Abstract
Mutations in presenilins 1 and 2 (PS1 and PS2) account for the majority of cases of early-onset familial Alzheimer's disease. However, the trafficking and interaction of PSs with other proteins in the early secretory pathways are poorly understood. Using co-immunoprecipitation, we found that PS bound to Syx5 (syntaxin 5), which is a target-soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor involved in endoplasmic reticulum (ER)–Golgi vesicular transport in vivo. Syx5 interacted only with the full-length PS holoproteins and not with the naturally occurring N- or C-terminal fragments. The PS holoproteins co-immunoprecipitated with the mutant Syx5, which localized to the ER and Golgi compartments, despite the substitution of the transmembrane region with that of syntaxin 1A. In contrast, the transmembrane deletion mutant that localized to the cytosol, but not to the ER or Golgi compartments, did not co-immunoprecipitate the PS holoproteins. The PS1 variant linked to familial Alzheimer's disease (PS1ΔE9), lacking the region that contains the endoproteolytic cleavage site in the cytoplasmic loop, showed markedly decreased binding to Syx5. Immunofluorescence and sucrose-density-gradient fractionation analyses showed that the full-length PS holoproteins co-localized with Syx5 to the ER and cis-Golgi compartments. Furthermore, Syx5 overexpression resulted in the accumulation of PS holoproteins and the β-amyloid precursor protein, and reduced the secretion of the Aβ (amyloid β) peptide in COS-7 cells. In summary, these results indicate that Syx5 binds to full-length PSs and affects the processing and trafficking of β-amyloid precursor protein in the early secretory compartments.
Keywords: Alzheimer's disease, β-amyloid, intracellular trafficking, presenilin, syntaxin 5
Abbreviations: AD, Alzheimer's disease; Aβ peptide, amyloid β peptide; βAPP, β-amyloid precursor protein; βCOP, β-coatmer-protein; CTF and NTF, C- and N-terminal fragments; ER, endoplasmic reticulum; ERGIC, ER–Golgi intermediate compartment; HA, haemagglutinin; PS, presenilin; SERCA, sarcoplasmic reticulum/ER Ca2+-ATPase; Syx5, syntaxin 5
INTRODUCTION
Mutations in the genes that encode presenilins 1 and 2 (PS1 and PS2) have been linked to familial forms of AD (Alzheimer's disease) (reviewed in [1,2]). PSs are expressed in neurons and glia [3–5], and are localized predominantly to the ER (endoplasmic reticulum), ERGIC (ER–Golgi intermediate compartment) and the Golgi compartment [5–9]. Full-length PSs are primarily ER-resident molecules that undergo endoproteolysis [10] within the ER, with the NTF and CTF (N- and C-terminal fragments respectively) being transported subsequently to the Golgi compartment followed by the formation of heterodimers [11]. The levels and stoichiometry of these endoproteolytic fragments are strongly regulated [10,12–14] and are incorporated into a complex of high molecular mass [15–17]. Although the precise mechanisms are unknown, proteolytic processing of PS1 is not associated with AD either in the presence or absence of PS1 mutations [18]. Recent studies have suggested that PS1 acts as the catalytic component of γ-secretase [19–22], and is closely linked to the processing of βAPP (β-amyloid precursor protein) to Aβ40 and Aβ42 (amyloid β40 and β42) peptides (reviewed in [23]). Cells that express mutant PSs that cause AD selectively produce increased levels of Aβ42, which is the major amyloid peptide in AD plaques, whereas PS gene knockouts have decreased production levels of both Aβ40 and Aβ42 [24,25]. The γ-secretase activity has been localized to multiple subcellular compartments, such as the ER, ERGIC, Golgi, post-Golgi, endosomes, lysosomes and the plasma membrane [8,26–30]. PS cofactors, such as nicastrin, APH-1 (anterior pharynx-1) and PEN-2 (presenilin enhancer-2), have been shown to play a role in the formation of the γ-secretase complex [31–36]. In addition, PSs have been shown to be involved in the intracellular trafficking of βAPP [28,37–40], β-catenin [41] and nicastrin [42–44] in the later secretory pathways. Although the precise mechanisms underlying these phenomena remain unknown, it appears that PSs are involved in the trafficking of familial AD-related proteins in the ER and Golgi compartments before they reach the later secretory pathways, since PSs are located primarily in the early secretory compartment. However, the determinants of PS progression along the secretory pathways and the mechanisms of intracellular trafficking are poorly understood.
Previous studies using the yeast two-hybrid system showed that HPC-1/syntaxin 1A interacts with the cytoplasmic loop region of PS1 [45]. HPC-1/syntaxin 1A is a member of the syntaxin family and is expressed in neuroendocrine cells [46–50]. Syntaxin 1A, a t-SNARE (target-soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor), functions as a central component of neurotransmitter release at the presynaptic plasma membrane, where it forms the ‘SNARE complex’ membrane-fusion machinery with other proteins (reviewed in [51]). Members of the mammalian syntaxin family have been identified; most of the individual isoforms localize to specific membrane compartments along the secretory and endocytotic pathways and are involved in the intracellular trafficking of vesicles [52–54]. However, soluble syntaxins, such as syntaxin 1C, also regulate intracellular transport [55]. In contrast with the localization of syntaxin 1A to the plasma membrane, full-length PS holoproteins are present mainly in the ER to Golgi compartment in the cell. Among the syntaxins, syntaxin 5 (referred to as Syx5) is expressed ubiquitously in many cell types and has been shown to be localized in the ER and Golgi compartment of the early secretory pathways [53,56–58], which represents the predominant localization of PS holoproteins. Since the overall secondary structures of syntaxins are well conserved among the syntaxin family proteins, we hypothesized that Syx5 is the primary candidate for interaction with PSs in the ER to Golgi compartment. In this context, we investigated the distribution patterns and in vivo interactions of PSs and Syx5 in the early secretory pathways of mammalian cells. In addition, we examined the effects of Syx5 overexpression on the production of PS holoproteins and Aβ. Syx5 interacted with PS holoproteins, but not with their endoproteolytic fragments, in the ER to Golgi compartment. Syx5 overexpression resulted in the accumulation of the PS and βAPP holoproteins and a reduction in the level of Aβ secretion.
EXPERIMENTAL
Materials and antibodies
Anti-PS antibodies were prepared as described previously [18,59]. The mouse anti-PS1 antibody PSN2 was raised against N-terminal residues 31–56 of PS1 (αPS1N). The rabbit polyclonal antibody PS2loop was raised against residues 320–352 within the putative hydrophilic loop region of PS2 (αPS2C). The rabbit polyclonal antibodies S182Nterm2nd (αPS1N) and AD3loop3rd (αPS1C) were raised against the NTF and CTF respectively. The mouse polyclonal antibody AD3L recognizes the peptide that corresponds to residues 295–325 of PS1 (αPS1C). The rabbit polyclonal antibody C40 was raised against the last 40 amino acid residues (IHHGVVEVDAAVTPEERHLSKMQQNGYENPTYKFFEQMQN) of human βAPP. The rat monoclonal anti-HA antibody 3F10 (where HA stands for haemagglutinin) was purchased from Roche Diagnostics (Indianapolis, IN, U.S.A.), the murine monoclonal antibodies against GM130 and γ-adaptin were from BD Transduction Laboratories (San Diego, CA, U.S.A.) and α-tubulin was from Sigma (St. Louis, MO, U.S.A.) The polyclonal rabbit anti-βCOP antibody (where βCOP stands for β-coatmer-protein) and the murine monoclonal anti-SERCA2 antibody (where SERCA stands for sarcoplasmic reticulum/ER Ca2+-ATPase) were from Affinity Bioreagents (Golden, CO, U.S.A.). Alexa Fluor 488 anti-mouse, anti-rat and anti-rabbit IgGs were purchased from Molecular Probes (Eugene, OR, U.S.A.). The Cy3-labelled anti-rat and anti-mouse IgGs were purchased from Jackson Immunoresearch Laboratories (West Grove, PA, U.S.A.). Rhodamine-labelled anti-rabbit IgG was purchased from Cappel (Aurora, OH, U.S.A.). The protease inhibitor cocktail was purchased from Wako Chemicals (Osaka, Japan).
Plasmid construction
Plasmids that contained the cDNAs for the full-length human syntaxin 1A and Syx5 proteins were prepared as described previously [53]. The cDNAs that encoded the full-length human syntaxins 1A and 2–4 were obtained by PCR amplification of the total RNA from the human neuroblastoma cell line NB-1 and from liver and kidney cDNA libraries (Invitrogen, Groningen, The Netherlands). The PCR products were inserted into the pcDNA3-HAN expression vector to create HA-tagged full-length syntaxin constructs (h1–h5). The full-length cDNA fragment that encoded the gene for the human Syx5 was inserted into the pcDNA3-HAN expression vector (h5-pcDNA3-HAN) [53] and was used to create transmembrane-truncated mutants (h5ΔTM-pcDNA3-HAN). The cDNA fragment for amino acids 1–279 of Syx5 was produced by PCR with a 5′-primer that contained an additional in-frame BamHI site and a 3′-primer that contained an additional stop codon sequence and a NotI site just downstream of the codon for amino acid 279. The PCR product was digested with BamHI and NotI and cloned into the BamHI and NotI sites of the pcDNA3-HAN vector to produce h5ΔTM-pcDNA3-HAN. A chimaeric mutant of HA-tagged Syx5, in which the transmembrane region was substituted with that of syntaxin 1A (h5-1ATMD-pcDNA3-HAN), was generated as described previously [53]. The cDNAs that encoded the full-length human PS1 and PS2 proteins were cloned into the expression vectors pCI (Promega, Madison, WI, U.S.A.) and pCMV (Clontech, Palo Alto, CA, U.S.A.), thereby generating PS1-pCI and PS2-pCMV respectively. The cDNA that encoded the PS1 variant that lacked exon 9 (PS1ΔE9) [10] was cloned into pCIneo (Promega) to generate the PS1ΔE9-pCIneo plasmid. The presence of the desired deletions and the identities of the inserted sequences were verified for all the constructs, both by direct sequencing using the ABI373A Sequencer with the BigDye filter (Applied Biosystems Japan, Tokyo, Japan) and by digestion with the appropriate restriction enzymes.
Cell culture, transfection and extract preparation
COS-7 and HeLa cells were cultured as described previously [60]. On the day before transfection, the cells were inoculated into 60 mm dishes for the immunoprecipitation experiments and into 35 mm dishes with attached coverslips for the immunocytochemical analyses. The cells were transfected using the FuGene6 Transfection Reagent (Roche Diagnostics). Cells that were co-transfected with wild-type or mutant PSs, together with wild-type or mutant forms of Syx5, were harvested with a cell scraper, recovered by centrifugation, lysed in extraction buffer (50 mM Tris/HCl, pH 7.5/0.15 M NaCl/1% Triton X-100) containing a protease inhibitor cocktail and then sonicated for 30 s. The insoluble material was removed by centrifugation at 15000 g for 10 min and the supernatant was used as the extract sample.
Immunoprecipitation, SDS/PAGE and Western-blot analysis
The extracts were precleared at 4 °C for 1 h using Protein G–Sepharose beads (Amersham Biosciences, Piscataway, NJ, U.S.A.) to remove the material that was non-specifically bound, and the samples were immunoprecipitated at 4 °C for 2 h with anti-PS polyclonal antibodies or the anti-HA antibody 3F10. The immunocomplexes were then precipitated overnight at 4 °C using Protein G–Sepharose beads. The beads were washed twice with the extraction buffer, followed by a single wash with 50 mM Tris/HCl (pH 7.5), and the bound proteins were solubilized in SDS sample buffer at 37 °C for 30 min. The samples were subjected to SDS/PAGE (12% gel) as described by Laemmli [61] and transferred on to Immobilon P membranes (Millipore, Bedford, MA, U.S.A.). The membranes were blocked with 5% (w/v) skimmed milk in PBS-T (PBS containing 0.1% Tween 20) for 1 h at room temperature (20 °C), and subsequently labelled with the primary antibody for 1.5 h. After washing with PBS-T, the blots were labelled with biotin-conjugated goat anti-IgG antibodies (Vector Laboratories, Burlingame, CA, U.S.A.) for 30 min, followed by the avidin–biotin complex for 30 min (Vector Laboratories). Immunoreactive bands were visualized on an X-ray film using enhanced chemiluminescence reagents (ECL®; Amersham Biosciences).
Discontinuous sucrose-density-gradient fractionation
On the day before transfection, the HeLa cells were inoculated into 100 mm dishes. Cells that were transfected with the wild-type or mutant PSs, together with wild-type or mutant forms of Syx5, were harvested with a cell scraper and recovered by centrifugation. To enrich ER- and Golgi-derived vesicles, discontinuous sucrose-density-gradient fractionation was performed by the methods of Greenfield et al. [26] and Gasparini et al. [62] with minor modifications. The transfected cells were homogenized in a homogenization medium (10 mM Tris/HCl, pH 7.4/0.25 M sucrose/1 mM MgCl2/the protease inhibitor cocktail) in a final mixture of 1 vol. of cell pellet to 5 vol. of homogenizing medium. The homogenate (1 ml) was loaded on top of a step gradient that comprised 1 ml of 2 M sucrose, 4 ml of 1.3 M sucrose, 3.5 ml of 1.16 M sucrose and 2.0 ml of 0.8 M sucrose. All the solutions contained 10 mM Tris/HCl (pH 7.4) and 1 mM MgCl2. The gradients were centrifuged for 2.5 h at 100000 g in a Hitachi P28S2 rotor. A total of 13 fractions (750 μl) were collected from the top of the gradient and assayed for total protein content by the Bradford method, using BSA as the standard.
Immunocytochemistry
Immunocytochemical analyses were performed essentially as described previously [60].
Quantification of Aβ using a sandwich ELISA
COS-7 cells were plated on 24-well plates (in 1 ml of medium) before the day of transfection. Cells were transfected with the indicated plasmids along with cDNA encoding APP695 (APP695-pCI) and the medium was collected 72 h after the transfection. Aβ40 and Aβ42 peptides secreted into the culture medium (100 μl) were quantified by sandwich ELISA specific for each peptide according to the manufacture's instructions (BioSource, Camarillo, CA, U.S.A.; Innogenetics, Ghent, Belgium respectively).
RESULTS
Intracellular localization of syntaxins and PSs
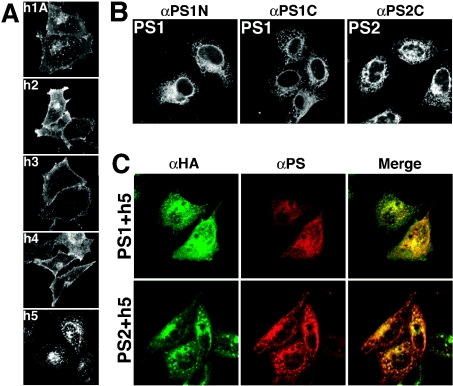
Syntaxins 1A and 2–4 have been localized to the plasma membrane [49,60,63–65]. On the other hand, Syx5 is located in the ER to Golgi compartment [56–58]. First, we compared the distribution patterns of the exogenous syntaxins and PSs that were expressed in HeLa cells. As expected, syntaxins 1–4 (h1–h4 respectively; Figure 1A) were located mainly in the plasma membrane, whereas Syx5 (h5) was located predominantly in the ER to Golgi compartment. Consistent with the observations made with other cell types [56–58], both endogenous (results not shown) and HA-tagged Syx5 co-localized with the cis-Golgi (marked by GM130), ERGIC, Golgi (marked by βCOP) and partially with the ER (marked by SERCA) in HeLa cells. Similar results were obtained for COS-7 cells (results not shown). Using specific antibodies for human PS1 (αPS1N and αPS1C) and PS2 (αPS2C), we showed that the transfected PS1 and PS2 proteins were present in intracellular membranes, such as the ER to Golgi compartment, in HeLa cells (Figure 1B), as has been reported for other cell types [5,6,11]. The PSs co-localized with βCOP and SERCA, which are known to be enriched in the Golgi and ERGIC and in the ER respectively (results not shown). These results suggest that Syx5 is located in the ER to Golgi compartment in the early secretory pathway. As shown in Figure 1(C), when Syx5 was co-expressed with the PSs, both PS1 and PS2 co-localized with Syx5 in the ER to Golgi compartment.
Figure 1. Localization of syntaxins and PSs.
(A) HA-tagged full-length syntaxins (syntaxins 1A and 2–4) were expressed in HeLa cells and fixed for 24 h after transfection. Intracellular localization of the syntaxins was studied immunocytochemically. Syntaxins 1A, 2, 3 and 4 (h1, h2, h3 and h4 respectively) are found in the plasma membrane, whereas HA-tagged full-length Syx5 (h5) is localized to intracellular membranes, such as the ER and ERGIC, and is also found in the cis-Golgi compartment. (B) The locations of expressed PS1 and PS2 were examined. PS1 was probed with anti-PS1 polyclonal antibodies directed against the N-terminal region of PS1 (αPS1N) or the C-terminal region of PS1 (αPS1C). PS2 was visualized with the anti-PS2 polyclonal antibody (αPS2C). Both PS1 and PS2 localize to intracellular membranes, such as the ER and ERGIC, and are also found in the cis-Golgi. (C) Cells were co-transfected with the h5-containing plasmid, together with either the PS1 or PS2 expression plasmid, and were fixed after 24 h of transfection. The cells were stained with the anti-HA antibody (green colour) in combination with anti-PS polyclonal antibodies (αPS1C for PS1 and αPS2C for PS2; red colour) using double-immunofluorescence methods, with co-localization indicated in yellow. Syx5 and PSs co-localize in the ER to Golgi compartment of the early secretory pathway when expressed in HeLa cells.
Interactions of PSs with Syx5 in vivo
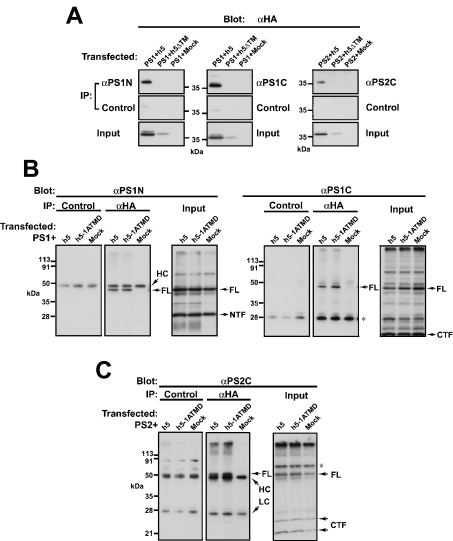
We examined the in vivo interactions of PSs with Syx5, using co-immunoprecipitation methods. For detecting the simultaneous presence of PSs and Syx5 in the intracellular compartment, the Syx5 mutant that lacked the putative transmembrane region (h5ΔTM) was used. This mutant Syx5 localized to the cytosol when it was expressed in the cells (results not shown). The cells were transfected with HA-tagged full-length Syx5 (h5) or h5ΔTM, together with PS1 or PS2, and immunoprecipitations were performed with specific antibodies against human PS1 and PS2. We found that Syx5 co-immunoprecipitated with both PS1 and PS2 when it was expressed in HeLa (Figure 2A, top panels) and COS-7 (results not shown) cells. In addition, PS1 and PS2 co-immunoprecipitated with Syx5 from the COS-7 (Figures 2B and 2C) and HeLa cells (results not shown) when the anti-HA antibody was used. As shown in Figure 2(B), the full-length holoprotein of PS1 (FL), which is approx. 50 kDa in mass, co-immunoprecipitated with Syx5, but not with either the proteolytically cleaved NTF or the CTF of PS1. Similarly, the full-length holoprotein of PS2, but not the CTF, co-immunoprecipitated with Syx5 (Figure 2C, middle panel). These results indicate that only PS holoproteins interact with Syx5 and the interaction domains in PSs probably map to the cytoplasmic loop region, which contains the cleavage site. Furthermore, this was also true for the Syx5 mutant, in which the transmembrane domain was substituted with that of syntaxin 1A (h5-1ATMD; Figures 2B and 2C, middle panel). This mutant was shown previously to localize to the Golgi compartment in a similar manner to that of the wild-type Syx5 [53]. The truncation mutant h5ΔTM, which lacks the transmembrane region and localizes to the cytosol, did not co-precipitate with PSs in HeLa cells (Figure 2A, top panels). Therefore it seems probable that the interactions between Syx5 and the full-length PSs rely more on the cytoplasmic regions than on the transmembrane domains and that these interactions occur in specific membrane compartments of the cells.
Figure 2. Binding of PS1 and PS2 to Syx5 in vivo.
(A) The PS1- and PS2-expressing plasmids were transfected into HeLa cells, along with the plasmid that carried either the HA-tagged full-length Syx5 (h5) or the transmembrane-truncated Syx5 (h5ΔTM). At 48 h post-transfection, the cells were harvested and immunoprecipitated (IP), and the immunocomplexes were subjected to SDS/PAGE and Western-blot analysis, as described in the Experimental section. The ‘Input’ panels show the h5 protein bands in the ‘Input’ fraction that was probed with αHA. PS precipitation in each sample was confirmed by immunoblotting of the same samples with the αPS1 or αPS2 murine antibodies (results not shown). Full-length Syx5 (h5) co-precipitates efficiently with PS1 and PS2 when αPS1 and αPS2 antibodies respectively are used. Truncation mutants that lack the transmembrane region (h5ΔTM) do not co-precipitate with PSs in HeLa cells. (B) The COS-7 cells were co-transfected with h5 or the h5 mutant, in which the transmembrane region was substituted with that of syntaxin 1A (h5-1ATMD), along with the PS1-expressing plasmid. The cells were then extracted and immunoprecipitated with either control IgGs or the anti-HA antibody and then subjected to SDS/PAGE and Western-blot analysis, as described in the Experimental section. The ‘Input’ panels show the PS protein bands in the ‘Input’ fraction that was probed with αPS1N or αPS1C. Syx5 precipitation was confirmed by immunoblotting of the same samples with the anti-HA antibody (results not shown). PS1 co-immunoprecipitates with h5 and h5-1ATMD. Note that only the full-length PS (FL) and not the NTF or CTF of PS co-immunoprecipitate with h5 and h5-1ATMD. (C) An experiment similar to that shown in (B) was performed with the PS2-expressing plasmid. The right panel shows the PS protein bands that were present in the input fraction. Syx5 precipitation was confirmed by immunoblotting of the same samples with the anti-HA antibody (results not shown). Full-length PS2 (FL), but not the CTFs, co-precipitated with h5 and h5-1ATMD (middle panel). The asterisk indicates non-specific bands. FL, full-length holoprotein; HC, immunoglobulin heavy chain; LC, immunoglobulin light chain.
Subcellular localization of PSs and Syx5
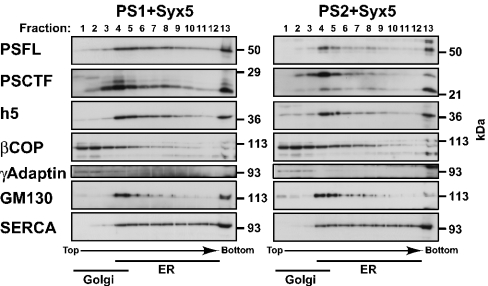
The full-length PS1 and PS2 proteins are proteolytically cleaved within cells to form stable complexes that consist of heteromers of NTF and CTF [10,14,66]. We have shown that PSs and Syx5 co-localize to the ER to Golgi compartment, using specific antibodies against PS1 and PS2 (Figure 1C). However, the immunocytochemical studies could not differentiate between the full-length PS proteins and fragments thereof. Therefore we performed subcellular fractionation experiments to examine further the precise distribution of nascent PSs and their endoproteolytic fragments in HeLa cells. To enrich for ER- and Golgi-derived vesicles, we used discontinuous sucrose-density-gradient fractionation and determined the distributions of full-length PSs and fragments of the PSs, together with the distribution of Syx5. As shown in Figure 3, full-length PS1 and PS2 proteins were observed primarily in the fractions (fractions 4–9) that were designated as light ER fractions, based on the presence of SERCA (an ER-derived marker) and calnexin (results not shown). With respect to the NTF and CTF of the PSs, they were distributed mainly in the dense Golgi fractions (fractions 2–5), as indicated by the presence of γ-adaptin, βCOP and GM130 (trans-Golgi network, ERGIC and cis-Golgi markers respectively). These results were essentially identical with those reported previously [11]. The subcellular localizations of the full-length PS1 and PS2 holoproteins paralleled specifically the distribution of Syx5. These results suggest that full-length PS holoproteins interact with Syx5 in specific intracellular compartments.
Figure 3. Sucrose-density-gradient fractionation of the cells that expressed PSs and Syx5.
HeLa cells that expressed both Syx5 (h5) and either PS1 or PS2 were fractionated on discontinuous sucrose-density gradients (see the Experimental section). Equal volumes from each fraction were separated by SDS/PAGE, followed by immunoblotting with the indicated antibodies. Fractions enriched for ER-derived vesicles, as assessed using the SERCA marker for the ER, were found in the heavy fractions (fractions 4–12). Fractions enriched for Golgi-derived vesicles, as assessed using three different Golgi markers (βCOP for ERGIC and Golgi, γ-adaptin for trans-Golgi network and GM130 for cis-Golgi), were present in the light fractions (fractions 1–4). The full-length PSs (PSFL) were broadly localized from the ER to the Golgi fractions, whereas their proteolytic fragments (PS1CTF and PS2CTF) were distributed predominantly in the Golgi fractions. The distribution of Syx5 (h5) paralleled that of the full-length PSs (PSFL).
Interaction of the PS1 variant (PS1ΔE9) with Syx5
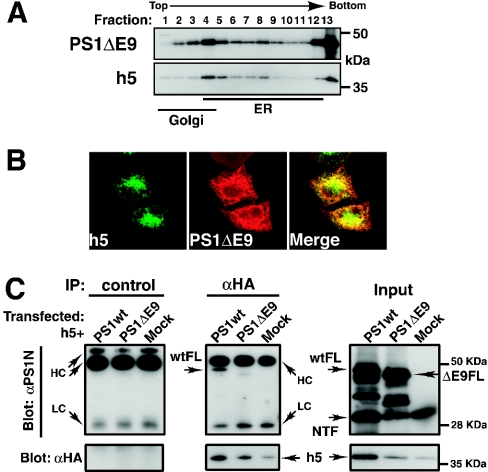
Since we have shown that the cytoplasmic region of Syx5 is necessary for interaction with PSs and only full-length PSs co-precipitate with Syx5 (Figure 2), it seems probable that the cytoplasmic loop regions of the PSs mediate the binding to Syx5, as has been observed for syntaxin 1A using two-hybrid systems in yeast. We took advantage of the familial AD-linked PS1 variant that lacks exon 9 (PS1ΔE9) [10] to examine whether the PS1 mutant could interact with Syx5. This mutant lacks the region that corresponds to amino acids 291–319 of PS1, which contains the endoproteolytic cleavage site of the cytoplasmic loop region. Immunocytochemical and fractionation studies were performed to compare the intracellular distribution pattern of the PS1ΔE9 holoprotein with that of the wild-type PS. As shown in Figure 4(A), sucrose-density-gradient fractionation analysis revealed that a major proportion of PS1ΔE9 was in the ER fraction (fractions 4–9), whereas PS1ΔE9 was located in the light Golgi fractions (fractions 1–3) in higher amounts when compared with the wild-type PS1 holoprotein (Figure 3, top panel). It should be noted that the Syx5 protein was not distributed in the light Golgi fractions (Figure 4A, lower panel). The increase in the amount of PS1ΔE9 in the Golgi fraction was examined further by immunocytochemistry (Figure 4B). Compared with the wild-type PS1 (Figure 1C), PS1ΔE9-associated immunofluorescence was absent from the cis-Golgi, but was more clearly evident in the late compartment of the Golgi (Figure 4B). Although the distribution of PS1ΔE9 overlapped with that of Syx5 (Figures 4A and 4B), the full-length PS1ΔE9 mutant protein showed a marked decrease in binding ability to Syx5 (Figure 4C, middle panel). Similarly, the binding of Syx5 decreased when it was immunoprecipitated with the anti-PS1 antibody (results not shown).
Figure 4. Interaction of the PS1 mutant with Syx5.
(A) HeLa cells that expressed both HA-tagged full-length Syx5 (h5) and PS1ΔE9 were fractionated on discontinuous sucrose-density gradients. Full-length PS1ΔE9 migrated with an apparent molecular mass of 47 kDa. (B) Cells were transfected with the h5-containing plasmid and the PS1ΔE9-expressing plasmid and fixed after 24 h of transfection. The cells were stained with the anti-HA antibody in combination with the anti-PS polyclonal antibodies (αPS1C). (C) Cells were transfected with h5, together with the PS1ΔE9-expressing plasmid. The proteins were extracted and immunoprecipitated (IP) with either control IgGs or the anti-HA antibody and then subjected to SDS/PAGE and Western-blot analysis, as described in the Experimental section. Results are representative of three independent experiments. The PS1ΔE9 mutant protein showed decreased binding to h5, despite the presence of considerable amounts of the full-length PS1ΔE9 in the input fraction (ΔE9FL). ΔE9FL, full-length holoprotein of PS1ΔE9; wtFL, full-length holoprotein of wild-type PS1.
Effect of Syx5 overexpression on the production of PS, APP holoproteins and Aβ
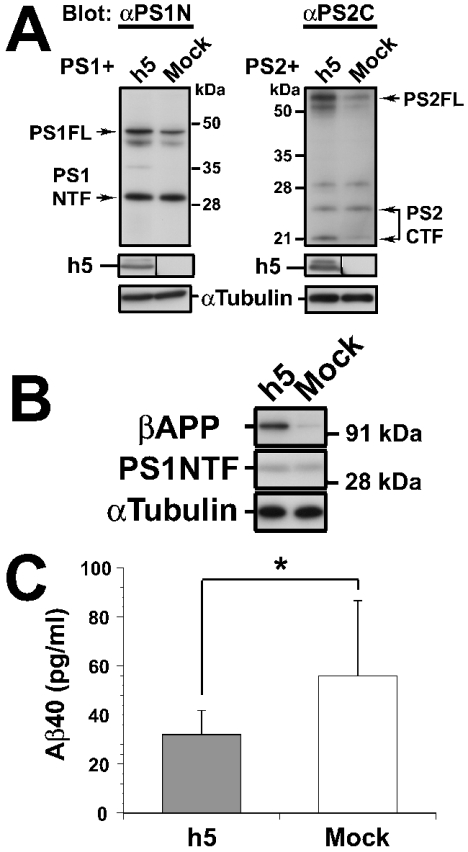
In a final series of experiments, we investigated the effect of Syx5 overexpression on the production of PS proteins, βAPP and Aβ in COS-7 cells. Cells in which Syx5 was overexpressed had higher levels of PS1 holoproteins when compared with the mock-transfected cells (Figure 5A, left panels). Similarly, PS2-expressing cells that simultaneously overexpressed Syx5 accumulated PS2 holoproteins (Figure 5A, right panels). The NTF of PS1 did not show significant changes in the levels of the expressed proteins (Figure 5A, left panels). Accumulation of PS1 holoproteins was also observed in HeLa cells that overexpressed Syx5 (results not shown). We also examined whether Syx5 overexpression influenced the level of the substrate for γ-secretase. COS-7 cells were transfected with h5 or mock-transfected, together with the cDNA that encodes human βAPP (APP695-pCI). The total levels of intracellular βAPP holoproteins were determined by immunoblotting. As shown in Figure 5(B, top panel), the h5-transfected cells that overexpressed Syx5 showed a 3.6-fold increase in the levels of intracellular βAPP holoproteins when compared with the mock-transfected cells (average of three independent trials; values were determined from densitometric analysis of ECL® films). The endogenous NTF of PS1 (PS1NTF) was not significantly altered by Syx5 overexpression (Figure 5B, middle panel). In parallel with the studies to detect the levels of intracellular βAPP and PS1NTF, the levels of Aβ secretion from the same cells were examined. The levels of Aβ40 and Aβ42 that were secreted in the medium were quantified by sandwich Aβ ELISAs, as described in the Experimental section. In cells that were transfected with APP695 alone, Aβ42 accounted for less than 10% of the total amount of Aβ (Aβ40 plus Aβ42) that was secreted into the medium (results not shown). Interestingly, as shown in Figure 5(C), the overexpression of Syx5 (h5) reduced Aβ40 secretion by 43% compared with that of the mock-transfected cells (32.0±9.9 and 56.0±30.5 pg/ml respectively; P<0.05). Since the amount of Aβ42 secreted from the COS-7 cells that overexpressed Syx5 was below the level of detection, it was difficult to assess the effect of Syx5 overexpression on the secretion of Aβ42. Nevertheless, these results indicate that Syx5, in co-operation with PSs, alters the production of Aβ peptides by affecting the processing and/or trafficking of βAPP.
Figure 5. Effect of Syx5 overexpression on PS, APP holoproteins and Aβ production.
(A) The PS1- and PS2-expressing plasmids were transfected into COS-7 cells, together with the plasmid that carries the HA-tagged full-length Syx5 (h5) or the empty vector alone (Mock). At 48 h post-transfection, the cells were harvested and the lysates were subjected to immunoblotting, as described in the Experimental section. An antibody against α-tubulin was used as the loading control. Overexpression of Syx5 results in increased expression of intracellular PS holoproteins (PS1FL, PS2FL) when compared with the mock-transfected cells. (B) The COS-7 cells were doubly transfected with the indicated plasmid along with the cDNA that encodes APP695 (APP695-pCI). The cells were harvested and extracted, 72 h after transfection. Equal amounts of protein were loaded in each lane and subjected to immunoblotting, as described in (A). A representative blot of three independent experiments is shown. Significant accumulation of βAPP is observed in the cells that overexpressed Syx5. (C) Culture supernatants were collected from the cells as described in (B). The amount of Aβ40 secreted was measured by selective sandwich ELISA. The values shown are the average of six (three independent ELISAs) and 12 samples (four independent ELISAs) for h5 and Mock respectively. The error bars indicate S.D. (*P<0.05; Student's t test). Mock, empty pcDNA3 vector.
DISCUSSION
Distribution and interactions of PSs with Syx5 in vivo
We have shown previously that syntaxin 1A is transported to the plasma membrane along a secretory pathway [60,67]. In this pathway, syntaxin 1A is first inserted into the ER and subsequently transported to the Golgi compartment, followed by trafficking to the plasma membrane. It has been proposed that PSs utilize a similar secretory pathway from the ER and Golgi compartments and that a small proportion of the total PS is targeted to the cell surface [28]. An in vitro experiment showed that syntaxin 1A interacts with the cytoplasmic loop region of PS1 [45]. This result suggests that this type of interaction occurs somewhere in the secretory pathway before reaching the plasma membrane. We do not know how syntaxin 1A interacts with PS1. Since syntaxin 1A interacts with various types of proteins [48,68–72] and has similar secondary structures to Syx5, syntaxin 1A and Syx5 may interact co-operatively or sequentially with full-length PS1 along the secretory pathway. Although the overall secondary structures of syntaxins are well conserved among the syntaxin family, as shown in Figure 1(A), they are found in specific compartments and contribute to the specificity of intracellular membrane fusion. Therefore, taking into account the predominant distribution patterns of the PSs and syntaxin proteins, we performed experiments to examine whether Syx5, which resides only in the early secretory pathway, is the major isoform of syntaxin that interacts with PSs within cells. We showed by immunocytochemical analysis that the PS and Syx5 distributions overlap in the early secretory pathway (Figure 1). We performed discontinuous sucrose-density-gradient fractionation to elucidate further the distribution patterns of the full-length PSs and fragments thereof (Figure 3). As reported previously [11], the full-length PSs are located mainly in the ER, in contrast with the preferred localizations of the NTF and CTF of PSs in the Golgi compartment (Figure 3). Consistent with the results obtained from the immunocytochemical analysis (Figure 1C), the subcellular localization of Syx5 paralleled PS distribution, particularly with respect to the full-length holoproteins (Figure 3). These results represent the basis for the interactions between these two proteins in vivo. Indeed, we showed that the full-length PS1 and PS2 holoproteins but not the naturally occurring NTF and CTF (PS1NTF, PS1CTF and PS2CTF) co-immunoprecipitated with Syx5 (Figures 2A–2C). The full-length PSs interacted with the mutant Syx5 (h5-1ATMD), in which the transmembrane region was substituted with that of syntaxin 1A (Figures 2B and 2C), but did not interact with the deletion mutant (h5ΔTM) that lacked the transmembrane region, which was found in the cytosol but not in the ER or Golgi (Figure 2A). Despite differences in the length and amino acid composition of the transmembrane regions between syntaxin 1A and Syx5, the h5-1ATMD mutant localizes to the ER and Golgi [53]. Since h5-1ATMD has binding properties similar to those of the wild-type Syx5 (Figures 2B and 2C), the transmembrane region of Syx5 is not highly specific for Syx5 binding to full-length PSs, but is necessary for the interactions that occur in specific membrane compartments in vivo. The fact that the PS1ΔE9 mutant showed a marked decrease in binding ability to Syx5 (Figure 4C) suggests that the interaction domain is near the site at which PS holoproteins are cleaved to generate active heteromers of the NTF and CTF. Considering these findings, it seems probable that Syx5 interacts with full-length PSs, at least via their cytoplasmic regions, and that these interactions rely more on the cytoplasmic regions than on the transmembrane domains.
Physiological significance of the interactions between PSs and Syx5
By overexpressing PS, we demonstrated successfully the binding of Syx5 to full-length PSs that are present at low levels within the cells (Figure 2). Significant accumulations of PS1 and PS2 holoproteins were observed in the cells that overexpressed Syx5 (Figure 5A). Since the full-length form of PS protein has been shown to be degraded rapidly via the ER-associated degradation pathway [13], interaction with Syx5 may protect the PS from ER-associated degradation. Since the abundance of endoproteolytic fragments was not significantly altered by Syx5 overexpression (Figures 5A and 5B), it seems unlikely that Syx5 has a profound effect on the activity of PS endoproteolysis.
Consistent with the results of a previous study [11], when the subcellular fractionation analysis in Figure 4(A) is compared with that in Figure 3 (left panel), it is noteworthy that in the Golgi fraction, PS1ΔE9 is more abundant compared with the wild-type full-length PS1. Although most of the wild-type PS1 and PS1ΔE9 holoproteins are localized to the ER, these results suggest that a larger fraction of the PS1ΔE9 holoprotein is transported to the late compartment of the Golgi compared with that of the wild-type PS, probably due to reduced interaction with Syx5. The failure of PS1ΔE9 to associate with Syx5 may cause the accumulation of the PS1ΔE9 holoprotein in the late compartment of the secretory pathways, in which the γ-secretase complex is activated. Although it lacks proteolytic processing [11,66], PS1ΔE9 is an active familial AD molecule that possesses γ-secretase activity [10,73–75]. Thus an increase in the amount of PS1ΔE9 in the late compartment of the secretory pathways may lead to increased levels of Aβ40 and Aβ42 production.
It has been reported that γ-secretase is active in the late secretory pathway and in the endosomal pathway, where little or no PS1 is detected [76–79]. Recent studies have demonstrated that APH-1 and PEN-2 associate with full-length PS1 and affect the endoproteolysis of PS1 [35,80]. It is noteworthy that these PS cofactors are reportedly localized to the ER and cis-Golgi, where Syx5 resides. Although it is not known if these factors form a complex with PS1 and Syx5, Syx5 may play a role in the trafficking and maturation of the PS complexes that are required for γ-secretase activity in the late secretory compartments. In the later secretory pathways, it has been shown that PS1 binds to nicastrin and serves as an essential component of the γ-secretase complex [28,81,82]. PS1 interacts preferentially with the mature form of nicastrin, which suggests that correct trafficking and co-localization of the PS complex components are essential for the execution of γ-secretase activity [42–44,81]. In addition, in studies using the PS1 mutant, it was reported that PS1 regulates the intracellular trafficking of βAPP and other type I membrane proteins as substrates for γ-secretase [28,37–40,83,84]. These reports suggest that the interaction with PSs is closely related to the intracellular localization and trafficking of PS cofactors and other proteins in the secretory pathway. However, it seems unlikely that PSs determine the fate of other proteins and contribute to their trafficking between the specific compartments. The correct trafficking of PSs, as well as that of other proteins related to AD, may be determined by interactions with proteins that are involved in vesicular transport between the specific compartments of the cells. In support of this idea, it has been shown that PS1 mutants have impaired kinesin-based axonal transport of vesicles that contain PS1, βAPP and glycogen synthase kinase 3β in neurons [85–87]. Furthermore, the PS1 mutant has been shown to disturb the membrane transport of APP-CTF in the late secretory pathway by affecting Rab8 metabolism in PC12D cells [88]. PS1 is suggested to regulate intracellular trafficking of full-length βAPP through the secretory pathways [28,39]. On the other hand, Syx5 is present only in the ER to Golgi compartment, and it is suggested to be involved in vesicular transport in the early secretory pathway [89–91]. Since we have demonstrated that Syx5 interacts with PSs within the pathway, Syx5 is unlikely to have a direct role in the catalytic activity of γ-secretase. Rather, it seems probable that Syx5, in co-operation with PSs, alters the production of Aβ peptides by affecting the processing and/or trafficking of βAPP, which is destined for the site in which active γ-secretase appears. This notion is supported by the finding that Syx5 overexpression reduced the amount of Aβ40 peptide secreted (Figure 5C). The reduction in Aβ secretion was not due to either the decreased expression of βAPP or a significant reduction in the total amount of NTF of PS1 (Figure 5B, middle panel), which is suggested to be stabilized in the γ-secretase complex [15–17]. Instead, the reduced secretion of the Aβ40 peptide (Figure 5C) was correlated with an increase in the amount of βAPP that was accumulated inside the cells (Figure 5B, top panel). The precise mechanisms underlying these phenomena are currently unknown. However, it is possible that Syx5 regulates the trafficking of multiple membrane proteins that are involved in AD and that the interactions between Syx5 and PSs define the intracellular localization of PS cofactors, as well as the substrates of γ-secretase. If this is the case, the impairment of Syx5-mediated transport of these proteins in the early secretory compartment may lead to changes in Aβ peptide production in the later secretory pathways. Further investigations of the Syx5 interaction with PS complexes may reveal new insights into the physiological roles of PSs as well as the pathological mechanisms of AD. More rigorous studies will demonstrate the roles of Syx5 in the interaction and trafficking of PSs and βAPP and in the generation of Aβ peptides within neuronal cell lines.
Acknowledgments
We thank K. Kasai (Gunma University, Maebashi, Gunma, Japan) for preparation of the plasmids and A. Saito for expert technical and secretarial assistance. K.A. was supported by a grant-in-aid from the Promotion and Mutual Aid Co-operation Program for private schools in Japan, and K.S. by a grant-in-aid for scientific research (B) (16700327) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Czech C., Tremp G., Pradier L. Presenilins and Alzheimer's disease: biological functions and pathogenic mechanisms. Prog. Neurobiol. 2000;60:363–384. doi: 10.1016/s0301-0082(99)00033-7. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe D. J. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 3.Kovacs D. M., Fausett H. J., Page K. J., Kim T. W., Moir R. D., Merriam D. E., Hollister R. D., Hallmark O. G., Mancini R., Felsenstein K. M., et al. Alzheimer-associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat. Med. 1996;2:224–229. doi: 10.1038/nm0296-224. [DOI] [PubMed] [Google Scholar]
- 4.Cook D. G., Sung J. C., Golde T. E., Felsenstein K. M., Wojczyk B. S., Tanzi R. E., Trojanowski J. Q., Lee V. M., Doms R. W. Expression and analysis of presenilin 1 in a human neuronal system: localization in cell bodies and dendrites. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9223–9228. doi: 10.1073/pnas.93.17.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lah J. J., Heilman C. J., Nash N. R., Rees H. D., Yi H., Counts S. E., Levey A. I. Light and electron microscopic localization of presenilin-1 in primate brain. J. Neurosci. 1997;17:1971–1980. doi: 10.1523/JNEUROSCI.17-06-01971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Culvenor J. G., Maher F., Evin G., Malchiodi-Albedi F., Cappai R., Underwood J. R., Davis J. B., Karran E. H., Roberts G. W., Beyreuther K., et al. Alzheimer's disease-associated presenilin 1 in neuronal cells: evidence for localization to the endoplasmic reticulum–Golgi intermediate compartment. J. Neurosci. Res. 1997;49:719–731. doi: 10.1002/(SICI)1097-4547(19970915)49:6<719::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 7.Annaert W. G., Levesque L., Craessaerts K., Dierinck I., Snellings G., Westaway D., George-Hyslop P. S., Cordell B., Fraser P., De Strooper B. Presenilin 1 controls γ-secretase processing of amyloid precursor protein in pre-Golgi compartments of hippocampal neurons. J. Cell Biol. 1999;147:277–294. doi: 10.1083/jcb.147.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cupers P., Bentahir M., Craessaerts K., Orlans I., Vanderstichele H., Saftig P., De Strooper B., Annaert W. The discrepancy between presenilin subcellular localization and γ-secretase processing of amyloid precursor protein. J. Cell Biol. 2001;154:731–740. doi: 10.1083/jcb.200104045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tekirian T. L., Merriam D. E., Marshansky V., Miller J., Crowley A. C., Chan H., Ausiello D., Brown D., Buxbaum J. D., Xia W., et al. Subcellular localization of presenilin 2 endoproteolytic C-terminal fragments. Brain Res. Mol. Brain Res. 2001;96:14–20. doi: 10.1016/s0169-328x(01)00250-9. [DOI] [PubMed] [Google Scholar]
- 10.Thinakaran G., Borchelt D. R., Lee M. K., Slunt H. H., Spitzer L., Kim G., Ratovitsky T., Davenport F., Nordstedt C., Seeger M., et al. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J., Kang D. E., Xia W., Okochi M., Mori H., Selkoe D. J., Koo E. H. Subcellular distribution and turnover of presenilins in transfected cells. J. Biol. Chem. 1998;273:12436–12442. doi: 10.1074/jbc.273.20.12436. [DOI] [PubMed] [Google Scholar]
- 12.Thinakaran G., Harris C. L., Ratovitski T., Davenport F., Slunt H. H., Price D. L., Borchelt D. R., Sisodia S. S. Evidence that levels of presenilins (PS1 and PS2) are coordinately regulated by competition for limiting cellular factors. J. Biol. Chem. 1997;272:28415–28422. doi: 10.1074/jbc.272.45.28415. [DOI] [PubMed] [Google Scholar]
- 13.Kim T. W., Pettingell W. H., Hallmark O. G., Moir R. D., Wasco W., Tanzi R. E. Endoproteolytic cleavage and proteasomal degradation of presenilin 2 in transfected cells. J. Biol. Chem. 1997;272:11006–11010. doi: 10.1074/jbc.272.17.11006. [DOI] [PubMed] [Google Scholar]
- 14.Capell A., Grunberg J., Pesold B., Diehlmann A., Citron M., Nixon R., Beyreuther K., Selkoe D. J., Haass C. The proteolytic fragments of the Alzheimer's disease-associated presenilin-1 form heterodimers and occur as a 100–150-kDa molecular mass complex. J. Biol. Chem. 1998;273:3205–3211. doi: 10.1074/jbc.273.6.3205. [DOI] [PubMed] [Google Scholar]
- 15.Seeger M., Nordstedt C., Petanceska S., Kovacs D. M., Gouras G. K., Hahne S., Fraser P., Levesque L., Czernik A. J., George-Hyslop P. S., et al. Evidence for phosphorylation and oligomeric assembly of presenilin 1. Proc. Natl. Acad. Sci. U.S.A. 1997;94:5090–5094. doi: 10.1073/pnas.94.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu G., Chen F., Levesque G., Nishimura M., Zhang D. M., Levesque L., Rogaeva E., Xu D., Liang Y., Duthie M., et al. The presenilin 1 protein is a component of a high molecular weight intracellular complex that contains β-catenin. J. Biol. Chem. 1998;273:16470–16475. doi: 10.1074/jbc.273.26.16470. [DOI] [PubMed] [Google Scholar]
- 17.Takasugi N., Takahashi Y., Morohashi Y., Tomita T., Iwatsubo T. The mechanism of γ-secretase activities through high molecular weight complex formation of presenilins is conserved in Drosophila melanogaster and mammals. J. Biol. Chem. 2002;277:50198–50205. doi: 10.1074/jbc.M205352200. [DOI] [PubMed] [Google Scholar]
- 18.Okochi M., Ishii K., Usami M., Sahara N., Kametani F., Tanaka K., Fraser P. E., Ikeda M., Saunders A. M., Hendriks L., et al. Proteolytic processing of presenilin-1 (PS-1) is not associated with Alzheimer's disease with or without PS-1 mutations. FEBS Lett. 1997;418:162–166. doi: 10.1016/s0014-5793(97)01378-1. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe M. S., Xia W., Ostaszewski B. L., Diehl T. S., Kimberly W. T., Selkoe D. J. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature (London) 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 20.Herreman A., Serneels L., Annaert W., Collen D., Schoonjans L., De Strooper B. Total inactivation of γ-secretase activity in presenilin-deficient embryonic stem cells. Nat. Cell Biol. 2000;2:461–462. doi: 10.1038/35017105. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z., Nadeau P., Song W., Donoviel D., Yuan M., Bernstein A., Yankner B. A. Presenilins are required for γ-secretase cleavage of β-APP and transmembrane cleavage of Notch-1. Nat. Cell Biol. 2000;2:463–465. doi: 10.1038/35017108. [DOI] [PubMed] [Google Scholar]
- 22.Esler W. P., Kimberly W. T., Ostaszewski B. L., Ye W., Diehl T. S., Selkoe D. J., Wolfe M. S. Activity-dependent isolation of the presenilin–γ-secretase complex reveals nicastrin and a γ substrate. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2720–2725. doi: 10.1073/pnas.052436599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haass C., Steiner H. Alzheimer disease γ-secretase: a complex story of GxGD-type presenilin proteases. Trends Cell Biol. 2002;12:556–562. doi: 10.1016/s0962-8924(02)02394-2. [DOI] [PubMed] [Google Scholar]
- 24.Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T. D., Hardy J., Hutton M., Kukull W., et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat. Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 25.De Strooper B., Saftig P., Craessaerts K., Vanderstichele H., Guhde G., Annaert W., Von Figura K., Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature (London) 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 26.Greenfield J. P., Tsai J., Gouras G. K., Hai B., Thinakaran G., Checler F., Sisodia S. S., Greengard P., Xu H. Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer β-amyloid peptides. Proc. Natl. Acad. Sci. U.S.A. 1999;96:742–747. doi: 10.1073/pnas.96.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soriano S., Chyung A. S., Chen X., Stokin G. B., Lee V. M., Koo E. H. Expression of β-amyloid precursor protein-CD3γ chimeras to demonstrate the selective generation of amyloid β(1–40) and amyloid β(1–42) peptides within secretory and endocytic compartments. J. Biol. Chem. 1999;274:32295–32300. doi: 10.1074/jbc.274.45.32295. [DOI] [PubMed] [Google Scholar]
- 28.Kaether C., Lammich S., Edbauer D., Ertl M., Rietdorf J., Capell A., Steiner H., Haass C. Presenilin-1 affects trafficking and processing of βAPP and is targeted in a complex with nicastrin to the plasma membrane. J. Cell Biol. 2002;158:551–561. doi: 10.1083/jcb.200201123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi R. H., Milner T. A., Li F., Nam E. E., Edgar M. A., Yamaguchi H., Beal M. F., Xu H., Greengard P., Gouras G. K. Intraneuronal Alzheimer aβ42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am. J. Pathol. 2002;161:1869–1879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasternak S. H., Bagshaw R. D., Guiral M., Zhang S., Ackerley C. A., Pak B. J., Callahan J. W., Mahuran D. J. Presenilin-1, nicastrin, amyloid precursor protein, and γ-secretase activity are co-localized in the lysosomal membrane. J. Biol. Chem. 2003;278:26687–26694. doi: 10.1074/jbc.m304009200. [DOI] [PubMed] [Google Scholar]
- 31.Yu G., Nishimura M., Arawaka S., Levitan D., Zhang L., Tandon A., Song Y. Q., Rogaeva E., Chen F., Kawarai T., et al. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and βAPP processing. Nature (London) 2000;407:48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- 32.Francis R., McGrath G., Zhang J., Ruddy D. A., Sym M., Apfeld J., Nicoll M., Maxwell M., Hai B., Ellis M. C., et al. aph-1 and pen-2 are required for Notch pathway signaling, γ-secretase cleavage of βAPP, and presenilin protein accumulation. Dev. Cell. 2002;3:85–97. doi: 10.1016/s1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- 33.Goutte C., Tsunozaki M., Hale V. A., Priess J. R. APH-1 is a multipass membrane protein essential for the Notch signaling pathway in Caenorhabditis elegans embryos. Proc. Natl. Acad. Sci. U.S.A. 2002;99:775–779. doi: 10.1073/pnas.022523499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaVoie M. J., Fraering P. C., Ostaszewski B. L., Ye W., Kimberly W. T., Wolfe M. S., Selkoe D. J. Assembly of the γ-secretase complex involves early formation of an intermediate subcomplex of Aph-1 and nicastrin. J. Biol. Chem. 2003;278:37213–37222. doi: 10.1074/jbc.M303941200. [DOI] [PubMed] [Google Scholar]
- 35.Luo W. J., Wang H., Li H., Kim B. S., Shah S., Lee H. J., Thinakaran G., Kim T. W., Yu G., Xu H. PEN-2 and APH-1 coordinately regulate proteolytic processing of presenilin 1. J. Biol. Chem. 2003;278:7850–7854. doi: 10.1074/jbc.C200648200. [DOI] [PubMed] [Google Scholar]
- 36.Takasugi N., Tomita T., Hayashi I., Tsuruoka M., Niimura M., Takahashi Y., Thinakaran G., Iwatsubo T. The role of presenilin cofactors in the γ-secretase complex. Nature (London) 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- 37.Naruse S., Thinakaran G., Luo J. J., Kusiak J. W., Tomita T., Iwatsubo T., Qian X., Ginty D. D., Price D. L., Borchelt D. R., et al. Effects of PS1 deficiency on membrane protein trafficking in neurons. Neuron. 1998;21:1213–1221. doi: 10.1016/s0896-6273(00)80637-6. [DOI] [PubMed] [Google Scholar]
- 38.Kim S. H., Leem J. Y., Lah J. J., Slunt H. H., Levey A. I., Thinakaran G., Sisodia S. S. Multiple effects of aspartate mutant presenilin 1 on the processing and trafficking of amyloid precursor protein. J. Biol. Chem. 2001;276:43343–43350. doi: 10.1074/jbc.M108245200. [DOI] [PubMed] [Google Scholar]
- 39.Cai D., Leem J. Y., Greenfield J. P., Wang P., Kim B. S., Wang R., Lopes K. O., Kim S. H., Zheng H., Greengard P., et al. Presenilin-1 regulates intracellular trafficking and cell surface delivery of β-amyloid precursor protein. J. Biol. Chem. 2003;278:3446–3454. doi: 10.1074/jbc.M209065200. [DOI] [PubMed] [Google Scholar]
- 40.Leem J. Y., Saura C. A., Pietrzik C., Christianson J., Wanamaker C., King L. T., Veselits M. L., Tomita T., Gasparini L., Iwatsubo T., et al. A role for presenilin 1 in regulating the delivery of amyloid precursor protein to the cell surface. Neurobiol. Dis. 2002;11:64–82. doi: 10.1006/nbdi.2002.0546. [DOI] [PubMed] [Google Scholar]
- 41.Nishimura M., Yu G., Levesque G., Zhang D. M., Ruel L., Chen F., Milman P., Holmes E., Liang Y., Kawarai T., et al. Presenilin mutations associated with Alzheimer disease cause defective intracellular trafficking of β-catenin, a component of the presenilin protein complex. Nat. Med. 1999;5:164–169. doi: 10.1038/5526. [DOI] [PubMed] [Google Scholar]
- 42.Leem J. Y., Vijayan S., Han P., Cai D., Machura M., Lopes K. O., Veselits M. L., Xu H., Thinakaran G. Presenilin 1 is required for maturation and cell surface accumulation of nicastrin. J. Biol. Chem. 2002;277:19236–19240. doi: 10.1074/jbc.C200148200. [DOI] [PubMed] [Google Scholar]
- 43.Yang D. S., Tandon A., Chen F., Yu G., Yu H., Arawaka S., Hasegawa H., Duthie M., Schmidt S. D., Ramabhadran T. V., et al. Mature glycosylation and trafficking of nicastrin modulate its binding to presenilins. J. Biol. Chem. 2002;277:28135–28142. doi: 10.1074/jbc.M110871200. [DOI] [PubMed] [Google Scholar]
- 44.Siman R., Velji J. Localization of presenilin-nicastrin complexes and γ-secretase activity to the trans-Golgi network. J. Neurochem. 2003;84:1143–1153. doi: 10.1046/j.1471-4159.2003.01616.x. [DOI] [PubMed] [Google Scholar]
- 45.Smith S. K., Anderson H. A., Yu G., Robertson A. G., Allen S. J., Tyler S. J., Naylor R. L., Mason G., Wilcock G. W., Roche P. A., et al. Identification of syntaxin 1A as a novel binding protein for presenilin-1. Brain Res. Mol. Brain Res. 2000;78:100–107. doi: 10.1016/s0169-328x(00)00079-6. [DOI] [PubMed] [Google Scholar]
- 46.Barnstable C. J., Akagawa K., Hofstein R., Horn J. P. Monoclonal antibodies that label discrete cell types in the mammalian nervous system. Cold Spring Harb. Symp. Quant. Biol. 1983;48:863–876. doi: 10.1101/sqb.1983.048.01.089. [DOI] [PubMed] [Google Scholar]
- 47.Barnstable C. J., Hofstein R., Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985;352:286–290. doi: 10.1016/0165-3806(85)90116-6. [DOI] [PubMed] [Google Scholar]
- 48.Bennett M. K., Calakos N., Scheller R. H. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 49.Inoue A., Obata K., Akagawa K. Cloning and sequence analysis of cDNA for a neuronal cell membrane antigen, HPC-1. J. Biol. Chem. 1992;267:10613–10619. [PubMed] [Google Scholar]
- 50.Tagaya M., Toyonaga S., Takahashi M., Yamamoto A., Fujiwara T., Akagawa K., Moriyama Y., Mizushima S. Syntaxin 1 (HPC-1) is associated with chromaffin granules. J. Biol. Chem. 1995;270:15930–15933. doi: 10.1074/jbc.270.27.15930. [DOI] [PubMed] [Google Scholar]
- 51.Brunger A. T. Structural insights into the molecular mechanism of Ca(2+)-dependent exocytosis. Curr. Opin. Neurobiol. 2000;10:293–302. doi: 10.1016/s0959-4388(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 52.Scales S. J., Chen Y. A., Yoo B. Y., Patel S. M., Doung Y. C., Scheller R. H. SNAREs contribute to the specificity of membrane fusion. Neuron. 2000;26:457–464. doi: 10.1016/s0896-6273(00)81177-0. [DOI] [PubMed] [Google Scholar]
- 53.Kasai K., Akagawa K. Roles of the cytoplasmic and transmembrane domains of syntaxins in intracellular localization and trafficking. J. Cell Sci. 2001;114:3115–3124. doi: 10.1242/jcs.114.17.3115. [DOI] [PubMed] [Google Scholar]
- 54.Jahn R., Lang T., Sudhof T. C. Membrane fusion. Cell (Cambridge, Mass.) 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 55.Nakayama T., Mikoshiba K., Yamamori T., Akagawa K. Activation of syntaxin 1C, an alternative splice-variant of HPC-1/syntaxin 1A, by phorbol 12-myristate 13-acetate (PMA) suppresses glucose transport into astroglioma cells via the glucose transporter-1 (GLUT-1) J. Biol. Chem. 2004;279:23728–23739. doi: 10.1074/jbc.M314297200. [DOI] [PubMed] [Google Scholar]
- 56.Dascher C., Matteson J., Balch W. E. Syntaxin 5 regulates endoplasmic reticulum to Golgi transport. J. Biol. Chem. 1994;269:29363–29366. [PubMed] [Google Scholar]
- 57.Hay J. C., Klumperman J., Oorschot V., Steegmaier M., Kuo C. S., Scheller R. H. Localization, dynamics, and protein interactions reveal distinct roles for ER and Golgi SNAREs. J. Cell Biol. 1998;141:1489–1502. doi: 10.1083/jcb.141.7.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chao D. S., Hay J. C., Winnick S., Prekeris R., Klumperman J., Scheller R. H. SNARE membrane trafficking dynamics in vivo. J. Cell Biol. 1999;144:869–881. doi: 10.1083/jcb.144.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sahara N., Yahagi Y., Takagi H., Kondo T., Okochi M., Usami M., Shirasawa T., Mori H. Identification and characterization of presenilin I-467, I-463 and I-374. FEBS Lett. 1996;381:7–11. doi: 10.1016/0014-5793(96)00054-3. [DOI] [PubMed] [Google Scholar]
- 60.Suga K., Yamamori T., Akagawa K. Identification of the carboxyl-terminal membrane-anchoring region of HPC-1/syntaxin 1A with the substituted-cysteine-accessibility method and monoclonal antibodies. J. Biochem. (Tokyo) 2003;133:325–334. doi: 10.1093/jb/mvg044. [DOI] [PubMed] [Google Scholar]
- 61.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 62.Gasparini L., Gouras G. K., Wang R., Gross R. S., Beal M. F., Greengard P., Xu H. Stimulation of β-amyloid precursor protein trafficking by insulin reduces intraneuronal β-amyloid and requires mitogen-activated protein kinase signaling. J. Neurosci. 2001;21:2561–2570. doi: 10.1523/JNEUROSCI.21-08-02561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bennett M. K., Garcia-Arraras J. E., Elferink L. A., Peterson K., Fleming A. M., Hazuka C. D., Scheller R. H. The syntaxin family of vesicular transport receptors. Cell (Cambridge, Mass.) 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- 64.Gaisano H. Y., Ghai M., Malkus P. N., Sheu L., Bouquillon A., Bennett M. K., Trimble W. S. Distinct cellular locations of the syntaxin family of proteins in rat pancreatic acinar cells. Mol. Biol. Cell. 1996;7:2019–2027. doi: 10.1091/mbc.7.12.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Low S. H., Chapin S. J., Weimbs T., Komuves L. G., Bennett M. K., Mostov K. E. Differential localization of syntaxin isoforms in polarized Madin–Darby canine kidney cells. Mol. Biol. Cell. 1996;7:2007–2018. doi: 10.1091/mbc.7.12.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ratovitski T., Slunt H. H., Thinakaran G., Price D. L., Sisodia S. S., Borchelt D. R. Endoproteolytic processing and stabilization of wild-type and mutant presenilin. J. Biol. Chem. 1997;272:24536–24541. doi: 10.1074/jbc.272.39.24536. [DOI] [PubMed] [Google Scholar]
- 67.Masaki R., Yamamoto A., Akagawa K., Tashiro Y. Important roles of the C-terminal portion of HPC-1/syntaxin 1A in membrane anchoring and intracellular localization. J. Biochem. (Tokyo) 1998;124:311–318. doi: 10.1093/oxfordjournals.jbchem.a022113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hata Y., Slaughter C. A., Sudhof T. C. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature (London) 1993;366:347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- 69.Naren A. P., Nelson D. J., Xie W., Jovov B., Pevsner J., Bennett M. K., Benos D. J., Quick M. W., Kirk K. L. Regulation of CFTR chloride channels by syntaxin and Munc18 isoforms. Nature (London) 1997;390:302–305. doi: 10.1038/36882. [DOI] [PubMed] [Google Scholar]
- 70.Fujita Y., Shirataki H., Sakisaka T., Asakura T., Ohya T., Kotani H., Yokoyama S., Nishioka H., Matsuura Y., Mizoguchi A., et al. Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron. 1998;20:905–915. doi: 10.1016/s0896-6273(00)80472-9. [DOI] [PubMed] [Google Scholar]
- 71.Wu M. N., Fergestad T., Lloyd T. E., He Y., Broadie K., Bellen H. J. Syntaxin 1A interacts with multiple exocytic proteins to regulate neurotransmitter release in vivo. Neuron. 1999;23:593–605. doi: 10.1016/s0896-6273(00)80811-9. [DOI] [PubMed] [Google Scholar]
- 72.Lao G., Scheuss V., Gerwin C. M., Su Q., Mochida S., Rettig J., Sheng Z. H. Syntaphilin: a syntaxin-1 clamp that controls SNARE assembly. Neuron. 2000;25:191–201. doi: 10.1016/s0896-6273(00)80882-x. [DOI] [PubMed] [Google Scholar]
- 73.Levitan D., Doyle T. G., Brousseau D., Lee M. K., Thinakaran G., Slunt H. H., Sisodia S. S., Greenwald I. Assessment of normal and mutant human presenilin function in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14940–14944. doi: 10.1073/pnas.93.25.14940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baumeister R., Leimer U., Zweckbronner I., Jakubek C., Grunberg J., Haass C. Human presenilin-1, but not familial Alzheimer's disease (FAD) mutants, facilitate Caenorhabditis elegans Notch signalling independently of proteolytic processing. Genes Funct. 1997;1:149–159. doi: 10.1046/j.1365-4624.1997.00012.x. [DOI] [PubMed] [Google Scholar]
- 75.Steiner H., Romig H., Grim M. G., Philipp U., Pesold B., Citron M., Baumeister R., Haass C. The biological and pathological function of the presenilin-1 Δexon 9 mutation is independent of its defect to undergo proteolytic processing. J. Biol. Chem. 1999;274:7615–7618. doi: 10.1074/jbc.274.12.7615. [DOI] [PubMed] [Google Scholar]
- 76.Hartmann T., Bieger S. C., Bruhl B., Tienari P. J., Ida N., Allsop D., Roberts G. W., Masters C. L., Dotti C. G., Unsicker K., et al. Distinct sites of intracellular production for Alzheimer's disease A β40/42 amyloid peptides. Nat. Med. 1997;3:1016–1020. doi: 10.1038/nm0997-1016. [DOI] [PubMed] [Google Scholar]
- 77.Petanceska S. S., Seeger M., Checler F., Gandy S. Mutant presenilin 1 increases the levels of Alzheimer amyloid β-peptide Aβ42 in late compartments of the constitutive secretory pathway. J. Neurochem. 2000;74:1878–1884. doi: 10.1046/j.1471-4159.2000.0741878.x. [DOI] [PubMed] [Google Scholar]
- 78.Iwata H., Tomita T., Maruyama K., Iwatsubo T. Subcellular compartment and molecular subdomain of β-amyloid precursor protein relevant to the Aβ42-promoting effects of Alzheimer mutant presenilin 2. J. Biol. Chem. 2001;276:21678–21685. doi: 10.1074/jbc.M007989200. [DOI] [PubMed] [Google Scholar]
- 79.Maltese W. A., Wilson S., Tan Y., Suomensaari S., Sinha S., Barbour R., McConlogue L. Retention of the Alzheimer's amyloid precursor fragment C99 in the endoplasmic reticulum prevents formation of amyloid β-peptide. J. Biol. Chem. 2001;276:20267–20279. doi: 10.1074/jbc.M007238200. [DOI] [PubMed] [Google Scholar]
- 80.Gu Y., Chen F., Sanjo N., Kawarai T., Hasegawa H., Duthie M., Li W., Ruan X., Luthra A., Mount H. T., et al. APH-1 interacts with mature and immature forms of presenilins and nicastrin and may play a role in maturation of presenilin–nicastrin complexes. J. Biol. Chem. 2003;278:7374–7380. doi: 10.1074/jbc.M209499200. [DOI] [PubMed] [Google Scholar]
- 81.Kopan R., Goate A. Aph-2/Nicastrin: an essential component of γ-secretase and regulator of Notch signaling and Presenilin localization. Neuron. 2002;33:321–324. doi: 10.1016/s0896-6273(02)00585-8. [DOI] [PubMed] [Google Scholar]
- 82.De Strooper B. Aph-1, Pen-2, and nicastrin with presenilin generate an active γ-secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 83.Annaert W. G., Esselens C., Baert V., Boeve C., Snellings G., Cupers P., Craessaerts K., De Strooper B. Interaction with telencephalin and the amyloid precursor protein predicts a ring structure for presenilins. Neuron. 2001;32:579–589. doi: 10.1016/s0896-6273(01)00512-8. [DOI] [PubMed] [Google Scholar]
- 84.Taniguchi Y., Kim S. H., Sisodia S. S. Presenilin-dependent ‘γ-secretase’ processing of deleted in colorectal cancer (DCC) J. Biol. Chem. 2003;278:30425–30428. doi: 10.1074/jbc.C300239200. [DOI] [PubMed] [Google Scholar]
- 85.Kamal A., Stokin G. B., Yang Z., Xia C. H., Goldstein L. S. Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-I. Neuron. 2000;28:449–459. doi: 10.1016/s0896-6273(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 86.Kamal A., Almenar-Queralt A., LeBlanc J. F., Roberts E. A., Goldstein L. S. Kinesin-mediated axonal transport of a membrane compartment containing β-secretase and presenilin-1 requires APP. Nature (London) 2001;414:643–648. doi: 10.1038/414643a. [DOI] [PubMed] [Google Scholar]
- 87.Pigino G., Morfini G., Pelsman A., Mattson M. P., Brady S. T., Busciglio J. Alzheimer's presenilin 1 mutations impair kinesin-based axonal transport. J. Neurosci. 2003;23:4499–4508. doi: 10.1523/JNEUROSCI.23-11-04499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kametani F., Usami M., Tanaka K., Kume H., Mori H. Mutant presenilin (A260V) affects Rab8 in PC12D cell. Neurochem. Int. 2003;44:313–320. doi: 10.1016/s0197-0186(03)00176-1. [DOI] [PubMed] [Google Scholar]
- 89.Hardwick K. G., Pelham H. R. SED5 encodes a 39-kDa integral membrane protein required for vesicular transport between the ER and the Golgi complex. J. Cell Biol. 1992;119:513–521. doi: 10.1083/jcb.119.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Banfield D. K., Lewis M. J., Rabouille C., Warren G., Pelham H. R. Localization of Sed5, a putative vesicle targeting molecule, to the cis-Golgi network involves both its transmembrane and cytoplasmic domains. J. Cell Biol. 1994;127:357–371. doi: 10.1083/jcb.127.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rabouille C., Kondo H., Newman R., Hui N., Freemont P., Warren G. Syntaxin 5 is a common component of the NSF- and p97-mediated reassembly pathways of Golgi cisternae from mitotic Golgi fragments in vitro. Cell (Cambridge, Mass.) 1998;92:603–610. doi: 10.1016/s0092-8674(00)81128-9. [DOI] [PubMed] [Google Scholar]