Abstract
Background
In nephrotic syndrome, protein leaks from the blood into the urine through the glomeruli, resulting in hypoproteinaemia and generalised oedema. While most children with nephrotic syndrome respond to corticosteroids, 80% experience a relapsing course. Corticosteroids have reduced the death rate to around 3%; however, corticosteroids have well‐recognised potentially serious adverse events such as obesity, poor growth, hypertension, diabetes mellitus, osteoporosis, cataracts, glaucoma and behavioural disturbances. This is an update of a review first published in 2000 and updated in 2002, 2005, 2007, 2015 and 2020.
Objectives
The aim of this review was to assess the benefits and harms of different corticosteroid regimens in children with steroid‐sensitive nephrotic syndrome (SSNS). The benefits and harms of therapy were studied in two groups of children: 1) children in their initial episode of SSNS and 2) children who experience a relapsing course of SSNS.
Search methods
We contacted the Information Specialist and searched the Cochrane Kidney and Transplant Register of Studies up to 9 July 2024 using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Registry Platform (ICTRP) Search Portal, and ClinicalTrials.gov.
Selection criteria
Randomised controlled trials (RCTs) performed in children (one to 18 years) during their initial or subsequent episode of SSNS, comparing different durations, total doses or other dose strategies using any corticosteroid agent.
Data collection and analysis
Summary estimates of effects were obtained using a random‐effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes and mean difference (MD) and 95% CI for continuous outcomes. Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results
In this 2024 update, we included five new studies, resulting in 54 studies randomising 4670 children.
Risk of bias methodology was often poorly performed, with only 31 studies and 28 studies respectively assessed to be at low risk for random sequence generation and allocation concealment. Ten studies were at low risk of performance bias (blinding of participants and personnel), and 12 studies were at low risk of detection bias (blinding of outcome assessment); nine of these studies were placebo‐controlled RCTs. Twenty‐seven studies (fewer than 50%) were at low risk for attrition bias, and 26 studies were at low risk for reporting bias (selective outcome reporting).
In studies at low risk of selection bias evaluating children in their initial episode of SSNS, there is little or no difference in the number of children with frequent relapses when comparing two months of prednisone with three months or more (RR 0.96, 95% CI 0.83 to 1.10; 755 children, 5 studies; I2 = 0%; high certainty evidence) or when comparing three months with five to seven months of therapy (RR 0.99, 95% CI 0.74 to 1.33; 376 children, 3 studies; I2 = 35%; high certainty evidence). In analyses of studies at low risk of selection bias, there is little or no difference in the number of children with any relapse by 12 to 24 months when comparing two months of prednisone with three months or more (RR 0.93, 95% CI 0.81 to 1.06; 808 children; 6 studies; I2 = 47%) or when comparing three months with five to seven months of therapy (RR 0.88, 95% CI 0.70 to 1.11; 377 children, 3 studies; I2 = 53%). Little or no difference was noted in adverse events between the different treatment durations.
Amongst children with relapsing SSNS, four small studies (177 children) utilising lower doses of prednisone compared with standard regimens found little or no differences between groups in the numbers with relapse (RR 1.01, 95% CI 0.85 to 1.20; I2 = 0%). A fifth study (117 children) reported little or no difference between two weeks and four weeks of alternate‐day prednisone after remission with daily prednisone.
A recent large, well‐designed study with 271 children found that administering daily prednisone compared with alternate‐day prednisone or no prednisone during viral infection did not reduce the risk of relapse. In contrast, four previous small studies in children with frequently relapsing disease had reported that daily prednisone during viral infections compared with alternate‐day prednisone or no treatment reduced the risk of relapse.
Authors' conclusions
There are four well‐designed studies randomising 823 children, which have demonstrated that there is no benefit of prolonging prednisone therapy beyond two to three months in the first episode of SSNS. Small studies in children with relapsing disease have identified no differences in efficacy using lower induction doses or shorter durations of prednisone therapy. Large, well‐designed studies are required to confirm these findings. While previous small studies had suggested that changing from alternate‐day to daily prednisone therapy at the onset of infection reduced the likelihood of relapse, a much larger and well‐designed study found no reduction in the number relapsing when administering daily prednisone at the onset of infection.
Keywords: Adolescent; Child; Child, Preschool; Humans; Infant; Adrenal Cortex Hormones; Adrenal Cortex Hormones/therapeutic use; Bias; Dexamethasone; Dexamethasone/therapeutic use; Glucocorticoids; Glucocorticoids/therapeutic use; Nephrotic Syndrome; Nephrotic Syndrome/drug therapy; Randomized Controlled Trials as Topic; Recurrence
Plain language summary
What are the benefits and risks of using steroids for treating nephrotic syndrome in children?
Key messages
• Nephrotic syndrome (a condition where the kidneys leak protein from the blood into the urine) is usually treated with steroid medication (powerful anti‐inflammatory medicines).
• Children experiencing nephrotic syndrome for the first time only need two to three months of prednisone (a type of steroid medication), as longer durations do not reduce the risk of a repeat episode or reduce the risk that the child will have frequent repeat episodes.
• Although the risk of side effects may be low, many of the studies did not report information that we could analyse.
What is nephrotic syndrome, and why should you treat it with steroids?
Nephrotic syndrome is a condition where the kidneys leak protein from the blood into the urine. When left untreated, children can suffer from serious infections. In most children with nephrotic syndrome, this protein leak is stopped with steroid therapy. Steroids are powerful anti‐inflammatory medicines and can be used for a wide range of conditions, but they can also have serious side effects. These side effects can include depression and anxiety, high blood pressure, eye disorders such as cataracts, increased risk of infection, weight gain and reduced growth. Children can also have repeat episodes of nephrotic syndrome, which is often triggered by viral infection.
What did we want to find out?
We wanted to find out the best treatment options for children with nephrotic syndrome to stop the leaking of protein from the blood into the urine and to avoid the harmful side effects of steroids.
What did we do?
We searched for all studies that compared the benefits and harms of randomly allocating steroid medicines to:
• children who experience nephrotic syndrome for the first time; or
• children who have frequent repeat episodes of nephrotic syndrome.
We compared and summarised the studies' results and rated our confidence in the information based on factors such as study methods and sizes.
What did we find?
We found 54 studies randomising 4670 children looking at a wide variety of steroid treatment options. Studies were conducted in countries around the world, and most were done in South Asia (23 studies). Twenty‐three studies compared giving the steroid prednisone for two or three months with longer durations (three to seven months) to children who experience nephrotic syndrome for the first time. The remaining studies looked at children who had frequent repeat episodes of nephrotic syndrome.
We found longer durations of prednisone (three to seven months) made little to no difference in the risk of children experiencing repeat episodes of nephrotic syndrome or in the number of children who have frequent repeat episodes compared to shorter durations of prednisone (two to three months). There may be no differences in the type and number of side effects with either longer or shorter durations of steroids; however, these were often not reported by the studies.
What are the limitations of the evidence?
We are confident that children experiencing nephrotic syndrome for the first time only need two to three months of prednisone, as longer durations do not reduce the risk of a repeat episode or reduce the risk that the child will have frequent repeat episodes.
We are less confident in the risk of side effects because many of the studies did not report information that we could use.
How up‐to‐date is the evidence?
The evidence is current to July 2024.
Summary of findings
Summary of findings 1. Steroid therapy for the first episode of nephrotic syndrome in children: 3 months or more versus 2 months of therapy.
| Steroid therapy for the first episode of nephrotic syndrome in children: 3 months or more versus 2 months of therapy | |||||
| Patient or population: children with nephrotic syndrome Setting: paediatric or paediatric nephrology services Intervention: 3 months or more of steroid therapy Comparison: 2 months of steroid therapy | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Risk with 2 months of therapy | Risk with 3 months or more of therapy | ||||
| Frequent relapses by 12 to 24 months | 450 per 1,000 | 387 per 1,000 (319 to 477) | RR 0.86 (0.71 to 1.06) | 976 (8) | ⊕⊕⊕⊝1 MODERATE |
| Relapsing by 12 to 24 months | 637 per 1,000 | 509 per 1,000 (433 to 611) | RR 0.80 (0.68 to 0.96) | 1279 (12) | ⊕⊕⊝⊝1,2 LOW |
| Frequent relapses by 12 to 24 months: low risk of selection bias | 480 per 1,000 | 461 per 1,000 (339 to 528) | RR 0.96 (0.83 to 1.10) | 756 (5) | ⊕⊕⊕⊕ HIGH |
| Number with frequent relapses by 12 to 24 months: unclear or high risk of selection bias | 333 per 1,000 | 150 per 1,000 (87 to 257) | RR 0.45 (0.26 to 0.77) | 220 (3) | ⊕⊕⊝⊝ 1,2 LOW |
| Adverse events: psychological disorders Median follow up: 2 years |
470 per 1,000 | 470 per 1,000 (249 to 894) | RR 1.00 (0.53 to 1.90) | 456 (4) | ⊕⊕⊝⊝2,3 LOW |
| Adverse events: hypertension Median follow up: 1.5 years |
50 per 1,000 | 89 per 1,000 (28 to 287) | RR 1.78 (0.55 to 5.73) | 548 (7) | ⊕⊕⊕⊝ 1 MODERATE |
| Adverse events: Cushingoid facies Median follow up: 2 years |
402 per 1,000 | 450 per 1,000 (305 to 663) | RR 1.12 (0.76 to 1.65) | 547 (5) | ⊕⊕⊕⊝1 MODERATE |
| Adverse events: eye disorders Median follow up: 2 years |
32 per 1,000 | 13 per 1,000 (3 to 48) |
RR 0.41 (0.11 to 1.52) |
623 (6) | ⊕⊕⊝⊝2,3 LOW |
| Adverse events: infections Median follow up: 1.75 years |
408 per 1,000 | 323 per 1,000 (216 to 478) |
RR 0.79 (0.53 to 1.17) |
172 (2) | ⊕⊕⊝⊝2,3 LOW |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect | |||||
1 Significant heterogeneity between studies
2 Some studies at high or unclear risk of bias
3 Few studies/events included in analyses
Summary of findings 2. Steroid therapy for the first episode of nephrotic syndrome in children: 5 to 7 months versus 3 months of therapy.
| Steroid therapy for the first episode of nephrotic syndrome in children: 5 to 7 months versus 3 months of therapy | |||||
| Patient or population: nephrotic syndrome in children Setting: paediatric or paediatric nephrology services Intervention: 5 to 7 months of steroid therapy Comparison: 3 months of steroid therapy | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Certainty of the evidence (GRADE) | |
| Risk with 3 months | Risk with 5 to 7 months | ||||
| Number with frequent relapses by 12 to 24 months | 387 per 1,000 | 282 per 1,000 (190 to 422) | RR 0.73 (0.49 to 1.09) | 706 (6) | ⊕⊕⊕⊝ Moderate 1 |
| Number relapsing by 12 to 24 months | 738 per 1,000 | 472 per 1,000 (369 to 598) | RR 0.64 (0.50 to 0.81) | 912 (8) | ⊕⊕⊝⊝ Low 1 2 |
| Number relapsing by 12 to 24 months: low risk of selection bias | 679 per 1,000 | 598 per 1,000 (469 to 754) | RR 0.88 (0.69 to 1.11) | 376 (3) | ⊕⊕⊕⊕ High |
| Number of children relapsing by 12 to 24 months: unclear of high risk of selection bias | 778 per 1,000 | 412 per 1,000 (319 to 537) | RR 0.53 (0.41 to 0.69) | 536 (5) | ⊕⊕⊝⊝ Low 1 2 |
| Adverse events: psychological disorders Median follow up: 2 years |
53 per 1,000 | 16 per 1,000 (3 to 96) |
RR 0.30 (0.05 to 1.83) |
505 (4) | ⊕⊕⊕⊝ Moderate 1 |
| Adverse events: hypertension Median follow up: 1.75 years |
126 per 1,000 | 140 per 1,000 (90 to 220) | RR 1.11 (0.71 to 1.74) | 752 (6) | ⊕⊕⊕⊝ Moderate 2 |
| Adverse events: Cushingoid appearance Median follow up: 1.75 years |
375 per 1,000 | 323 per 1,000 (225 to 461) |
RR 0.86 (0.60 to 1.23) |
762 (6) | ⊕⊕⊕⊝ Moderate 1 |
| Adverse events: eye complications Median follow up: 1.5 years |
36 per 1,000 | 17 per 1,000 (6 to 42) | RR 0.46 (0.18 to 1.17) | 614 (5) | ⊕⊕⊕⊝ Moderate 2 |
| Adverse events: infections Median follow up: 1.5 years |
185 per 1,000 | 181 per 1,000 (120 to 270) | RR 0.98 (0.65 to 1.46) | 702 (5) | ⊕⊕⊕⊝ Moderate 1 2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect | |||||
1 Significant heterogeneity between studies
2 Some studies at high or unclear risk of bias
Background
Description of the condition
Nephrotic syndrome is the most common acquired childhood kidney disease. Its characteristic features, including oedema, proteinuria, and hypoalbuminaemia, result from alterations in the selectivity of the permeability barrier of the glomerular capillary wall.
Based on a comprehensive meta‐analysis of 27 studies, the overall incidence of idiopathic nephrotic syndrome was 2.92 (95% confidence intervals (CI) 0.00 to 6.51) per 100,000 children per year (Veltkamp 2021), with no significant variation in incidence between 1945 and 2011. There are marked differences in the incidence of nephrotic syndrome depending on ethnicity, with proportions ranging from 1.15 to 16.9/100,000 children, with the highest incidence in children from South Asia (Noone 2018). Most children have minimal change disease, in which changes seen on light microscopy are minor or absent, and respond to corticosteroid agents. The histological variant seen and the response to immunosuppressive treatment varies with ethnicity. Steroid‐sensitive nephrotic syndrome (SSNS) is less common in African and African‐American children, and in South Africa, only 6.7% of 236 African children had SSNS compared with 65% of 286 Indian children (Bhimma 1997). The pathogenesis of SSNS remains unknown but appears to be related to abnormalities in T‐cell and B‐cell regulation, leading to injury of the podocyte, a key component of the glomerular filtration barrier.
Many children who respond to corticosteroids experience a relapsing course with recurrent episodes of oedema and proteinuria (Koskimies 1982; Tarshish 1997). While the incidence of idiopathic nephrotic syndrome has not changed, the risk of relapse between 1945 and 2011 has fallen from 87.4% to 66.2% based on the meta‐analysis of 54 studies (Veltkamp 2021). The complications of nephrotic syndrome are related to the effects of the disease itself and to adverse effects related to corticosteroid therapy and corticosteroid‐sparing agents. Children with nephrotic syndrome with frequent relapses, steroid dependence, or late resistance to therapy are at increased risk of bacterial infection (characteristically resulting in peritonitis, cellulitis, or septicaemia), thromboembolic phenomena and acute kidney injury. Before antibiotics became available, two‐thirds of children with nephrotic syndrome died. Death rates fell to 35% with the introduction of sulphonamides and penicillin (Arneil 1971) and fell further with the use of corticosteroid medications (Arneil 1956).
Description of the intervention
Corticosteroids have been used to treat childhood nephrotic syndrome since 1950 when large doses of adrenocorticotrophic hormone (ACTH) and cortisone given for two to three weeks were found to induce diuresis with loss of oedema and proteinuria (Arneil 1956; Arneil 1971). Corticosteroid usage has reduced the death rate in childhood nephrotic syndrome to around 3%, with infection remaining the most important cause of death (ISKDC 1984). Of children who present with their first episode of nephrotic syndrome, approximately 80% will achieve remission with corticosteroid therapy (Koskimies 1982). Because of this dramatic before‐after treatment evidence, oral corticosteroids are the first‐line treatment of a child presenting with idiopathic nephrotic syndrome and no randomised controlled trials (RCTs) of corticosteroids compared to placebo were carried out. The achievement of remission with corticosteroid therapy determines long‐term prognosis for kidney function irrespective of kidney histology (Niaudet 2009). However, corticosteroids have well‐documented adverse effects in children. Major complications related to prolonged corticosteroid use in nephrotic syndrome include growth impairment, particularly with steroid therapy administered daily (Hyams 1988), cataracts (Aydin 2019; Ng 2001), arterial hypertension (Aydin 2019) and excessive weight gain or obesity (Ruth 2005). Two studies (Mishra 2010; Neuhaus 2010) highlight the impact of psychological and behavioural abnormalities related to corticosteroid therapy. Anxiety, depression, emotional lability, aggressive behaviour and attention problems had already developed with the completion of 12 weeks of therapy (Mishra 2010). Neuhaus 2010 demonstrated that family background, particularly maternal distress, reduced the quality of life (QoL) and psychosocial adjustment. Patients and families report challenges in living with the disease because the condition is poorly understood and the clinical course is uncertain (Beanlands 2017). Adverse effects are particularly prevalent in those children who relapse frequently and require multiple courses of corticosteroids.
How the intervention might work
Glucocorticoids are potent anti‐inflammatory and immunosuppressant drugs. The effects of glucocorticoids are known to be mediated by both genomic and non‐genomic mechanisms (Schijvens 2019). It is widely believed the main effect is through the regulation of nuclear gene expression via the cytosolic glucocorticoid receptor, which activates genes for anti‐inflammatory cytokines and suppresses genes for pro‐inflammatory cytokines (Kadmiel 2013; Kirshcke 2014; Ponticelli 2018). Glucocorticoids are lipid soluble and can easily pass through cell membranes. This process takes several hours. More recently, research has identified corticosteroid effects, which are independent of nuclear gene transcription and occur earlier (Ramamoorthy 2016). These are mediated via interactions of various kinases with cytosolic or membrane‐bound glucocorticoid receptors and do not require protein synthesis. At high glucocorticoid doses, suppression of T‐cell function occurs. Corticosteroids also act directly to stabilise the podocyte cytoskeleton (Guess 2010; Ohashi 2011).
Why it is important to do this review
The original treatment schedules for childhood nephrotic syndrome were developed in an ad hoc manner more than 50 years ago. The International Study of Kidney Disease in Children (ISKDC) was established in 1966 and determined by consensus a regimen of daily corticosteroids for four weeks followed by corticosteroids given on three consecutive days out of seven for four weeks (Arneil 1971). Since then, many physicians have used regimens involving periods of daily followed by alternate‐day or intermittent therapy, and RCTs have investigated different durations and total corticosteroid therapy doses in an effort to delineate the optimal doses and durations of corticosteroid therapy, balancing efficacy and toxicity. These have been evaluated in previous versions of this systematic review. However, despite these data, there remains no consensus on the most appropriate corticosteroid regimen to achieve and maintain remission with the least adverse effects. Observational data (Raja 2017) and small RCTs (Borovitz 2020; Kansal 2019; Kainth 2021; Sheikh 2019; Tu 2022) suggest that children can be successfully treated with smaller doses and durations of corticosteroid therapy. Therefore, this 2024 update was undertaken to identify whether new RCTs, which evaluate different corticosteroid regimens in the initial episode of SSNS and in relapsing disease, provide additional information on the most effective corticosteroid therapy regimens for SSNS in children.
Objectives
The aim of this review was to assess the benefits and harms of different corticosteroid regimens in children with SSNS. The benefits and harms of therapy were studied in two groups of children:
Children in their initial episode of SSNS
Children who experience a relapsing course of SSNS.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) were included in which different doses, dose strategies, routes of administration and durations of treatment with prednisone, prednisolone or other corticosteroid agents are compared in the treatment of SSNS in children.
Types of participants
Inclusion criteria
Children aged one to 18 years with SSNS (i.e. become oedema‐free with urine protein ≤ 1+ on dipstick, urinary protein‐creatinine ratio (UPCR) ≤ 20 mg/mmol or ≤ 4 mg/m2/hour for three consecutive days while receiving corticosteroid therapy). A kidney biopsy diagnosis of minimal change disease was not required for inclusion in the study.
Children with an initial episode of SSNS
Children with relapsing SSNS.
Exclusion criteria
Children with steroid‐resistant nephrotic syndrome (SRNS) (failure to achieve remission following four weeks or more of prednisone at 60 mg/m2/day) or congenital or infantile nephrotic syndrome
Children with other kidney or systemic forms of nephrotic syndrome defined on kidney biopsy, clinical features or serology (e.g. idiopathic membranous glomerulonephritis, mesangiocapillary glomerulonephritis, post‐infectious glomerulonephritis, IgA vasculitis, systemic lupus erythematosus).
Types of interventions
Prednisone, prednisolone, or other corticosteroid medication given orally or intravenously (IV) without additional non‐corticosteroid medications, including but not limited to alkylating agents, calcineurin inhibitors, mycophenolic acid derivatives, levamisole, Chinese medications, montelukast and monoclonal Anti‐CD20 antibodies.
The following aspects of the corticosteroid regimens were considered.
Shorter durations compared with two months or more of corticosteroid treatment
Longer durations compared with two months or less of corticosteroid treatment
Comparisons of different doses of corticosteroid medication given for induction of a remission
Comparisons of other regimens of corticosteroid therapy
Different corticosteroid agents (e.g. deflazacort, methylprednisolone) compared with standard agents (e.g. prednisone, prednisolone)
Comparisons of daily, alternate‐day or intermittent administration of corticosteroid medication. Intermittent administration refers to the administration of corticosteroids on three consecutive days out of seven days
Single daily dose compared with divided daily doses of corticosteroid medication.
Types of outcome measures
Primary outcomes
The numbers of children with and without relapse at six to 24 months or more after completion of treatment.
The number of children who developed frequently relapsing nephrotic syndrome (FRNS) or steroid‐dependent nephrotic syndrome (SDNS).
Secondary outcomes
Mean relapse rates
Serious adverse events, including psychological disturbances, hypertension, Cushing's Syndrome, eye complications (e.g. cataracts, glaucoma), infections, reduced growth rates, thromboses and osteoporosis
Cumulative corticosteroid dosage.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 9 July 2024 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Searches of kidney and transplant journals and the proceedings and abstracts from major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Monthly searches of the International Clinical Trials Registry Platform (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies, and clinical practice guidelines.
Contacting relevant individuals/organisations seeking information about unpublished or incomplete studies.
Conference proceedings of meetings of the International Pediatric Nephrology Association and European Society for Paediatric Nephrology.
Data collection and analysis
Selection of studies
The initial review was undertaken by four authors. The titles and abstracts were screened by two authors who discarded studies that were not relevant (i.e. studies of lipid‐lowering agents), although studies and reviews that could have included relevant data or information on studies were retained initially. Three authors independently assessed abstracts and, if necessary, the full text to determine which studies satisfied the characteristics required for inclusion. Updates in 2003, 2005, 2007 and 2015 were undertaken by three or four authors (DH, EH, NW, JC). The 2020 update was undertaken by three authors (DH, SS, EH), with a final review by two other authors (NW and JC). This 2024 update was undertaken by three authors (EH, DH, NW), with a final review by two authors (SS, JC).
Data extraction and management
Two authors used standardised data extraction forms to extract data and assess the risk of bias. Studies in languages other than English were translated before data extraction. Where more than one report of a study was identified, data were extracted from all reports. Where there were discrepancies between reports, data from the primary source was used. Study authors were contacted for additional information about studies where possible.
Assessment of risk of bias in included studies
For this update, the following items were assessed independently by two authors using the risk of bias assessment tool (Higgins 2022) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Was incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. relapse or no relapse, adverse events), the risk ratio (RR) for individual studies was calculated with 95% CI. Where continuous scales of measurement were used to assess the effects of treatment (e.g. cumulative steroid therapy, relapse rate), these data were analysed as mean differences (MD) or standardised mean difference (SMD) if different scales had been used. The time to relapse was not included since many children did not experience relapse, so the data would be biased.
Unit of analysis issues
Data from cross‐over studies were included in the meta‐analyses if separate data for the first part of the study were available. Otherwise, the results of cross‐over studies were reported in the text only.
Dealing with missing data
We aimed to analyse available data in meta‐analyses using intention‐to‐treat (ITT) data. However, where ITT data were not provided or additional information could not be obtained from authors, available published data were used in the analyses.
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. We then quantified statistical heterogeneity using the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). The following is a guide to the interpretation of I2 values.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P value from the Chi2 test or a 95% CI for I2) (Higgins 2022).
Assessment of reporting biases
The search strategy used aimed to reduce publication bias caused by lack of publication of studies with negative results. Where there were several publications of the same study, all reports were reviewed to ensure that all details of methods and results were included to reduce the risk of selective outcome reporting bias.
Data synthesis
Data were combined using the random‐effects model for dichotomous and continuous data.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to investigate between‐study differences based on the risk of bias, differences between definitions of FRNS and different durations of treatment in the experimental group in studies of initial treatment with different durations of prednisone.
Sensitivity analysis
Where a single study differed considerably from the other studies in the meta‐analysis, it was temporarily excluded to determine whether its removal altered the meta‐analysis's results.
Summary of findings and assessment of the certainty of the evidence
We have presented the main results of the review in summary of findings tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2022a). The summary of findings tables also include an overall grading of the evidence related to each of the main outcomes using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2022b). We presented the following outcomes in the summary of findings tables.
Number with relapse
Number with frequent relapse (total and stratified for risk of bias)
Adverse effects (psychological disturbances, hypertension, Cushing's Syndrome, eye complications, infections).
Results
Description of studies
The following section contains broad descriptions of the studies considered in this review. For further details on each individual study, see, Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
For this update, we searched the Cochrane Kidney and Transplant Register of Studies (up to 9 July 2024) and identified 21 new reports. Four new studies (five reports) were included, and eight studies (eight reports) were excluded. We also identified two new reports of two existing included studies and one report of an existing ongoing study. We reassessed and reclassified two ongoing studies (five new reports) as included studies (Kainth 2021; PREDNOS 2 2022).
A total of 54 studies were included (103 reports, 4670 randomised children), 10 studies were excluded, and there are four ongoing studies.
Search results are shown in Figure 1.
1.
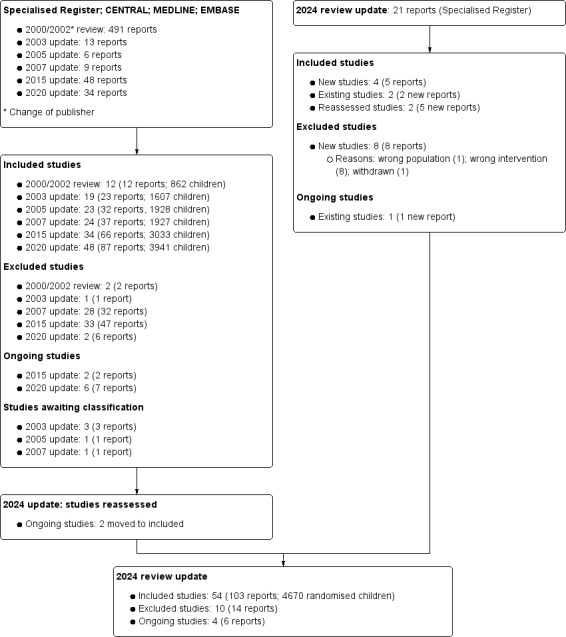
Flow diagram for 2024 review update study selection
Included studies
The majority of the studies were performed in South Asia (23 studies), Europe (14 studies), Asia (9 studies), and other regions, including the Middle East, South America and the USA (8 studies).
The number of children randomised ranged from 11 to 271 (median number: 66).
The 54 included studies were divided into groups according to the comparisons of corticosteroid regimens. Most studies used prednisone or prednisolone. For ease of reading, the term "prednisone" has been used in the text for both medications.
Three months or more versus two months of therapy in the initial episode of SSNS
Thirteen studies (1465 randomised children) compared durations of two months with three months or more of prednisone therapy (APN 1993; Bagga 1999; Jayantha 2000; Ksiazek 1995 (groups 1 and 3); Moundekhel 2012; Norero 1996; Paul 2014; PREDNOS 2019; PREDNOS PILOT 2019; Satomura 2001; Ueda 1988; Yoshikawa 1998; Yoshikawa 2015). Except for Satomura 2001, all these studies increased the duration of treatment, resulting in an increased total prednisone dose compared with the control group. Satomura 2001 compared three months of treatment with two months using the same total dose of prednisone in each group. In Ksiazek 1995, which compared three different regimens, data from the two‐month therapy group (group 3) and the group treated for six months (group 1) were included in the meta‐analysis. Norero 1996 excluded children who became steroid‐dependent. In this update, Yoshikawa 1998, which compared two months of prednisone with 4.5 months with both groups receiving the Chinese herb, Sairei‐to, was included in this analysis on the assumption that the effect of the herb would be the same in both treatment groups. Data from Paul 2014 could not be included in meta‐analyses because of differential loss to follow‐up, with loss to follow‐up of 15/47 children (33%) in the 12‐week treatment group compared with 6/46 children (13%) in the eight‐week treatment group.
Five to seven months versus three months of therapy in the initial episode of SSNS
Ten studies (1142 randomised children) compared five to seven months with three months of prednisone therapy (Al Talhi 2018; Anand 2013; Hiraoka 2003; Jamshaid 2022; Ksiazek 1995 (groups 1 and 2); Mishra 2012; Pecoraro 2003; Sharma 2002; Sinha 2015; Teeninga 2013). Anand 2013 (60 children) did not report the number of children treated in each group, so data from only nine studies could be included in the meta‐analyses. Increased duration of prednisone treatment led to increased total prednisone dose compared with the three‐month group in all studies except Teeninga 2013, who compared three months with six months therapy, using the same total dose of prednisone in both groups. From Ksiazek 1995, data from the groups treated for three months (group 2) and six months (group 1) were included in this analysis. Pecoraro 2003 included three groups ‐ a control group treated for three months and two groups treated for six months with different total doses of prednisone. Only the control group and treatment group 1 (total prednisone dose 5235 mg/m2) were included in the meta‐analysis.
Daily prednisone treatment during viral infections in children with relapsing SSNS
Five studies (503 randomised children) evaluated the effect of daily prednisone for five to seven days at the onset of infection to prevent relapse (Abeyagunawardena 2008; Abeyagunawardena 2017; Gulati 2011; Mattoo 2000; PREDNOS 2 2022). Three studies (Abeyagunawardena 2008; Gulati 2011; Mattoo 2000) compared daily with alternate‐day prednisone to prevent relapse during viral infections in children with SSNS. Abeyagunawardena 2017 compared daily prednisone with a placebo to prevent relapse during viral upper respiratory tract infections (URTI) in children not receiving prednisone. PREDNOS 2 2022 included four groups of children with SSNS, as shown below.
Not on alternate‐day prednisone
Receiving alternate‐day prednisone
Receiving alternate‐day prednisone and other immunosuppressive agents
Receiving other immunosuppressive agents but not alternate‐day prednisone.
Deflazacort versus prednisone therapy in children with relapsing or an initial episode of SSNS
Four studies (118 randomised children) explored different deflazacort regimens versus prednisone.
Agarwal 2010 compared deflazacort with prednisone in children with the initial episode of SSNS, but the details of the intervention were not reported.
Broyer 1997 compared deflazacort with an equivalent dose of prednisone with reducing doses over 12 months in children with steroid‐dependent SSNS.
Liern 2008 compared deflazacort with methylprednisolone for 12 weeks in children with relapsing SSNS in a cross‐over study.
Singhal 2015 compared deflazacort with prednisone for 12 weeks in children with the initial episode of SSNS.
Oral methylprednisolone regimens in children with the initial episode of SSNS
Three studies (113 randomised children) compared different regimens of methylprednisolone with prednisone.
Imbasciati 1985 compared six months of treatment commencing with methylprednisolone and then prednisone with six months of prednisone.
Mocan 1999 compared 14 days of high‐dose methylprednisolone with six months of prednisone.
Zhang 2007d compared six months of treatment involving methylprednisolone with six months of prednisone. The details of interventions were not reported.
One versus two months of therapy in the initial episode of SSNS
APN 1988 (61 randomised children) compared less than two months of prednisone with two months.
Five versus 12 months of therapy in the initial episode of SSNS
Kleinknecht 1982 (58 randomised children) compared five months of prednisone with 12 months; the timing of the follow‐up period in relation to the duration of initial therapy was not reported.
Different total doses of prednisone given for three months in the initial episode of SSNS
Hiraoka 2000 (68 randomised children) compared a higher dose of prednisone given for three months with a conventional dose.
Alternate‐day versus intermittent therapy in relapsing SSNS
APN 1981 (64 randomised children) compared an alternate‐day prednisone regimen with a three out of seven‐day regimen to maintain remission.
Daily versus intermittent therapy in relapsing SSNS
ISKDC 1979 (64 randomised children) compared a daily prednisone regimen with a three out of seven‐day regimen to maintain remission.
Intravenous then oral therapy versus oral therapy alone
Imbasciati 1985 (64 children) compared IV methylprednisolone for three days, then oral prednisone (daily for four weeks, then alternate‐day for four months) with oral prednisone alone.
Single versus multiple daily doses in relapsing nephrotic syndrome
Ekka 1997, Khan 2023, Li 1994 and Weerasooriya 2023 (314 randomised children) compared a single daily dose of prednisone with two or three times/day dosing to achieve remission.
Low versus conventional dose prednisone in relapsing nephrotic syndrome
Five studies (303 randomised children) compared low versus conventional dose prednisone in relapsing nephrotic syndrome.
Borovitz 2020 compared two reduced daily doses (1 mg/kg/day; 1.5 mg/kg/day) with conventional daily dose of prednisone (2 mg/kg/day) to achieve remission.
Sheikh 2019 compared a reduced daily dose (1 mg/kg/day) of prednisone with a conventional daily dose (2 mg/kg/day) to achieve remission.
Tu 2022 compared a reduced daily dose (30 mg/m2/day) of prednisone with a conventional daily dose of prednisone (60 mg/m2/day) to achieve remission.
Kansal 2019 compared different alternate‐day prednisone doses in the second month of initial treatment to maintain remission.
Kainth 2021 compared two weeks of alternate‐day prednisone (40 mg/m2) with four weeks after participants achieved remission with a conventional daily dose of prednisone (60 mg/m2/day).
Daily versus alternate‐day prednisone in relapsing nephrotic syndrome
Yadav 2019 (62 randomised children) compared daily with alternate‐day prednisone for one year in children with frequently relapsing SSNS
Weight‐based versus body surface area‐based dosing of prednisone in the initial episode of SSNS
Two studies (160 randomised children) compared weight‐based dosing with body surface area (BSA)‐based dosing in children with their initial episode of SSNS and with a relapse of SSNS (Basu 2020; Raman 2016).
Alternate‐day prednisone for four weeks versus an eight‐week weaning regimen in relapsing nephrotic syndrome
PROPINE 2020 (78 randomised children) compared 18 doses of prednisone (40 mg/m2) given on alternate days over 36 days with a tapering dose (36 doses) over 72 days using the same cumulative prednisone dose in each group.
Three months or more versus two months of therapy in relapsing nephrotic syndrome
Jayantha 2002b (129 randomised children) compared two months of prednisone with seven months in children with relapsing nephrotic syndrome.
Cortisol addition to prednisone regimen versus no cortisol addition in relapsing nephrotic syndrome
Leisti 1978 (13 randomised children) compared the addition of cortisol supplementation with no cortisol in children with relapsing nephrotic syndrome and a subnormal response to a 2‐hour ACTH test one to 12 days after completing prednisone.
Excluded studies
In this update, eight (eight reports) new studies were excluded. One study (Xu 2020b) was abandoned due to a lack of funding. Five studies investigated prednisone therapy together with Chinese medications (Hou 2021; Wu 2022; Yang 2022a), montelukast (Javidi 2021) or vitamin D (Zhou 2021). Two studies (Zhang 2021b; Zhu 2021a) used intensive regimens in difficult‐to‐manage children, including children with SRNS.
In total, 10 studies were excluded (APN 2006; Hou 2021; Javidi 2021; Wu 2022; Xu 2020b; Yang 2022a; Zhang 2014; Zhang 2021b; Zhou 2021; Zhu 2021a).
Ongoing studies
There are four ongoing studies.
CTRI/2018/05/013634 will compare alternate‐day prednisone (1 mg/kg versus 1.5 mg/kg) for relapse in children with nephrotic syndrome.
CTRI/2018/05/014075 will compare 1 mg/kg/day versus 2 mg/kg/day till remission in children with SSNS presenting with relapse.
RESTERN 2017 will compare daily prednisone till remission, then alternate days for two weeks versus six weeks in children with SSNS presenting with relapse.
Sinha 2016 will compare tapering prednisone over 12 weeks versus stop therapy in children initially treated with standard therapy for 12 weeks.
Risk of bias in included studies
Risk of bias assessments were performed using Cochrane's risk of bias assessment tool (Appendix 2). Figure 2 summarises the overall risks of bias in the studies, and Figure 3 reports the risks of bias in each individual study.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randon sequence generation
Random sequence generation was considered at low risk of bias in 31 studies (Abeyagunawardena 2008; Abeyagunawardena 2017; Agarwal 2010; APN 1993; Bagga 1999; Basu 2020; Broyer 1997; Gulati 2011; Hiraoka 2003; Imbasciati 1985; Jayantha 2000; Jayantha 2002b; Kainth 2021; Khan 2023; Kleinknecht 1982; Liern 2008; Mishra 2012; PREDNOS 2019; PREDNOS PILOT 2019; PREDNOS 2 2022; PROPINE 2020; Raman 2016; Sharma 2002; Sheikh 2019; Singhal 2015; Sinha 2015; Teeninga 2013; Weerasooriya 2023; Yadav 2019; Yoshikawa 1998; Yoshikawa 2015) and high risk in seven studies (Borovitz 2020; Li 1994; Mattoo 2000; Mocan 1999; Moundekhel 2012; Pecoraro 2003; Satomura 2001). Sequence generation methods were assessed as unclear in the remaining 16 studies.
Allocation concealment
Allocation concealment was considered to be at low risk of bias in 28 studies (Abeyagunawardena 2008; Abeyagunawardena 2017; Al Talhi 2018; APN 1981; APN 1988; APN 1993; Bagga 1999; Basu 2020; Broyer 1997; Gulati 2011; Hiraoka 2003; Imbasciati 1985; Kainth 2021; Khan 2023; Kleinknecht 1982; Liern 2008; PREDNOS 2019; PREDNOS PILOT 2019; PREDNOS 2 2022; PROPINE 2020; Raman 2016; Sheikh 2019; Sinha 2015; Teeninga 2013; Weerasooriya 2023; Yadav 2019; Yoshikawa 1998; Yoshikawa 2015) and at high risk of bias in nine studies (Borovitz 2020; Ksiazek 1995; Li 1994; Mattoo 2000; Mocan 1999; Moundekhel 2012; Norero 1996; Pecoraro 2003; Satomura 2001). Ksiazek 1995 stated that parents could influence which treatment group their child was assigned. Allocation concealment methods were assessed as unclear in the remaining 17 studies.
Blinding
Ten studies were considered to be at low risk of performance bias because they were placebo‐controlled studies (Abeyagunawardena 2008; Abeyagunawardena 2017; Broyer 1997; Leisti 1978; Liern 2008; PREDNOS 2019; PREDNOS PILOT 2019; PREDNOS 2 2022; Sinha 2015; Teeninga 2013). These ten studies, together with Basu 2020 and Yoshikawa 2015 were at low risk of detection bias. Basu 2020 and Yoshikawa 2015 were open‐label studies, so they were at high risk of performance bias but were at low risk of detection bias. For Kansal 2019, the risk for both performance and detection bias was unclear. The remaining studies were at high risk of both performance and detection bias. Most studies reported the primary outcome of relapse using the ISKDC definition of relapse (ISKDC 1970).
Incomplete outcome data
We assessed 27 studies to be at low risk of attrition bias because they reported fewer than 10% of participants lost to follow‐up or excluded from analysis (Al Talhi 2018; APN 1993; Bagga 1999; Basu 2020; Borovitz 2020; Broyer 1997; Hiraoka 2000; Hiraoka 2003; Imbasciati 1985; Kainth 2021; Khan 2023; Ksiazek 1995; Leisti 1978; Mattoo 2000; Mishra 2012; PREDNOS 2019; PREDNOS 2 2022; PREDNOS PILOT 2019; PROPINE 2020; Raman 2016; Sheikh 2019; Singhal 2015; Sinha 2015; Teeninga 2013; Tu 2022; Yadav 2019; Yoshikawa 2015). Fourteen studies were considered at high risk of attrition bias because 10% or more of participants were lost to follow‐up or excluded from the analysis (Abeyagunawardena 2008; Abeyagunawardena 2017; APN 1981; APN 1988; Ekka 1997; Gulati 2011; ISKDC 1979; Jayantha 2000; Jayantha 2002b; Mocan 1999; Norero 1996; Paul 2014; Sharma 2002; Yoshikawa 1998). The remaining 13 studies were considered to be unclear risk of attrition bias.
Selective reporting
Studies were deemed to be at risk of reporting bias if outcome data did not include one or more outcomes of FRNS, relapse rate and adverse events. Studies were also considered to be at high risk of bias if data were provided in a format which could not be entered into the meta‐analyses. Cross‐over studies were considered to be at high risk of bias if data from the first and second parts of the study were not separable. Twenty‐six studies were at low risk of reporting bias (Al Talhi 2018; APN 1981; APN 1993; Bagga 1999; Basu 2020; Broyer 1997; Ekka 1997; Gulati 2011; Hiraoka 2000; Hiraoka 2003; Imbasciati 1985; Jayantha 2000; Kainth 2021; Norero 1996; PREDNOS 2019; PREDNOS 2 2022; PREDNOS PILOT 2019; PROPINE 2020; Sharma 2002; Singhal 2015; Sinha 2015; Teeninga 2013; Tu 2022; Yadav 2019; Yoshikawa 2015; Ueda 1988). There were 22 studies at high risk of selective reporting bias (Abeyagunawardena 2008; Abeyagunawardena 2017; APN 1988; Borovitz 2020; ISKDC 1979; Jamshaid 2022; Jayantha 2002b; Khan 2023; Kleinknecht 1982; Ksiazek 1995; Leisti 1978; Li 1994; Liern 2008; Mattoo 2000; Mocan 1999; Moundekhel 2012; Paul 2014; Pecoraro 2003; Raman 2016; Sheikh 2019; Weerasooriya 2023; Yoshikawa 1998). The remaining six studies were at unclear risk of selective reporting bias.
Other potential sources of bias
Nineteen studies were considered at low risk of potential bias as they were funded by educational or philanthropic organisations or stated that they received no funding (Abeyagunawardena 2008; APN 1981; APN 1988; Bagga 1999; Basu 2020; Gulati 2011; Kainth 2021; Khan 2023; Leisti 1978; Norero 1996; PREDNOS 2019; PREDNOS 2 2022; PREDNOS PILOT 2019; PROPINE 2020; Sinha 2015; Teeninga 2013; Ueda 1988; Yadav 2019; Yoshikawa 2015). One study was considered to be at high risk of bias as it was funded by industry, and no full‐text publication was identified 10 years after the first conference abstract (Pecoraro 2003). The remaining 34 studies were deemed unclear of other risks of bias as no information on funding sources was provided.
In Ueda 1988, the calculated total protocol dose (4620 mg/m2) exceeded the dose administered (3132 ± 417 mg/m2), suggesting that the protocol was not adhered to in all patients. In three studies (Jayantha 2000; Ksiazek 1995; Ueda 1988), the numbers of children in the treatment and control groups differed markedly.
Effects of interventions
Three months or more versus two months of therapy in the initial episode of SSNS
Therapy for three months or more probably makes little or no difference to the number of children with frequent relapses by 12 to 24 months compared to two months of therapy (Analysis 1.1: RR 0.86, 95% CI 0.71 to 1.06; 976 children, 8 studies; I2 = 33%; moderate certainty evidence).
-
Therapy for three months or more may reduce the number of children relapsing by 12 to 24 months (Analysis 1.2: RR 0.80, 95% CI 0.68 to 0.96; 1279 children, 12 studies; I2 = 70%; low certainty evidence).
In subgroup analysis of studies at low risk of selection bias, there is little or no difference in the number with frequent relapses between the two groups (Analysis 1.3.1: RR 0.96, 95% CI 0.83 to 1.10; 755 children; 5 studies; I2 = 0%) or the number of children relapsing by 12 to 24 months (Analysis 1.4.1: RR 0.93, 95% CI 0.81 to 1.06; 808 children; 6 studies; I2 = 36%) (high certainty evidence) (Figure 4).
In contrast, in subgroup analysis of studies at unclear or high risk of selection bias, longer duration of prednisone therapy probably reduces the number of children with frequent relapses (Analysis 1.3.2: RR 0.45, 95% CI 0.26 to 0.77; 220 children, 3 studies; I2 = 0%) (moderate certainty evidence) and the number of children relapsing by 12 to 24 months (Analysis 1.4.2: RR 0.69, 95% CI 0.49 to 0.98; 471 children, 6 studies; I2 = 72%).
Similar differences in results were shown when data were stratified according to risk of bias for detection and performance bias or for attrition bias (data not shown).
There may be little or no difference in adverse events between the two groups (Analysis 1.5) (low or moderate certainty evidence). In Yoshikawa 2015, results were reported as events, not patients, so they could not be included in the meta‐analyses. The authors reported that the frequency and severity of adverse events were similar in both groups.
1.1. Analysis.

Comparison 1: Steroid therapy in first episode: ≥ 3 months versus 2 months, Outcome 1: Number with frequent relapses by 12 to 24 months
1.2. Analysis.

Comparison 1: Steroid therapy in first episode: ≥ 3 months versus 2 months, Outcome 2: Number relapsing by 12 to 24 months
1.3. Analysis.

Comparison 1: Steroid therapy in first episode: ≥ 3 months versus 2 months, Outcome 3: Number with frequent relapses by 12 to 24 months: stratified by risk of selection bias
1.4. Analysis.

Comparison 1: Steroid therapy in first episode: ≥ 3 months versus 2 months, Outcome 4: Number relapsing by 12 to 24 months: stratified by risk of selection bias
4.

Forest plot of comparison: 1 Steroid therapy in first episode: ≥ 3 months versus 2 months therapy, outcome: 1.3 Number with frequent relapses by 12 to 24 months stratified by risk of bias for selection bias.
1.5. Analysis.

Comparison 1: Steroid therapy in first episode: ≥ 3 months versus 2 months, Outcome 5: Adverse events
Results were downgraded for medium to high levels of heterogeneity between studies and for risk of bias issues (Table 1). The heterogeneity between studies was explained by the risk of bias issues (Analysis 1.3.1; Analysis 1.3.2; Analysis 1.4.1; Analysis 1.4.2) but not by the inclusion or exclusion of patients with steroid‐dependent disease, different durations of prednisone (two months versus three months or more) or different definitions of FRNS (ISKDC definition compared with other definitions) (data not shown).
Five to seven months versus three months of therapy in the initial episode of SSNS
Five to seven months of therapy probably makes little or no difference to the number of children with frequent relapses by 12 to 24 months compared to three months of therapy (Analysis 2.1: RR 0.73, 95% CI 0.49 to 1.09; 707 children, 6 studies; I2 = 68%; moderate certainty evidence).
-
Five to seven months of therapy may reduce the number of children relapsing by 12 to 24 months compared to three months of therapy (Analysis 2.2: (RR 0.64, 95% CI 0.50 to 0.81; 912 children; 8 studies; I2 = 80%; low certainty evidence).
In subgroup analysis of studies at low risk of selection bias, there is little or no difference in the number with frequent relapses (Analysis 2.3.1: RR 0.99, 95% CI 0.74 to 1.33; 376 children, 3 studies; I2 = 35%; high certainty evidence) or in the number relapsing by 12 to 24 months (Analysis 2.4.1: RR 0.88, 95% CI 0.69 to 1.11; 376 children, 3 studies; I2 = 53%) (Figure 5).
In contrast, in subgroups of studies at high or unclear risk of selection bias, five to seven months of therapy probably reduces the risk of FRNS (Analysis 2.3.2: RR 0.48, 95% CI 0.32 to 0.72; 330 children, 3 studies; I2 = 0% moderate certainty evidence) and the number relapsing by 12 to 24 months (Analysis 2.4.2: RR 0.53, 95% CI 0.41 to 0.69; 536 children; 5 studies; I2 = 60%).
Similar differences in results were shown when data were stratified according to risk of bias for detection and performance bias or for attrition bias (data not shown).
There may be little or no difference in adverse events, including psychological disorders, growth retardation, hypertension, cataracts/glaucoma, osteoporosis, infections or Cushingoid features (Analysis 2.5; low or moderate certainty evidence).
Anand 2013 reported that the number relapsing at 12 months was lower with six months of prednisone compared with three months. Data could not be included in the meta‐analysis as the numbers in each group were not provided.
2.1. Analysis.

Comparison 2: Steroid therapy in first episode: 5 to 7 months versus 3 months, Outcome 1: Number with frequent relapses by 12 to 24 months
2.2. Analysis.

Comparison 2: Steroid therapy in first episode: 5 to 7 months versus 3 months, Outcome 2: Number relapsing by 12 to 24 months
2.3. Analysis.

Comparison 2: Steroid therapy in first episode: 5 to 7 months versus 3 months, Outcome 3: Number with frequent relapses: stratified by risk of selection bias
2.4. Analysis.

Comparison 2: Steroid therapy in first episode: 5 to 7 months versus 3 months, Outcome 4: Number relapsing by 12 to 24 months: stratified by risk of selection bias
5.

Forest plot of comparison: 2 Steroid therapy in first episode: 5 to 7 months versus 3 months, outcome: 2.3 Number with frequent relapses stratified by risk of selection bias.
2.5. Analysis.

Comparison 2: Steroid therapy in first episode: 5 to 7 months versus 3 months, Outcome 5: Adverse events
Results were downgraded for medium to high levels of heterogeneity between studies and for risk of bias issues (Table 2). The heterogeneity between studies was explained by the risk of bias issues (Analysis 2.3.1; Analysis 2.3.2; Analysis 2.4.1; Analysis 2.4.2) but not by inclusion or exclusion of patients with steroid‐dependent disease, different durations of prednisone (three months versus five to seven months), or different definitions of FRNS (ISKDC definition compared with other definitions) (Data not shown).
One versus two months of therapy in the initial episode of SSNS
APN 1988 reported two months of therapy compared to one month may reduce the risk of relapse at six to 12 months (Analysis 3.1: RR 1.60, 95% CI 1.01 to 2.54; 61 children) and 12 to 24 months (Analysis 3.2: RR 1.46, 95% CI 1.01 to 2.12; 60 children).
APN 1988 reported two months of therapy compared to one month had uncertain effects on the number of children with frequent relapses (Analysis 3.3: RR 1.48, 95%CI 0.85 to 2.59; 61 children).
3.1. Analysis.

Comparison 3: Steroid therapy in the first episode: 1 month versus 2 months, Outcome 1: Number relapsing by 6 to 12 months
3.2. Analysis.

Comparison 3: Steroid therapy in the first episode: 1 month versus 2 months, Outcome 2: Number relapsing by 12 to 24 months
3.3. Analysis.

Comparison 3: Steroid therapy in the first episode: 1 month versus 2 months, Outcome 3: Number with frequent relapses
Twelve versus five months of therapy in the initial episode of SSNS
Ksiazek 1995 reported that 12 months of therapy compared to five months had uncertain effects on the number of children relapsing (Analysis 4.1: RR 0.76, 95% CI 0.51 to 1.13; 58 children).
4.1. Analysis.

Comparison 4: Steroid therapy in the first episode: 12 months versus 5 months, Outcome 1: Number with relapse
Different total doses of prednisone given for three months in the initial episode of SSNS
Hiraoka 2000 reported a higher dose may reduce the number of children relapsing by 12 months (Analysis 5.1: RR 0.65, 95% CI 0.43 to 0.98; 60 children) but may make little or no difference to the number with frequent relapses (Analysis 5.2: RR 0.69, 95% CI 0.35 to 1.37; 60 children).
Hiraoka 2000 reported psychological disorders, hypertension, and Cushing's Syndrome may not differ between the groups (Analysis 5.3).
5.1. Analysis.

Comparison 5: Steroid therapy in the first episode: different total doses given over the same duration, Outcome 1: Relapse at 12 months
5.2. Analysis.

Comparison 5: Steroid therapy in the first episode: different total doses given over the same duration, Outcome 2: Number with FRNS
5.3. Analysis.

Comparison 5: Steroid therapy in the first episode: different total doses given over the same duration, Outcome 3: Adverse events
Oral methylprednisolone in children with relapsing or initial episodes of SSNS
Methylprednisolone, compared with prednisolone, may reduce the time to remission (Analysis 6.1: MD ‐5.54 days, 95% CI ‐8.46 to ‐2.61; 38 children, 2 studies; I2 = 0%).
Imbasciati 1985 reported methylprednisolone, compared with prednisolone, may make little or no difference to the number of children who relapse (Analysis 6.2: RR 1.00, 95% CI 0.75 to 1.52; 62 children).
6.1. Analysis.

Comparison 6: Steroid therapy in first episode: methylprednisone versus prednisolone, Outcome 1: Time to remission [days]
6.2. Analysis.

Comparison 6: Steroid therapy in first episode: methylprednisone versus prednisolone, Outcome 2: Number with relapse
Daily prednisone treatment during viral infections in children with relapsing or initial episodes of SSNS
Daily prednisone at the onset of URTI compared with placebo probably makes little or no difference to the risk of relapse (Analysis 7.1.1: RR 0.81, 95% CI 0.44 to 1.49; 310 children; 2 studies; I2 = 45%) (moderate certainty evidence).
-
Subgroup analyses
PREDNOS 2 2022 reported that in those not initially receiving any prednisone, administering daily prednisone at the onset of URTI may make little or no difference to the risk of relapse (Analysis 7.1.2: RR 1.11, 95% CI 0.72 to 1.71; 60 children).
In children receiving alternate‐day prednisone, changing to daily prednisone at the onset of URTI may make little or no difference to the risk of relapse (Analysis 7.1.3: RR 0.75, 95% CI 0.47 to 1.19; 110 children; 2 studies; I2 = 0%).
PREDNOS 2 2022 reported that in those receiving alternate‐day prednisone and other immunosuppressive agents, changing to daily prednisone at the onset of URTI may make little or no difference to the risk of relapse (Analysis 7.1.4: RR 0.86, 95% CI 0.50 to 1.50; 89 children).
PREDNOS 2 2022 reported that in those receiving other immunosuppressive agents without prednisone, commencing daily prednisone at the onset of URTI may make little or no difference to the risk of relapse (Analysis 7.1.5: RR 1.01, 95% CI 0.44 to 2.28; 43 children).
Gulati 2011 reported daily prednisone therapy, administered at the onset of URTI, may reduce the infection‐related relapses/patient‐years (Analysis 7.2.1: MD ‐0.70, 95% CI ‐0.87 to ‐0.53; 95 children) and the total number of relapses/patient/year (Analysis 7.2.2: MD ‐0.90, 95% CI ‐1.08 to ‐0.72; 95 children).
Mattoo 2000 reported daily prednisone, administered at the onset of URTI, may reduce total relapse episodes/patient at two years compared with alternate‐day prednisone (Analysis 7.3: MD ‐3.30, 95% CI ‐4.03 to ‐2.57; 36 children).
In a cross‐over study in children who had not received alternate‐day prednisone for at least three months, Abeyagunawardena 2017 reported daily prednisone administered at the onset of URTI resulted in 11 relapses associated with 115 episodes of URTI in 33 children compared with 25 relapses associated with 101 episodes of URTI in 33 children completing two years.
7.1. Analysis.

Comparison 7: Daily prednisolone treatment during viral infections, Outcome 1: Number with relapse with infection
7.2. Analysis.

Comparison 7: Daily prednisolone treatment during viral infections, Outcome 2: Number of relapses/patient
7.3. Analysis.

Comparison 7: Daily prednisolone treatment during viral infections, Outcome 3: Number of relapses/patient at 2 years
Deflazacort versus prednisone therapy in children with relapsing or initial episodes of SSNS
Deflazacort, compared with prednisone, may make little or no difference to the number of children achieving remission (Analysis 8.1: RR 1.08, 95% CI 0.94 to 1.24; 67 children, 2 studies; I2 = 0%).
Deflazacort, compared with prednisone, may reduce the number of children with relapses by nine to 12 months (Analysis 8.2: RR 0.46, 95% CI 0.27 to 0.78; 63 children, 2 studies; I2 = 0%).
No differences in time to remission or time to relapse in 11 children treated with deflazacort or methylprednisolone were found in a cross‐over study by Liern 2008.
8.1. Analysis.

Comparison 8: Deflazacort versus prednisolone, Outcome 1: Number with remission
8.2. Analysis.

Comparison 8: Deflazacort versus prednisolone, Outcome 2: Number with relapse by 9 to 12 months
Alternate‐day or daily therapy versus intermittent therapy in relapsing SSNS
-
Number relapsing during therapy.
APN 1981 reported uncertain effects between alternate‐day therapy and intermittent therapy on the number of children relapsing during therapy (Analysis 9.1.1: RR 0.60, 95% CI 0.36 to 1.02; 48 children).
ISKDC 1979 reported daily therapy may reduce the number of children relapsing during therapy compared to intermittent therapy (Analysis 9.1.2: RR 0.20, 95% CI 0.05 to 0.82; 50 children)
-
Number with relapses by nine to 12 months.
APN 1981 reported little or no difference in the number of children with relapses by nine to 12 months between alternate‐day and intermittent therapy (Analysis 9.2.1: RR 1.20, 95% CI 0.93 to 1.55; 48 children).
ISKDC 1979 reported little or no difference in the number of children with relapses by nine to 12 months between daily and intermittent therapy (Analysis 9.2.2: RR 1.00, 95% CI 0.89 to 1.12; 50 children).
9.1. Analysis.

Comparison 9: Alternate‐day or daily steroid regimens versus intermittent dosing to prevent relapse, Outcome 1: Number relapsing during therapy
9.2. Analysis.

Comparison 9: Alternate‐day or daily steroid regimens versus intermittent dosing to prevent relapse, Outcome 2: Number with relapses by 9 to 12 months
Intravenous then oral therapy versus oral therapy alone
Imbasciati 1985 reported little or no difference in the number of children relapsing by six months between IV then oral therapy and oral therapy alone (Analysis 10.1: RR 1.06, 95% CI 0.75 to 1.52; 64 children).
10.1. Analysis.

Comparison 10: Intravenous then oral therapy versus oral therapy alone, Outcome 1: Relapse by 6 months
Single versus multiple daily doses in relapsing nephrotic syndrome
There may be little or no difference between single daily doses versus divided daily dosing in the number with relapse (Analysis 11.1: RR 1.05, 95% CI 0.77 to 1.42; 151 children; 2 studies; I2 = 0%)
Ekka 1997 reported little or no difference between single daily doses versus divided daily dosing in the mean relapse rate (Analysis 11.2: MD ‐0.20, 95% CI ‐0.64 to 0.24; 94 children).
There may be little or no difference in the mean time to remission between single daily doses and divided daily dosing (Analysis 11.3: MD 0.70 days, 95% CI ‐0.56 to 1.96; 242 children; 3 studies; I2 = 60%).
There may be little or no difference in the mean time to remission between single daily and divided daily dosing according to age group (Analysis 11.4)
Serious adverse events may be less common with single daily doses compared with divided daily dosing (Analysis 11.5: RR 0.41, 95% CI 0.18 to 0.91; 138 children, 2 studies; I2 = 0%).
Ekka 1997 reported no differences in the cumulative steroid dose between single daily doses versus divided daily dosing (Analysis 11.6: MD ‐0.05 mg/kg, 95% CI ‐0.68 to 0.58; 94 children).
Khan 2023 reported that the number of children with HPA suppression did not differ between groups (Analysis 11.7)
11.1. Analysis.

Comparison 11: Single versus divided daily doses of prednisone to prevent relapse, Outcome 1: Number with relapse
11.2. Analysis.

Comparison 11: Single versus divided daily doses of prednisone to prevent relapse, Outcome 2: Mean relapse rate
11.3. Analysis.

Comparison 11: Single versus divided daily doses of prednisone to prevent relapse, Outcome 3: Number of days to remission
11.4. Analysis.

Comparison 11: Single versus divided daily doses of prednisone to prevent relapse, Outcome 4: Number of days to remission according to age group
11.5. Analysis.

Comparison 11: Single versus divided daily doses of prednisone to prevent relapse, Outcome 5: Serious adverse events
11.6. Analysis.

Comparison 11: Single versus divided daily doses of prednisone to prevent relapse, Outcome 6: Cumulative steroid dose [mg/kg]
11.7. Analysis.

Comparison 11: Single versus divided daily doses of prednisone to prevent relapse, Outcome 7: Number with HPA suppression
Reduced versus standard prednisone doses in relapsing nephrotic syndrome
There may be little or no difference in time to remission between reduced (1 mg/kg) and standard prednisone doses (2 mg/kg) (Analysis 12.1: MD 0.72 days, 95% CI ‐0.43 to 1.88; 75 children; 2 studies; I2 = 11%).
There may be little or no difference in the number relapsing between reduced and standard prednisone doses (Analysis 12.2: RR 1.01, 95% CI 0.85 to 1.20; 177 children; 4 studies; I2 = 0%).
Kainth 2021 reported there may be little or no difference in the number developing FRNS or SDNS between reduced duration (two weeks) and standard duration (four weeks) of alternate‐day prednisone (Analysis 12.3: RR 0.97, 95% CI 0.49 to 1.91; 117 children).
Borovitz 2020 reported that compared to a dose of 2 mg/kg/day, the cumulative dose of prednisone to achieve remission may be less in children treated with a dose of 1 mg/kg/day (Analysis 12.4: MD ‐20.60 mg/kg, 95% CI ‐25.65 to ‐15.55; 20 children).
-
Adverse events
Tu 2022 reported that there may be no difference in growth or steroid adverse events with low versus conventional dose prednisone (Analysis 12.5).
Borovitz 2020 reported that none of the included children had treatment‐related complications.
Kansal 2019 reported that prednisone adverse events were more common in the standard dose group compared with the low dose group.
Kainth 2021 identified no differences in the number of days to relapse or in steroid adverse effects between reduced duration and standard duration of alternate‐day prednisone.
Sheikh 2019 did not provide any information on adverse effects.
12.1. Analysis.

Comparison 12: Reduced versus standard steroid doses to prevent relapse, Outcome 1: Time to remission
12.2. Analysis.

Comparison 12: Reduced versus standard steroid doses to prevent relapse, Outcome 2: Number with relapse
12.3. Analysis.

Comparison 12: Reduced versus standard steroid doses to prevent relapse, Outcome 3: Number with FRNS or SDNS at 12 months
12.4. Analysis.

Comparison 12: Reduced versus standard steroid doses to prevent relapse, Outcome 4: Cumulative prednisone dose to achieve remission [mg/kg]
12.5. Analysis.

Comparison 12: Reduced versus standard steroid doses to prevent relapse, Outcome 5: Adverse events
Daily versus alternate‐day prednisone in relapsing nephrotic syndrome
Yadav 2019 reported daily, compared with alternate‐day prednisone, may reduce the number of relapses during 12 months of therapy (Analysis 13.1: MD ‐0.90 relapses/year, 95% CI ‐1.33 to ‐0.47; 62 children).
Yadav 2019 reported there may be little or no difference in the frequency of Cushingoid facies or cataracts (Analysis 13.2).
13.1. Analysis.

Comparison 13: Daily versus alternate‐day prednisone to prevent relapse, Outcome 1: Number of relapses in 12 months [number/year]
13.2. Analysis.

Comparison 13: Daily versus alternate‐day prednisone to prevent relapse, Outcome 2: Adverse events
Short‐duration alternate‐day prednisone without taper of dose versus long‐duration alternate‐day prednisone with tapering dose in relapsing nephrotic syndrome
PROPINE 2020 reported administering alternate‐day prednisone for 36 days compared with a tapering dose given over 72 days using the same cumulative prednisone dose in each group may make little or no difference to the risk of relapsing during treatment (Analysis 14.1: RR 0.07, 95% CI 0.00 to 1.19; 78 children) or at six months (Analysis 14.2: RR 0.73, 95% CI 0.46 to 1.16; 78 children).
PROPINE 2020 reported there may be little or no difference in the frequency of viral infection, bacterial infection, or urticaria (Analysis 14.3).
14.1. Analysis.

Comparison 14: Short (36 days) versus long (72 days) duration alternate‐day prednisone to prevent relapse, Outcome 1: Number with relapse during treatment
14.2. Analysis.

Comparison 14: Short (36 days) versus long (72 days) duration alternate‐day prednisone to prevent relapse, Outcome 2: Number with relapse by 6 months
14.3. Analysis.

Comparison 14: Short (36 days) versus long (72 days) duration alternate‐day prednisone to prevent relapse, Outcome 3: Adverse events
Weight‐based versus body surface area‐based dosing of prednisone in relapsing nephrotic syndrome
Weight‐based dosing may make little or no difference to the number relapsing at six months compared to BSA‐based dosing (Analysis 15.1: RR 1.03, 95% CI 0.71 to 1.49; 146 children; 2 studies; I2 = 0%).
Weight‐based dosing may make little or no difference to the risk of adverse events compared to BSA‐based dosing (Cushingoid features, serious infections, eye changes, hypertension) (Analysis 15.2: 144 children; 2 studies).
Basu 2020 reported that the mean prednisone dose for both the induction dose over six months and the cumulative dose over six months was lower in the weight‐based dosing group compared with the BSA‐based dosing group (Analysis 15.3.1: ‐32.00 g/kg, 95% CI ‐57.21 to ‐6.79; Analysis 15.3.2: ‐30.00 g/kg, 95% CI ‐54.34 to ‐5.66).
15.1. Analysis.

Comparison 15: Weight‐based versus body surface area (BSA)‐based dosing of prednisolone, Outcome 1: Relapse at 6 months
15.2. Analysis.

Comparison 15: Weight‐based versus body surface area (BSA)‐based dosing of prednisolone, Outcome 2: Adverse events
15.3. Analysis.

Comparison 15: Weight‐based versus body surface area (BSA)‐based dosing of prednisolone, Outcome 3: Prednisone dose
Prolonged steroid therapy for children with relapsing SSNS
Jayantha 2002b reported seven months of prednisone may reduce the number of relapses at six months (Analysis 16.1.1: RR 0.04, 95% CI 0.01 to 0.25; 90 children), 12 months (Analysis 16.1.2: RR 0.43, 95% CI 0.29 to 0.65; 76 children) and 24 months (Analysis 16.1.3: RR 0.60, 95% CI 0.45 to 0.80; 64 children) compared to two months of therapy.
Jayantha 2002b reported the relapse rate/patient/year was lower with seven months of prednisone compared to two months at 12 months (Analysis 16.2.1: MD ‐1.78, 95% CI ‐2.30 to ‐1.26; 72 children), 24 months (Analysis 16.2.2: MD ‐1.79, 95% CI ‐2.39 to ‐1.19; 55 children), and 36 months (Analysis 16.2.3: ‐RR 1.74, 95% CI ‐2.39 to ‐1.09; 41 children).
Jayantha 2002b reported the number with FRNS or SDNS was lower with seven months of prednisone (Analysis 16.3: RR 0.43, 95% CI 0.19 to 0.95; 72 children) compared to two months of prednisone.
Jayantha 2002b reported the cumulative prednisone dose was lower at one year with two months of treatment (Analysis 16.4.1: MD 0.59 g/kg, 95% CI 0.02 to 1.16; 72 children), but there was little or no difference at two years (Analysis 16.4.2: MD ‐0.32 g/kg, 95% CI ‐1.52 to 0.88; 55 children) or three years (Analysis 16.4.3: MD ‐1.13, 95% CI ‐3.08 to 0.82; 41 children) compared to seven months of prednisone.
Jayantha 2002b reported there may be little or no difference in hypertension or growth failure between seven months and two months of prednisone (Analysis 16.5).
16.1. Analysis.

Comparison 16: Prolonged steroid therapy (7 months) for relapsing nephrotic syndrome, Outcome 1: Number with relapses
16.2. Analysis.

Comparison 16: Prolonged steroid therapy (7 months) for relapsing nephrotic syndrome, Outcome 2: Relapse rate/patient/year
16.3. Analysis.

Comparison 16: Prolonged steroid therapy (7 months) for relapsing nephrotic syndrome, Outcome 3: Number with FRNS or SDNS
16.4. Analysis.

Comparison 16: Prolonged steroid therapy (7 months) for relapsing nephrotic syndrome, Outcome 4: Cumulative steroid dose
16.5. Analysis.

Comparison 16: Prolonged steroid therapy (7 months) for relapsing nephrotic syndrome, Outcome 5: Adverse events
Cortisol supplementation in children with relapsing nephrotic syndrome and adrenocortical suppression
In a cross‐over study by Leisti 1978, cortisol substitution may result in fewer children with post‐prednisone adrenocortical suppression relapsing during a six‐month period. After three months of treatment, 5/13 children (38%) receiving cortisol had relapsed compared with 12/13 receiving placebo (92%) (Chi2 = 4.0, P = 0.05), and at six months, 9/13 children receiving cortisol had relapsed compared with 12/13 receiving placebo.
Discussion
Summary of main results
We have added five new included studies to this 2024 update, bringing the total number of included studies to 54, which randomised 4670 children.
Prednisone in the first episode of SSNS
In earlier iterations of this review (2000 to 2007), we concluded that prednisone administered for longer durations compared with two or three months reduced the risk of relapse and of FRNS in the initial episode of SSNS. In practice, considerable variation exists amongst paediatric nephrologists in the duration of prednisone used in the initial episode of nephrotic syndrome, reflecting in part the poor quality of the evidence from earlier RCTs (MacHardy 2009; Samuel 2013). The 2015 update of this review (Hahn 2015) included three well‐designed and adequately powered studies (Sinha 2015; Teeninga 2013; Yoshikawa 2015), which clearly demonstrated that there was no benefit of prolonging prednisone therapy beyond two or three months. In the 2020 update (Hahn 2020) a further well‐designed study (PREDNOS 2019) also concluded that there was no benefit of prolonging prednisone therapy beyond two months. In our analysis of factors which might account for the differences in results, we concluded that, in studies at low risk of selection or performance bias, we could identify no benefit of extending prednisone therapy beyond eight or 12 weeks. In contrast, studies at high risk for these biases found a benefit of longer durations of therapy. In the 2020 update we included four studies (Al Talhi 2018; Anand 2013; Moundekhel 2012; Paul 2014) which evaluated longer durations of prednisone compared with two or three months. All four studies concluded that there was a benefit from a longer duration of prednisone therapy. All four studies were at high risk of selection and performance bias. In this 2024 update, an additional study (Jamshaid 2022) also found that longer‐duration prednisone reduced the risk of relapse, but this study was at high risk of bias (selection bias, performance bias). Since there are already four well‐designed studies randomising 823 children with nephrotic syndrome, which clearly demonstrate that there is no benefit for durations of prednisone exceeding two or three months, resources should not be wasted on further studies to evaluate different durations of prednisone in the initial episode of SSNS.
Prednisone in relapsing SSNS
Four studies enrolling 204 children found that daily prednisone, administered at the onset of viral infections, compared with alternate‐day prednisone therapy, reduced the rate of relapse. However, a much larger study (PREDNOS 2 2022) involving 271 children, including both children already on alternate‐day and those not on alternate‐day prednisone, did not support this finding. This study was at low risk of bias for all attributes in contrast to the earlier studies (Figure 6), which were at high risk of bias and showed a benefit of changing to daily prednisone therapy at the onset of URTI. Studies at high risk of bias may overestimate the benefit of the intervention (Moher 1998; Schulz 1995). Although PREDNOS 2 2022 does not support changing from alternate to daily prednisone at the onset of a viral infection, such an approach might be considered in children with relapsing SSNS who are already taking low‐dose alternate‐day prednisone or considered to be at a greater risk of a URTI triggering relapse (IPNA 2023).
6.

Forest plot of comparison: 7 Daily prednisolone treatment during viral infections, outcome: 7.2 Number of relapses/patient.
In the 2020 update, nine small studies evaluated different interventions using prednisone in relapsing disease. Yadav 2019 (62 children) found that daily prednisone, compared with alternate‐day prednisone in children with FRNS, was associated with a reduced risk of relapse. Both the KDIGO 2012 and the KDIGO 2021 guidelines suggest that alternate‐day prednisone rather than daily prednisone should be used. Two studies (Basu 2020; Raman 2016) found no difference in the risk for relapse between weight‐based and surface area‐based dosing of prednisone. Two studies (Ekka 1997; Khan 2023) found no difference in time to remission using a single daily dose or multiple daily doses of prednisone. Many clinicians have tapered the dose of prednisone before discontinuing it. PROPINE 2020 found no differences in the risk for relapse or in adverse events in children managed without tapering of the dose of alternate‐day prednisone compared with slow tapering of the dose. The KDIGO 2021 and IPNA 2023 guidelines on SSNS in children conclude that tapering the alternate‐day dose before cessation of prednisone is not necessary and that prednisone can be given as a single daily dose.
Five small studies (Borovitz 2020; Kainth 2021; Kansal 2019; Sheikh 2019; Tu 2022) evaluated regimens using lower doses or shorter durations of prednisone for relapsing SSNS and suggested that smaller doses or shorter durations were as effective as the conventional regimen for relapse of prednisone 60 mg/m2/day till remission followed by four weeks of alternate‐day prednisone at 40 mg/m2. Much larger studies are needed to confirm these findings.
Overall completeness and applicability of evidence
Four well‐designed studies randomising 823 children in their first episode of SSNS have confirmed that the optimum duration of prednisone therapy is two or three months, with no additional benefit found with a longer duration of therapy in reducing the number of relapses. Now that we have these data, there is no requirement for further RCTs evaluating the duration of prednisone therapy involving children of all ages with their first episode of SSNS. However, post hoc analyses in two studies (PREDNOS 2019; Sinha 2015) suggested that the benefit of longer‐duration therapy in young children has not been completely excluded, and this is being assessed in an ongoing study enrolling children below four years of age (Sinha 2016). There are currently no RCTs assessing whether lower doses and/or shorter durations of prednisone can be used in the first episode of SSNS.
Four small studies (Abeyagunawardena 2008; Abeyagunawardena 2017; Gulati 2011; Mattoo 2000) reported that daily prednisone initiated at the onset of infection reduced the risk of relapse associated with infective episodes. However, a new, large, and well‐designed study (PREDNOS 2 2022) has not confirmed these findings. Based on these new data, the IPNA 2023 guidelines do not recommend the routine use of daily prednisone at the onset of infection.
Five small studies (Borovitz 2020; Kainth 2021; Kansal 2019; Sheikh 2019; Tu 2022) are the first randomised studies to examine whether lower total doses of prednisone can be used to treat relapsing nephrotic syndrome. It is imperative that a large study is undertaken to confirm that lower doses of prednisone are as effective in achieving and maintaining remission as the conventional dose regimens, which have been used for 50 years; otherwise, there is a risk that clinicians will try using smaller doses of prednisone in relapsing nephrotic syndrome without data from adequately powered RCTs to support such a change in management.
Although adverse effects of medications were reported in more detail in the four recent high‐quality studies (PREDNOS 2019; Sinha 2015; Teeninga 2013; Yoshikawa 2015), generally, there was limited reporting of adverse effects. Amongst 23 studies evaluating increased duration or dose in the initial episode of SSNS, hypertension, ophthalmological disorders and Cushing's syndrome were reported in 14, 11 and 12 studies, respectively. Prednisone therapy is known to be associated with significant behavioural and psychological adverse effects (Mishra 2010; Neuhaus 2010). However, only eight studies reported this outcome. In PREDNOS 2019, a detailed analysis of quantitative data collected using the Achenbach child behaviour checklist found no differences in behaviour scores between the two durations of prednisone, although parents reported more poor behaviour in children treated for two months. No studies have reported on the burden of having a chronic kidney condition on the child or their family (Beanlands 2017).
The studies included the major ethnic groups, but there are no separable data on efficacy and safety for African‐American or African children. These groups of children, who are known to have a higher incidence of initial and late SRNS (Gipson 2011; Kim 2005), may show different responses in studies of increased dose or duration of prednisone. The four recent high‐quality studies were carried out in Europe, Japan, and India, so few African children were included in the studies.
Quality of the evidence
Of the 54 included studies, only 31 (57%) and 28 (52%) studies reported adequate random sequence generation and allocation concealment, respectively.
Only 10 studies (19%) were at low risk of bias for performance bias (blinding of participants and personnel) and 12 for detection bias (blinding of outcome assessment) since these studies were placebo‐controlled studies. Yoshikawa 2015 was an open‐label study and was at high risk of performance bias, but this study was at low risk of detection bias. The remaining studies were at high risk of bias for both performance and detection bias. Studies without blinding are considered at high risk of bias because knowledge of treatment groups could influence both patient management and reporting of remission and relapse (Moher 1998; Schulz 1995).
Fewer than 50% of studies were at low risk for both attrition bias (incomplete reporting of outcome data) and reporting bias (selective outcome reporting). Nineteen studies were considered at low risk of other potential biases as they were funded by educational or philanthropic organisations.
In the summary of findings tables (Table 1; Table 2), the certainty of the evidence was considered moderate or low for efficacy outcomes related to risk of bias and heterogeneity between studies. When studies were separated into subgroups according to the risk of selection bias, the certainty of the evidence was assessed as high for the primary efficacy outcomes of FRNS in seven well‐designed studies, while the certainty of the evidence was judged low or moderate for these outcomes in studies at high or uncertain risk of selection bias. The quality of studies for the adverse effects was considered moderate or low because of the inclusion of some studies at high risk of bias, and few included studies.
Only 18 of the 54 studies were included in the summary of findings tables and all compared treatment regimens in the first episode of nephrotic syndrome. The remaining studies were mostly single studies of an intervention or data were reported differently for each study, so they could not be included in the meta‐analyses. There were four small studies which evaluated the number of relapses after lower prednisone doses compared to standard doses for relapsing nephrotic syndrome. However, these studies only included 177 children, so a summary of the findings table was not created for this limited dataset.
Potential biases in the review process
A detailed search using the Cochrane Kidney and Transplant Register of Studies was last undertaken in July 2024. The Cochrane Kidney and Transplant Register of Studies contains conference abstracts as well as published studies, and there is no language restriction. This minimised the risk that eligible studies were omitted, although more recently published eligible studies and eligible studies in some congress proceedings not searched could have been missed. There were 10 (19%) included studies that were only available in abstract form with limited information on study methods and outcomes. Failure to include these studies could result in an overestimation of treatment effects, since it is known that negative studies are less likely to be published or may be published later than positive studies (Hopewell 2007). Alternately, some authors have argued that the inclusion of these studies could result in overestimation of treatment effect through selective outcome reporting and incomplete reporting of the number of patients completing follow‐up (Egger 2001).
Many studies were small and had incomplete information related to study methodology and results, and further information, particularly from older studies, could not be obtained despite contacting authors. Of the 54 included studies, 16 were published in or before 2000 ‐ before the CONSORT checklist was first published in 1996 would be likely to influence study methodology and reporting (Moher 2001).
This is an extensive review; each step was completed independently by at least two authors, thus minimising the risks of errors in determining study eligibility, data extraction, risk of bias assessment, and data synthesis.
Agreements and disagreements with other studies or reviews
Studies at low risk of bias included in this review indicate that there is no benefit in prolonging the corticosteroid treatment of all children for more than eight to 12 weeks in the initial episode of SSNS. These data have been incorporated into recent guidelines from KDIGO (KDIGO Executive Conclusions 2019; KDIGO 2021) and IPNA (IPNA 2023), which recommend that daily prednisone be given for four or six weeks followed by alternate‐day prednisone for four or six weeks. Since there are no RCTs comparing an eight‐week regimen with a 12‐week regimen, these guidelines have included both regimens.
This review identified four older studies reporting that increasing prednisone administration from alternate‐day to daily or giving prednisone to children not on prednisone at the onset of an intercurrent viral infection reduced the risk of relapse. However, a much larger study (PREDNOS 2 2022) involving 271 children, including both children already on alternate‐day prednisone and children not on alternate‐day prednisone, did not support this finding. IPNA 2023 does not recommend the routine use of daily prednisone at the onset of URTI.
Current guidelines (Gipson 2009; IPNA 2023; IPNG‐IAP 2008; KDIGO 2021) recommend that alternate‐day prednisone therapy be used to reduce the risk of relapse in children with FRNS. However, a small study (Yadav 2019) showed that the number of relapses was lower in children treated with low‐dose daily prednisone compared with alternate‐day dosing, with the total dose/48 hours not differing between treatment groups. There were no differences in adverse events. The IPNA 2023 guidelines recommend that the initial treatment of children with FRNS should be with either alternate‐day or daily prednisone. Guidelines also recommend that children be dosed with prednisone according to BSA rather than weight in children weighing less than 30 kg because the calculation of dose by weight results in a lower dose compared with a calculation based on BSA. However, two studies (Basu 2020; Raman 2016) found no differences in the number of relapses or adverse events between the two dosing schedules. IPNA 2023 guidelines suggest that the dose of prednisone can be calculated using either weight or BSA.
The listed guidelines emphasise the use of non‐corticosteroid immunosuppressive medications in children with FRNS or SDNS. These medications are the subject of another Cochrane systematic review (Larkins 2020).
Authors' conclusions
Implications for practice.
Prolongation of prednisone therapy beyond two to three months in the initial episode of SSNS does not reduce the risk of relapse, as demonstrated in four large, well‐designed studies at low risk of bias (PREDNOS 2019; Sinha 2015; Teeninga 2013; Yoshikawa 2015).
Older studies undertaken in lower‐middle income countries found that daily prednisone therapy during a URTI or other infection reduced the risk of relapse compared with continuing alternate‐day prednisone or no prednisone (Abeyagunawardena 2008; Abeyagunawardena 2017; Gulati 2011; Mattoo 2000). However, a newly published and large study, which assessed this intervention in European children, where the pattern of intercurrent infections may be different, found no benefit of changing to daily prednisone at the onset of infection in reducing the risk of relapse (PREDNOS 2 2022). Therefore, the most recent guidelines (IPNA 2023) do not recommend that prednisone dosing be changed routinely from alternate daily to daily at the onset of infection.
The most important question to be answered currently is whether a lower dose of prednisone is as effective in achieving remission compared with the conventional dose of 60 mg/m2/day (2 mg/kg/day). This study should assess the time to remission, time to relapse and the number of relapses to determine if children with SSNS can be safely and effectively treated with lower doses of prednisone. Initially, such a study should be carried out in children with the relapsing disease before lower doses of prednisone are evaluated in new‐onset disease.
Implications for research.
Four studies randomising 823 children have demonstrated that there is no benefit from prolonging prednisone therapy beyond two to three months in the first episode of SSNS. Therefore, no further studies are required to evaluate the duration of therapy, so scarce resources for RCTs should not be used to look further at the duration of treatment. However, all studies evaluating the duration of prednisone have used similar daily and alternate daily doses of prednisone based on the empirical regimens established by ISDKC and Arbeitsgemeinschaft für Pädiatrische Nephrologie in the 1970s and 1980s, so we still do not know whether the same results could be obtained with lower total doses of prednisone. Five small studies included in the review update examined lower doses or shorter durations of prednisone with the currently recommended doses and found no differences in efficacy or adverse effects. Therefore, the most important question to be answered is whether a lower dose of prednisone is as effective in achieving remission compared with the conventional dose of 60 mg/m2/day (2 mg/kg/day). This study should assess the time to remission, time to relapse and the number of relapses to determine if children with SSNS can be safely and effectively treated with lower doses of prednisone. Initially, such a study should be carried out in children with the relapsing disease before lower doses of prednisone are evaluated in new‐onset disease.
Adverse events, including hypertension, ophthalmological disorders and behavioural or psychological effects, were not reported in all studies. Recently published studies have provided additional information on adverse effects. PREDNOS 2019 identified no differences in behavioural effects between different treatment durations but found that extended‐duration therapy resulted in a small improvement in QoL. PREDNOS 2 2022 found no differences in behavioural effects or in the QoL scores between different treatment groups. All new studies should include evaluations of the QoL for the child and their family.
Current guidelines recommend that children with FRNS receive prolonged treatment with alternate‐day prednisone, although there is no RCT data to support this recommendation. A small RCT shows that low‐dose daily prednisone reduced the number of relapses compared with alternate‐day therapy during a one‐year follow‐up (Yadav 2019). Further RCTs with longer periods of follow‐up are required to evaluate the relative efficacy and safety of using alternate‐day compared with daily prednisone to prevent relapse.
Limited evidence from a small cross‐over study (Leisti 1978) suggests that children with SSNS may suffer post‐prednisone adrenal insufficiency and that this state may predispose them to relapse. Our review of the four well‐designed RCTs (PREDNOS 2019; Sinha 2015; Teeninga 2013; Yoshikawa 2015) identified a single report of adrenal insufficiency. The frequency of adrenal insufficiency and the efficacy of cortisol substitution in such children require examination in further RCTs.
What's new
| Date | Event | Description |
|---|---|---|
| 21 August 2024 | New search has been performed | New studies added |
| 21 August 2024 | New citation required but conclusions have not changed | Conclusions unchanged from previous update |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 30 May 2020 | New search has been performed | 16 new studies added to review |
| 16 September 2015 | Amended | Minor amendment to forest plot description 2.8.2 ‐ changed from 'Low risk...' to "High risk..." |
| 11 March 2015 | New citation required and conclusions have changed | 10 new studies included |
| 11 March 2015 | New search has been performed | New studies identified |
| 13 May 2009 | Amended | Contact details updated. |
| 23 September 2008 | Amended | Converted to new review format. |
| 21 August 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We are grateful to Dr John F Knight who contributed to the design, quality assessment, data collection, entry, analysis and interpretation, and writing of early versions of this review (Hodson 2000; Hodson 2005).
The authors would like to thank Professor A Bagga, Professor A Abeyagunawardena, Professor PF Hoyer, Professor UK Jayantha, Dr C Kleinknecht, Professor M Liern, Professor TE Mattoo, Professor O Mishra, Professor RK Sharma, Professor Nicholas Webb and Professor N Yoshikawa for the information that they provided about their studies. The authors wish to thank Professors Barratt, Brodehl, Broyer and Ponticelli for responding to our requests for information about unpublished studies.
The authors are grateful to the following peer reviewers for their time and comments for this review update: William Wong (Director of Paediatric Nephrology, Clinical Director, Paediatric Medical Specialties, Starship Children's Hospital, New Zealand); Damien Noone (Division of Nephrology, The Hospital for Sick Children, Toronto, Canada).
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available. but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Steroid therapy in first episode: ≥ 3 months versus 2 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Number with frequent relapses by 12 to 24 months | 8 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.71, 1.06] |
| 1.2 Number relapsing by 12 to 24 months | 12 | 1279 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.68, 0.96] |
| 1.3 Number with frequent relapses by 12 to 24 months: stratified by risk of selection bias | 8 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.71, 1.06] |
| 1.3.1 Low risk of selection bias | 5 | 756 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.10] |
| 1.3.2 Unclear or high risk of selection bias | 3 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.26, 0.77] |
| 1.4 Number relapsing by 12 to 24 months: stratified by risk of selection bias | 12 | 1279 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.68, 0.96] |
| 1.4.1 Low risk of selection bias | 6 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.06] |
| 1.4.2 Unclear or high risk of selection bias | 6 | 471 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.49, 0.98] |
| 1.5 Adverse events | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.5.1 Psychological disorders | 4 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.53, 1.90] |
| 1.5.2 Hypertension | 7 | 548 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.55, 5.73] |
| 1.5.3 Cushingoid facies | 5 | 547 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.76, 1.65] |
| 1.5.4 Cataracts/eye disorders | 6 | 623 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.11, 1.52] |
| 1.5.5 Infections | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.53, 1.17] |
| 1.5.6 Retarded growth | 4 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.25, 1.18] |
| 1.5.7 Osteoporosis | 3 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.06, 3.38] |
Comparison 2. Steroid therapy in first episode: 5 to 7 months versus 3 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Number with frequent relapses by 12 to 24 months | 6 | 706 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.49, 1.09] |
| 2.2 Number relapsing by 12 to 24 months | 8 | 912 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.50, 0.81] |
| 2.3 Number with frequent relapses: stratified by risk of selection bias | 6 | 706 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.49, 1.09] |
| 2.3.1 Low risk of selection bias | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.33] |
| 2.3.2 High or unclear risk of selection bias | 3 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.32, 0.72] |
| 2.4 Number relapsing by 12 to 24 months: stratified by risk of selection bias | 8 | 912 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.50, 0.81] |
| 2.4.1 Low risk of selection bias | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.69, 1.11] |
| 2.4.2 High or unclear risk of selection bias | 5 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.41, 0.69] |
| 2.5 Adverse events | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.5.1 Psychological disorders | 4 | 505 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.05, 1.83] |
| 2.5.2 Hypertension | 6 | 752 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.71, 1.74] |
| 2.5.3 Cushingoid appearance | 6 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.60, 1.23] |
| 2.5.4 Eye complications | 5 | 614 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.18, 1.17] |
| 2.5.5 Infections | 5 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.65, 1.46] |
| 2.5.6 Growth | 3 | 436 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.36, 1.48] |
| 2.5.7 Addisonian crisis | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 5.39] |
| 2.5.8 Gastrointestinal bleeding | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.26, 8.70] |
Comparison 3. Steroid therapy in the first episode: 1 month versus 2 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Number relapsing by 6 to 12 months | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.01, 2.54] |
| 3.2 Number relapsing by 12 to 24 months | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.01, 2.12] |
| 3.3 Number with frequent relapses | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.85, 2.59] |
Comparison 4. Steroid therapy in the first episode: 12 months versus 5 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Number with relapse | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.51, 1.13] |
Comparison 5. Steroid therapy in the first episode: different total doses given over the same duration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Relapse at 12 months | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.43, 0.98] |
| 5.2 Number with FRNS | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.35, 1.37] |
| 5.3 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.3.1 Psychological disorders | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.33, 27.23] |
| 5.3.2 Hypertension | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.33, 5.45] |
| 5.3.3 Cushing's Syndrome | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.90, 10.01] |
Comparison 6. Steroid therapy in first episode: methylprednisone versus prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Time to remission [days] | 2 | 38 | Mean Difference (IV, Random, 95% CI) | ‐5.54 [‐8.46, ‐2.61] |
| 6.2 Number with relapse | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.75, 1.52] |
Comparison 7. Daily prednisolone treatment during viral infections.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Number with relapse with infection | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1.1 All children | 2 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.44, 1.49] |
| 7.1.2 Subgroup analysis: children not on alternate‐day prednisone | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.72, 1.71] |
| 7.1.3 Subgroup analysis: children receiving alternate‐day prednisone | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.47, 1.19] |
| 7.1.4 Subgroup analysis: children receiving alternate‐day prednisone and other immunosuppression | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.50, 1.50] |
| 7.1.5 Subgroup analysis: children receiving other immunosuppression without alternate‐day prednisone | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.44, 2.28] |
| 7.2 Number of relapses/patient | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.2.1 Number of infection‐related relapses/patient‐years | 1 | 95 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐0.87, ‐0.53] |
| 7.2.2 Total relapses (episodes/patient/year) | 1 | 95 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.08, ‐0.72] |
| 7.3 Number of relapses/patient at 2 years | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐4.03, ‐2.57] |
Comparison 8. Deflazacort versus prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Number with remission | 2 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.94, 1.24] |
| 8.2 Number with relapse by 9 to 12 months | 2 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.27, 0.78] |
Comparison 9. Alternate‐day or daily steroid regimens versus intermittent dosing to prevent relapse.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Number relapsing during therapy | 2 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.14, 1.25] |
| 9.1.1 Alternate‐day versus intermittent therapy (6 months therapy) | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.36, 1.02] |
| 9.1.2 Daily versus intermittent therapy (2 months therapy) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.05, 0.82] |
| 9.2 Number with relapses by 9 to 12 months | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.2.1 Alternate‐day therapy versus intermittent dose therapy (6 months therapy) | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.93, 1.55] |
| 9.2.2 Daily versus intermittent therapy (2 months therapy) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.12] |
| 9.3 Mean relapse rate/patient/year | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.3.1 Daily versus intermittent therapy (2 months therapy) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.50, 1.58] |
9.3. Analysis.

Comparison 9: Alternate‐day or daily steroid regimens versus intermittent dosing to prevent relapse, Outcome 3: Mean relapse rate/patient/year
Comparison 10. Intravenous then oral therapy versus oral therapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Relapse by 6 months | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.75, 1.52] |
Comparison 11. Single versus divided daily doses of prednisone to prevent relapse.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Number with relapse | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.77, 1.42] |
| 11.2 Mean relapse rate | 1 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.64, 0.24] |
| 11.3 Number of days to remission | 3 | 242 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.56, 1.96] |
| 11.4 Number of days to remission according to age group | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.4.1 Children aged 1 to 5 years | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 2.45 [0.59, 4.31] |
| 11.4.2 Children aged 5 to 10 years | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐1.04, 1.28] |
| 11.4.3 Children aged 10 to 14 years | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 1.88 [0.03, 3.73] |
| 11.5 Serious adverse events | 2 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.18, 0.91] |
| 11.6 Cumulative steroid dose [mg/kg] | 1 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.68, 0.58] |
| 11.7 Number with HPA suppression | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.70, 0.99] |
Comparison 12. Reduced versus standard steroid doses to prevent relapse.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Time to remission | 2 | 75 | Mean Difference (IV, Random, 95% CI) | 0.72 [‐0.43, 1.88] |
| 12.2 Number with relapse | 4 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.85, 1.20] |
| 12.3 Number with FRNS or SDNS at 12 months | 1 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.49, 1.91] |
| 12.4 Cumulative prednisone dose to achieve remission [mg/kg] | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐20.60 [‐25.87, ‐15.33] |
| 12.5 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.5.1 Reduced growth in height | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.36, 4.22] |
| 12.5.2 Steroid adverse events | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.47, 1.72] |
Comparison 13. Daily versus alternate‐day prednisone to prevent relapse.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Number of relapses in 12 months [number/year] | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.33, ‐0.47] |
| 13.2 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.2.1 Cushingoid facies | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.69, 1.45] |
| 13.2.2 Cataracts | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.00] |
Comparison 14. Short (36 days) versus long (72 days) duration alternate‐day prednisone to prevent relapse.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Number with relapse during treatment | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.19] |
| 14.2 Number with relapse by 6 months | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.46, 1.16] |
| 14.3 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.3.1 Viral infection | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.23] |
| 14.3.2 Bacterial infection | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.01, 8.35] |
| 14.3.3 Urticaria | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.01, 8.35] |
Comparison 15. Weight‐based versus body surface area (BSA)‐based dosing of prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Relapse at 6 months | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.71, 1.49] |
| 15.2 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.2.1 Hypertension | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.05, 1.73] |
| 15.2.2 Cushingoid features | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.58, 2.32] |
| 15.2.3 Eye changes | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.05, 5.57] |
| 15.2.4 Serious infections | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.20, 1.66] |
| 15.3 Prednisone dose | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.3.1 Induction dose | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐32.00 [‐57.21, ‐6.79] |
| 15.3.2 Cumulative dose over 6 months | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐30.00 [‐54.34, ‐5.66] |
Comparison 16. Prolonged steroid therapy (7 months) for relapsing nephrotic syndrome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Number with relapses | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1.1 Relapse by 6 months | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.01, 0.25] |
| 16.1.2 Relapse by 12 months | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.29, 0.65] |
| 16.1.3 Relapse by 2 years | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.45, 0.80] |
| 16.1.4 Relapse by 3 years | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.56, 0.90] |
| 16.2 Relapse rate/patient/year | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.2.1 Relapse rate at 1 year | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐1.78 [‐2.30, ‐1.26] |
| 16.2.2 Relapse rate at 2 years | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐1.79 [‐2.39, ‐1.19] |
| 16.2.3 Relapse rate at 3 years | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐1.74 [‐2.39, ‐1.09] |
| 16.3 Number with FRNS or SDNS | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.19, 0.95] |
| 16.4 Cumulative steroid dose | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.4.1 After 1 year | 1 | 72 | Mean Difference (IV, Random, 95% CI) | 0.59 [0.02, 1.16] |
| 16.4.2 After 2 years | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐1.52, 0.88] |
| 16.4.3 After 3 years | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐3.08, 0.82] |
| 16.5 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.5.1 Hypertension | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 2.40 [0.86, 6.73] |
| 16.5.2 Growth failure | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.62, 2.50] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abeyagunawardena 2008.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
Randomised at onset of URTI to receive one of the interventions; at next URTI received alternate therapy |
|
| Outcomes | Outcomes relevant to the review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated, sealed envelopes, sequential patients |
| Allocation concealment (selection bias) | Low risk | Randomly allocated, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators and parents blinded to contents of containers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators and parents blinded to contents of containers |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8/48 excluded from study (17%) for need for additional immunosuppression (4), no second viral infection (3), number without further relapses (1) |
| Selective reporting (reporting bias) | High risk | Not all the review's pre‐specified outcomes were recorded; no mention of adverse events |
| Other bias | Low risk | The study appears to be free of other source of bias |
Abeyagunawardena 2017.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "At the time of enrolment in the study, the patients were randomised into two groups using the envelope method" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "At the time of enrolment in the study, the patients were randomised into two groups using the envelope method" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Group 1 patients were provided a bottle labeled "Drug A" containing 100 5‐mg tablets and group 2 patients received "Drug B" containing 100 5‐mg tablets." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Group 1 patients were provided a bottle labeled "Drug A" containing 100 5‐mg tablets and group 2 patients received "Drug B" containing 100 5‐mg tablets." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15/48 (31%) did not complete both parts of the 2 year cross‐over study |
| Selective reporting (reporting bias) | High risk | No report of adverse effects; cross‐over study and no separate results available for first part of the study so results could not be included in meta‐analyses |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Agarwal 2010.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Not all prespecified outcomes mentioned but only abstract available |
| Other bias | Unclear risk | insufficient information to permit judgement |
Al Talhi 2018.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes provided to each centre QUOTE: "One opened when patient qualified to enter the study" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients appear accounted for; 4 patients (3%) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Anand 2013.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Relapse defined by urinalysis done by family/staff |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
APN 1981.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (alternate)
Intervention group 2
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation to permit judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes provided to each centre QUOTE: "One opened when patient qualified to enter the study" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16/64 withdrawn: steroid toxicity (8); incorrect treatment or uncooperative parents (6); late non‐response (1); one patient unaccounted for in the text |
| Selective reporting (reporting bias) | Low risk | Recorded the review's pre‐specified outcomes (number with relapse, frequency of relapses, adverse events) |
| Other bias | Low risk | Supported by grants from the VW Foundation |
APN 1988.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (4 weeks)
Intervention group 2 (8 weeks)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation to permit judgement |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Central random allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | QUOTE: "77 patients were initially recruited into the trial, but 16 had to be removed at an early stage due to steroid resistance (8), or early deviations from the treatment protocol (8)" QUOTE: "34 patients completed the study for the full 2 years. Data for the other 27 patients were included for the period that they remained in the study protocol. Of the 27, 5 patients of the short‐course group and 4 from the standard group were removed when they required other immunosuppressive agents; 2 patients from each group left the country during the course of the study; 7 children from the short‐course group, and 3 from the standard group, were lost to follow‐up due to failure of continuous parental cooperation; and late treatment faults were observed in 3 cases after short‐course treatment, and in 1 patient after standard therapy. The full course was completed by 15 patients receiving the short course and by 19 receiving standard treatment." |
| Selective reporting (reporting bias) | High risk | Did not report all the review's pre‐specified outcomes. No report on number of FRNS |
| Other bias | Low risk | Supported by grants from the VW Foundation |
APN 1993.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (3 months)
Intervention group 2 (2 months)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central random allocation |
| Allocation concealment (selection bias) | Low risk | Central random allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7.7% excluded for protocol violation. This proportion of missing outcomes are not sufficient to impact results |
| Selective reporting (reporting bias) | Low risk | Reported the review's pre‐specified outcomes |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Bagga 1999.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (4 months)
Intervention group 2 (2 months)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information from author that sequence generation was random |
| Allocation concealment (selection bias) | Low risk | Information from author that allocation occurred after child had entered study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal/lost to follow‐up: 6/51; steroid resistance (4); poor compliance (2) |
| Selective reporting (reporting bias) | Low risk | All the review's pre‐specified outcomes have been reported |
| Other bias | Low risk | Research grant from the All India Institute of Medical Sciences, New Delhi, India The study appears to be free of other source of bias |
Basu 2020.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (weight‐based dosing)
Intervention group 2 (BSA‐based dosing)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation with variable blocks; stratified for sex, initial episode versus IFR SSNS |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes Investigators responsible for enrolment, randomisation, group assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was relapse and this was confirmed by laboratory measurement of urinary protein/creatinine ratio, which is unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed 6 months follow‐up |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | Authors stated that they received no external funding for the study |
Borovitz 2020.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
Intervention group 3
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | QUOTE: "Patients were divided into three prednisone treatment groups in running order of enrolment, as follows: first patient, 2 mg/kg/day; second, 1.5 mg/kg/day; third, 1 mg/kg; and so forth. Patients and clinicians were informed about prednisone dose only after randomization" |
| Allocation concealment (selection bias) | High risk | QUOTE: "Patients were divided into three prednisone treatment groups in running order of enrolment, as follows: first patient, 2 mg/kg/day; second, 1.5 mg/kg/day; third, 1 mg/kg; and so forth. Patients and clinicians were informed about prednisone dose only after randomization" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and lack of blinding could influence patient management |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and lack of blinding could influence assessment of time to remission |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reported participants accounted for |
| Selective reporting (reporting bias) | High risk | No report of adverse events |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Broyer 1997.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Blocks of 10 packages containing equal numbers of each intervention in order determined by random code" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Block randomisation and sealed packages, lots of 10" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured QUOTE: "Medication in identical bottles and identical tablets" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured QUOTE: "Blinded until end of study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal/lost to follow‐up: 2/40 (loss to follow‐up (1); protocol treatment deviation (1)) |
| Selective reporting (reporting bias) | Low risk | All the review's pre‐specified outcomes have been reported (cannot report on SDNS, as all remained on steroids as per protocol). |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Ekka 1997.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (single dose)
Intervention group 2 (divided dose)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised " insufficient information about sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal/lost to follow‐up: 12/106; did not report for follow‐up (11); steroid resistant (1) |
| Selective reporting (reporting bias) | Low risk | All the review's pre‐specified outcomes have been reported. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Gulati 2011.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation. "randomised by stratified randomisation" on basis of therapy with or without levamisole |
| Allocation concealment (selection bias) | Low risk | QUOTE: "allocation was concealed with opaque sealed envelopes opened at inclusion" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11/100 (11%) patients excluded or lost to follow‐up; lost to follow‐up (5), discontinued treatment (6) |
| Selective reporting (reporting bias) | Low risk | All the review's pre‐specified outcomes have been reported |
| Other bias | Low risk | Funded by the Indian Council of Medical Research |
Hiraoka 2000.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (high dose)
Intervention group 2 (standard)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated " ‐ insufficient information about sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated but no information on method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data Withdrawal/lost to follow‐up: 8/68 excluded for steroid resistance |
| Selective reporting (reporting bias) | Low risk | All the review's pre‐specified outcomes have been reported |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Hiraoka 2003.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (7 months)
Intervention group 2 (3 months)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated" ‐ sealed envelopes |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Simple randomisation using sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal/ lost to follow‐up: 3/73; steroid resistance (3) |
| Selective reporting (reporting bias) | Low risk | All the review's pre‐specified outcomes have been reported |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Imbasciati 1985.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Randomly assigned from a table with random numbers" |
| Allocation concealment (selection bias) | Low risk | Central randomisation centre |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. All 89 randomised patients followed for 12‐24 months |
| Selective reporting (reporting bias) | Low risk | All the review's pre‐specified outcomes have been reported. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
ISKDC 1979.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated " ‐ insufficient information about sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated but no information on method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14/64 (22%) not included in analyses because of protocol violations or loss to follow up |
| Selective reporting (reporting bias) | High risk | Not all of the review's pre‐specified primary outcomes have been reported. Adverse events not reported |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Jamshaid 2022.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
Co‐interventions
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: “These children were allocated into short‐ and long‐term therapy groups, randomly” |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: “These children were allocated into short‐ and long‐term therapy groups, randomly” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All reported participants accounted for but unclear whether the study reported on all participants included |
| Selective reporting (reporting bias) | High risk | Incomplete reporting on relapse rates and no report of adverse effects |
| Other bias | Unclear risk | Unclear what this statement in report means "This Quasi‐experimental study was conducted in the Department of Paediatric Nephrology at the Children’s Hospital Lahore" |
Jayantha 2000.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (7 months)
Intervention group 2 (ISKDC)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random allocation table" ‐ notes received from author |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal/loss to follow‐up: 46/135 (34%) lost to follow‐up at 2 years |
| Selective reporting (reporting bias) | Low risk | Reported on all of review's pre‐specified outcomes |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Jayantha 2002b.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (7 months)
Intervention group 2 (2 months)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random allocation table". Information from author |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24% lost to follow‐up at 1 year (23/95) |
| Selective reporting (reporting bias) | High risk | Not all the review's pre‐specified outcomes have been reported. No report on adverse effects |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Kainth 2021.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (short duration)
Intervention group 2 (standard duration)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: “Random numbers were computer generated for 1:1 assignment using variable block size (4 or 6)" QUOTE: "Stratified for FRNS/SDNS before IFR course & for age <4 yrs" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Sequentially numbered, opaque, sealed envelopes were designed to ensure allocation concealment. Preparation and safekeeping of the randomization list were ensured by staff not involved in study" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients and investigators were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients and investigators were not blinded. Urine protein diary provided by patient. Dipstick to confirm at follow‐up |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/117 (5.2%) did not complete the study |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported though some could not be included in analyses as reported as IQR |
| Other bias | Low risk | Funded by Dept of Biotech, Gov of India N BT/PR11030/MED/97/1644/2016 |
Kansal 2019.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not mentioned and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not mentioned and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | insufficient information to permit judgement |
| Other bias | Unclear risk | insufficient information to permit judgement |
Khan 2023.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation (reported in trial registration) |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, opaque envelopes (reported in trial registration) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study (reported in trial registration) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study (reported in trial registration) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for according to abstract |
| Selective reporting (reporting bias) | High risk | Outcomes reported as medians and IQR, unable to meta‐analyse |
| Other bias | Low risk | Study apppears free of other biases |
Kleinknecht 1982.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (13 months)
Intervention group 2 (6 months)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Sealed closed number envelopes in series of ten" Information obtained from author |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Not all review's pre‐specified outcomes have been reported No data on adverse effect |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Ksiazek 1995.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (6 months)
Intervention group 2 (3 months)
Intervention group 3 (2 months)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", insufficient information about sequence generation to permit judgement |
| Allocation concealment (selection bias) | High risk | QUOTE: "Parents had an influence on assignment, favouring Protocol C" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed for 2 years |
| Selective reporting (reporting bias) | High risk | Not all review's pre‐specified outcomes have been reported No data on numbers with FRNS |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Leisti 1978.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention
Group 1
Group 2
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "allotted". No other information |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated but no information on allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants and personnel blinded. Tablets were of identical taste and appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participants and personnel blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed study |
| Selective reporting (reporting bias) | High risk | Not all review's pre‐specified outcomes have been reported No data on adverse events |
| Other bias | Low risk | Sigrid Juselius Foundation financial support, Medica OY, Helsenki drug preparations |
Li 1994.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (single dose)
Intervention group 2 (divided dose)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated by alternation |
| Allocation concealment (selection bias) | High risk | Patients allocated by alternation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient data to permit judgement |
| Selective reporting (reporting bias) | High risk | Not all review's pre‐specified outcomes have been reported No data on frequent relapses |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Liern 2008.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer generated table (information received from author) |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind to patients and medical caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind to patients and medical caregivers |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if all patients completed both arms of the study |
| Selective reporting (reporting bias) | High risk | Not all review's pre‐specified outcomes have been reported No data on relapse, frequent relapses and minimal data on adverse effects |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Mattoo 2000.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study information
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Data received from authors; alternate patients allocated to groups |
| Allocation concealment (selection bias) | High risk | QUOTE: "alternate patients allocated to groups" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Each patient was followed for a period of two years |
| Selective reporting (reporting bias) | High risk | Not all review's pre‐specified outcomes have been reported No data on adverse events. Only steroid dependent patients included |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Mishra 2012.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (prolonged treatment)
Intervention group 2 (standard treatment)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/80 (6.3%) lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Did not reported on all of review's pre‐specified outcomes The number of patients with at least one relapse is unclear |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Mocan 1999.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (high dose group)
Intervention group 2 (standard therapy)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | QUOTE: "Children arbitrarily randomised into two groups" |
| Allocation concealment (selection bias) | High risk | QUOTE: "Children arbitrarily randomised into two groups" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/21 excluded; 4/21 (21%) lost to follow‐up and this could influence results; 2/21 SRNS |
| Selective reporting (reporting bias) | High risk | Reported on adverse events, relapse rate but not number with FRNS |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Moundekhel 2012.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (3 months)
Intervention group 2 (2 months)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients said to be "randomly divided"; equal numbers in each group suggests that alternation used |
| Allocation concealment (selection bias) | High risk | Patients said to be "randomly divided"; equal numbers in each group suggests that alternation used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No report of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if all included patients were reported |
| Selective reporting (reporting bias) | High risk | No report of FRNS/SDNS & limited report of adverse effects |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Norero 1996.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (3 months)
Intervention group 2 (2 months)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | High risk | Patients allocated by odd or even numbers |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number excluded or lost to follow‐up: 56/96 completed follow‐up. Of 40 excluded patients, 19 had SRNS. Remaining 21 excluded inappropriately: SDNS (5); deviation from protocol (3); duration of follow‐up insufficient (11); loss to follow‐up (2) |
| Selective reporting (reporting bias) | Low risk | Reported on all of review's pre‐specified outcomes |
| Other bias | Low risk | Grant No 1940506 from FONDECYT (National Scientific and Technology Foundation) |
Paul 2014.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (12 weeks)
Intervention group 2 (8 weeks)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Lottery method" |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Lottery method" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No report of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/93 (7.5%) excluded from 6‐month analysis (2 died, 3 deviated from protocol, 3 lost to follow‐up). 72 (77%) completed 1 year follow‐up and reported data on number (%) with relapse and FRNS refers to 72 who completed 12 months |
| Selective reporting (reporting bias) | High risk | Expected outcomes of relapse, FRNS, steroid dose and adverse effects reported but only for patients completing 12 months |
| Other bias | Unclear risk | No information provided |
Pecoraro 2003.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
Intervention group 3
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Information from authors suggests "alternation" was used |
| Allocation concealment (selection bias) | High risk | 'Alternation" was used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Said that all patients completed follow‐up but unclear whether any patients had been excluded |
| Selective reporting (reporting bias) | High risk | Not all review's pre‐specified outcomes have been reported No data on frequent relapses |
| Other bias | High risk | Educational grant from Fresenius |
PREDNOS 2 2022.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Children were randomized in a 1:1 ratio, minimized by background therapy at recruitment, using a secure 24‐hour internet‐based randomization service or by a telephone call to the University of Birmingham Clinical Trials Unit." |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Children were randomized in a 1:1 ratio, minimized by background therapy at recruitment, using a secure 24‐hour internet‐based randomization service or by a telephone call to the University of Birmingham Clinical Trials Unit." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: “Both families and the clinical investigation teams were blinded to the allocation of the trial medication”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Parents were provided with written information as well as a fridge magnet aide‐memoire for this definition (URTI) and a tympanometric electronic thermometer to accurately record their child’s temperature. They were asked to contact their local research team shortly after commencing treatment" Relapse: the diagnosis of relapse was based on a standard definition of proteinuria (3+) for 3 days based on home urine testing and was not confirmed by a hospital visit |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for. Numbers lost to follow‐up were reported. Numbers included in each analysis varied but were reported |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | Project funded by NIHR Health Technology Assessment Programme |
PREDNOS 2019.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Randomisation online via a secure 24 hour internet based randomisation service or by a telephone call to the Birmingham Clinical Trials Unit." 1:1 ratio using minimisation algorithm to balance ethnicity (South Asian, White, Other) and age (≤ 5, ≥ 6 years). Randomisation took place when child considered to be in remission |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Randomisation online via a secure 24 hour internet based randomisation service or by a telephone call to the Birmingham Clinical Trials Unit" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment was open‐label for first 4 weeks. Then blinded for participants/personnel for 12 weeks with matching placebo in the control group. Blinded trial drugs were dispensed from a central pharmacy in blister packs |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants/personnel for 12 weeks after initial 4 weeks of therapy. Parents tested urine and when relapse occurred, they commenced treatment and informed the trial co‐ordinators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes (relapse, FRNS, adverse effects) reported |
| Other bias | Low risk | National Institute of Health Research's Health Technology Assessment programme |
PREDNOS PILOT 2019.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Randomisation online via a secure 24 hour Internet based randomisation service or by a telephone call to the Birmingham Clinical Trials Unit" 1:1 ratio using minimisation algorithm to balance ethnicity (South Asian, White, Other) and age (≤ 5, ≥ 6 years). Randomisation took place when child considered to be in remission |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Randomisation online via a secure 24 hour Internet based randomisation service or by a telephone call to the Birmingham Clinical Trials Unit" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment was open‐label for first 4 weeks. Then blinded for participants/personnel for 12 weeks with matching placebo in the control group. Blinded trial drugs were dispensed from a central pharmacy in blister packs |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants/personnel for 12 weeks after initial 4 weeks of therapy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes (relapse, FRNS, adverse effects) reported |
| Other bias | Low risk | Kidney Research UK and Kid’s Kidney Research |
PROPINE 2020.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Both groups received 60 mg/m2 (maximum dose 60 mg) till remission for 5 days and then randomised Intervention group 1 (short)
Intervention group 2 (long)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Patients were randomized by blocks, using a block size of 6. The randomization protocol was stratified per center and for the prescription of PDN maintenance therapy. An online, encrypted, password‐protected case report form was generated on the Phebo platform (GPI SpA, Trento, Italy), a commercial e‐health platform specializing in remote monitoring of biometric parameters. Patients were randomized after communicating the relapse to the coordinating center" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Patients were randomized by blocks, using a block size of 6. The randomization protocol was stratified per center and for the prescription of PDN maintenance therapy. An online, encrypted, password‐protected case report form was generated on the Phebo platform (GPI SpA, Trento, Italy), a commercial e‐health platform specializing in remote monitoring of biometric parameters. Patients were randomized after communicating the relapse to the coordinating center" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study and outcomes could be influenced by lack of blinding Patients measured urinary albumin and reported to physicians who made changes if required |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study and outcomes could be influenced by lack of blinding Patients measured urinary albumin and reported to physicians who made changes if required |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | Primary data presented as absolute numbers; expected outcomes reported |
| Other bias | Low risk | Funding source: Italian Medicines agency |
Raman 2016.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (Body weight‐based)
Intervention group 2 (BSA‐based)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Block randomization using 20 blocks of two block sizes (4 and 6) was generated using random allocation software version 2.0 (Informer Technologies, Inc.) to allocate the enrolled subjects into one of two groups (BW‐based or BSA‐based prednisolone regimen) in an allocation ratio of 1:1 by a person not directly involved with data collection, analysis or interpretation. This randomization list was concealed from the investigators carrying out the study" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Allocation was concealed placing individual assignments (folded twice) in serially numbered, sealed opaque envelopes by a person not involved in the trial" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinicians not blinded but statistician was blinded to treatment groups |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | QUOTE: The clinicians were not blinded but "the statistician was blinded to the assigned interventions until initial analysis and preparation of the first draft of manuscript" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 44/49 analysed for primary outcome. 7/100 (7%) not analysed |
| Selective reporting (reporting bias) | High risk | Outcomes presented as medians with ranges and not able to add to meta‐ analyses |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Satomura 2001.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (high dose)
Intervention group 2 (low dose)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients assigned "alternately" |
| Allocation concealment (selection bias) | High risk | "Alternation" used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not reported and outcome measurement likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient data to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data to permit judgement |
| Other bias | Unclear risk | Insufficient data to permit judgement |
Sharma 2002.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (6 months)
Intervention group 2 (3 months)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "table of random numbers". Randomisation at 12 weeks after the beginning of initial therapy. Information provided by authors |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "table of random numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded and outcome is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16/156 excluded (10.3%); 160 consecutive patients, 4 refused consent. Of 156 entered, 10 were non‐compliant and 6 lost to follow‐up and their results were excluded from analysis |
| Selective reporting (reporting bias) | Low risk | All the reviews pre‐specified outcomes have been reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Sheikh 2019.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (low dose)
Intervention group 2 (conventional dose)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "A computer‐generated block randomization sequence of varying block sizes (2 2 2 4 8 4 2 2 2 8 4 4 8 4 4; seed 2384) was created by a physician not involved in the study” |
| Allocation concealment (selection bias) | Low risk | QUOTE: “Allocation concealment was achieved by sequentially numbered, opaque, sealed envelopes, which were opened after taking informed consent from the attendants of the enrolled patients.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. Not blinded and outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study. Not blinded and outcome is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients appear accounted for |
| Selective reporting (reporting bias) | High risk | None |
| Other bias | Unclear risk | Insufficient information to permit judgement. No report on funding |
Singhal 2015.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding may influence outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded and lack of blinding may influence outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes mentioned |
| Other bias | Unclear risk | Insufficient data to permit judgement |
Sinha 2015.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (3 months)
Intervention group 2 (6 months)
Co‐interventions
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. Randomly assigned 1:1 in permuted blocks of four |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Procedures for randomisation and packing and distribution were conducted at this centre by individuals, who were not involved in trial implementation" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "External pharmacy manufactured identical‐appearing sugar coated tablets of prednisolone and placebo, packaged in matching blister packs of 10 tablets each" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Investigators, patients and outcome assessors were blinded to randomisation schedule. Masking was maintained during data analysis, following which the randomisation code was broken" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/181 (3%) excluded (SRNS 1, loss to follow‐up 5) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Funded by Indian Council of Medical Research |
Teeninga 2013.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (3 months)
Intervention group 2 (6 months)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central pharmacy with a computer generated random number table |
| Allocation concealment (selection bias) | Low risk | Central pharmacy, controlled allocation concealment with a computer generated random number table. Provided prepackaged medications, with fixed and blinded dose |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, health care providers, data collectors and researchers were blinded to group allocation. Identical tasteless capsules containing prednisolone or placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, health care providers, data collectors and researchers were blinded to group allocation. Randomisation code broken September 2011 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients with consent and not SRNS were included and followed up (13 withdrew consent, 11 steroid resistant) |
| Selective reporting (reporting bias) | Low risk | All the review's pre‐specified outcomes have been reported |
| Other bias | Low risk | No disclosures. Trial registered Netherlands Trial Registry number 255. Funded by Dutch Kidney Foundation Grant C03 and by Vrienden van het Sophia Foundation |
Tu 2022.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (moderate dose of prednisolone)
Intervention group 2 (full dose of prednisolone)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: “They were randomly divided into a moderate‐dose GC group (32 children) and a full‐dose GC group (35 children).” |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: “They were randomly divided into a moderate‐dose GC group (32 children) and a full‐dose GC group (35 children).” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No report of blinding so open label presumed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No report of blinding so open label presumed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions occurred before randomisation, all randomised patients were included in analyses |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | No information provided about monetary support |
Ueda 1988.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (prolonged)
Intervention group 2 (standard)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "allocated randomly", insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned, randomisation stated but no information on method used available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded and outcome is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether any patients, who were randomised, were not included in analysis; complete 1 year follow‐up |
| Selective reporting (reporting bias) | Low risk | The pre‐specified outcomes of the review have been reported |
| Other bias | Low risk | Supported by a grant from the Ministry of Health and Welfare in Japan |
Weerasooriya 2023.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "grouped and randomised according to a computer‐generated system" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "grouped and randomised according to a computer‐generated system" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessment of remission based on urinalysis |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All patients accounted for |
| Selective reporting (reporting bias) | High risk | Not all relvant outcomes reported |
| Other bias | Unclear risk | Funding source not reported |
Yadav 2019.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
All patients received daily supplements
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permutated block randomisation; stratified for steroid dependence. Computer generated allocation Consecutive patients enrolled |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sequentially numbered sealed, opaque envelopes, by personnel not involved in the randomisation process; envelopes were opened following informed written parental consent |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessment of relapse based on urinalysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | The pre‐specified outcomes of the review have been reported |
| Other bias | Low risk | Funding by Indian Council of Medical Research (No 5/5/1090/2013‐RHN) |
Yoshikawa 1998.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (prolonged)
Intervention group 2 (standard)
Co‐interventions
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: 'randomly assigned, concealed envelopes' |
| Allocation concealment (selection bias) | Low risk | QUOTE: 'randomly assigned, concealed envelopes' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded and outcome is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25/196 (13%) did not complete study |
| Selective reporting (reporting bias) | High risk | Not all the reviews, pre‐specified outcomes were reported. No reports of adverse effects of steroids |
| Other bias | Unclear risk | Insufficient data to permit judgment |
Yoshikawa 2015.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (6 months)
Intervention group 2 (2 months)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated sequence in 1:1 ratio, stratified for age (1 to 10 years or 11 to 15 years), sex and institution |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Patients were randomly assigned....at the Japan Clinical Research Support Unit" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label, study patients, guardians, treating physicians and individuals were data were not blinded to treatment groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Apart from trial statistician and data monitoring committee, all treating physicians and other investigators remained blinded to the trial results until follow up was completed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Excluded 9/255 (3%): early relapses after remission (5), 3 no follow‐up data available (3), withdrew consent before allocated study medication (1) |
| Selective reporting (reporting bias) | Low risk | All studies pre‐specified outcomes mentioned |
| Other bias | Low risk | Grant from the Ministry of Health, Labour and Welfare, Japan |
Zhang 2007d.
| Study characteristics | ||
| Methods | Study design
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (3 months)
Intervention group 2 (2 months)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be randomised; insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No evidence that study was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Clinical outcomes could be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
APN: Arbetsgemeinschaft für Pädiatrische Nephrologie; BMD: bone mineral density; BMI: body mass index; BSA: body surface area; CNI: calcineurin inhibitors; CPA: cyclophosphamide; CrCl: creatinine clearance; CPA: cyclophosphamide; CSA: cyclosporin; eGFR: estimated glomerular filtration rate; FRNS: frequently relapsing steroid‐sensitive nephrotic syndrome; HIV: human immunodeficiency virus; HSP: Henoch‐Schönlein purpura; IFR: infrequently relapsing; INS: idiopathic nephrotic syndrome; IQR: interquartile range; ISKDC: International Study of Kidney Disease in Children; LFT: liver function test/s; M/F: male/female; PedsQL: Pediatric Quality of Life Inventory; QoL: quality of life; RCT: randomised controlled trial; SCr: serum creatinine; SD: standard deviation; SDNS: steroid‐dependent nephrotic syndrome; SSNS: steroid‐sensitive nephrotic syndrome; SRNS: steroid‐resistant nephrotic syndrome; TB: tuberculosis; UPCR: urinary protein/creatinine ratio; URTI: upper respiratory tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| APN 2006 | Wrong intervention: RCT comparing cyclosporin with prednisone. Transferred to Cochrane Review on "Non‐corticosteroid immunosuppressive agents for steroid‐sensitive nephrotic syndrome in children" |
| Hou 2021 | Wrong intervention: evaluating Chinese herbal medicine with prednisolone in children with primary nephrotic syndrome |
| Javidi 2021 | Wrong intervention: combination therapy of monteleukast with prednisolone |
| Wu 2022 | Wrong intervention: RCT evaluating Chinese herbal medicine and corticosteroids in children with SSNS |
| Xu 2020b | Study withdrawn: placebo‐controlled RCT planned to compare 6 months with 3 months of prednisolone in the first episode of SSNS in children aged 1‐6 years. Withdrawn because of lack of funding |
| Yang 2022a | Wrong intervention: evaluating Chinese medicine with nursing care |
| Zhang 2014 | Wrong intervention: RCT comparing azithromycin with prednisone. Transferred to Cochrane Review on "Non‐corticosteroid immunosuppressive agents for steroid‐sensitive nephrotic syndrome in children" |
| Zhang 2021b | Wrong population: SRNS |
| Zhou 2021 | Wrong intervention: combination of prednisolone and vitamin D |
| Zhu 2021a | Wrong intervention: multiple medications used |
RCT: randomised controlled trial; SRNS: steroid‐resistant nephrotic syndrome; SSNS: steroid‐sensitive nephrotic syndrome
Characteristics of ongoing studies [ordered by study ID]
CTRI/2018/05/013634.
| Study name | A randomised controlled clinical trial to compare the efficacy of standard dose of steroids vs reduced dose in treating relapses in children with steroid sensitive nephrotic syndrome |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group
Control group
|
| Outcomes | Planned outcomes
|
| Starting date | 1/6/2018 |
| Contact information | Associate Professor Suprita Kalra Email: kalrasuprita@gmail.com |
| Notes | Children with SRNS or SSNS commenced on steroid sparing agent in past 6 months because of FRNS or SDNS |
CTRI/2018/05/014075.
| Study name | A comparison of two doses of prednisolone for relapses in children with steroid sensitive nephrotic syndrome: a randomised controlled non inferiority trial |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group
Control group
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | 01/06/2018 |
| Contact information | Kirtisudha Mishra Email: kirtisen@gmail.com |
| Notes | Children receiving non‐corticosteroid immunosuppressive agents are excluded |
RESTERN 2017.
| Study name | Steroid treatment reduction in relapsing childhood nephrotic syndrome: a new nationwide randomised controlled trial in the Netherlands ‐ the RESTERN study |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group 1
Intervention group 2
|
| Outcomes | Planned outcomes
|
| Starting date | December 2016 |
| Contact information | Dr Anne Schijivens, Radboudume Amalia Children's Hospital, Nijmegen, The Netherlands Email: anne.schijvens@radboudumc.nl |
| Notes |
Sinha 2016.
| Study name | Randomised controlled trial to compare efficacy of 3‐months versus 6‐months therapy with prednisolone for the first episode of idiopathic nephrotic syndrome in children <4‐yr‐old |
| Methods | Study design
|
| Participants | Study characteristics
Baseline characteristics
|
| Interventions | Initial treatment
Intervention group 1
Intervention group 2
|
| Outcomes | Planned outcomes
|
| Starting date | July 2015 |
| Contact information | Dr Aditi Sinha Email: aditisinhaaiims@gmail.com |
| Notes |
FRNS: frequently relapsing nephrotic syndrome; MMF: mycophenolate mofetil; RCT: randomised controlled trial; SDNS: steroid‐dependent nephrotic syndrome; SSNS: steroid‐sensitive nephrotic syndrome; URTI: upper respiratory tract infection
Differences between protocol and review
Risk of bias assessment tool has replaced the quality assessment checklist list used in the previous versions of this review.
Contributions of authors
Deirdre Hahn: Study selection, quality appraisal, data extraction, data analysis, writing review, updating review.
Susan Samuel: Study selection, data extraction, updating review
Narelle Willis: Literature search, obtaining articles, organising translation, data extraction, data analysis, data display, updating review.
Jonathan Craig: Data analysis, writing review, updating review.
Elisabeth Hodson: Study selection, quality appraisal, data extraction, data analysis, writing review, updating review.
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
Deirdre Hahn: No relevant interests were disclosed
Susan Samuel: No relevant interests were disclosed
Narelle Willis: No relevant interests were disclosed
Jonathan Craig: No relevant interests were disclosed
Elisabeth Hodson: No relevant interests were disclosed
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abeyagunawardena 2008 {published data only}
- Abeyagunawardena AS, Trompeter RS. Increasing the dose of prednisolone during viral infections reduces the risk of relapse in nephrotic syndrome: a randomised controlled trial. Archives of Disease in Childhood 2008;93(3):226-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Abeyagunawardena 2017 {published and unpublished data}
- Abeyagunawardena A, Thalgahagoda S, Illangasekera Y, Trompeter R. A short course of prednisolone during an upper respiratory tract infection reduces the risk of relapse in childhood nephrotic syndrome [abstract no: P12]. Pediatric Nephrology 2014;29(9):1689. [EMBASE: 71662409] [DOI] [PubMed] [Google Scholar]
- Abeyagunawardena AS, Thalgahagoda RS, Dissanayake PV, Abeyagunawardena S, Illangasekera YA, Karunadasa UI, et al. Short courses of daily prednisolone during upper respiratory tract infections reduce relapse frequency in childhood nephrotic syndrome. Pediatric Nephrology 2017;32(8):1377-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Abeyagunawardena AS, Thalgahagoda S, Illangasekera Y, Trompeter RS. Short course of daily prednisolone during upper respiratory tract infection reduces the risk of relapse in childhood nephrotic syndrome [abstract no: G376(P)]. Archives of Disease in Childhood 2014;99(Suppl 1):A155-6. [EMBASE: 71566356] [Google Scholar]
Agarwal 2010 {published data only}
- Agarwal I, Gemson JA, Moses PD, Mathew L, Prashanth P. Open randomized clinical study to evaluate efficacy and safety of deflazacort versus prednisolone in idiopathic nephrotic syndrome [abstract no: 558]. Pediatric Nephrology 2010;25(9):1906. [EMBASE: 70438660] [Google Scholar]
Al Talhi 2018 {published data only}
- Al Talhi A, Al Saran K, Osman ET, Al Shatri A, Osman M, Mirza K. A randomized study on a 3-month versus a 7-month prednisolone regimen for the initial episode of childhood idiopathic nephrotic syndrome at a large Saudi center. International Journal of Pediatrics & Adolescent Medicine 2018;5(1):18-23. [EMBASE: 620760413] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anand 2013 {published data only}
- Anand P, Pruthi PK, Anand K. Standard versus long term corticosteroid therapy in the treatment of initial episode of nephrotic syndrome in children [abstract no: P-SUN228]. Pediatric Nephrology 2013;28(8):1609-10. [EMBASE: 620760413] [Google Scholar]
APN 1981 {published data only}
- Anonymous. Alternate-day prednisone is more effective than intermittent prednisone in frequently relapsing nephrotic syndrome. A report of "Arbeitsgemeinschaft fur Padiatrische Nephrologie". European Journal of Pediatrics 1981;135(3):229-37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Anonymous. Alternate-day versus intermittent prednisone in frequently relapsing nephrotic syndrome. A report of "Arbetsgemeinschaft fur Padiatrische Nephrologie". Lancet 1979;1(8113):401-3. [MEDLINE: ] [PubMed] [Google Scholar]
- Brodehl J, Krohn HP. Steroid trial in frequently relapsing nephrotic syndrome in children. In: Glomerulonephritis. International Conference on Pathogenesis, Pathology and Treatment. New York: Wiley, 1977:210-5. [Google Scholar]
APN 1988 {published data only}
- Anonymous. Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft fur Padiatrische Nephrologie. Lancet 1988;1(8582):380-3. [MEDLINE: ] [PubMed] [Google Scholar]
- Brodehl J, Krohn HP, Ehrich JH. The treatment of minimal change nephrotic syndrome (lipoid nephrosis): cooperative studies of the Arbeitsgemeinschaft fur Padiatrische Nephrologie (APN). Klinische Padiatrie 1982;194(3):162-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ehrich JH, Arbeitsgemeinschaft fur Padiatrische Nephrologie (APN). Initial treatment of idiopathic nephrotic syndrome: short vs standard prednisone [abstract]. In: 10th International Congress of Nephrology; 1987 Jul 26-31; London, UK. 1987:59. [CENTRAL: CN-00644361]
- Ehrich JH, Arbeitsgemeinschaft fur Padiatrische Nephrologie (APN). Short initial prednisone therapy versus standard prednisone therapy in the steroid responsive nephrotic syndrome [abstract no: F5-1]. Pediatric Nephrology 1987;1(1):C28. [CENTRAL: CN-00445202] [Google Scholar]
APN 1993 {published data only}
- Ehrich JH, Brodehl J. Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft fur Padiatrische Nephrologie. European Journal of Pediatrics 1993;152(4):357-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ehrich JH, Arbeitsgemeinschaft fur Padiatrische Nephrologie. Minimal change nephrotic syndrome: long prednisone therapy vs standard prednisone therapy [abstract]. Nephrology Dialysis Transplantation 1991;6(10):771. [CENTRAL: CN-00260613] [Google Scholar]
Bagga 1999 {published and unpublished data}
- Bagga A, Hari P, Srivastava RN. Long (LP) versus standard (SP) initial prednisolone treatment for idiopathic nephrotic syndrome (NS) [abstract no: P225]. Pediatric Nephrology 1998;12(1):C155. [CENTRAL: CN-00583944] [Google Scholar]
- Bagga A, Hari P, Srivastava RN. Long (LP) versus standard (SP) initial prednisolone treatment for nephrotic syndrome (NS) [abstract no: FP23]. In: 3rd Congress. Nephrology Urology Transplantation Society (NUTS) of SAARC; 1999 Feb 18 - 21; Colombo, Sri Lanka. 1999:158. [CENTRAL: CN-00460325]
- Bagga A, Hari P, Srivastava RN. Prolonged versus standard prednisolone therapy for initial episode of nephrotic syndrome. Pediatric Nephrology 1999;13(9):824-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Basu 2020 {published data only}2015/03/005655
- Basu B, Bhattacharyya S, Barua S, Naskar A, Roy B. Efficacy of body weight vs body surface area-based prednisolone regimen in nephrotic syndrome. Clinical & Experimental Nephrology 2020;24(7):622-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Borovitz 2020 {published data only}
- Borovitz Y, Alfandary H, Haskin O, Levi S, Kaz S, Davidovits M, et al. Lower prednisone dosing for steroid-sensitive nephrotic syndrome relapse: a prospective randomized pilot study. European Journal of Pediatrics 2020;179(2):279-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Borovitz Y, Haskin O, Levi S, Kaz S, Alfandary H, Davidovits M, et al. Lower prednisone dosing for nephrotic syndrome relapse: a prospective randomized study [abstract no: O-07]. Pediatric Nephrology 2017;32(9):1647. [EMBASE: 618119422] [DOI] [PubMed] [Google Scholar]
Broyer 1997 {published data only}
- Broyer M, Terzi F, Gagnadoux MF, Guest G, Niaudet P. A randomized double blind study of deflazacort (D) versus prednisone (P) in the treatment of idiopathic nephrotic syndrome (INS) [abstract no: 1548]. Journal of the American Society of Nephrology 1995;6(3):414. [CENTRAL: CN-00483349] [Google Scholar]
- Broyer M, Terzi F, Lehnert A, Gagnadoux MF, Guest G, Niaudet P. A controlled study of deflazacort in the treatment of idiopathic nephrotic syndrome. Pediatric Nephrology 1997;11(4):418-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ekka 1997 {published data only}
- Bagga A, Ekka BK, Srivastava RN. Single versus divided dose prednisolone therapy for relapses of nephrotic syndrome (NS) [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A113. [CENTRAL: CN-00261391] [DOI] [PubMed] [Google Scholar]
- Ekka BK, Bagga A, Srivastava RN. Single- versus divided-dose prednisolone therapy for relapses of nephrotic syndrome. Pediatric Nephrology 1997;11(5):597-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gulati 2011 {published data only}2008/091/000245
- Gulati A, Math A, Sreenivas V, Hari P, Bagga A. Administration of daily corticosteroids prevents infection-associated relapses in frequently relapsing NS [abstract]. Clinical Nephrology 2010;74(7 Suppl 1):S158-9. [EMBASE: 70344148] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati A, Math A, Sreeniwas V, Hari P, Bagga A. Administration of daily corticosteroids prevents infection associated relapses in frequently relapsing nephrotic syndrome [abstract no: SU714]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009. [CENTRAL: CN-01912377]
- Gulati A, Sinha A, Hari P, Bagga A. Efficacy of daily corticosteroids to prevent infection associated relapses in frequently relapsing nephrotic syndrome: A randomized controlled trial [abstract]. Pediatric Nephrology 2010;25(9):1874. [EMBASE: 70438498] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati A, Sinha A, Sreenivas V, Math A, Hari P, Bagga A. Daily corticosteroids reduce infection-associated relapses in frequently relapsing nephrotic syndrome: a randomized controlled trial. Clinical Journal of the American Society of Nephrology: CJASN 2011;6(1):63-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hiraoka 2000 {published data only}
- Hiraoka M, Sudo M, West Japanese Cooperative Study of Kidney Disease in Children. Low versus standard dosage of prednisolone for initial treatment of idiopathic nephrotic syndrome in children [abstract no: A0445]. Journal of the American Society of Nephrology 1996;7(9):1335. [Google Scholar]
- Hiraoka M, Tsukahara H, Haruki S, Hayashi S, Takeda N, Miyagawa K, et al. Older boys benefit from higher initial prednisolone therapy for nephrotic syndrome. The West Japan Cooperative Study of Kidney Disease in Children. Kidney International 2000;58(3):1247-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hiraoka M, Tsukahara H, Haruki S, Hayashi S, Takeda N, Miyagawa K, et al. Older boys benefit from intensive initial prednisolone therapy for nephrotic syndrome [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):103A. [CENTRAL: CN-00550605] [Google Scholar]
Hiraoka 2003 {published data only}
- Hiraoka M, Tsukahara H, Matsubara K, Tsurusawa M, Takeda N, Haruki S, et al. A randomized study of two long-course prednisolone regimens for nephrotic syndrome in children. American Journal of Kidney Diseases 2003;41(6):1155-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Imbasciati 1985 {published data only}
- Imbasciati E, Gusmano R, Edefonti A, Zucchelli P, Pozzi C, Grassi C, et al. Controlled trial of methylprednisolone pulses and low dose oral prednisone for the minimal change nephrotic syndrome. British Medical Journal Clinical Research Ed 1985;291(6505):1305-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISKDC 1979 {published data only}
- Anonymous. Nephrotic syndrome in children: a randomized trial comparing two prednisone regimens in steroid-responsive patients who relapse early. Report of the International Study of Kidney Disease in Children. Journal of Pediatrics 1979;95(2):239-43. [MEDLINE: ] [PubMed] [Google Scholar]
Jamshaid 2022 {published data only}
- Jamshaid AA, Akhtar N, Adnan A, Perveen S, Chaudhry A, Fatima T. Outcome of short and long duration steroid therapy in childhood nephrotic syndrome in terms of frequency of relapse rate. Journal of Ayub Medical College, Abbottabad: JAMC 2022;34(2):300-3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jayantha 2000 {published and unpublished data}
- Jayantha UK. Comparison of ISKDC regime with a 7 months steroid regime in the first attack of nephrotic syndrome [abstract no: OPFC26]. Pediatric Nephrology 2004;19(9):C81. [CENTRAL: CN-00583710] [Google Scholar]
- Jayantha UK. Comparison of ISKDC regime with a six month steroid regime in the treatment of steroid sensitive nephrotic syndrome [abstract no: FP2B]. In: 7th Asian Congress of Pediatric Nephrology; 2000 Nov 1-4; Singapore. 2000:28. [CENTRAL: CN-00583708]
- Jayantha UK. Comparison of ISKDC regime with a six month steroid regime in the treatment of steroid sensitive nephrotic syndrome. Data on file 2002.
Jayantha 2002b {published and unpublished data}
- Jayantha UK. Comparison of ISKDC regime with 6 month regime in patients with relapsing nephrotic syndrome. Data on file 2002.
- Jayantha UK. Prolong versus standard steroid therapy for children with relapsing course of nephrotic syndrome [abstract no: P026]. Pediatric Nephrology 2004;19(9):C99. [CENTRAL: CN-00583706] [Google Scholar]
Kainth 2021 {published data only}2015/11/006345
- Kainth D, Hari P, Sinha A, Pandey S, Bagga A. Short-duration prednisolone in children with nephrotic syndrome relapse: a noninferiority randomized controlled trial. Clinical Journal of the American Society of Nephrology: CJASN 2021;16(2):225-32. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kansal 2019 {published and unpublished data}
- Kansal A, Mantan M, Yadav S. Effectiveness of a low dose prednisolone regimen for treatment of relapses in children with SSNS [abstract no: IPN10471- 84]. Pediatric Nephrology 2019;34(10):1871. [Google Scholar]
Khan 2023 {published data only}2021/11/037940
- Khan T, Akhtar S, Mukherjee D, Basu S, Tse Y, Sinha R. Single- versus divided-dose prednisolone for the first episode of nephrotic syndrome in children: an open-label RCT. Clinical Journal of the American Society of Nephrology: CJASN 2023;18(10):1294‐9. [DOI: 10.2215/CJN.0000000000000216] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T, Basu S, Akhtar S, Sarkar S. A randomized control trial comparing safety and efficacy of single dose versus divided daily dose prednisolone in first episode of childhood nephrotic syndrome [abstract no: PI-122]. Pediatric Nephrology 2021;36(10):3353. [Google Scholar]
Kleinknecht 1982 {published and unpublished data}
- Kleinknecht C, Broyer M, Parchoux B, Loriat C, Nivet H, Palcoux JB, et al. Comparison of short and long treatment at onset of steroid sensitive nephrosis (SSN). Preliminary results of a multicenter controlled trial for the French Society of Pediatric Nephrology [abstract]. International Journal of Pediatric Nephrology 1982;3(1):45. [CENTRAL: CN-00550480] [Google Scholar]
Ksiazek 1995 {published data only}
- Ksiazek J, Wyszynska T. Short versus long initial prednisone treatment in steroid-sensitive nephrotic syndrome in children. Acta Paediatrica 1995;84(8):889-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leisti 1978 {published data only}
- Leisti S, Koskimies O, Perheentupa J, Vilska J, Hallman N. Idiopathic nephrotic syndrome: prevention of early relapse. British Medical Journal 1978;1(6117):892. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 1994 {published data only}
- Li X, Li Z, Cheng Z. Treatment of children with simple nephrotic syndrom using prednison once per day. Acta Academiae Medicinae Hubei 1994;15(4):386-8. [EMBASE: 25005617] [Google Scholar]
Liern 2008 {published data only}
- Liern M, Dieguez S, Canepa C. Recovery of total immunoglobulin and immunoglobulin subclasses in nephrotic syndrome: deflazacort vs methylprednisone [Recuperacion de la inmnoglobulina total y sus subclases en el sindrome nefrotico: deflazacort vs metilprednisona]. Nefrologia 2008;28(5):563. [MEDLINE: ] [PubMed] [Google Scholar]
Mattoo 2000 {published data only}
- Mattoo TK, Mahmoud MA. Increased maintenance corticosteroids during upper respiratory infection decrease the risk of relapse in nephrotic syndrome. Nephron 2000;85(4):343-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mishra 2012 {published data only}
- Mishra OP, Thakur N, Mishra RN, Prasad R. Prolonged versus standard prednisolone therapy for initial episode of idiopathic nephrotic syndrome. Journal of Nephrology 2012;25(3):394-400. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mocan 1999 {published data only}
- Mocan H, Erduran E, Karaguzel G. High dose methylprednisolone therapy in nephrotic syndrome. Indian Journal of Pediatrics 1999;66(2):171-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mocan H, Mocan MZ, Erduran E, Karaguzel G, Aslan Y. The effect of high dose methylprednisolone therapy in patients with minimal change nephrotic syndrome [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A74. [CENTRAL: CN-00261380] [Google Scholar]
Moundekhel 2012 {published data only}
- Moundekhel S, Khan GS, Afridi U. Management of nephrotic syndrome: ISKDC versus APN. Pakistan Journal of Medical & Health Sciences 2012;6(1):212-5. [EMBASE: 368679233] [Google Scholar]
Norero 1996 {published data only}
- Norero C, Delucchi A, Lagos E, Rosati P. Initial therapy of primary nephrotic syndrome in children: evaluation in a period of 18 months of two prednisone treatment schedules. Chilean Co-operative Group of Study of Nephrotic Syndrome in Children [Cuadro inicial del sindrome nefrosico primario del nino: evaluacion a 18 meses de dos esquemas de tratamiento con prednisona. Groupo Cooperativo Chileno de Estudio del Sindrome Nefrosico del Nino]. Revista Medica de Chile 1996;124(5):567-72. [MEDLINE: ] [PubMed] [Google Scholar]
- Norero C, Delucchi A, Lagos E, Rosati P. Long term evaluation of two prednisone treatments in initial idiopathic nephrotic syndrome (INS) in children. Preliminary findings [abstract no: P135]. Pediatric Nephrology 1995;9(6):C88. [CENTRAL: CN-00550669] [Google Scholar]
Paul 2014 {published data only}
- Paul SK, Muinuddin G, Jahan S, Begum A, Rahman MH, Hossain MM. Long versus standard initial prednisolone therapy in children with idiopathic nephrotic syndrome. Mymensingh Medical Journal: MMJ 2014;23(2):261-7. [MEDLINE: ] [PubMed] [Google Scholar]
Pecoraro 2003 {published data only}
- Pecoraro C, Caropreso MR, Malgieri G, Ferretti A, Nuzzi F. Therapy of first episode steroid responsive nephrotic syndrome (FESRNS): a randomised controlled trial [abstract no: MP087]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v230. [CENTRAL: CN-00644161] [Google Scholar]
- Pecoraro C, Caropreso MR, Malgieri G, Ferretti AV, Raddi G, Piscitelli A, et al. Therapy of first episode of steroid responsive nephrotic syndrome: a randomised controlled trial [abstract no: OFC41]. Pediatric Nephrology 2004;19(9):C72. [CENTRAL: CN-00644160] [Google Scholar]
- Pecoraro C, Caropreso MR, Passaro G, Ferretti AV, Malgieri G. Therapy of first episode of steroid responsive nephrotic syndrome: a randomised controlled trial [abstract no: M199]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):63. [CENTRAL: CN-00447140] [Google Scholar]
PREDNOS 2019 {published data only}2010‐022489‐2916645249
- Webb N, Trompeter R, Cummins C, Wheatley K, Frew E. Long-term tapering versus standard prednisolone (steroid) therapy for the treatment of the initial episode of childhood nephrotic syndrome: national multicentre randomised double blind trial [protocol]. PREDNOS Clinical Trial Protocol Version 2.1 2013 Sep 1.
- Webb N, Wooley R, Brettell E, Cummins C, Trompeter R, Barsoum E, et al. Standard vs extended course prednisolone therapy for the presenting episode of steroid sensitive nephrotic syndrome: the PREDNOS study [abstract no: O-08]. Pediatric Nephrology 2017;32(9):1647-8. [EMBASE: 618119435] [Google Scholar]
- Webb NJ, Woolley RL, Lambe T, Frew E, Brettell EA, Barsoum EN, et al. Long term tapering versus standard prednisolone treatment for first episode of childhood nephrotic syndrome: phase III randomised controlled trial and economic evaluation. BMJ 2019;365:1800. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb NJ, Woolley RL, Lambe T, Frew E, Brettell EA, Barsoum EN, et al. Sixteen-week versus standard eight-week prednisolone therapy for childhood nephrotic syndrome: the PREDNOS RCT. Health Technology Assessment (Winchester, England) 2019;23(26):1-108. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
PREDNOS 2 2022 {published data only}2012‐003476‐3910900733
- Christian M, Webb N, Mehta S, Nafsika A, Woolley R, Frew, et al. Short course daily low-dose prednisolone at the time of upper respiratory tract infection (URTI) in non-selected children with relapsing steroid sensitive nephrotic syndrome does not prevent URTI-related relapse: The PREDNOS 2 trial [abstract no: FC132]. Nephrology Dialysis Transplantation 2021;36(Suppl 1):i90. [EMBASE: 635917789] [Google Scholar]
- Christian M, Webb N, Mehta S, Woolley R, Brettell E, Khan A, et al. Low-dose prednisolone at the time of an URTI does not prevent relapses in steroid sensitive nephrotic syndrome but may have a health economic benefit: the PREDNOS 2 trial [abstract no: OP-50]. Pediatric Nephrology 2021;36(10):3305. [Google Scholar]
- Christian MT, Webb NJ, Woolley RL, Afentou N, Mehta S, Frew E, et al. Daily low-dose prednisolone to prevent relapse of steroid-sensitive nephrotic syndrome in children with an upper respiratory tract infection: PREDNOS2 RCT. Health Technology Assessment (Winchester, England) 2022;26(3):1-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Christian MT, Webb NJA, Mehta S, Woolley RL, Afentou N, Frew E, et al. Evaluation of daily low-dose prednisolone during upper respiratory tract infection to prevent relapse in children with relapsing steroid-sensitive nephrotic syndrome: the PREDNOS 2 randomized clinical trial. JAMA Pediatrics 2022;176(3):236-43. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb NJ, Frew E, Brettell EA, Milford DV, Bockenhauer D, Saleem MA, et al. Short course daily prednisolone therapy during an upper respiratory tract infection in children with relapsing steroid-sensitive nephrotic syndrome (PREDNOS 2): protocol for a randomised controlled trial. Trials [Electronic Resource] 2014;15:147. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
PREDNOS PILOT 2019 {published data only}2004‐001813‐33
- Webb NJ, Woolley RL, Lambe T, Frew E, Brettell EA, Barsoum EN, et al. Sixteen-week versus standard eight-week prednisolone therapy for childhood nephrotic syndrome: the PREDNOS RCT. Health Technology Assessment (Winchester, England) 2019;23(26):1-108. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
PROPINE 2020 {published data only}2012‐004326‐16
- Gargiulo A, Massella L, Ruggiero B, Rava L, Ciofi Degli Atti M, Materassi M, et al. Results of the PROPINE randomized controlled study suggest tapering of prednisone treatment for relapses of steroid sensitive nephrotic syndrome is not necessary in children. Kidney International 2021;99(2):475-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gargiulo A, Vivarelli M, Pecoraro C, Pennesi M, Pasini A, Edefonti A, et al. Short versus long courses of prednisone for the treatment of relapses of steroid sensitive nephrotic syndrome. results of the PROPINE study [abstract no: O-4]. Pediatric Nephrology 2018;33(10):1810. [EMBASE: 618119435] [Google Scholar]
Raman 2016 {published data only}2014/04/004541
- Raman V, Krishnamurthy S, Harichandrakumar KT. Body weight-based prednisolone versus body surface area-based prednisolone regimen for induction of remission in children with nephrotic syndrome: a randomized, open-label, equivalence clinical trial. Pediatric Nephrology 2016;31(4):595-604. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Satomura 2001 {published data only}
- Satomura K, Yamaoka K, Shima M, Tanaka Y, Ashino N, Nakagawa K, et al. Standard vs low initial dose of prednisolone therapy for first episodes of nephrotic syndrome in children [abstract no: P238]. Pediatric Nephrology 2001;16(8):C117. [CENTRAL: CN-00447593] [Google Scholar]
Sharma 2002 {unpublished data only}
- Gulati S, Ahmed M, Sharma RK, Gupta A, Pokhariyal S. Comparison of abrupt withdrawal versus slow tapering regimen of prednisolone therapy in the management of first episode of steroid responsive childhood idiopathic nephrotic syndrome [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A87. [CENTRAL: CN-00445595] [Google Scholar]
- Sharma RK, Ahmed M, Gulati S, Gupta A, Pokhariyal S. Comparison of abrupt withdrawal versus slow tapering regimen of prednisolone therapy in the management of first episode of steroid responsive childhood idiopathic nephrotic syndrome. Data on file 2002.
- Sharma RK, Ahmed M, Gupta A, Gulati S, Sharma AP. Comparison of abrupt withdrawal versus slow tapering regimens of prednisolone therapy in management of first episode of steroid responsive childhood idiopathic nephrotic syndrome [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):97A. [CENTRAL: CN-00550434] [Google Scholar]
Sheikh 2019 {published data only}
- Sheikh S, Mishra K, Kumar M. Low versus conventional dose prednisolone for nephrotic syndrome relapses: randomised-controlled, non-inferiority-trial [abstract no: IPN10923-86]. Pediatric Nephrology 2019;34(10):1980. [DOI] [PubMed] [Google Scholar]
- Sheikh S, Mishra K, Kumar M. Low-dose versus conventional-dose prednisolone for nephrotic syndrome relapses: a randomized controlled non-inferiority trial. Pediatric Nephrology 2021;36(10):3143–50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Singhal 2015 {published data only}2011/12/002208
- Singhal R, Pandit S, Dhawan N. Deflazacort versus prednisolone: randomized controlled trial in treatment of children with idiopathic nephrotic syndrome. Iranian Journal of Pediatrics 2015;25(2):e510. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinha 2015 {published and unpublished data}2010/091/001095
- Bagga A, Sinha A, Saha A, Kumar M, Afzal K, Mehta A. Randomized double blind, placebo controlled trial to compare the efficacy of 3-months versus 6-months therapy with prednisolone for the first episode of idiopathic nephrotic syndrome (CTRI/2010/091/001095) [abstract no: P139]. Pediatric Nephrology 2012;27(9):1707. [EMBASE: 71386357] [Google Scholar]
- Sinha A, Bagga A, Sharma S, Saha A, Kumar M, Afzal K, et al. Randomized, double blind, placebo controlled trial to compare the efficacy of 3-months versus 6-months therapy with prednisolone for the first episode of idiopathic nephritic syndrome [abstract no: O-40]. Pediatric Nephrology 2013;28(8):1361-2. [EMBASE: 71126981] [Google Scholar]
- Sinha A, Saha A, Kumar M, Sharma S, Afzal K, Mehta A, et al. Extending initial prednisolone treatment in a randomized control trial from 3 to 6 months did not significantly influence the course of illness in children with steroid- sensitive nephrotic syndrome. Kidney International 2015;87(1):217-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Teeninga 2013 {published data only}27871415255
- Teeninga N, Guan Z, Stevens J, Kist-Van Holthe JE, Ackermans MT, Heijden AJ, et al. Population pharmacokinetics of prednisolone in relation to clinical outcome in children with nephrotic syndrome. Therapeutic Drug Monitoring 2016;38(4):534-45. [EMBASE: 610346284] [DOI] [PubMed] [Google Scholar]
- Teeninga N, Kist-Van Holthe JE, De Mos NI, Peters N, Hop WC, Heijden AJ, et al. Optimal treatment of steroid sensitive nephrotic syndrome in children: a multicenter randomised controlled trial [abstract no: 450]. Pediatric Nephrology 2010;25(9):1884. [EMBASE: 70438552] [Google Scholar]
- Teeninga N, Kist-Van Holthe JE, De Mos NI, Van Rijswijk-Peeters N, Hop WC, Nauta J. Optimal treatment of steroid sensitive nephrotic syndrome in children: a randomised controlled trial [abstract no: P090]. Pediatric Nephrology 2008;23(9):1619. [CENTRAL: CN-01657514] [Google Scholar]
- Teeninga N, Kist-Van Holthe JE, Van Ruswuk N, De Mos NI, Hop WC, Wetzels JF, et al. Effect of extended prednisolone treatment from three to six months, with equal cumulative doses, on childhood nephrotic syndrome: a nation-wide randomised controlled trial [abstract no: OP61]. Pediatric Nephrology 2012;27(9):1637-8. [EMBASE: 71386208] [Google Scholar]
- Teeninga N, Kist-van Holthe JE, Rijswijk N, Mos NI, Hop WC, Wetzels JF, et al. Extending prednisolone treatment does not reduce relapses in childhood nephrotic syndrome. Journal of the American Society of Nephrology 2013;24(1):149-59. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeninga N, Kist-van Holthe JE, den Akker EL, Kersten MC, Boersma E, Krabbe HG, et al. Genetic and in vivo determinants of glucocorticoid sensitivity in relation to clinical outcome of childhood nephrotic syndrome. Kidney International 2014;85(6):1444-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tu 2022 {published data only}
- Tu J, Chen CY, Geng HY, Li HR, Xia H, Lin Y, et al. Clinical assessment of moderate-dose glucocorticoid in the treatment of recurrence of primary nephrotic syndrome in children: a prospective randomized controlled trial. Zhongguo Dangdai Erke Zazhi 2022;24(5):466-71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ueda 1988 {published data only}
- Ueda N, Chihara M, Kawaguchi S, Niimomi Y, Nonada T, Matsumoto J, et al. Intermittent versus long-term tapering prednisolone for initial therapy in children with idiopathic nephrotic syndrome. Journal of Pediatrics 1988;112(1):122-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ueda N, Chihara M, Kawaguchi S, Niinomi Y, Yasaki T. Intermittent versus long-term tapering prednisolone regimens as initial therapy in children with steroid-responsive nephrotic syndrome [abstract]. In: 10th International Congress of Nephrology; 1987 Jul 26-31; London, UK. 1987:98. [CENTRAL: CN-01658375]
Weerasooriya 2023 {published data only}
- Weerasooriya WA, Abeyagunawardena AS, Thalgahagoda RS. Single vs split dose of prednisolone in the treatment of relapses of childhood nephrotic syndrome. European Journal of Pediatrics 2023;182(4):1741‐7. [DOI: 10.1007/s00431-023-04804-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yadav 2019 {published data only}2012/12/003194
- Yadav M, Sinha A, Hari P, Bagga A. Efficacy of low-dose daily versus alternate day prednisone in children with frequently relapsing nephrotic syndrome (FRNS): open-label randomized controlled trial (RCT) [abstract no: FP-S25-09]. Pediatric Nephrology 2016;31(10):1752. [EMBASE: 612479637] [DOI] [PubMed] [Google Scholar]
- Yadav M, Sinha A, Khandelwal P, Hari P, Bagga A. Efficacy of low-dose daily versus alternate-day prednisolone in frequently relapsing nephrotic syndrome: an open-label randomized controlled trial. Pediatric Nephrology 2019;34(5):829-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yoshikawa 1998 {published data only}
- Ito H, Yoshikawa N. Multicenter controlled trial in nephrotic syndrome in Japanese children [abstract no: S-13.5]. In: 9th Congress. International Pediatric Nephrology Association; 1992 Aug 30 - Sep 4; Jerusalem, Israel. 1992:C37. [CENTRAL: CN-00484469]
- Nakanishi K, Iijima K, Ishikura K, Hataya H, Nakazato H, Sasaki S, et al. Two-year outcome of the ISKDC regimen and frequent-relapsing risk in children with idiopathic nephrotic syndrome. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(5):756-62. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekoshi Y. Treatment of idiopathic nephrotic syndrome [abstract no: S-1]. Pediatric Nephrology 1996;10(1):C3. [CENTRAL: CN-00402800] [Google Scholar]
- Yoshikawa N, Ito H, Takehoshi Y, Honda M, Awazu M, Iijima K, et al. Standard versus long-term prednisolone with sairei-to for initial therapy in childhood steroid-responsive nephrotic syndrome: a prospective controlled study. Nippon Jinzon Gakkai Shi [Japanese Journal of Nephrology] 1998;40(8):587-90. [MEDLINE: ] [PubMed] [Google Scholar]
Yoshikawa 2015 {published and unpublished data}000000747
- Yoshikawa N, Nakanishi K, Oba MS, Sako M, Ohashi Y, Iijima K. Increased duration and dose of prednisolone (PSL) treatment does not reduce relapses in childhood nephrotic syndrome [abstract no: SA-PO1080]. Journal of the American Society of Nephrology 2014;24(Abstracts):3B. [CENTRAL: CN-01912375] [Google Scholar]
- Yoshikawa N, Nakanishi K, Sako M, Oba MS, Mori R, Ota E, et al. A multicenter randomized trial indicates initial prednisolone treatment for childhood nephrotic syndrome for two months is not inferior to six-month treatment. Kidney International 2015;87(1):225-32. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2007d {published data only}
- Zhang Y, Huang JP, Xiao HJ, Ding J, Yao Y, Yang JY. Prospective clinical randomized controlled trial of pulse methylprednisolone therapy in children with steroid sensitive nephrotic syndrome [abstract no: 797 (P)]. Pediatric Nephrology 2007;22(9):1609. [CENTRAL: CN-01912552] [Google Scholar]
References to studies excluded from this review
APN 2006 {published data only}
- Arbeitsgemeinschaft fur Padiatrische Nephrologie. Results of the nephrotic syndrome study VIII of the APN: new standard treatment versus new standard treatment plus 8 weeks cyclosporin A [abstract]. Pediatric Nephrology 1999;13:C26. [CENTRAL: CN-00636143] [Google Scholar]
- Hoyer PF, Brodehl J. Initial treatment of idiopathic nephrotic syndrome in children: prednisone versus prednisone plus cyclosporine A: a prospective, randomized trial. Journal of the American Society of Nephrology 2006;17(4):1151–7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hoyer PF. Results of the Nephrotic Syndrome Study VIII of the APN: new standard treatment versus new standard treatment plus 8 weeks cyclosporin A [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):104A. [CENTRAL: CN-0055522] [Google Scholar]
- Hoyer PF, Arbeitsgemeinschaft fur Padiatrische Nephrologie. The initial treatment of idiopathic nephrotic syndrome with prednisone and cyclosporin A: preliminary results of a therapeutic trial [abstract no: P147]. Pediatric Nephrology 1995;9(6):C91. [CENTRAL: CN-00401335] [Google Scholar]
Hou 2021 {published data only}
- Hou X, Xu M, Li J, Li R, Zhang J, Ju J. Study of the therapeutic effects of Chinese herbal decoction combined with glucocorticoid in treating primary nephrotic syndrome in children. Evidence-based Complementary & Alternative Medicine: eCAM 2021;2:4434504. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Javidi 2021 {published data only}
- Javidi F, Yousefichaijan P, Dorreh F, Arjmand A, Rezagholizamenjany M. Using montelukast as an add-on treatment in nephrotic syndrome pediatrics: a randomized clinical trial study. Nephro-Urology Monthly 2021;13(4):1-4. [EMBASE: 2016084908] [Google Scholar]
Wu 2022 {published data only}
- Wu H, Zhang L, Liu Q, Ren B, Li J. Clinical efficacy of adjuvant treatment of primary nephrotic syndrome in pediatric patients with Chinese medicine. Journal of Healthcare Engineering 2022;2022:1516633. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2020b {published data only}
- Xu H. Study of initial steroid treatment in young children with nephrotic syndrome. clinicaltrials.gov/show/NCT04536181 (first received 2 September 2020).
Yang 2022a {published data only}
- Yang G, Yang H, Cui S, Shan J. Effect of Huaiqihuang Granules combined with comprehensive nursing on children with primary nephrotic syndrome. Journal of Healthcare Engineering 2022;2022:3279503. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang B, Liu T, Wang W, Zhang X, Fan S, Liu Z, et al. A prospective randomly controlled clinical trial on azithromycin therapy for induction treatment of children with nephrotic syndrome. European Journal of Pediatrics 2014;173(4):509-15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zhang BL, Liu T. A prospective clinical randomized controlled trial on azithromycin therapy in induction treatment in children with nephrotic syndrome [abstract no: O-44]. Pediatric Nephrology 2013;28(8):1364. [EMBASE: 71126985] [Google Scholar]
Zhang 2021b {published data only}
- Zhang Y, Wu F, Huang T, Fu T. Efficacy of triple regimen of tacrolimus combined with low-dose glucocorticoid and low-dose sirolimus in treatment of steroid-resistant nephrotic syndrome in children. Pharmaceutical Care & Research 2021;21(3):181-4. [EMBASE: 2017478751] [Google Scholar]
Zhou 2021 {published data only}
- Zhou G, Kong X. Study on the effect of combination of prednisone and vitamin D in the treatment of primary nephrotic syndrome in children. Journal of Healthcare Engineering 2021;2021:7932721. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Zhu 2021a {published data only}
- Zhu H, Zhang H, Chen HD, Wu HL. Effect of intensive drug regimen on laboratory indexes and safety of children with nephrotic syndrome. Chinese Journal of Pharmaceutical Biotechnology 2021;28(3):272-4. [EMBASE: 2015848399] [Google Scholar]
References to ongoing studies
CTRI/2018/05/013634 {published data only}2018/05/013634
- Kalra S. A randomized controlled clinical trial to compare the efficacy of standard dose of steroids vs reduced dose in treating relapses in children with steroid sensitive nephrotic syndrome. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=25815 (first received 3 May 2018).
CTRI/2018/05/014075 {published data only}2018/05/014075
- Mishra K. A comparison of two doses of prednisolone for relapses in children with steroid sensitive nephrotic syndrome: a randomized controlled non inferiority trial. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=26036 (first received 23 May 2018).
RESTERN 2017 {published data only}2016‐002430‐765670
- Schijvens A, Schreuder M. Steroid treatment reduction in relapsing childhood nephrotic syndrome: a new nationwide randomized controlled trial in the Netherlands - the RESTERN study [abstract no: P-163]. Pediatric Nephrology 2017;32(9):1729. [EMBASE: 618120212] [Google Scholar]
- Schijvens AM, Dorresteijn EM, Roeleveld N, Ter Heine R, Wijk JA, Bouts AH, et al. REducing STEroids in Relapsing Nephrotic syndrome: the RESTERN study- protocol of a national, double-blind, randomised, placebo-controlled, non-inferiority intervention study. BMJ Open 2017;7(9):e018148. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinha 2016 {published data only}2015/06/005939
- Sinha A, Gipson D, Wong C, Massengil S, Ahmad A, Hari P, et al. 6-months versus 3-months prednisolone for initial therapy of steroid sensitive nephrotic syndrome: open label RCT [abstract no: IPN11430-80]. Pediatric Nephrology 2019;34(10):2076. [Google Scholar]
- Sinha A, Hari P, Bagga A. Randomized controlled trial to compare efficacy of 3-months versus 6-months therapy with prednisolone for the first episode of idiopathic nephrotic syndrome in children <4-yr-old [abstract no: PO-321]. Pediatric Nephrology 2016;31(10):1867-8. [EMBASE: 612479470] [Google Scholar]
Additional references
Arneil 1956
- Arneil GC. Treatment of nephrosis with prednisolone. Lancet 1956;270(6920):409-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arneil 1971
- Arneil GC. The nephrotic syndrome. Pediatric Clinics of North America 1971;18(2):547-59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Aydin 2019
- Aydin M, Franke I, Kurylowicz L, Ganshow R, Lentze M, Born M, et al. The long-term outcome of childhood nephrotic syndrome in Germany: a cross sectional study. Clinical & Experimental Nephrology 2019;23(5):676-88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Beanlands 2017
- Beanlands H, Maione M, Poulton C, Herreshoff E, Hladunewich MA, Hailperin M, et al. Learning to live with nephrotic syndrome: experiences of adult patients and parents of children with nephrotic syndrome. Nephrology Dialysis Transplantation 2017;32(Suppl 1):i98-105. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhimma 1997
- Bhimma R, Coovadia HM, Adhikari M. Nephrotic syndrome in South African children: changing perspectives over 20 years. Pediatric Nephrology 1997;11(4):429-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 2001
- Egger M, Dickersin K, Davey Smith G. Problems and limitations in conducting systematic reviews. In: Egger M, Davey Smith G, Altman D, editors(s). Systematic Reviews in Health care: Meta-analysis in context. Second edition. London: BMJ Publishing Group, 2001. [ISBN-13: 978-0727914880] [Google Scholar]
Gipson 2009
- Gipson DS, Massengill SF, Yoa L, Nagaraj S, Smoyer WE, Mahan JD, et al. Management of childhood onset nephrotic syndrome. Pediatrics 2009;124(2):747-57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gipson 2011
- Gipson DS, Trachtman H, Kaskel FJ, Radeva MK, Gassman J, Greene TH, et al. Clinical trials treating focal segmental glomerulosclerosis should measure patient quality of life. Kidney International 2011;79(6):678-85. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guess 2010
- Guess A, Agrawal S, Wei CC, Ransom RF, Benndorf R, Smoyer WE. Dose- and time-dependent glucocorticoid receptor signaling in podocytes. American Journal of Physiology - Renal Physiology 2010;299(4):F845-53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Hopewell 2007
- Hopewell S, McDonald S, Clarke M, Egger M. Grey literature in meta-analysis of randomized trials of health care interventions. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: MR000010. [DOI: 10.1002/14651858.MR000010.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyams 1988
- Hyams JS, Carey DE. Corticosteroids and growth. Journal of Pediatrics 1988;113(2):249-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
IPNA 2023
- Trautmann A, Boyer O, Hodson E, Bagga A, Gipson DS, Samuel S, et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid‑sensitive nephrotic syndrome. Pediatric Nephrology 2023;38(3):877-919. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
IPNG‐IAP 2008
- Bagga A, Ali U, Banerjee S, Kanitkar M, Phadke KD, Senguttuvan P, et al. Management of steroid sensitive nephrotic syndrome: revised guidelines. Indian Pediatrics 2008;45(3):203-14. [MEDLINE: ] [PubMed] [Google Scholar]
ISKDC 1970
- Abramowicz M, Barnett HL, Edelmann CM Jr, Greifer I, Kobayashi O, Arneil GC, et al. Controlled trial of azathioprine in children with nephrotic syndrome: a report for the International Study of Kidney Disease in Children. Lancet 1970;1(7654):959-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ISKDC 1984
- Anonymous. Minimal change nephrotic syndrome in children: deaths during the first 5 to 15 years' observation. Report of the International Study of Kidney Disease in Children. Pediatrics 1984;73(4):497-501. [MEDLINE: ] [PubMed] [Google Scholar]
Kadmiel 2013
- Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends in Pharmacological Sciences 2013;34(9):518-30. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
KDIGO 2012
- Lombel RM, Gipson DS, Hodson EM, Kidney Disease: Improving Global Outcomes. Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatric Nephrology 2013;28(3):415-26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDIGO 2021
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney International 2021;100(4S):S1-276. [PMID: ] [DOI] [PubMed] [Google Scholar]
KDIGO Executive Conclusions 2019
- Rovin BH, Caster DJ, Catran DC, Gibson KL, Hogan JJ, Moeller MJ, et al. Management and treatment of glomerular diseases (part 2): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney International 2019;95(2):281-95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2005
- Kim JS, Bellew CA, Silverstein DM, Aviles DH, Boineau FG, Vehaskari VM. High incidence of initial and late steroid resistance in childhood nephrotic syndrome. Kidney International 2005;68(3):1275-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kirshcke 2014
- Kirschke E, Goswami D, Southworth D, Griffin PR, Agard D. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaparone cycles. Cell 2014;157(7):1685-97. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koskimies 1982
- Koskimies O, Vilska J, Rapola J, Hallman N. Long-term outcome of primary nephrotic syndrome. Archives of Disease in Childhood 1982;57(7):544-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Larkins 2020
- Larkins NG, Liu ID, Willis NS, Craig JC, Hodson EM. Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD002290. [DOI: 10.1002/14651858.CD002290.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
MacHardy 2009
- MacHardy N, Miles PV, Massengill SF, Smoyer WE, Mahan JD, Greenbaum L, et al. Management patterns of childhood-onset nephrotic syndrome. Pediatric Nephrology 2009;24(11):2193-201. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mishra 2010
- Mishra OM, Basu B, Upadhyay SK, Prasad R, Schaefer F. Behavioural abnormalities in children with nephrotic syndrome. Nephrology Dialysis Transplantation 2010;25(8):2537-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352(9128):609-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Jones A, Lepage L, CONSORT Group (Consolidated Standards for Reporting of Trials). Use of the CONSORT statement and quality of reports of randomized trials: a comparative before-and-after evaluation. JAMA 2001;285(15):1992-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Neuhaus 2010
- Neuhaus TJ, Langlois V, Licht C. Behavioural abnormalities in children with nephrotic syndrome--an underappreciated complication of a standard treatment? Nephrology Dialysis Transplantation 2010;25(8):2397-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ng 2001
- Ng JS, Wong W, Law RW, Hui J, Wong EN, Lam DS. Ocular complications of paediatric patients with nephrotic syndrome. Clinical & Experimental Ophthalmology 2001;29(4):239-43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Niaudet 2009
- Niaudet P. Long-term outcome of children with steroid-sensitive idiopathic nephrotic syndrome. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(10):1457-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Noone 2018
- Noone DG, Iijima K, Parekh R. Idiopathic nephrotic syndrome in children. Lancet 2018;392(10141):61-74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ohashi 2011
- Ohashi T, Uchida K, Uchida S, Sasaki S, Nitta K. Dexamethasone increases the phosphorylation of nephrin in cultured podocytes. Clinical & Experimental Nephrology 2011;15(5):688-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ponticelli 2018
- Ponticelli C, Locatelli F. Glucocorticoids in the treatment of glomerular diseases. Clinical Journal of The American Society of Nephrology: CJASN 2018;13(5):815-22. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raja 2017
- Raja K, Parikh A, Webb H, Hothi D. Use of a low-dose prednisolone regimen to treat a relapse of steroid-sensitive nephrotic syndrome in children. Pediatric Nephrology 2017;32(2):99-105. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ramamoorthy 2016
- Ramamoorthy S, Cidlowski JA. Corticosteroids: mechanisms of action in health and disease. Rheumatic Diseases Clinics of North America 2016;42(1):15-31. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruth 2005
- Rüth EM, Kemper M, Leumann EP, Laube GF, Neuhaus TJ. Children with steroid sensitive nephrotic syndrome come of age: long-term outcome. Journal of Pediatrics 2005;147(2):202-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Samuel 2013
- Samuel S, Morgan CJ, Bitzan N, Mammen C, Dart AB, Manns BJ, et al. Substantial practice variation exists in the management of childhood nephrotic syndrome. Pediatric Nephrology 2013;28(12):2289-98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schijvens 2019
- Schijvens AM, ter Heine R, Wildt SN, Schreuder MF. Pharmacology and pharmacogenetics of prednisone and prednisolone in patients with nephrotic syndrome. Pediatric Nephrology 2019;34(3):389-403. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2022a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Schünemann 2022b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Tarshish 1997
- Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr. Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. Journal of the American Society of Nephrology 1997;8(5):769-76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Veltkamp 2021
- Veltkamp F, Rensma LR, Bouts AH. Incidence and relapse of idiopathic nephrotic syndrome: meta-analysis. Pediatrics 2021;148(1):e2020029249. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hahn 2015
- Hahn D, Hodson EM, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD001533. [DOI: 10.1002/14651858.CD001533.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hahn 2020
- Hahn D, Samuel SM, Willis NS, Craig JC, Hodson EM. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD001533. [DOI: 10.1002/14651858.CD001533.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodson 2000
- Hodson EM, Knight JF, Willis NS, Craig JC. Corticosteroid therapy in nephrotic syndrome: a meta-analysis of randomised controlled trials. Archives of Disease in Childhood 2000;83(1):45-51. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodson 2002
- Hodson EM, Knight JF, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2002, Issue 3. Art. No: CD001533. [DOI: 10.1002/14651858.CD001533] [DOI] [PubMed] [Google Scholar]
Hodson 2003
- Hodson EM, Knight JF, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2003, Issue 2. Art. No: CD001533. [DOI: 10.1002/14651858.CD001533.pub2] [DOI] [PubMed] [Google Scholar]
Hodson 2005
- Hodson EM, Knight JF, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2005, Issue 1. Art. No: CD001533. [DOI: 10.1002/14651858.CD001533.pub3] [DOI] [PubMed] [Google Scholar]
Hodson 2007
- Hodson EM, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD001533. [DOI: 10.1002/14651858.CD001533.pub4] [DOI] [PubMed] [Google Scholar]


