru-6-P and ATP [45], thereby excluding water to prevent ATP hydrolysis. Therefore the PFK-2 domain is a bi-lobed structure (Figure 5) and induced-fit changes on substrate binding are a likely feature, in common with other kinases.
Recently, the crystal structure of the human liver PFK-2/FBPase-2 isoenzyme was solved [12]. Superimposition of the structures of the liver and testis isoenzymes indicated that the overall folding was similar, as expected. However, the sequence differences (26%) cause conformational differences, including a twist of the FBPase-2 domain relative to the PFK-2 domain. This allows new dimeric interactions between the FBPase-2 domains of the liver isoenzyme, increasing their conformational stability and FBPase-2 activity. Sequence differences in the FBPase-2 binding pocket of the liver isoenzyme would also explain its higher affinity for Fru-2,6-P2 relative to the testis isoenzyme. Like in the testis isoenzyme, the PFK-2/FBPase-2 interface of the liver isoenzyme includes hydrophobic interactions between a β-hairpin (residues 215–230) and the following helix α7 of the PFK-2 domain, which in turn interacts with helix α13 in the FBPase-2 domain (Figure 4A). Part of this structure also forms the dimeric interface by which the PFK-2 domains come together head-to-head (Figure 4B). Sequence differences in this hairpin (T224Q, A225S and Q232A, liver compared with testis isoenzyme; numbering refers to the human liver isoenzyme) could impart a twist in the FBPase-2 domain [12]. Arg225 in the liver isoenzyme forms a salt bridge with Glu208 (see Figure 4B) participating in the rotation of the hairpin structure relative to the testis isoenzyme, a rotation that triggers the swing of the FBPase-2 domains. Interestingly, we showed that Arg225 in the liver isoenzyme was labelled by the group-specific reagent, phenylglyoxal, and proposed that this residue might be involved in Fru-6-P-binding in the PFK-2 domain [59]. Indeed, mutation of Arg225 to serine in the liver isoenzyme increased the Km of PFK-2 for Fru-6-P and decreased the Vmax [47]. However, the Arg225→Ser mutant displayed an increase in the Vmax of FBPase-2, contrary to its proposed role from the crystal structure. This might be because other hydrogen-bonding interactions via Thr224 and Gln232 would be maintained and that mutation of all three residues would be necessary to change the kinetics of FBPase-2 into those characteristic of the testis isoenzyme. Lastly, it is perhaps noteworthy that phosphorylation by PKA decreased the labelling and PFK-2 inactivation by phenylglyoxal [59]. An interesting possibility is that the phosphorylated Ser32 might interact with Arg225 and in some way transmit conformational changes to the FBPase-2 and PFK-2 domains (see below).
EVOLUTION OF PFK-2/FBPase-2
Mammalian PFK-2/FBPase-2 sequences have been derived from cDNAs obtained from liver (rat [60,61], human [62,63], bovine [64] and mouse [65]), skeletal muscle (rat [66]), heart (rat [67], bovine [68], human [23] and mouse [65]), placenta/brain (human [25,69], bovine [24] and rat [70]) and testis (human [16] and rat [11]). Sequences are also available from birds (chicken liver [71]), amphibians (frog muscle and frog liver [72]), fish (seabream, Sparus aurata [73]), plants (Arabidopsis thaliana [74], potato [75] and spinach [76]), baker's yeast [77–79] and from various genome projects, such as Caenorhabditis elegans, Drosophila melanogaster, Neurospora crassa, Schizosaccharomyces pombe and trypanosomatids. Interestingly, a search of 272 eubacterial genomes in the NCBI database with the full-length rat liver PFK-2/FBPase-2 isoenzyme sequence revealed the presence of a homologue in Desulfovibrio desulfuricans (see below). The FBPase-2 domain, the phosphoglycerate mutases and the acid phosphatases probably diverged from a common ancestor [6,7,34,35], whereas the PFK-2 domain is related to a superfamily of mononucleotide binding proteins including AK, p21 Ras, EF-Tu, the mitochondrial ATPase β-subunits and myosin ATPase, all of which contain the Walker A and B motifs and have a similar fold ([41,45] and see above). As most of the PFK-2/FBPase-2 sequences contain an N-terminal PFK-2 domain and a C-terminal FBPase-2 domain in the same polypeptide, the bifunctional enzyme must have arisen by gene fusion followed by duplication of this primordial gene. The PFK-2 and FBPase-2 domains can be flanked by variable extensions, which, in the mammalian isoenzymes, correspond to regulatory domains often containing phosphorylation sites for protein kinases. The plant [74–76], Neurospora and yeast pfk26 [77] sequences possess a long N-terminal end responsible for an increase in molecular mass of the enzyme subunits from about 55 kDa to 90 kDa. The two catalytic cores have also evolved into inactive domains in lower organisms. For example, the yeast pfk26 is devoid of FBPase-2 activity, principally by mutation of the active site histidine in the RHG motif to serine [77], while yeast fbp26 lacks PFK-2 activity ([79], and see above). The yeast pfk27 is essentially a PFK-2 domain (subunit molecular mass of approx. 40 kDa) [78] with a small C-tail (Figure 1).
Fru-2,6-P2 and PFK-2 and FBPase-2 activities have also been detected in other simple eukaryotes: in the unicellular photosynthetic flagellate Dunaliella sp. (phylum Chlorophyta) and in the slime mould Dictyostelium discoideum (only Fru-2,6-P2 detected) (reviewed in [5]). No Fru-2,6-P2, PFK-2 or FBPase-2 could be detected in the protists Tritrichomonas foetus and Trichomonas vaginalis (phylum Parabasalia) and Isotricha prostoma (phylum Ciliaphora) [5]. In trypanosomatids, most of the glycolytic pathway is compartmentalized within the glycosome, where PFK-1 is insensitive to Fru-2,6-P2 [80]. In contrast, cytosolic pyruvate kinase is stimulated by Fru-2,6-P2, consistent with the presence of PFK-2 and FBPase-2 in the cytosol [80]. By searching trypanosomatid databases, four genes potentially encoding PFK-2/FBPase-2 isoenzymes were found in both Trypanosoma brucei and Leishmania major, and were termed Tb1/Lm1 to Tb4/Lm4 (N. Chevalier, L. Bertrand, M. H. Rider, F. R. Opperdoes, D. J. Rigden and P. A. M. Michels, unpublished work). Lm1, Tb1 and Lm4 are predicted to contain long N-terminal extensions, whereas the molecular masses of Lm2, Lm3 and Tb3 are predicted to be more akin to the subunit molecular masses of the mammalian isoforms. Tb1 and Lm1 would possess PFK-2 activity and expressed recombinant Tb1 was indeed active. The PFK-2 domain of Lm2 could be active, although some important residues for catalysis (Lys54, Asp130 and Arg195) are mutated. The FBPase-2 domains of Tb1, Lm1 and Lm2 contain several mutated key residues and would be inactive. Therefore Tb1, Lm1 and Lm2 would be monofunctional PFK-2 isoenzymes. Lm3 and Tb3, on the other hand, are predicted to be monofunctional FBPase-2s. Lm4 and Tb4 would be inactive in the PFK-2 domains, but could have FBPase-2 activity, where the role of the nucleophilic histidine residue in the RHG motif, replaced by proline or asparagine respectively, would be fulfilled by another residue.
To look more closely at evolutionary relationships, phylogenetic trees were made from alignments of the separate PFK-2 and FBPase-2 catalytic core sequences taken from databases with AK and phosphoglycerate mutase as outgroups respectively (Figure 6). The mammalian isoenzymes cluster into the liver, heart, brain/placenta and testis groups, reflecting the four different genes, while in plants, only a single gene has been detected. The evolution of PFK-2/FBPase-2 in yeasts and trypanosomatids is not easy to deduce from the phylogenetic trees. This is due to low levels of conservation in the two domains and the fact that in these organisms, one or both domains have been inactivated and possibly subjected to a high evolution rate. Most of the isoenzymes of fungi have probably arisen from gene duplications within that group. Also, in the tree of the FBPase-2 domain, all the trypanosomatid sequences cluster together, separated from the sequences of all other organisms (but containing the outgroup). The cluster comprises two sets of two subgroups containing putative PFK-2s (Lm1/Tb1/Tc1 and Lm2; Tc1 is from Trypanosoma cruzi) and FBPase-2s (Lm3/Tb3 and Lm4/Tb4) respectively. The situation for trypanosomatids in the PFK-2 tree is very similar, except for the presence of yeast pfk27 in the cluster. The phylogenetic analysis indicates that the four isoenzymes in trypanosomatids already existed in the ancestor of Trypanosoma and Leishmania.
Figure 6. Phylogenetic trees constructed from sequences of the separate PFK-2 and FBPase-2 domains.
Sequences of PFK-2 and/or FBPase-2s were aligned using the computer program ClustalX [208]. From this alignment, sub-alignments of either the PFK-2 domain or the FBPase-2 domain were made. The residues were from 42 to 248 for the PFK-2 domain and from 249 to 438 for the FBPase-2 domain (numbering refers to the rat liver isoenzyme). The sequences were yeast PFK-2 27 (no. Q12471), yeast PFK-2 26 (no. P40433), Neurospora crassa PFK-2/FBPase-2 (no. Q9P522), Schizosaccharomyces pombe PFK-2/FBPase-2 (nos. Q9UTE1, Q8TFH0), spinach (Spinacea oleracea) PFK-2/FBPase-2 (GenBank® accession no. AF041848), Arabidopsis thaliana PFK-2/FBPase-2 (GenBank® accession no. AF190739), potato (Solanum tuberosum) PFK-2/FBPase-2 (GenBank® accession no. AF073830), Leishmania major PFK-2 designated ‘Lm1’ (no. Q9NKN9), yeast FBPase-2 26 (no. P32604), Caenorhabditis elegans PFK-2/FBPase-2 (no. Q21122), Drosophila melanogaster PFK-2/FBPase-2 (no. Q9VWH7), human testis PFK-2/FBPase-2 (no. Q16877), rat testis PFK-2/FBPase-2 (no. P25114), bovine brain PFK-2/FBPase-2 (no. Q28901), human placenta PFK-2/FBPase-2 (no. Q16875), mouse placenta PFK-2/FBPase-2 (no. Q9ESY2), rat brain PFK-2/FBPase-2 (no. O35552), bovine heart PFK-2/FBPase-2 (no. P26285), human heart PFK-2/FBPase-2 (no. O60825), mouse heart PFK-2/FBPase-2 (no. P70265), rat heart PFK-2/FBPase-2 (no. Q9JJH5), chicken liver PFK-2/FBPase-2 (no. Q91348), seabream liver PFK-2/FBPase-2 (GenBank® accession no. U84724), bullfrog liver PFK-2/FBPase-2 (no. Q91309), rat liver PFK-2/FBPase-2 (no. P07953), bovine liver PFK-2/FBPase-2 (no. P49872), human liver PFK-2/FBPase-2 (no. P16118). The trypanosomatid sequences were retrieved from the relevant genome databases. The Desulphovibrio desulfuricans sequence (accession no. ZP_00131027) was taken from a BLAST search of the NCBI eubacterial genomes with the full-length rat liver PFK-2/FBPase-2 sequence. Accession numbers are for Swiss-Prot/TrEMBL unless stated otherwise. Phylogenetic trees were created using the tree option of the ClustalX program after exclusion of positions with gaps. Arabidopsis thaliana AK (no. Q9ZUU1) and E. coli phosphoglycerate mutase (no. P36942) were taken as outgroups to root the PFK-2 and FBPase-2 trees respectively. Horizontal bars represent ten substitutions per 100 residues.
A PFK-2/FBPase-2 sequence in the completed genome of Desulfovibrio desulfuricans raises interesting possibilities as to the evolutionary origin of the bifunctional enzyme, assuming that its presence is not due to contamination. The gene neighbours of the putative bifunctional enzyme in Desulfovibrio are genuinely bacterial with no evidence that a eukaryotic DNA could have been introduced. The sequence would be expected to code for an enzyme that would be functional in both domains, based on the conservation of residues for substrate binding and catalysis. Desulfovibrio species are strict anaerobes that can oxidize low-molecular-mass compounds such as lactate, pyruvate, fumarate, ethanol and glycerol. However, they can also store and synthesize polyglucose which can be catabolized in the absence of exogenous substrates, and the resulting NADH can be utilized for the reduction of sulphate, thiosulphate or nitrite. It remains to be seen whether Fru-2,6-P2 would be present in these organisms, for example to control PFK-1 during polyglucose degradation. The gene does not seem to be part of an operon, although the downstream neighbour is 4-α-glucanotransferase (amylomaltase). The fact that a PFK-2/FBPase-2 is found in only a single bacterium out of over 300, also including archaea, makes it unlikely that the bifunctional enzyme arose in a bacterium. Its existence could be due to horizontal gene transfer from a eukaryote. The phylogenetic trees show that the Desulfovibrio sequences branch at the root of the tree and are not close to any of the other organisms (Figure 6). Therefore the donor of the gene was most likely to be a protist unrelated to trypanosomatids. The presence of a PFK-2/FBPase-2 sequence in Desulfovibrio, with its likely origin from a protist, and the predicted domain structure of the isoenzymes in the trypanosomatids, indicates that the gene fusion giving rise to a bifunctional enzyme occurred early in eukaryotic evolution in an ancestral unicellular organism.
The occurrence of multiple isoforms of PFK-2/FBPase-2 in mammals can be understood as a need for enzymes with different kinetic and regulatory mechanisms to regulate glycolysis (gluconeogenesis) in different tissues under various hormonal and nutritional states. In yeast, pfk26 is activated by PKA [77,82], whereas the synthesis of pfk27 is induced by fermentable carbon sources [78]. Neither is essential for growth [78,83], but there is evidence for distinct roles for the two yeast PFK-2s from metabolomic analysis of mutants [84]. The reason for the existence of four isoenzymes in trypanosomatids could be to adapt to radically different nutritional environments encountered by the parasite in its complicated life cycle between insects and mammals. Why the bifunctional PFK-2/FBPase-2 evolved into monofunctional enzymes in yeasts and trypanosomatids is unclear. It might represent an adaptation to the specific requirements of glucose metabolism in these unicellular organisms, different from the requirements in the ancestral eukaryote where the fusion of the PFK-2 and FBPase-2 domains in a single enzyme occurred. One may wonder why, in the monofunctional enzymes, an inactive PFK-2 or FBPase-2 domain has been retained. Only in the yeast pfk27 has the FBPase-2 domain diverged considerably and become truncated. For the mammalian PFK-2/FBPase-2s, the PFK-2 domains are intimately associated in the 3D structure (see above), whereas the FBPase-2 domains are independent in the testis isoenzyme [45], but they do form contacts in the liver isoenzyme [12]. Also, when expressed separately, the PFK-2 domain forms inactive aggregates [85], while the FBPase-2 domain can be active as a monomer [86]. Therefore the inactive domains have been kept probably to maintain the active dimeric structure of the enzyme. For the mammalian isoenzymes, the advantages of bifunctionality are simplicity in short-term control, such as by phosphorylation or by allosteric effectors, and simplicity in long-term control (one mRNA per bifunctional polypeptide). One reason why certain micro-organisms at a later stage of evolution again uncoupled PFK-2 and FBPase-2 could be to give increased flexibility to adapt to different growth conditions. Different combinations of monofunctional PFK-2 and FBPase-2 enzymes might become expressed in trypanosomatids growing in different environments, and this could be studied by following the expression of the different enzymes throughout the life cycle of the parasite.
TRANSCRIPTIONAL CONTROL OF MAMMALIAN PFK-2/FBPase-2
Regulatory sequences that account for some of the mechanisms involved in the long-term hormonal control and tissue-specific expression of PFK-2/FBPase-2 have been identified in the PFKFB1, PFKFB2 and PFKFB3 genes.
PFKFB1 gene
This gene has been extensively studied, since its expression is stimulated by glucocorticoids, an effect inhibited by insulin, by glucose and by mitogenic stimuli, and because its L promoter is liver- and adipose-tissue-specific. Glucocorticoids and insulin control PFKFB1 via a GRU (glucocorticoid-response unit) located in its first intron [87]. A combination of in vitro DNA-binding assays, transfection experiments and in vivo genomic footprinting showed that this GRU behaves as an enhancer in an open chromatin conformation [88] and binds the transcription factor NF-1 (nuclear factor 1) in unstimulated cells. Binding of glucocorticoids to their GR (glucocorticoid receptor) on the GRU induces binding of HNF (hepatocyte nuclear factor)-3, which then co-operates with the GR [89]. Insulin targets the ligand-binding domain of the GR, whose action is thereby inhibited, via a signalling cascade that is distinct from the PI3K (phosphoinositide 3-kinase) or MAPK (mitogen-activated protein kinase) pathways [90], but appears to involve the JNK (c-Jun N-terminal kinase)/SAPK (stress-activated protein kinase) pathway [91].
The F promoter binds the ets (proto)oncogene product, which stimulates its activity [17,18]. F promoter activity is inhibited when cells differentiate, through its binding of the E2F transcription factor. F promoter activity is stimulated at the G1/S transition of the cell cycle by growth factors [e.g. EGF (epidermal growth factor)] and oncogene (e.g. v-src and E1A) products, which relieve the E2F-mediated inhibition [92]. This effect of EGF is inhibited by cAMP and could involve MAPK [93] and PI3K/PKB (protein kinase B) [94] signalling. Moreover, F precursor mRNA splicing is blocked in G0 and this block requires the E2F-binding site [95]. These data shed light on the mechanisms that switch-off transcription from the F promoter, e.g. in adipoblasts when they differentiate into adipocytes, which use instead the M and L promoters, and in myoblasts when they differentiate into adult muscle, which uses the M promoter.
Glucose increases the concentration of the F and L mRNAs in hepatic cell lines. This effect of glucose is mimicked by xylitol, a precursor of intermediates of the pentose phosphate and glycolytic pathways, and is inhibited by okadaic acid, an inhibitor of protein phosphatases. Transfection experiments showed that the F promoter region is a target of the glucose effect. Another region of the gene, located between the F and L promoters, behaves as a glucose-sensitive enhancer of both the F and the L promoters. This region corresponds to a cluster of DNase-I hypersensitive sites that are induced in chromatin following glucose treatment [96]. Its sequence organization resembles that of the glucose-sensitive insulin gene promoter. The transcription factor(s) involved in this glucose effect have not been identified.
The regulation of the M promoter is poorly understood. Its TATA box region binds the transcription factor C/EBP (CCAAT/enhancer-binding protein), which stimulates its activity [97]. This could play a role in white adipose tissue, since C/EBP is an important transcriptional activator of genes that are expressed in adipocytes. The DNA-binding and transactivation properties of C/EBP are stimulated through its direct interaction with the tumour suppressor protein Rb and this is crucial for C/EBP-dependent terminal differentiation of fibroblasts into adipocytes. Fibroblasts express PFK-2 mRNA from the F promoter and this depends on the transcription factor E2F. The latter is inhibited by Rb, which switches off the F promoter. Thus C/EBP could be an important component of the F-to-M promoter switch during differentiation [98].
The basal activity of the L promoter, which is controlled by the GRU discussed above, was found to depend on its binding of both ubiquitous (NF-1 and Oct-1) and liver-specific (HNF-3 and C/EBP) transcription factors [99,100]. Further study of regulatory protein binding to this promoter led to the discovery [100] and cloning [101] of HNF-6.
HNF-6 is the prototype of a new class, called Onecut (OC) [102], of homoeoproteins conserved from nematodes, ascidians, flies and zebrafish to mammals, with three members in humans, namely HNF-6 or OC-1, OC-2 and OC-3 [103,104]. In adult liver, HNF-6 stimulates the transcription of genes coding for secreted proteins [105], for P450 cytochromes [106] and for enzymes of glucose metabolism, including PFK-2/FBPase-2, PEPCK (phosphoenolpyruvate carboxykinase) [107], glucose-6-phosphatase [108] and glucokinase [109]. Interestingly, the Hnf6 gene is controlled by growth hormone [106,110]. HNF-6 can also counteract the stimulatory action of glucocorticoids on transcription of the PFKFB1 and PEPCK genes, by interacting with the GR on the DNA of these target genes [107]. As the enzymes controlled by HNF-6 are involved in both gluconeogenesis and glycolysis, the effect of HNF-6 might be to provide the hepatocyte with an adequate enzyme level for short-term regulation by other signals rather than influence the direction of carbohydrate metabolism. In any case, Hnf6-knockout mice are diabetic [109].
In the embryo, HNF-6 is expressed in developing liver and pancreas, where it regulates genes coding for transcription factors that control cell differentiation and morphogenetic programmes. Studies on Hnf6-knockout mice showed that HNF-6 controls the timing of pancreas specification from the endoderm by inducing the transcription factor Pdx-1 [111]. Later on, HNF-6 delineates in the pancreatic epithelium a subset of cells, corresponding to endocrine and ductal precursors, by inducing the expression of HNF-1β [112]. Finally, HNF-6 stimulates the differentiation of endocrine precursors, via induction of the transcription factor Ngn-3 [113]. Type II diabetic patients were screened for mutations in the HNF6 gene, but none have been identified so far. Hnf6-knockout mice also lack a gall bladder and their extrahepatic bile ducts are abnormal. Their liver is cholestatic and displays anomalies that resemble human biliary diseases called ‘ductal plate malformations’. Indeed, the differentiation of cholangiocytes and morphogenesis of the intrahepatic bile ducts are perturbed. These disorders involve the transcription factor HNF-1β, which is a target of HNF-6 [114]. Recent data from promoter microarrays following chromatin immunoprecipitation suggest that HNF-6 serves as a master regulator for feedforward loops in liver and pancreas [115]. As OC-2 and OC-3 are also expressed in midgut endoderm, the discovery of the OC class of transcription opens exciting perspectives in the field of developmental biology.
PFKFB2 gene
The 5′ flanking sequence of PFKFB2 contains regions that are conserved between the human, bovine and rat genes. One finds in these regions potential binding sites for the Sp1, HNF-1 and βHLH (helix–loop–helix) (E boxes) transcription factors and for the GR [20,22,23], but a factor binding to these sites has not been reported.
PFKFB3 gene
Expression of the ubiquitous isoform is induced by progestins [26] and by insulin [116]. Potential binding sites for the progesterone receptor and for SREBP (sterol-regulatory-element-binding protein)-1c, a transcription factor known to mediate insulin action, are present in the 5′ flanking sequence of the gene, which also contains consensus sequences for binding the ubiquitous factors Sp-1, AP-2 (activating protein 2), and NF-1 [15].
The concentration of the mRNA coding for the inducible isoform increases in human monocytes when they are exposed to lipopolysaccharide. This could result from increased stability of the mRNA, whose 3′ untranslated region contains typical AUUUA regulatory sequences [27]. Transcription of the PFKFB3 gene also increases following PKC (protein kinase C) and PKA stimulation [15] and in response to hypoxia, an effect that requires the HIF-1 (hypoxia-inducible transcription factor-1) [117]. However, these data were obtained with mRNA probes that do not allow distinction between the ubiquitous and the inducible isoforms.
MECHANISMS OF CONTROL OF PFK-2 AND FBPase-2 BY PROTEIN PHOSPHORYLATION
Although protein phosphorylation has been recognized for more than 40 years as the main mechanism for controlling enzyme activity and protein function, we know surprisingly little about how the introduction of a phosphate group on specific serine, threonine and tyrosine residues leads to its biological effects. Historically, the first 3D structures revealing mechanisms of phosphorylation-induced changes in enzyme activity came from those of rabbit muscle glycogen phosphorylase [118] and Escherichia coli isocitrate dehydrogenase [119]. Crystal structures of phosphorylase in its phosphorylated and dephosphorylated forms showed that the phosphorylation of Ser14 at the subunit interface induces the N-terminus to adopt a α-helical conformation. This change is transmitted to the active site some 30 Å (1 Å=0.1 nm) away and results in the T-to-R transition [118]. On the other hand, phosphorylation of E. coli isocitrate dehydrogenase at Ser113 inactivates the enzyme not via a conformational change [119], but by charge repulsion between the phosphorylated serine and the negatively charged substrate [120]. Phosphorylation of protein kinases, such as the insulin receptor kinase or the AGC kinases in the so-called ‘activation loop’, leads to activation by electrostatic interaction between the negatively charged phosphate group and positively charged residues in the upper lobe, which stabilizes the closed conformation [121]. Both conformational changes and/or direct electrostatic effects in the substrate-binding sites could be responsible for the control of PFK-2 and FBPase-2 activities by phosphorylation.
Rat liver PFK-2/FBPase-2
Although the human liver isoenzyme has recently been crystallized for 3D structural analysis, the N-terminus was not solved [12], possibly because of its high flexibility. Phosphorylation of the liver isoenzyme at Ser32 by PKA [122] leads to PFK-2 inactivation, mainly by increasing the Km for Fru-6-P [123–125]. Therefore movement of the phosphorylated Ser32 into the PFK-2 domain, where it could repel the negatively charged phosphate group of the substrate, might explain the increase in Km for Fru-6-P. At the same time, a conformational change transmitted to the FBPase-2 domain would explain how phosphorylation of Ser32 by PKA activates FBPase-2 by increasing the Vmax [124,126,127]. Mutation of Ser32 to aspartic acid in the rat liver isoenzyme [128], or introduction of a phosphorylation site motif in the N-terminus of the testis isoenzyme and its subsequent phosphorylation by PKA [129], inactivated PFK-2 and activated FBPase-2 as expected.
FBPase-2 activity seems to be repressed by the PFK-2 domain, since partial proteolysis of the liver isoenzyme, which removes most of the PFK-2 domain, leads to a 2-fold increase in Vmax of FBPase-2 [130]. Regulation of the PFK-2 and FBPase-2 activities by PKA-induced phosphorylation appears to involve both the N- and C-termini. Deletion of the N-terminal 22 residues of the liver isoenzyme increased the kcat of FBPase-2 4-fold and decreased the affinity of PFK-2 for Fru-6-P 20-fold [131]. This deletion also blunted the PKA-induced effects of phosphorylation on both activities. Truncation of the C-terminal 25–30 residues from the rat liver [132] and chicken liver [133] isoenzymes increased the kcat of FBPase-2 and abolished the effect of PKA-mediated phosphorylation to activate FBPase-2. A sequence His444-Arg-Glu-Arg447 was identified in chicken liver PFK-2/FBPase-2, which mediates the repressive effect of the N-terminal PFK-2 domain on FBPase-2 activity [133]. It was proposed that phosphorylation by PKA could activate FBPase-2 by relieving the repression by the PFK-2 domain that is mediated by the two basic residues, His444 and Arg445, in this sequence [133]. However, it is unlikely that this His-Arg-Glu-Arg sequence interacts directly with the N-terminus or could form the serine-phosphate recognition site, since, assuming the chicken liver bifunctional enzyme structure is the same as that of the testis isoenzyme, the N-terminus would be located too far away (some 50 Å). In the rat liver isoenzyme, this motif corresponds to His446-Arg-Asp-Lys449 and in the testis isoenzyme to His444-Arg-Asp-Arg447, which in the crystal structure would be located in the FBPase-2 domain. As in glycogen phosphorylase, phosphorylation could induce a long-range conformational change by introducing new charge–charge interactions resulting in a more catalytically competent active site.
Bovine heart PFK-2/FBPase-2
The mechanism of control of PFK-2 by phosphorylation of the heart isoenzyme is also difficult to explain in the absence of a crystal structure of the phosphorylated isoenzyme. Phosphorylation of Ser466 and Ser483 at the C-terminal end of the bovine heart isoenzyme by PKA [134–137] and insulin-stimulated protein kinases [137] activates PFK-2 by decreasing the Km for Fru-6-P and by increasing the Vmax without affecting FBPase-2. Mutagenesis of Ser466 to glutamic acid and Ser483 to glutamic acid, to mimic the effects of phosphorylation, indicated that Ser466 phosphorylation is responsible for the increase in Vmax, whereas both Ser466 and Ser483 phosphorylation are necessary to decrease the Km for Fru-6-P [138]. The C-terminal ten residues in the 3D structure of the rat testis isoenzyme are indicated by arrows in Figure 4(A). In the bovine heart isoenzyme, the C-terminus is 60 residues longer and could conceivably stretch into the PFK-2 domain. The role of the C-terminus in the bovine heart isoenzyme was demonstrated by deletion mutagenesis. Deletion of the 49 C-terminal residues, removing Ser483, but leaving the sequence around Ser466 intact, led to a slight increase in Vmax, slightly decreased the Km for Fru-6-P and increased the kcat/Km of PFK-2 2-fold without affecting FBPase-2 [139]. Furthermore, the Vmax of PFK-2 of the truncated enzyme was increased following phosphorylation by PKA. Deletion of 78 C-terminal residues, which removes both PKA phosphorylation sites, led to a 2.5-fold increase in Vmax, a 4.5-fold decrease in Km for Fru-6-P and 12-fold increased kcat/Km of PFK-2 [139]. This longer deletion also activated FBPase-2 through a 3-fold increase in kcat/Km. A possible interpretation is that the C-terminal end represses the PFK-2 and FBPase-2 domains and that phosphorylation by PKA at Ser466 and Ser483 relieves the inhibition of PFK-2 either by displacing the C-terminus from the PFK-2 domain or via the transmission of conformational changes.
PHOSPHORYLATION OF PFK-2/FBPase-2 ISOENZYMES AND THE CONTROL OF GLYCOLYSIS
Lower eukaryotes
In yeast incubated with high concentrations of glucose, cAMP concentrations rise, PKA is stimulated and then phosphorylates and activates PFK-2 [82]. When the yeast pfk26 isoform was cloned and sequenced, it was suggested that phosphorylation at Ser644 in the C-terminal sequence Arg-Arg-Tyr-Ser would be responsible for PFK-2 activation [83], by analogy with the heart isoenzyme. However, the yeast pfk26 Ser644→Ala mutant was still phosphorylated in vitro by PKA or in vivo in response to glucose, and a second PKA site was suggested as Thr157 [140]. In vitro, both Ser644 and Thr157 were phosphorylated by PKA; however, in vivo, only Ser644 phosphorylation was the result of PKA stimulation [141]. In yeast, lowering glucose concentrations activates Snf1 kinase, the homologue of AMPK (AMP-activated protein kinase) in mammals [142]. AMPK phosphorylates and activates heart PFK-2 and was suggested to be involved in the Pasteur effect in this tissue (see below). However, it is unlikely that Snf1 would be involved in the regulation of glycolysis in yeast in response to glucose, unless phosphorylation at a site different from the PKA site would inactivate PFK-2. Under hypotonic conditions, yeast PFK-2 becomes inactivated via phosphorylation at Ser652, probably by yeast PKC1 [143].
In plants, Fru-2,6-P2 levels change diurnally and the targets of Fru-2,6-P2 are the pyrophosphate:fructose-6-phosphate 1-phosphotransferase, an alternative PFK-1 homologue, and FBPase-1 [144,145]. Low Fru-2,6-P2 concentrations favour sucrose synthesis from photosynthetically assimilated carbon, whereas increased Fru-2,6-P2 concentrations promote starch synthesis. In A. thaliana, a principally serine-phosphorylated form of PFK-2/FBPase-2 has been detected [146]. However, the protein kinase(s) were not identified, and although the phosphorylated form was predominant at the beginning of the dark period, it is not known whether phosphorylation would result in changes in PFK-2 or FBPase-2 activity.
PFK-2 has been purified to homogeneity from the hepatopancreas of an anoxia-tolerant marine whelk as an Mr 67000 subunit dimer with negligible FBPase-2 activity [147]. Anoxia depressed Fru-2,6-P2 concentrations and PFK-2 activity in all organs of this mollusc via a phosphorylation mechanism, but PKA or PKC were not involved. An intriguing possibility is that AMPK might be implicated; however, if so, this would have the opposite effect on PFK-2 activity as that described for the heart enzyme (see below).
Mammals
The liver and muscle isoenzymes
In the liver, glucagon stimulates PKA, which phosphorylates Ser32 of the liver isoenzyme, leading to PFK-2 inactivation and FBPase-2 activation (reviewed in [1–7]). The subsequent fall in Fru-2,6-P2 concentration decreases glycolysis and increases gluconeogenic flux. Together with the PKA-induced phosphorylation and inactivation of pyruvate kinase, this is the basis of the effect of glucagon to block hepatic glycolysis and stimulate gluconeogenesis during starvation. Apart from control by PKA, no other meaningful phosphorylations of the liver isoenzyme have been described. Indeed, in intact hepatocytes, PFK-2/FBPase-2 was phosphorylated by glucagon, but not by treatment with vasopressin, phorbol ester or calcium ionophore [148]. In contrast, an increase in Fru-2,6-P2 was observed in hepatocytes incubated with vasopressin, probably as a result of an increase in glycogen breakdown [149].
Insulin stimulates glycolysis in white adipose tissue and skeletal muscle. The muscle isoenzyme is a splice variant of the liver isoenzyme with a different N-terminus, where the first 32 residues, including the PKA phosphorylation site, are replaced by a peptide devoid of phosphorylation sites (see above). White adipose tissue [19] and skeletal muscle [150] contain both the liver and muscle isoenzymes. In skeletal muscle, Fru-2,6-P2 concentrations are increased by insulin [151], adrenaline [151–153] and contraction induced by exercise [154,155] or electrical stimulation [152,156,157], probably secondary to a rise in Fru-6-P. In white adipose tissue, insulin stimulates glycolysis without increasing Fru-2,6-P2 concentrations or affecting PFK-2 activity [158]. The stimulation of glycolysis by insulin in white adipose tissue and skeletal muscle is thus likely to be mainly due to the well-known stimulation of glucose transport [159].
The heart isoenzyme
Insulin stimulates glycolysis in heart by a combination of an increase in glucose transport and the activation of PFK-2 [8,9]. Rat heart PFK-2 is activated by insulin in vivo through a 2-fold increase in Vmax, with no change in Km for Fru-6-P [160]. The heart can oxidize different fuels to sustain contraction. Lipid fuels (fatty acids and ketone bodies) are preferentially used, which helps to conserve glucose in the fasting state. However, when blood glucose concentrations rise following a meal, the subsequent rise in insulin would allow the heart to switch to glucose utilization by stimulating glycolysis [8]. It is not clear why heart, and not adipose tissue and skeletal muscle, should have evolved with an insulin-activated PFK-2. A possible explanation is that following insulin-stimulated glucose transport, glucose 6-phosphate is directed towards glycogen in skeletal muscle [161], whereas in heart, glucose 6-phosphate would be mainly routed towards pyruvate [8]. An interesting parallel can be drawn with acetyl-CoA carboxylase (ACC), where an isoform is present in heart and skeletal muscle (ACC2) [162] that is different from the liver and adipose tissue isoenzyme (ACC1) [163]. Heart and skeletal muscle have little lipogenic capacity and the role of ACC2 is probably just to control fatty acid oxidation through changes in malonyl-CoA concentrations [164,165].
Considerable advances towards the understanding of insulin action [166–169] have been made since we first described heart PFK-2 activation by insulin many years ago [160]. There are several signalling pathways which can be triggered following the binding of insulin to its receptor. Insulin receptor binding leads to autophosphorylation and activation of its receptor kinase, which in turn phosphorylates members of the IRS family, Shc and Cbl [169]. Cbl phosphorylation in lipid rafts has been proposed to be involved in GLUT4 (glucose transporter 4) vesicle translocation to the plasma membrane. The adaptor proteins Grb2 and Sos lead to activation of the MAPK cascade via Ras stimulation, while stimulation of the PI3K pathway leads to the activation of p70S6k (p70 ribosomal protein S6 kinase) and PKB [167,168]. PKB is activated in response to insulin via phosphorylation in the activation loop at Thr308 by PDK (phosphoinositide-dependent protein kinase)-1 and by phosphorylation in the hydrophobic motif at Ser473 by PDK2. The involvement of the insulin signalling cascades can be studied by the careful use of inhibitors. In rat cardiomyocytes, we showed that the insulin-induced activation of PFK-2 was blocked by wortmannin, a PI3K inhibitor, but was insensitive to rapamycin or PD098059, which prevent the activation of p70S6k and the MAPK cascade respectively [170]. These experiments with inhibitors suggested that PI3K, but not p70S6k, is involved in the activation of PFK-2 in response to insulin. In vitro experiments with purified enzyme preparations showed that PKB phosphorylated and activated recombinant heart PFK-2 [137]. The phosphorylation sites were identified as Ser466 and Ser483 in the bovine heart isoenzyme. Interestingly, 14-3-3 proteins bind to phosphorylated Ser483 and this might contribute towards PFK-2 activation [171]. To understand further the mechanism for the activation of heart PFK-2 by insulin, HEK-293 (human embryonic kidney) cells were transfected with protein kinase constructs. In cells overexpressing heart PFK-2, insulin activated PFK-2 by phosphorylation of Ser466 and Ser483 [138]. Co-transfection with dominant-negative PDK1 abolished PFK-2 activation by insulin. However, transfection of dominant-negative PKB failed to prevent insulin-induced PFK-2 activation [138]. Therefore PDK1 appears to be necessary for the insulin-induced activation of heart PFK-2, whereas PKB is not essential. This was supported further by the failure of insulin to activate heart PFK-2 in vivo in PDK1-deficient mice [172]. Purification of insulin-stimulated PFK-2s from perfused hearts revealed the presence of a wortmanninsensitive, insulin-stimulated protein kinase, which we called ‘WISK’ and which did not correspond to PKB or PDK1 [173]. The nature of this protein kinase is currently under investigation.
Bovine heart PFK-2 is also a substrate of PKC [10,134,135], which phosphorylates Ser84, Ser466 and Ser475 [135,136]. The preparations of PKC used in these early experiments were from rat brain and were not separated into the various PKC isoforms. Therefore these preparations were probably mixtures of ‘conventional’ PKC isoforms, i.e. principally PKCα, PKCβ and PKCγ [174]. Although PKC phosphorylated sites in the C-terminal domain (Ser466 and Thr475), phosphorylation did not lead to a change in PFK-2 activity [10,135]. This might be due to the fact that phosphorylation of a third site in the PFK-2 domain (Ser84) would counteract the effects of phosphorylation at the activating C-tail sites. Site-directed mutagenesis of the PKC sites to glutamic acid/aspartic acid will be required to solve this issue. At present, therefore, the physiological significance of the phosphorylation of heart PFK-2 by PKCs is unclear, although distinct isoforms, such as members of the ‘atypical’ PKCs, might phosphorylate Ser466 specifically and activate PFK-2. This could be relevant to the activation of heart PFK-2 by insulin, as the WISK preparation might contain an atypical PKC isoform such as PKCζ (V. Mouton, D. Vertommen, L. Bertrand, L. Hue and M. H. Rider, unpublished work).
Adrenaline, a positive inotropic hormone, increases Fru-2,6-P2 concentrations and stimulates glycolysis in heart [175]. The phosphorylation and activation of heart PFK-2 by PKA could participate in this effect. Indeed, phosphorylation of heart PFK-2 by PKA in vitro activated PFK-2 by virtue of an increase in Vmax and a decrease in Km for Fru-6-P [134,135,137]. Moreover, the phosphorylation sites were identified as Ser466 and Ser483 in the bovine heart isoenzyme [136], i.e. the same sites phosphorylated by PKB and other insulin-stimulated protein kinases [137]. However, the involvement of PKA in the stimulation of heart glycolysis by adrenaline is questionable, because the activation of PFK-2 is difficult to demonstrate (C. Beauloye, L. Bertrand, M. H. Rider and L. Hue, unpublished work). The rise in Fru-2,6-P2 might be secondary to an increase in Fru-6-P concentration [175]; however, such an increase has not always been observed [152].
Heart glycolysis is also stimulated when the oxygen supply is restricted, as in myocardial ischaemia or anoxia (the Pasteur effect). Under these conditions, the only source of ATP is glycolysis, and thus the only substrates the heart can use are glucose and glycogen. Anaerobic conditions cause ATP concentrations to fall and AMP rises in parallel. These changes in adenine nucleotide levels lead to AMPK activation [142], which stimulates glycolysis by increasing GLUT4 translocation [176] and by activating heart PFK-2 [177]. This contrasts with the situation in liver where Fru-2,6-P2 concentrations decrease during anoxia [178]. Heart PFK-2 is a good substrate for AMPK, and phosphorylation leads to an increase in Vmax with no change in Km for Fru-6-P. The phosphorylation site was identified as Ser466 [177]. This observation is consistent with the presence of hydrophobic residues at −4 and +4 relative to the phosphorylated serine residue. In addition, there are arginine residues at −3 and −2 for phosphorylation by PKA and arginine residues at −5 and −3 plus a hydrophobic residue at +1 for recognition by insulin-stimulated protein kinases (Figure 7).
Figure 7. Conserved C-terminal domains in the heart and brain/placenta PFK-2/FBPase-2 isoenzymes.
The C-terminal sequence of human heart PFK-2/FBPase-2 containing the phosphorylation sites, Ser466 and Ser483, was aligned by eye with C-terminal sequences of the heart isoenzyme from other species and with the brain/placenta isoenzyme. The residue corresponding to Ser466 is in a suitable consensus for phosphorylation by AMPK in all sequences, whereas the brain/placenta isoenzyme lacks the −5 arginine residue for phosphorylation by insulin-stimulated protein kinases, although phosphorylation by PKA and PKC would still be expected (see text).
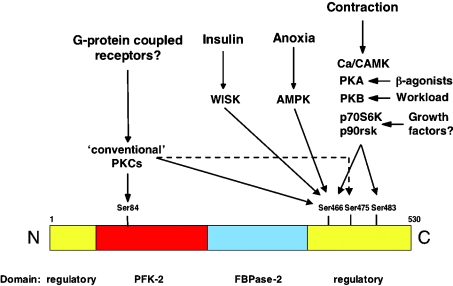
Glycolysis in heart also increases in response to increasing the workload [179,180]. Under these conditions, Fru-2,6-P2 concentrations rise and heart PFK-2 is activated. We originally proposed that phosphorylation and activation of heart PFK-2 by Ca/CAMK (Ca2+/calmodulin-activated protein kinase), secondary to a rise in cytoplasmic Ca2+, could explain this effect [179]. However, recent studies from our laboratory indicate that this might not be the whole story. Surprisingly, submitting hearts to an increased workload did not activate AMPK [180]. However, increasing the workload did activate PKB, but not p70S6k. Furthermore, the activation of PKB and PFK-2 due to increasing the workload was blocked by wortmannin, but was rapamycin-insensitive [180]. Therefore a possible scenario is that PFK-2 activation could be due to shear-stress-induced PI3K activation and activation of PKB. A summary of the various protein kinases that converge on the phosphorylation of heart PFK-2 is depicted in Figure 8.
Figure 8. Potential protein kinase cascades converging on heart PFK-2/FBPase-2.
The numbering of residues refers to the bovine heart isoenzyme. The signalling pathways leading to Ser466 phosphorylation of the heart isoenzyme could also impinge on Ser461 of the brain/placenta isoenzyme, but not on the equivalents of Ser84 or Thr475, which are replaced by non-phosphorylatable residues in the brain/placenta isoenzyme.
Brain/placenta isoenzyme
Another PFK-2/FBPase-2 that contains the equivalent of Ser466 in a suitable motif for AMPK is the brain/placenta isoenzyme with its ‘ubiquitous’ and ‘inducible’ (iPFK-2, see above) isoforms (Figure 7). In iPFK-2, Ser461 corresponds to Ser466 and phosphorylation by AMPK activates PFK-2, as expected [181]. The expression of iPFK-2 is induced in monocytes exposed to lipopolysaccharide, upon which it can be phosphorylated and activated by AMPK [181]. The stimulation of monocyte glycolysis via the AMPK-induced phosphorylation and activation of iPFK-2 could be important for furnishing ATP to sustain cytokine synthesis in infected anaerobic tissues. iPFK-2 is also expressed constitutively in several cancer cell lines [27], which display high rates of glycolysis, even in the presence of oxygen (Warburg effect). Since tumours contain anoxic zones, the activation of AMPK in cancer cells in these areas could stimulate glycolysis further by phosphorylating iPFK-2. It would be interesting to see whether insulin could activate the brain/placenta PFK-2 isoenzyme, in which the consensus around Ser461 is similar to that around Ser466 phosphorylated in response to insulin in the heart isoenzyme (Figure 7). Indeed, Ser461 of the brain/placenta isoenzyme is phosphorylated by PKA, PKC [182] and AMPK [181] like Ser466 in heart PFK-2/FBPase-2 [137,178]. The major PFK-2/FBPase-2 isoform in astrocytes is iPFK-2 [183]. In these cells, but not in neurons, NO stimulated glycolysis and increased Fru-2,6-P2 concentrations. This effect was attributed to mitochondrial respiratory chain inhibition at cytochrome oxidase by NO, activation of AMPK and phosphorylation and activation of iPFK-2 [183]. The resulting cytoprotective effect of stimulating glycolysis by NO in astrocytes was absent in neurons, where iPFK-2 protein was undetectable.
Isoenzymes in proliferating cells
PFK-2/FBPase-2 present in various cells and cell lines including cancer cells [27,184–187], foetal liver [188], regenerating liver [189] and lymphocytes [190] has properties similar to those of the heart and brain/placenta isoenzymes. Foetal liver was subsequently shown to contain equal amounts of the L and M mRNAs [191] and regenerating liver was shown to contain the F mRNA [192]. Fru-2,6-P2 concentrations and PFK-2 activity were increased in chick embryo fibroblasts upon transformation by retroviruses carrying the v-src oncogene [193]. The oncogene-dependent transformation seems to involve PKC and both RNA and protein synthesis. Therefore pp60 v-src, acting via PKC, could change the isoenzyme expression of PFK-2/FBPase-2 [194]. Phorbol esters increase Fru-2,6-P2 concentrations in chick embryo fibroblasts [195], in human fibroblasts [196], in human platelets [197], in differentiating megakaryocytes [198], in human B-lymphocytes [199], in mouse spleen lymphocytes and purified B-cells [190], and in foetal rat hepatocytes [200]. The effect of phorbol esters to increase Fru-2,6-P2 in these systems could be attributed to a rise in hexose 6-phosphates and in some cases to PFK-2 activation. In foetal liver, although PFK-2/FBPase-2 was phosphorylated by PKC, the activity of PFK-2 was unaffected [188], as described above for the heart isoenzyme. Therefore, in foetal hepatocytes, phorbol esters probably do not activate PFK-2 via PKC-induced phosphorylation. In chick embryo fibroblasts, the activation of PFK-2 by phorbol esters involves the increased synthesis of an isoenzyme of PFK-2/FBPase-2, which might be identical with the heart isoenzyme or iPFK-2 (see above). Supposing that the heart or brain/placenta isoenzyme is expressed in these systems, PFK-2 activation via phorbol ester/PKC activation might be secondary to the activation of the MAPK cascade [201] and phosphorylation by MAPKAPK-1 (MAPK-activated protein kinase-1) [137]. The increase in Fru-2,6-P2 concentration and PFK-2 activity in thrombin-stimulated platelets [197], and in fibroblasts stimulated by growth factors [196] might also be mediated by this pathway. In A431 cells treated with EGF, the increase in Fru-2,6-P2 and PFK-2 activity was not due to an increase in transcription or translation [202]. Furthermore, PKC did not appear to be involved in the signalling pathway, and a role for Ca2+, possibly acting via the multifunctional Ca/CAMK, was postulated [202]. Lastly, insulin has been shown to increase Fru-2,6-P2 concentrations and to activate PFK-2 in several cultured cells, including human fibroblasts [203], chick embryo fibroblasts [195], chick embryo hepatocytes [204] and foetal rat hepatocytes [200]. These effects could be mediated by a novel protein kinase of the insulin signalling cascades, as described above for the heart isoenzyme.
CONCLUDING REMARKS
The 3D structure of PFK-2/FBPase-2 revealed that the PFK-2 domains in the homodimer come together in a head-to-head fashion with the FBPase-2 domains being almost independent. Differences in the PFK-2/FBPase-2 activity ratio between the mammalian isoenzymes could be explained by differences in flexibility at the PFK-2/FBPase-2 domain interface and interactions with the different N- and C-termini and the catalytic sites. The PFK-2 domain has a similar fold to AK and is not related to PFK-1, as originally proposed.
The presence of a PFK-2/FBPase-2 sequence in Desulfovibrio raises the possibility that it was horizontally transferred from a unicellular eukaryote. Gene fusion between the PFK-2 and FBPase-2 catalytic domains must have occurred early in eukaryotic evolution, in the common ancestor of this gene donor, the trypanosomatids and all other eukaryotes. In trypanosomatids, monofunctional isoenzymes have arisen in which either the PFK-2 or the FBPase-2 domain has become inactive. These isoenzymes might become expressed at different stages of the parasite's life cycle to enable the organism to adjust to different environments. It will be interesting to see if ‘primitive’ protist lineages can be found in which monofunctional, single domain PFK-2 and FBPase-2 enzymes occur or in which no traces of these enzymes and Fru-2,6-P2 can be found.
Studies on both the long- and short-term control of the PFK-2/FBPase-2 system have proved to be fruitful areas of research, leading to the identification of protein kinase cascades that converge on the heart and brain/placenta isoenzymes and of the novel transcription factor, HNF-6. Investigations on the mechanism of heart PFK-2 activation by insulin could result in the recognition of new elements in insulin signalling to metabolic pathways that may not necessarily be mediated by PKB. Studies on the phosphorylation of the heart and brain/placenta isoenzymes by AMPK have provided new explanations for the old observations of the Pasteur and Warburg effects in terms of a protein phosphorylation mechanism. It is likely that other protein kinases will be found that impinge on the heart and brain/placenta isoenzymes. Good candidates are members of the AGC family, e.g. protein kinase G and the serum- and glucocorticoid-activated protein kinases. Clearly, more in vivo studies will be needed to assess the physiological relevance of heart and brain/placenta PFK-2 phosphorylation.
Interesting new avenues of research are isoenzyme overexpression and tissue-specific gene knockouts. Overexpression of PFK-2/FBPase-2 in mouse liver has been shown to lower blood glucose levels by increasing Fru-2,6-P2 and lowering hepatic glucose production [205]. Another field of interest is control by protein–protein interactions. The liver and brain/placenta isoenzymes were shown to bind to glucokinase [206]. This could be involved in channelling glycolytic intermediates. In pancreatic islets, the interaction between PFK-2/FBPase-2 and glucokinase stimulates glucose phosphorylation and its subsequent metabolism, which could be involved in glucose-induced insulin secretion [207].
Acknowledgments
We thank Professor F. R. Opperdoes [Institute of Cellular Pathology (ICP)/Université Catholique de Louvain (UCL), Brussels] for help with the phylogenetic analysis and Professor E. Van Schaftingen (ICP/UCL, Brussels) for pointing out the Desulfovibrio sequence. Work in the authors' laboratory was supported by grants from the Interuniversity Attraction Poles Program – Belgian Science Policy, by the Directorate General Higher Education and Scientific Research, French Community of Belgium, by the Fund for Medical Scientific Research (Belgium) and by EU contract no. QLG1-CT-2001-01488 (AMPDIAMET).
References
- 1.Van Schaftingen E. Fructose 2,6-bisphosphate. Adv. Enzymol. 1987;59:315–395. doi: 10.1002/9780470123058.ch7. [DOI] [PubMed] [Google Scholar]
- 2.Hue L., Rider M. H. Role of fructose 2,6-bisphosphate in the control of glycolysis in mammalian tissues. Biochem. J. 1987;245:313–324. doi: 10.1042/bj2450313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilkis S. J., El-Maghrabi M. R., Claus T. H. Hormonal regulation of hepatic gluconeogenesis and glycolysis. Annu. Rev. Biochem. 1988;57:755–783. doi: 10.1146/annurev.bi.57.070188.003543. [DOI] [PubMed] [Google Scholar]
- 4.Hue L., Rider M. H., Rousseau G. G. Fructose-2,6-bisphosphate in extra hepatic tissues. In: Pilkis S. J., editor. Fructose-2,6-bisphosphate. Boca Raton: CRC Press; 1990. pp. 173–193. [Google Scholar]
- 5.Van Schaftingen E., Mertens E., Opperdoes F. R. Fructose-2,6-bisphosphate in primitive systems. In: Pilkis S. J., editor. Fructose-2,6-bisphosphate. Boca Raton: CRC Press; 1990. pp. 229–244. [Google Scholar]
- 6.Pilkis S. J., Claus T. H., Kurland I. J., Lange A. J. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase: a metabolic signaling enzyme. Annu. Rev. Biochem. 1995;64:799–835. doi: 10.1146/annurev.bi.64.070195.004055. [DOI] [PubMed] [Google Scholar]
- 7.Okar D. A., Manzano A., Navarro-Sabate A., Riera L., Bartrons R., Lange A. J. PFK-2/FBPase-2: maker and breaker of the essential biofactor fructose-2,6-bisphosphate. Trends Biochem. Sci. 2001;26:30–35. doi: 10.1016/s0968-0004(00)01699-6. [DOI] [PubMed] [Google Scholar]
- 8.Depré C., Rider M. H., Hue L. Mechanisms of control of heart glycolysis. Eur. J. Biochem. 1998;258:277–290. doi: 10.1046/j.1432-1327.1998.2580277.x. [DOI] [PubMed] [Google Scholar]
- 9.Hue L., Beauloye C., Marsin A.-S., Bertrand L., Horman S., Rider M. H. Insulin and ischaemia stimulate glycolysis by acting on the same targets through different and opposing signaling pathways. J. Mol. Cell. Cardiol. 2002;34:1091–1097. doi: 10.1006/jmcc.2002.2063. [DOI] [PubMed] [Google Scholar]
- 10.Rider M. H., Hue L. Phosphorylation of purified bovine heart and rat liver 6-phosphofructo-2-kinase by protein kinase C and comparison of the fructose-2,6-bisphosphatase activity of the two enzymes. Biochem. J. 1986;240:57–61. doi: 10.1042/bj2400057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakata J., Abe Y., Uyeda K. Molecular cloning of the DNA and expression and characterization of rat testis fructose-6-phosphate,2-kinase:fructose-2,6-bisphosphatase. J. Biol. Chem. 1991;266:15764–15770. [PubMed] [Google Scholar]
- 12.Lee H.-T., Li Y., Uyeda K., Hasemann C. A. Tissue-specific structure/function differentiation of the liver isoform of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. J. Biol. Chem. 2002;278:523–530. doi: 10.1074/jbc.M209105200. [DOI] [PubMed] [Google Scholar]
- 13.Darville M. I., Crepin K. M., Hue L., Rousseau G. G. 5′ flanking sequence and structure of a gene encoding rat 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Proc. Natl. Acad. Sci. U.S.A. 1989;86:6543–6547. doi: 10.1073/pnas.86.17.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darville M. I., Chikri M., Lebeau E., Hue L., Rousseau G. G. A rat gene encoding heart 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. FEBS Lett. 1991;288:91–94. doi: 10.1016/0014-5793(91)81009-w. [DOI] [PubMed] [Google Scholar]
- 15.Navarro-Sabate A., Manzano A., Riera L., Rosa J. L., Ventura F., Bartrons R. The human ubiquitous 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene (PFKFB3): promoter characterization and genomic structure. Gene. 2001;264:131–138. doi: 10.1016/s0378-1119(00)00591-6. [DOI] [PubMed] [Google Scholar]
- 16.Manzano A., Perez J. X., Nadal M., Estivill X., Lange A., Bartrons R. Cloning, expression and chromosomal localization of a human testis 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene. Gene. 1999;229:83–89. doi: 10.1016/s0378-1119(99)00037-2. [DOI] [PubMed] [Google Scholar]
- 17.Darville M. I., Antoine I. V., Rousseau G. G. Characterization of an enhancer upstream from the muscle-type promoter of a gene encoding 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Nucleic Acids Res. 1992;20:3575–3583. doi: 10.1093/nar/20.14.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupriez V. J., Darville M. I., Antoine I. V., Gegonne A., Ghysdael J., Rousseau G. G. Characterization of a hepatoma mRNA transcribed from a third promoter of a 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-encoding gene and controlled by ets oncogene-related products. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8224–8228. doi: 10.1073/pnas.90.17.8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruni P., Vandoolaeghe P., Rousseau G. G., Hue L., Rider M. H. Expression and regulation of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase isozymes in white adipose tissue. Eur. J. Biochem. 1999;259:756–761. doi: 10.1046/j.1432-1327.1999.00104.x. [DOI] [PubMed] [Google Scholar]
- 20.Chikri M., Rousseau G. G. Rat gene coding for heart 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: characterization of an unusual promoter region and identification of four mRNAs. Biochemistry. 1995;34:8876–8884. doi: 10.1021/bi00027a040. [DOI] [PubMed] [Google Scholar]
- 21.Vidal H., Crepin K. M., Rider M. H., Hue L., Rousseau G. G. Cloning and expression of novel isoforms of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase from bovine heart. FEBS Lett. 1993;330:329–333. doi: 10.1016/0014-5793(93)80898-5. [DOI] [PubMed] [Google Scholar]
- 22.Tsuchiya Y., Uyeda K. Bovine heart fructose 6-P,2-kinase:fructose 2,6-bisphosphatase mRNA and gene structure. Arch. Biochem. Biophys. 1994;310:467–474. doi: 10.1006/abbi.1994.1194. [DOI] [PubMed] [Google Scholar]
- 23.Heine-Suner D., Diaz-Guillen M. A., Lange A. J., Rodriguez de Cordoba S. Sequence and structure of the human 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase heart isoform gene (PFKFB2) Eur. J. Biochem. 1998;254:103–110. doi: 10.1046/j.1432-1327.1998.2540103.x. [DOI] [PubMed] [Google Scholar]
- 24.Ventura F., Ambrosio S., Bartrons R., El-Maghrabi M. R., Lange A. J., Pilkis S. J. Cloning and expression of a catalytic core bovine brain 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Biochem. Biophys. Res. Commun. 1995;209:1140–1148. doi: 10.1006/bbrc.1995.1616. [DOI] [PubMed] [Google Scholar]
- 25.Sakai A., Kato M., Fukasawa M., Ishiguro M., Furuya E., Sakakibara R. Cloning of cDNA encoding for a novel isozyme of fructose 6-phosphate, 2-kinase/fructose 2,6-bisphosphatase from human placenta. J. Biochem. 1996;119:506–511. doi: 10.1093/oxfordjournals.jbchem.a021270. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton J. A., Callaghan M. J., Sutherland R. L., Watts C. K. Identification of PRG1, a novel progestin-responsive gene with sequence homology to 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Mol. Endocrinol. 1997;11:490–502. doi: 10.1210/mend.11.4.9909. [DOI] [PubMed] [Google Scholar]
- 27.Chesney J., Mitchell R., Benigni F., Bacher M., Spiegel L., Al-Abed Y., Han J. H., Metz C., Bucala R. An inducible gene product for 6-phospho-fructo-2-kinase with an AU-rich instability element: role in tumor cell glycolysis and the Warburg effect. Proc. Natl. Acad. Sci. U.S.A. 1999;96:3047–3052. doi: 10.1073/pnas.96.6.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe F., Furuya E. Tissue-specific alternative splicing of rat brain fructose 6-phosphate 2-kinase/fructose 2,6-bisphosphatase. FEBS Lett. 1999;458:304–308. doi: 10.1016/s0014-5793(99)01174-6. [DOI] [PubMed] [Google Scholar]
- 29.Kessler R., Eschrich K. Splice isoforms of ubiquitous 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase in human brain. Mol. Brain Res. 2001;87:190–195. doi: 10.1016/s0169-328x(01)00014-6. [DOI] [PubMed] [Google Scholar]
- 30.Sakata J., Abe Y., Uyeda K. Molecular cloning of the DNA and expression and characterization of rat testes fructose-6-phosphate,2-kinase:fructose-2,6-bisphosphatase. J. Biol. Chem. 1991;266:15764–15770. [PubMed] [Google Scholar]
- 31.Kitajima S., Sakakibara R., Uyeda K. Kinetic studies of fructose 6-phosphate,2-kinase and fructose 2,6-bisphosphatase. J. Biol. Chem. 1984;259:6896–6903. [PubMed] [Google Scholar]
- 32.Kretschmer M., Hofmann E. Inhibition of rat liver phosphofructokinase-2 by phosphoenolpyruvate and ADP. Biochem. Biophys. Res. Commun. 1984;124:793–796. doi: 10.1016/0006-291x(84)91027-1. [DOI] [PubMed] [Google Scholar]
- 33.Kountz P. D., Freeman S., Cook A. G., El-Maghrabi M. R., Knowles J. R., Pilkis S. J. The stereochemical course of phospho group transfer catalyzed by rat liver 6-phosphofructo-2-kinase. J. Biol. Chem. 1988;263:16069–16072. [PubMed] [Google Scholar]
- 34.Bazan J. F., Fletterick R. J., Pilkis S. J. Evolution of a bifunctional enzyme: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Proc. Natl. Acad Sci. U.S.A. 1989;86:9642–9646. doi: 10.1073/pnas.86.24.9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bazan J. F., Fletterick R. J. Tertiary structure modelling of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: structural, functional and evolutionary design of a bifunctional enzyme. In: Pilkis S. J., editor. Fructose-2,6-bisphosphate. Boca Raton: CRC Press; 1990. pp. 125–171. [Google Scholar]
- 36.Crepin K. M., Vertommen D., Dom G., Hue L., Rider M. H. Rat muscle 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: study of the kinase domain by site-directed mutagenesis. J. Biol. Chem. 1993;268:15277–15284. [PubMed] [Google Scholar]
- 37.Vertommen D., Bertrand L., Sontag B., Di Pietro A., Louckx M. P., Vidal H., Hue L., Rider M. H. The ATP binding site in the 2-kinase domain of liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase study of the role of Lys-54 and Thr-55 by site-directed mutagenesis. J. Biol. Chem. 1996;271:17875–17880. doi: 10.1074/jbc.271.30.17875. [DOI] [PubMed] [Google Scholar]
- 38.Rider M. H., Crepin K. M., De Cloedt M., Bertrand L., Hue L. Site-directed mutagenesis of rat muscle 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: role of Asp-130 in the 2-kinase domain. Biochem. J. 1994;300:111–115. doi: 10.1042/bj3000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Traut T. W. The functions and consensus motifs of nine types of peptide segments that form different types of nucleotide-binding sites. Eur. J. Biochem. 1994;222:9–19. doi: 10.1111/j.1432-1033.1994.tb18835.x. [DOI] [PubMed] [Google Scholar]
- 41.Bertrand L., Vertommen D., Depiereux E., Hue L., Rider M. H., Feytmans E. Modelling the 2-kinase domain of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase on adenylate kinase. Biochem. J. 1997;321:615–621. doi: 10.1042/bj3210615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertrand L., Vertommen D., Feytmans E., Di Pietro A., Rider M. H., Hue L. Mutagenesis of charged residues in a conserved sequence in the 2-kinase domain of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Biochem. J. 1997;321:609–614. doi: 10.1042/bj3210609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertrand L., Deprez J., Vertommen D., Di Pietro A., Hue L., Rider M. H. Site-directed mutagenesis of Lys-174, Asp-179 and Asp-191 in the 2-kinase domain of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Biochem. J. 1997;321:623–627. doi: 10.1042/bj3210623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertrand L., Vertommen D., Freeman P. M., Wouters J., Depiereux E., Di Pietro A., Hue L., Rider M. H. Mutagenesis of the fructose 6-phosphate binding site in the 2-kinase domain of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Eur. J. Biochem. 1998;254:490–496. doi: 10.1046/j.1432-1327.1998.2540490.x. [DOI] [PubMed] [Google Scholar]
- 45.Hasemann C. A., Istvan E. S., Uyeda K., Deisenhofer J. The crystal structure of the bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase reveals distinct domain homologies. Structure. 1996;4:1017–1029. doi: 10.1016/s0969-2126(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 46.Li L., Lin K., Kurland I. J., Correia J. J., Pilkis S. J. Site-directed mutagenesis in rat liver 6-phosphofructo-2-kinase: mutation at the fructose 6-phosphate binding site affects phosphate activation. J. Biol. Chem. 1992;267:4386–4393. [PubMed] [Google Scholar]
- 47.Rider M. H., Crepin K. M., De Cloedt M., Bertrand L., Vertommen D., Hue L. Study of the roles of Arg-104 and Arg-225 in the 2-kinase domain of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase by site-directed mutagenesis. Biochem. J. 1995;309:341–346. doi: 10.1042/bj3090341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai M.-D., Yan H. Mechanism of adenylate kinase: site-directed mutagenesis versus X-ray and NMR. Biochemistry. 1991;30:6806–6818. doi: 10.1021/bi00242a002. [DOI] [PubMed] [Google Scholar]
- 49.Frech M., Darden T. A., Pederson L. G., Foley C. K., Charifson P. S., Anderson M. W., Wittinghofer A. Role of glutamine-61 in the hydrolysis of GTP by p21H-ras: an experimental and theoretical study. Biochemistry. 1994;33:3237–3244. doi: 10.1021/bi00177a014. [DOI] [PubMed] [Google Scholar]
- 50.Gideon P., John J., Frech M., Lautwein A., Clark R., Scheffer J. E., Wittinghofer A. Mutational and kinetic analyses of the GTPase-activating protein (GAP)–p21 interaction: the C-terminal domain of GAP is not sufficient for full activity. Mol. Cell. Biol. 1992;12:2050–2056. doi: 10.1128/mcb.12.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kosloff M., Selinger Z. Substrate-assisted catalysis – application to G proteins. Trends Biochem. Sci. 2001;26:161–166. doi: 10.1016/s0968-0004(00)01748-5. [DOI] [PubMed] [Google Scholar]
- 52.Knowles J. R. Enzyme-catalyzed phosphoryl transfer reactions. Annu. Rev. Biochem. 1980;49:877–919. doi: 10.1146/annurev.bi.49.070180.004305. [DOI] [PubMed] [Google Scholar]
- 53.Matte A., Tari L. W., Delbaere L. T. J. How do kinases transfer phosphoryl groups? Structure. 1998;6:413–419. doi: 10.1016/s0969-2126(98)00043-4. [DOI] [PubMed] [Google Scholar]
- 54.Maegley K. A., Admiraal S. J., Hershlag D. Ras-catalysed hydrolysis of GTP: a new perspective from model studies. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8160–8166. doi: 10.1073/pnas.93.16.8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wittinghofer A., Pai E. F. The structure of Ras protein: a model for a universal molecular switch. Trends Biochem. Sci. 1991;16:382–387. doi: 10.1016/0968-0004(91)90156-p. [DOI] [PubMed] [Google Scholar]
- 56.Scheffzek K., Ahmadian M. R., Kabsch W., Wiesmüller L., Lautwein A., Schmitz F., Wittinghofer A. The Ras–RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997;277:333–338. doi: 10.1126/science.277.5324.333. [DOI] [PubMed] [Google Scholar]
- 57.Anderson C. M., Zucker F. H., Steitz T. A. Space-filling models of kinase clefts and conformational changes. Science. 1979;204:375–380. doi: 10.1126/science.220706. [DOI] [PubMed] [Google Scholar]
- 58.Schulz G. E., Müller C. W., Diederichs K. Induced-fit movements in adenylate kinases. J. Mol. Biol. 1990;213:627–630. doi: 10.1016/S0022-2836(05)80250-5. [DOI] [PubMed] [Google Scholar]
- 59.Rider M. H., Hue L. Inactivation of liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase by phenylglyoxal: evidence for essential arginine residues. Eur. J. Biochem. 1992;285:405–411. doi: 10.1111/j.1432-1033.1992.tb17131.x. [DOI] [PubMed] [Google Scholar]
- 60.Colosia A. D., Lively M. O., El-Maghrabi M. R., Pilkis S. J. Isolation of a cDNA clone for rat liver 6-phosphofructo 2-kinase/fructose 2,6-bisphosphatase. Biochem. Biophys. Res. Commun. 1987;143:1092–1098. doi: 10.1016/0006-291x(87)90364-0. [DOI] [PubMed] [Google Scholar]
- 61.Darville M. I., Crepin K. M., Vandekerckhove J., Van Damme J., Octave J.-N., Rider M. H., Marchand M. J., Hue L., Rousseau G. G. Complete nucleotide sequence coding for rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase derived from a cDNA clone. FEBS Lett. 1987;224:317–321. doi: 10.1016/0014-5793(87)80476-3. [DOI] [PubMed] [Google Scholar]
- 62.Algaier J., Uyeda K. Molecular cloning, sequence analysis, and expression of a human liver cDNA coding for fructose-6-P,2-kinase: fructose-2,6-bisphosphatase. Biochem. Biophys. Res. Commun. 1988;153:328–333. doi: 10.1016/s0006-291x(88)81226-9. [DOI] [PubMed] [Google Scholar]
- 63.Lange A. J., Pilkis S. J. Sequence of human liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Nucleic Acids Res. 1990;18:3652–3652. doi: 10.1093/nar/18.12.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lange A. J., El-Maghrabi M. R., Pilkis S. J. Isolation of bovine liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase cDNA: bovine liver and heart forms of the enzyme are separate gene products. Arch. Biochem. Biophys. 1991;290:258–263. doi: 10.1016/0003-9861(91)90617-r. [DOI] [PubMed] [Google Scholar]
- 65.Batra R. S., Brown R. M., Brown G. K., Craig I. W. Molecular cloning and tissue-specific expression of mouse kidney 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. FEBS Lett. 1996;393:167–173. doi: 10.1016/0014-5793(96)00878-2. [DOI] [PubMed] [Google Scholar]
- 66.Crepin K. M., Darville M. I., Michel A., Hue L., Rousseau G. G. Cloning and expression in Escherichia coli of a rat hepatoma cell cDNA coding for 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Biochem. J. 1989;264:151–160. doi: 10.1042/bj2640151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watanabe F., Sakai A., Furuya E., Uyeda K. Molecular cloning and tissue specific expression of fructose 6-phosphate,2-kinase:fructose 2,6-bisphosphatase of rat brain. Biochem. Biophys. Res. Commun. 1994;198:335–340. doi: 10.1006/bbrc.1994.1047. [DOI] [PubMed] [Google Scholar]
- 68.Sakata J., Uyeda K. Bovine heart fructose-6-phosphate 2-kinase/fructose-2,6-bisphosphatase:complete amino acid sequence and localization of phosphorylation sites. Proc. Natl. Acad. Sci. U.S.A. 1990;87:4951–4955. doi: 10.1073/pnas.87.13.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Manzano A., Rosa J. L., Ventura F., Perez J. X., Nadal M., Estivill X., Ambrosio S., Gil J., Bartrons R. Molecular cloning, expression, and chromosomal localization of a ubiquitously expressed human 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene (PFKFB3) Cytogenet. Cell Genet. 1998;83:214–217. doi: 10.1159/000015181. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe F., Sakai A., Furuya E. Novel isoforms of rat brain fructose 6-phosphate 2-kinase/fructose 2,6-bisphosphatase are generated by tissue-specific alternative splicing. J. Neurochem. 1997;69:1–9. doi: 10.1046/j.1471-4159.1997.69010001.x. [DOI] [PubMed] [Google Scholar]
- 71.Li L., Lange A. J., Pilkis S. J. Isolation of a cDNA for chicken liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Biochem. Biophys. Res. Commun. 1993;190:397–405. doi: 10.1006/bbrc.1993.1061. [DOI] [PubMed] [Google Scholar]
- 72.Sakai A., Watanabe F., Furuya E. Cloning of cDNAs for fructose 6-phosphate 2-kinase/fructose 2,6-bisphosphatase from frog skeletal muscle and liver, and their expression in skeletal muscle. Biochem. Biophys. Res. Commun. 1994;198:1099–1106. doi: 10.1006/bbrc.1994.1156. [DOI] [PubMed] [Google Scholar]
- 73.Meton I., Caseras A., Mediavilla D., Fernandez F., Baanante I. V. Molecular cloning of a cDNA encoding 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase from liver of Sparus aurata: nutritional regulation of enzyme expression. Biochim. Biophys. Acta. 1999;1444:153–165. doi: 10.1016/s0167-4781(98)00270-x. [DOI] [PubMed] [Google Scholar]
- 74.Villadsen D., Rung J. H., Draborg H., Nielsen T. H. Structure and hererologous expression of a gene encoding fructose-6-phosphate,2-kinase/fructose-2,6-bisphosphatase from Arabidopsis thaliana. Biochim. Biophys. Acta. 2000;1492:406–413. doi: 10.1016/s0167-4781(00)00134-2. [DOI] [PubMed] [Google Scholar]
- 75.Draborg H., Villadsen D., Nielsen T. H. Cloning, characterization and expression of a bifunctional fructose-6-phosphate, 2-kinase/fructose-2,6-bisphosphatase from potato. Plant Mol. Biol. 1999;39:709–720. doi: 10.1023/a:1006102412693. [DOI] [PubMed] [Google Scholar]
- 76.Markham J. E., Kruger N. J. Kinetic properties of bifunctional 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase from spinach leaves. Eur. J. Biochem. 2002;269:1267–1277. doi: 10.1046/j.1432-1033.2002.02771.x. [DOI] [PubMed] [Google Scholar]
- 77.Kretschmer M., Tempst P., Fraenkel D. G. Identification and cloning of yeast phosphofructokinase 2. Eur. J. Biochem. 1991;197:367–372. doi: 10.1111/j.1432-1033.1991.tb15920.x. [DOI] [PubMed] [Google Scholar]
- 78.Boles E., Gohlmann H. W., Zimmermann F. K. Cloning of a second gene encoding 6-phosphofructo-2-kinase in yeast and characterization of mutant strains without fructose-2,6-bisphosphate. Mol. Microbiol. 1996;20:65–76. doi: 10.1111/j.1365-2958.1996.tb02489.x. [DOI] [PubMed] [Google Scholar]
- 79.Paravicini G., Kretschmer M. The yeast FBP26 gene codes for a fructose-2,6-bisphosphatase. Biochemistry. 1992;31:7126–7133. doi: 10.1021/bi00146a014. [DOI] [PubMed] [Google Scholar]
- 80.Van Schaftingen E., Opperdoes F. R., Hers H.-G. Stimulation of Trypanosoma brucei pyruvate kinase by fructose 2,6-bisphosphate. Eur. J. Biochem. 1985;153:403–406. doi: 10.1111/j.1432-1033.1985.tb09316.x. [DOI] [PubMed] [Google Scholar]
- 81. Reference deleted.
- 82.François J., Van Schaftingen E., Hers H.-G. The mechanism by which glucose increases fructose 2,6-bisphosphate concentration in Saccharomyces cerevisiae: a cyclic-AMP-dependent activation of phosphofructokinase 2. Eur. J. Biochem. 1984;145:187–193. doi: 10.1111/j.1432-1033.1984.tb08539.x. [DOI] [PubMed] [Google Scholar]
- 83.Kretschmer M., Fraenkel D. G. Yeast 6-phosphofructo-2-kinase: sequence and mutant. Biochemistry. 1991;30:10663–10672. doi: 10.1021/bi00108a009. [DOI] [PubMed] [Google Scholar]
- 84.Raamsdonk L. M., Teusink B., Broadhurst D., Zhang N., Hayes A., Walsh M. C., Berden J. A., Brindle K. M., Kell D. B., Rowland J. J., et al. A functional genomic strategy that uses metabolome data to reveal the phenotype of silent mutations. Nat. Biotechnol. 2001;19:45–50. doi: 10.1038/83496. [DOI] [PubMed] [Google Scholar]
- 85.Tauler A., Lange A. J., El-Maghrabi M. R., Pilkis S. J. Expression of rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase and its kinase domain in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 1989;86:7316–7320. doi: 10.1073/pnas.86.19.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tauler A., Rosenberg A. H., Colosia A., Studier F. W., Pilkis S. J. Expression of the bisphosphatase domain of rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 1988;85:6642–6646. doi: 10.1073/pnas.85.18.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lange A. J., Espinet C., Hall R., El-Maghrabi M. R., Vargas A. M., Miksicek R. J., Granner D. K., Pilkis S. J. Regulation of gene expression of rat skeletal muscle/liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: isolation and characterization of a glucocorticoid response element in the first intron of the gene. J. Biol. Chem. 1992;267:15673–15680. [PubMed] [Google Scholar]
- 88.Zimmermann P. L., Rousseau G. G. Liver-specific DNase I-hypersensitive sites and DNA methylation pattern in the promoter region of a 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene. Eur. J. Biochem. 1994;220:183–191. doi: 10.1111/j.1432-1033.1994.tb18613.x. [DOI] [PubMed] [Google Scholar]
- 89.Zimmermann P. L., Pierreux C. E., Rigaud G., Rousseau G. G., Lemaigre F. P. In vivo protein–DNA interactions on a glucocorticoid response unit of a liver-specific gene: hormone-induced transcription factor binding to constitutively open chromatin. DNA Cell Biol. 1997;16:713–723. doi: 10.1089/dna.1997.16.713. [DOI] [PubMed] [Google Scholar]
- 90.Pierreux C. E., Urso B., De Meyts P., Rousseau G. G., Lemaigre F. P. Inhibition by insulin of glucocorticoid-induced gene transcription: involvement of the ligand-binding domain of the glucocorticoid receptor and independence from the phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways. Mol. Endocrinol. 1998;12:1343–1354. doi: 10.1210/mend.12.9.0172. [DOI] [PubMed] [Google Scholar]
- 91.De Los Pinos E., Fernandez De Mattos S., Joaquin M., Tauler A. Insulin inhibits glucocorticoid-stimulated L-type 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene expression by activation of the c-Jun N-terminal kinase pathway. Biochem. J. 2001;353:267–273. doi: 10.1042/0264-6021:3530267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Darville M. I., Antoine I. V., Mertens-Strijthagen J. R., Dupriez V. J., Rousseau G. G. An E2F-dependent late-serum-response promoter in a gene that controls glycolysis. Oncogene. 1995;11:1509–1517. [PubMed] [Google Scholar]
- 93.Joaquin M., Salvado C., Bellosillo B., Lange A. J., Gil J., Tauler A. Effect of growth factors on the expression of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase in Rat-1 fibroblasts. J. Biol. Chem. 1997;272:2846–2851. doi: 10.1074/jbc.272.5.2846. [DOI] [PubMed] [Google Scholar]
- 94.Fernandez de Mattos S., De Los Pinos E. E., Joaquin M., Tauler A. Activation of phosphatidylinositol 3-kinase is required for transcriptional activity of F-type 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: assessment of the role of protein kinase B and p70 S6 kinase. Biochem. J. 2000;349:59–65. doi: 10.1042/0264-6021:3490059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Darville M. I., Rousseau G. G. E2F-dependent mitogenic stimulation of the splicing of transcripts from an S phase-regulated gene. Nucleic Acids Res. 1997;25:2759–2765. doi: 10.1093/nar/25.14.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dupriez V. J., Rousseau G. G. Glucose response elements in a gene that codes for 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. DNA Cell Biol. 1997;16:1075–1085. doi: 10.1089/dna.1997.16.1075. [DOI] [PubMed] [Google Scholar]
- 97.Vandoolaeghe P., Rousseau G. G. C/EBP binds over the TATA box and can activate the M promoter of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Biochem. Biophys. Res. Commun. 1997;232:247–250. doi: 10.1006/bbrc.1997.6266. [DOI] [PubMed] [Google Scholar]
- 98.Vandoolaeghe P., Gueuning M. A., Rousseau G. G. M-type 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase is the product of a late muscle differentiation gene. Biochem. Biophys. Res. Commun. 1999;259:250–254. doi: 10.1006/bbrc.1999.0767. [DOI] [PubMed] [Google Scholar]
- 99.Lemaigre F. P., Durviaux S. M., Rousseau G. G. Identification of regulatory sequences and protein-binding sites in the liver-type promoter of a gene encoding 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Mol. Cell. Biol. 1991;11:1099–1106. doi: 10.1128/mcb.11.2.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lemaigre F. P., Durviaux S. M., Rousseau G. G. Liver-specific factor binding to the liver promoter of a 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene. J. Biol. Chem. 1993;268:19896–19905. [PubMed] [Google Scholar]
- 101.Lemaigre F. P., Durviaux S. M., Truong O., Lannoy V. J., Hsuan J. J., Rousseau G. G. Hepatocyte nuclear factor 6, a transcription factor that contains a novel type of homeodomain and a single cut domain. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9460–9464. doi: 10.1073/pnas.93.18.9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lannoy V. J., Burglin T. R., Rousseau G. G., Lemaigre F. P. Isoforms of hepatocyte nuclear factor-6 differ in DNA-binding properties, contain a bifunctional homeodomain, and define the new ONECUT class of homeodomain proteins. J. Biol. Chem. 1998;273:13552–13562. doi: 10.1074/jbc.273.22.13552. [DOI] [PubMed] [Google Scholar]
- 103.Jacquemin P., Lannoy V. J., Rousseau G. G., Lemaigre F. P. OC-2, a novel mammalian member of the ONECUT class of homeodomain transcription factors whose function in liver partially overlaps with that of hepatocyte nuclear factor-6. J. Biol. Chem. 1999;274:2665–2671. doi: 10.1074/jbc.274.5.2665. [DOI] [PubMed] [Google Scholar]
- 104.Vanhorenbeeck V., Jacquemin P., Lemaigre F. P., Rousseau G. G. OC-3, a novel mammalian member of the ONECUT class of transcription factors. Biochem. Biophys. Res. Commun. 2002;292:848–854. doi: 10.1006/bbrc.2002.6760. [DOI] [PubMed] [Google Scholar]
- 105.Nacer-Cherif H., Bois-Joyeux B., Rousseau G. G., Lemaigre F. P., Danan J. L. Hepatocyte nuclear factor-6 stimulates transcription of the α-fetoprotein gene and synergizes with the retinoic acid receptor-related orphan receptor α-4. Biochem. J. 2003;369:583–591. doi: 10.1042/BJ20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lahuna O., Fernandez L., Karlsson H., Maiter D., Lemaigre F. P., Rousseau G. G., Gustafsson J., Mode A. Expression of hepatocyte nuclear factor 6 in rat liver is sex-dependent and regulated by growth hormone. Proc. Natl. Acad. Sci. U.S.A. 1997;94:12309–12313. doi: 10.1073/pnas.94.23.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pierreux C. E., Stafford J., Demonte D., Scott D. K., Vandenhaute J., O'Brien R. M., Granner D. K., Rousseau G. G., Lemaigre F. P. Antiglucocorticoid activity of hepatocyte nuclear factor-6. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8961–8966. doi: 10.1073/pnas.96.16.8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Streeper R. S., Hornbuckle L. A., Svitek C. A., Goldman J. K., Oeser J. K., O'Brien R. M. Protein kinase A phosphorylates hepatocyte nuclear factor-6 and stimulates glucose-6-phosphatase catalytic subunit gene transcription. J. Biol. Chem. 2001;276:19111–19118. doi: 10.1074/jbc.M101442200. [DOI] [PubMed] [Google Scholar]
- 109.Lannoy V. J., Decaux J. F., Pierreux C. E., Lemaigre F. P., Rousseau G. G. Liver glucokinase gene expression is controlled by the onecut transcription factor hepatocyte nuclear factor-6. Diabetologia. 2002;45:1136–1141. doi: 10.1007/s00125-002-0856-z. [DOI] [PubMed] [Google Scholar]
- 110.Lahuna O., Rastegar M., Maiter D., Thissen J. P., Lemaigre F. P., Rousseau G. G. Involvement of STAT5 (signal transducer and activator of transcription 5) and HNF-4 (hepatocyte nuclear factor 4) in the transcriptional control of the hnf6 gene by growth hormone. Mol. Endocrinol. 2000;14:285–294. doi: 10.1210/mend.14.2.0423. [DOI] [PubMed] [Google Scholar]
- 111.Jacquemin P., Lemaigre F. P., Rousseau G. G. The Onecut transcription factor HNF-6 (OC-1) is required for timely specification of the pancreas and acts upstream of Pdx-1 in the specification cascade. Dev. Biol. 2003;258:105–116. doi: 10.1016/s0012-1606(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 112.Maestro M. A., Boj S. F., Luco R. F., Pierreux C. E., Cabedo J., Servitja J. M., German M. S., Rousseau G. G., Lemaigre F. P., Ferrer J. Hnf6 and Tcf2 (MODY5) are linked in a gene network operating in a precursor cell domain of the embryonic pancreas. Hum. Mol. Genet. 2003;12:3307–3314. doi: 10.1093/hmg/ddg355. [DOI] [PubMed] [Google Scholar]
- 113.Jacquemin P., Durviaux S. M., Jensen J., Godfraind C., Gradwohl G., Guillemot F., Madsen O. D., Carmeliet P., Dewerchin M., Collen D., et al. Transcription factor hepatocyte nuclear factor 6 regulates pancreatic endocrine cell differentiation and controls expression of the proendocrine gene ngn3. Mol. Cell. Biol. 2000;20:4445–4454. doi: 10.1128/mcb.20.12.4445-4454.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clotman F., Lannoy V. J., Reber M., Cereghini S., Cassiman D., Jacquemin P., Roskams T., Rousseau G. G., Lemaigre F. P. The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development. 2002;129:1819–1828. doi: 10.1242/dev.129.8.1819. [DOI] [PubMed] [Google Scholar]
- 115.Odom D. T., Zizlsperger N., Gordon D. B., Bell G. W., Rinaldi N. J., Murray H. L., Volkert T. L., Schreiber J., Rolfe P. A., Gifford D. K., et al. Control of pancreas and liver gene expression by HNF transcription factors. Science. 2004;303:1378–1381. doi: 10.1126/science.1089769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Riera L., Manzano A., Navarro-Sabate A., Perales J. C., Bartrons R. Insulin induces PFKFB3 gene expression in HT29 human colon adenocarcinoma cells. Biochim. Biophys. Acta. 2002;1589:89–92. doi: 10.1016/s0167-4889(02)00169-6. [DOI] [PubMed] [Google Scholar]
- 117.Minchenko A., Leshchinsky I., Opentanova I., Sang N., Srinivas V., Armstead V., Caro J. Hypoxia-inducible factor-1-mediated expression of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) gene: its possible role in the Warburg effect. J. Biol. Chem. 2002;277:6183–6187. doi: 10.1074/jbc.M110978200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barford D., Hu S. H., Johnson L. N. Structural mechanism for glycogen phosphorylase control by phosphorylation and AMP. J. Mol. Biol. 1991;218:233–260. doi: 10.1016/0022-2836(91)90887-c. [DOI] [PubMed] [Google Scholar]
- 119.Hurley J. H., Dean A. M., Thorsness P. E., Koshland D. E., Jr, Stroud R. M. Regulation of isocitrate dehydrogenase by phosphorylation involves no long-range conformational change in the free enzyme. J. Biol. Chem. 1990;265:3599–3602. doi: 10.2210/pdb4icd/pdb. [DOI] [PubMed] [Google Scholar]
- 120.Hurley J. H., Dean A. M., Sohl J. L., Koshland D. E., Jr, Stroud R. M. Regulation of an enzyme by phosphorylation at the active site. Science. 1990;249:1012–1016. doi: 10.1126/science.2204109. [DOI] [PubMed] [Google Scholar]
- 121.Johnson L. N., Lowe E. D., Noble M. E. M., Owen D. J. The structural basis for substrate recognition and control by protein kinases. FEBS Lett. 1998;430:1–11. doi: 10.1016/s0014-5793(98)00606-1. [DOI] [PubMed] [Google Scholar]
- 122.Murray K. J., El-Maghrabi M. R., Kountz P. D., Lukas T. J., Soderling T. R., Pilkis S. J. Amino acid sequence of the phosphorylation site of rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. J. Biol. Chem. 1984;259:7673–7681. [PubMed] [Google Scholar]
- 123.El-Maghrabi M. R., Claus T. H., Pilkis J., Pilkis S. J. Regulation of 6-phosphofructo-2-kinase activity by cyclic AMP-dependent phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 1982;79:315–319. doi: 10.1073/pnas.79.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sakakibara R., Kitajima S., Uyeda K. Differences in kinetic properties of phospho and dephospho forms of fructose-6-phosphate, 2-kinase and fructose 2,6-bisphosphatase. J. Biol. Chem. 1984;259:41–46. [PubMed] [Google Scholar]
- 125.Van Schaftingen E., Davies D. R., Hers H. G. Inactivation of phosphofructokinase 2 by cyclic AMP-dependent protein kinase. Biochem. Biophys. Res. Commun. 1981;103:362–368. doi: 10.1016/0006-291x(81)91701-0. [DOI] [PubMed] [Google Scholar]
- 126.Van Schaftingen E., Davies D. R., Hers H. G. Fructose-2,6-bisphosphatase from rat liver. Eur. J. Biochem. 1982;124:143–149. doi: 10.1111/j.1432-1033.1982.tb05917.x. [DOI] [PubMed] [Google Scholar]
- 127.El-Maghrabi M. R., Claus T. H., Pilkis J., Fox E., Pilkis S. J. Regulation of rat liver fructose 2,6-bisphosphatase. J. Biol. Chem. 1982;257:7603–7607. [PubMed] [Google Scholar]
- 128.Kurland I. J., El-Maghrabi M. R., Correia J. J., Pilkis S. J. Rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: properties of phospho- and dephospho-forms and of two mutants in which Ser32 has been changed by site-directed mutagenesis. J. Biol. Chem. 1992;267:4416–4423. [PubMed] [Google Scholar]
- 129.Abe Y., Uyeda K. Effect of adding phosphorylation sites for cAMP-dependent protein kinase to rat testis 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Biochemistry. 1994;33:5766–5771. doi: 10.1021/bi00185a013. [DOI] [PubMed] [Google Scholar]
- 130.El-Maghrabi M. R., Pate T. M., Murray K. J., Pilkis S. J. Differential effects of proteolysis and protein modification on the activities of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. J. Biol. Chem. 1984;259:13096–13103. [PubMed] [Google Scholar]
- 131.Kurland I., Li L., Lange A. J., Correia J. J., El-Maghrabi M. R., Pilkis S. J. Regulation of rat 6-phosphofructo-2-kinase/fructose-2,6-bisphosphate: role of the NH2-terminal region. J. Biol. Chem. 1993;268:14056–14064. [PubMed] [Google Scholar]
- 132.Lin K., Kurland I. J., Li L., Lee Y. H., Okar D., Marecek J. F., Pilkis S. J. Evidence for NH2- and COOH-terminal interactions in rat 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. J. Biol. Chem. 1994;269:16953–16960. [PubMed] [Google Scholar]
- 133.Zhu Z., Ling S., Yang Q.-H., Li L. Involvement of the chicken liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase sequence His444-Arg-Glu-Arg in modulation of the bisphosphatase activity by its kinase domain. Biochem. J. 2001;357:513–520. doi: 10.1042/0264-6021:3570513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kitamura K., Kangawa K., Matsuo H., Uyeda K. Phosphorylation of myocardial fructose-6-phosphate,2-kinase:fructose-2,6-bisphosphatase by cAMP-dependent protein kinase and protein kinase C: activation by phosphorylation and amino acid sequences of the phosphorylation sites. J. Biol. Chem. 1988;263:16796–16801. [PubMed] [Google Scholar]
- 135.Rider M. H., Vandamme J., Lebeau E., Vertommen D., Vidal H., Rousseau G. G., Vandekerckhove J., Hue L. The two forms of bovine heart 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase result from alternative splicing. Biochem. J. 1992;285:405–411. doi: 10.1042/bj2850405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rider M. H., Van Damme J., Vertommen D., Michel A., Vandekerckhove J., Hue L. Evidence for new phosphorylation sites for protein kinase C and cyclic AMP-dependent protein kinase in bovine heart 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. FEBS Lett. 1992;310:139–142. doi: 10.1016/0014-5793(92)81315-d. [DOI] [PubMed] [Google Scholar]
- 137.Deprez J., Vertommen D., Alessi D. R., Hue L., Rider M. H. Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signalling cascades. J. Biol. Chem. 1997;272:17269–17275. doi: 10.1074/jbc.272.28.17269. [DOI] [PubMed] [Google Scholar]
- 138.Bertrand L., Alessi D. R., Deprez J., Deak M., Viaene E., Rider M. H., Hue L. Heart 6-phosphofructo-2-kinase activation by insulin results from Ser-466 and Ser-483 phosphorylation and requires 3-phosphoinositide-dependent kinase-1, but not protein kinase B. J. Biol. Chem. 1999;274:30927–30933. doi: 10.1074/jbc.274.43.30927. [DOI] [PubMed] [Google Scholar]
- 139.Abe Y., Minami Y., Li Y., Nguyen C., Uyeda K. Expression of bovine heart fructose 6-phosphate,2-kinase:fructose 2,6-bisphosphatase and determination of the role of the carboxyl terminus by mutagenesis. Biochemistry. 1995;34:2553–2559. doi: 10.1021/bi00008a020. [DOI] [PubMed] [Google Scholar]
- 140.Kessler R., Eschrich K. Ser644 is important for catalytic activity but is not involved in cAMP-dependent phosphorylation of yeast 6-phosphofructo-2-kinase. FEBS Lett. 1996;395:225–227. doi: 10.1016/0014-5793(96)01045-9. [DOI] [PubMed] [Google Scholar]
- 141.Dihazi H., Kessler R., Eschrich K. Glucose-induced stimulation of the Ras-cAMP pathway in yeast leads to multiple phosphorylations and activation of 6-phosphofructo-2-kinase. Biochemistry. 2003;42:6275–6282. doi: 10.1021/bi034167r. [DOI] [PubMed] [Google Scholar]
- 142.Hardie D. G., Carling D., Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 143.Dihazi H., Kessler R., Eschrich K. Phosphorylation and inactivation of yeast 6-phosphofructo-2-kinase contribute to the regulation of glycolysis under hypoxic stress. Biochemistry. 2001;40:14669–14678. doi: 10.1021/bi0155549. [DOI] [PubMed] [Google Scholar]
- 144.Macdonald F. D., Buchanan B. B. The role of fructose-2,6-bisphosphate in plant tissues. In: Pilkis S. J., editor. Fructose-2,6-bisphosphate. Boca Raton: CRC Press; 1990. pp. 193–210. [Google Scholar]
- 145.Stitt M. Fructose-2,6-bisphosphate as a regulatory molecule in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990;41:153–185. [Google Scholar]
- 146.Furumoto T., Teramoto M., Inada N., Ito M., Nishida I., Watanabe A. Phosphorylation of a bifunctional enzyme, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 2-phosphatase, is regulated physiologically and developmentally in rosette leaves of Arabidopsis thaliana. Plant Cell Physiol. 2001;42:1044–1048. doi: 10.1093/pcp/pce161. [DOI] [PubMed] [Google Scholar]
- 147.Boscá L., Storey K. B. Inactivation of 6-phosphofructo-2-kinase during anaerobiosis in the marine whelk Busycon canaliculatum. Am. J. Physiol. 1991;260:R1168–R1175. doi: 10.1152/ajpregu.1991.260.6.R1168. [DOI] [PubMed] [Google Scholar]
- 148.Garrison J. C., Johnsen D. E., Campanile C. P. Evidence for the role of phosphorylase kinase, protein kinase C, and other Ca2+-sensitive protein kinases in the response of hepatocytes to angiotensin II and vasopressin. J. Biol. Chem. 1984;259:3283–3292. [PubMed] [Google Scholar]
- 149.Hue L., Van Schaftingen E., Blackmore P. F. Stimulation of glycolysis and accumulation of a stimulator of phosphofructokinase in hepatocytes incubated with vasopressin. Biochem. J. 1981;194:1023–1026. doi: 10.1042/bj1941023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Crepin K. M., De Cloedt M., Vertommen D., Foret D., Michel A., Rider M. H., Rousseau G. G., Hue L. Molecular forms of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase expressed in rat skeletal muscle. J. Biol. Chem. 1992;267:21698–21704. [PubMed] [Google Scholar]
- 151.Bosca L., Challiss R. A., Newsholme E. A. The effect of fructose 2,6-bisphosphate on muscle fructose-1,6-bisphosphatase activity. Biochim. Biophys. Acta. 1985;828:151–154. doi: 10.1016/0167-4838(85)90051-2. [DOI] [PubMed] [Google Scholar]
- 152.Hue L., Blackmore P. F., Shikama H., Robinson-Steiner A., Exton J. H. Regulation of fructose-2,6-bisphosphate content in rat hepatocytes, perfused hearts, and perfused hindlimbs. J. Biol. Chem. 1982;257:4308–4313. [PubMed] [Google Scholar]
- 153.Jones J. P., MacLean P. S., Winder W. W. Correlation between fructose 2,6-bisphosphate and lactate production in skeletal muscle. J. Appl. Physiol. 1994;76:2169–2176. doi: 10.1152/jappl.1994.76.5.2169. [DOI] [PubMed] [Google Scholar]
- 154.Winder W. W., Duan C. Control of fructose 2,6-diphosphate in muscle of exercising fasted rats. Am. J. Physiol. 1992;262:E919–E924. doi: 10.1152/ajpendo.1992.262.6.E919. [DOI] [PubMed] [Google Scholar]
- 155.Krause U., Wegener G. Control of glycolysis in vertebrate skeletal muscle during exercise. Am. J. Physiol. 1996;270:R821–R829. doi: 10.1152/ajpregu.1996.270.4.R821. [DOI] [PubMed] [Google Scholar]
- 156.Minatogawa Y., Hue L. Fructose 2,6-bisphosphate in rat skeletal muscle during contraction. Biochem. J. 1984;223:73–79. doi: 10.1042/bj2230073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Green H. J., Cadefau J., Pette D. Altered glucose 1,6-bisphosphate and fructose 2,6-biphosphate levels in low-frequency stimulated rabbit fast-twitch muscle. FEBS Lett. 1991;282:107–109. doi: 10.1016/0014-5793(91)80455-c. [DOI] [PubMed] [Google Scholar]
- 158.Rider M. H., Hue L. Regulation of fructose 2,6-bisphosphate concentration in white adipose tissue. Biochem. J. 1985;225:421–428. doi: 10.1042/bj2250421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pessin J. E., Thurmond D. C., Elmendorf J. S., Coker K. J., Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. J. Biol. Chem. 1999;274:2593–2596. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- 160.Rider M. H., Hue L. Activation of rat heart phosphofructokinase-2 by insulin in vivo. FEBS Lett. 1984;176:484–488. doi: 10.1016/0014-5793(84)81223-5. [DOI] [PubMed] [Google Scholar]
- 161.Larner J., Lawrence J. C., Walkenbach R. J., Roach P. J., Hazen R. J., Huang L. C. Insulin control of glycogen synthesis. Adv. Cyclic Nucleotide Res. 1978;9:425–439. [PubMed] [Google Scholar]
- 162.Ha J., Lee J.-K., Kim K.-S., Witters L. A., Kim K.-H. Cloning of human acetyl-CoA carboxylase-β and its unique features. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11466–11470. doi: 10.1073/pnas.93.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lopez-Casillas F., Bai D.-H., Luo X., Kong I.-S., Hermodson M. A., Kim K.-H. Structure of the coding sequence and primary amino acid sequence of acetyl-coenzyme A carboxylase. Proc. Natl. Acad. Sci. U.S.A. 1988;85:5784–5788. doi: 10.1073/pnas.85.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Saddik M., Gamble J., Witters L. A., Lopaschuk G. D. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J. Biol. Chem. 1993;268:25836–25845. [PubMed] [Google Scholar]
- 165.Awan M. M., Saggerson E. D. Malonyl-CoA metabolism in cardiac myocytes and its relevance to the control of fatty acid oxidation. Biochem. J. 1993;295:61–66. doi: 10.1042/bj2950061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.White M. F. The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol. Cell. Biochem. 1998;182:3–11. [PubMed] [Google Scholar]
- 167.Lizcano J. M., Alessi D. R. The insulin signalling pathway. Current Biol. 2002;12:R236–R238. doi: 10.1016/s0960-9822(02)00777-7. [DOI] [PubMed] [Google Scholar]
- 168.Vanhaesebroeck B., Alessi D. R. The PI3K–PDK1 connection: more than just a road to PKB. Biochem. J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- 169.Saltiel A. R., Kahn C. R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature (London) 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 170.Lefebvre V., Méchin M.-C., Louckx M. P., Rider M. H., Hue L. Signaling pathway involved in the activation of heart 6-phosphofructo-2-kinase by insulin. J. Biol. Chem. 1996;271:22289–22292. doi: 10.1074/jbc.271.37.22289. [DOI] [PubMed] [Google Scholar]
- 171.Pozuelo Rubio M., Peggie M., Wong B. H., Morrice N., MacKintosh C. 14-3-3s regulate fructose-2,6-bisphosphate levels by binding to PKB-phosphorylated cardiac fructose-2,6-bisphosphate kinase/phosphatase. EMBO J. 2003;22:3514–3523. doi: 10.1093/emboj/cdg363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mora A., Davies A. M., Bertrand L., Sharif I., Budas G. R., Jovanovic S., Mouton V., Kahn C. R., Lucocq J. M., Gray G. A., et al. Deficiency of PDK1 in cardiac muscle results in heart failure and increased sensitivity to hypoxia. EMBO J. 2003;22:4666–4676. doi: 10.1093/emboj/cdg469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Deprez J., Bertrand L., Alessi D. R., Krause U., Hue L., Rider M. H. Partial purification of a wortmannin-sensitive and insulin-stimulated protein kinase that activates heart 6-phosphofructo-2-kinase. Biochem. J. 2000;347:305–312. [PMC free article] [PubMed] [Google Scholar]
- 174.Newton A. C. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem. Rev. 2001;101:2353–2369. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 175.Narabayashi H., Lawson J. W., Uyeda K. Regulation of phosphofructokinase in perfused rat heart: requirement for fructose 2,6-bisphosphate and a covalent modification. J. Biol. Chem. 1985;260:9750–9758. [PubMed] [Google Scholar]
- 176.Russell R. R., Bergeron R., Shulman G. I., Young L. H. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am. J. Physiol. 1999;277:H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 177.Marsin A.-S., Bertrand L., Rider M. H., Deprez J., Beauloye C., Vincent M. F., Van Den Berghe G., Carling D., Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr. Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 178.Hue L. Role of fructose 2,6-bisphosphate in the stimulation of glycolysis by anoxia in isolated hepatocytes. Biochem. J. 1982;206:359–365. doi: 10.1042/bj2060359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Depré C., Rider M. H., Veitch K., Hue L. Role of fructose 2,6-bisphosphate in the control of heart glycolysis. J. Biol. Chem. 1993;268:13274–13279. [PubMed] [Google Scholar]
- 180.Beauloye C., Marsin A. S., Bertrand L., Vanoverschelde J. L., Rider M. H., Hue L. The stimulation of heart glycolysis by increased workload does not require AMP-activated protein kinase but a wortmannin-sensitive mechanism. FEBS Lett. 2002;531:324–328. doi: 10.1016/s0014-5793(02)03552-4. [DOI] [PubMed] [Google Scholar]
- 181.Marsin A. S., Bouzin C., Bertrand L., Hue L. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J. Biol. Chem. 2002;277:30778–30783. doi: 10.1074/jbc.M205213200. [DOI] [PubMed] [Google Scholar]
- 182.Okamura N., Sakakibara R. A common phosphorylation site for cyclic AMP-dependent protein kinase and protein kinase C in human placental 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Biosci. Biotechnol. Biochem. 1998;62:2039–2042. doi: 10.1271/bbb.62.2039. [DOI] [PubMed] [Google Scholar]
- 183.Almeida A., Moncada S., Bolanos J. P. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat. Cell Biol. 2004;6:45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- 184.Loiseau A. M., Rider M. H., Foret D., Rousseau G. G., Hue L. Rat hepatoma (HTC) cell 6-phosphofructo-2-kinase differs from that in liver and can be separated from fructose 2,6-bisphosphatase. Eur. J. Biochem. 1988;175:27–32. doi: 10.1111/j.1432-1033.1988.tb14161.x. [DOI] [PubMed] [Google Scholar]
- 185.Denis-Pouxviel C., Gauthier T., Daviaud D., Murat J. C. Phosphofructokinase 2 and glycolysis in HT29 human colon adenocarcinoma cell line: regulation by insulin and phorbol esters. Biochem. J. 1990;268:465–470. doi: 10.1042/bj2680465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nissler K., Petermann H., Wenz I., Brox D. Fructose 2,6-bisphosphate metabolism in Ehrlich ascites tumour cells. J. Cancer Res. Clin. Oncol. 1995;121:739–745. doi: 10.1007/BF01213320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hirata T., Kato M., Okamura N., Fukasawa M., Sakakibara R. Expression of human placental-type 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase in various cells and cell lines. Biochem. Biophys. Res. Commun. 1998;242:680–684. doi: 10.1006/bbrc.1997.8024. [DOI] [PubMed] [Google Scholar]
- 188.Martin-Sanz P., Cascales M., Bosca L. Characterization of 6-phosphofructo-2-kinase from foetal-rat liver. Biochem. J. 1992;281:457–463. doi: 10.1042/bj2810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rosa J. L., Ventura F., Carreras J., Bartrons R. Fructose 2,6-bisphosphate and 6-phosphofructo-2-kinase during liver regeneration. Biochem. J. 1990;270:645–649. doi: 10.1042/bj2700645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bosca L., Mojena M., Diaz-Guerra J. M., Marquez C. Phorbol 12,13-dibutyrate and mitogens increase fructose 2,6-bisphosphate in lymphocytes. Comparison of lymphocyte and rat-liver 6-phosphofructo-2-kinase. Eur. J. Biochem. 1988;175:317–323. doi: 10.1111/j.1432-1033.1988.tb14199.x. [DOI] [PubMed] [Google Scholar]
- 191.Casado M., Bosca L., Martin-Sanz P. Multiple forms of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase are expressed in perinatal rat liver. Am. J. Physiol. 1996;270:E244–E250. doi: 10.1152/ajpendo.1996.270.2.E244. [DOI] [PubMed] [Google Scholar]
- 192.Joaquin M., Rosa J. L., Bartrons R., Tauler A. Expression of the F-type 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase mRNA during liver regeneration. Biochim. Biophys. Acta. 1997;1334:256–260. doi: 10.1016/s0304-4165(96)00105-5. [DOI] [PubMed] [Google Scholar]
- 193.Bosca L., Mojena M., Ghysdael J., Rousseau G. G., Hue L. Expression of the v-src or v-fps oncogene increases fructose 2,6-bisphosphate in chick-embryo fibroblasts: novel mechanism for the stimulation of glycolysis by retroviruses. Biochem. J. 1986;236:595–599. doi: 10.1042/bj2360595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Marchand M. J., Maisin L., Hue L., Rousseau G. G. Activation of 6-phosphofructo-2-kinase by pp60v-src is an indirect effect. Biochem. J. 1992;285:413–417. doi: 10.1042/bj2850413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bosca L., Rousseau G. G., Hue L. Phorbol 12-myristate 13-acetate and insulin increase the concentration of fructose 2,6-bisphosphate and stimulate glycolysis in chicken embryo fibroblasts. Proc. Natl. Acad Sci. U.S.A. 1985;82:6440–6444. doi: 10.1073/pnas.82.19.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Meacci E., Vasta V., Vannini F., Farnararo M., Bruni P. Bradykinin stimulates fructose 2,6-bisphosphate metabolism in human fibroblasts. Biochim. Biophys. Acta. 1994;1221:233–237. doi: 10.1016/0167-4889(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 197.Vasta V., Bruni P., Farnararo M. Mechanism of thrombin-induced rise in platelet fructose 2,6-bisphosphate content: studies using phorbol myristate acetate, dioctanoylglycerol and ionophore A23187. Biochem. J. 1987;244:547–551. doi: 10.1042/bj2440547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Meacci E., Vasta V., Farnararo M., Bruni P. Fructose 2,6-bisphosphate metabolism during megakaryocytic differentiation of K562 and MEG-01 cells. Mol. Cell. Biochem. 1996;156:125–130. doi: 10.1007/BF00426334. [DOI] [PubMed] [Google Scholar]
- 199.Colomer D., Vives Corrons J. L., Bartrons R. Effect of TPA on fructose 2,6-bisphosphate levels and protein kinase C activity in B-chronic lymphocytic leukemia (B-CLL) Biochim. Biophys. Acta. 1991;1097:270–274. doi: 10.1016/0925-4439(91)90080-s. [DOI] [PubMed] [Google Scholar]
- 200.Cascales M., Martin-Sanz P., Bosca L. Phorbol esters, bombesin and insulin elicit differential responses on the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase system in primary cultures of foetal and adult rat hepatocytes. Eur. J. Biochem. 1992;207:391–397. doi: 10.1111/j.1432-1033.1992.tb17062.x. [DOI] [PubMed] [Google Scholar]
- 201.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 2000;351:289–305. [PMC free article] [PubMed] [Google Scholar]
- 202.Baulida J., Onetti R., Bassols A. Effects of epidermal growth factor on glycolysis in A431 cells. Biochem. Biophys. Res. Commun. 1992;183:1216–1223. doi: 10.1016/s0006-291x(05)80320-1. [DOI] [PubMed] [Google Scholar]
- 203.Farnararo M., Vasta V., Bruni P., D'Alessandro A. The effect of insulin on Fru-2,6-P2 levels in human fibroblasts. FEBS Lett. 1984;171:117–120. doi: 10.1016/0014-5793(84)80470-6. [DOI] [PubMed] [Google Scholar]
- 204.Hamer M. J., Dickson A. J. Control of glycolysis in cultured chick embryo hepatocytes: fructose 2,6-bisphosphate content and phosphofructokinase-1 activity are stimulated by insulin and epidermal growth factor. Biochem. J. 1990;269:685–690. doi: 10.1042/bj2690685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wu C., Okar D. A., Newgard C. B., Lange A. J. Overexpression of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase in mouse liver lowers blood glucose by suppressing hepatic glucose production. J. Clin. Invest. 2001;107:91–98. doi: 10.1172/JCI11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Baltrusch S., Lenzen S., Okar D. A., Lange A. J., Tiedge M. Characterization of glucokinase-binding protein epitopes by a phage-displayed peptide library: identification of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase as a novel interaction partner. J. Biol. Chem. 2001;276:43915–43923. doi: 10.1074/jbc.M105470200. [DOI] [PubMed] [Google Scholar]
- 207.Baltrusch S., Okar D. A., Lange A. J., Lenzen S., Tiedge M. Interaction of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase with glucokinase activates glucose phosphorylation and glucose metabolism in insulin-producing cells. Diabetes. 2004;4:1020–1029. doi: 10.2337/diabetes.53.4.1020. [DOI] [PubMed] [Google Scholar]
- 208.Thompson J. D., Gibson T. J., Plewniak J., Jeanmougin F., Higgins D. G. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]