Abstract
δ Crystallin, a taxon-specific crystallin present in avian eye lenses, is homologous to the urea cycle enzyme ASL (argininosuccinate lyase). Although there are two δ crystallin isoforms in duck lenses, dδc1 (duck δ1 crystallin) and dδc2 (duck δ2 crystallin), only dδc2 is catalytically active. Previous structural studies have suggested that residues Ser283 and His162 in the multi-subunit active site of dδc2/ASL are the putative catalytic acid/base, while the highly conserved, positively charged Lys289 is thought to help stabilize the carbanion intermediate. The strict conservation of a small hydroxy-containing residue (Thr or Ser) at position 161 adjacent to the putative catalytic base, as well as its proximity to the substrate in the S283A dδc2 enzyme–substrate complex, prompted us to investigate further the role this residue. Structures of the active T161S and inactive T161D dδc2 mutants, as well as T161D complexed with argininosuccinate, have been determined to 2.0 Å resolution. The structures suggest that a hydroxy group is required at position 161 to help correctly position the side chain of Lys289 and the fumarate moiety of the substrate. Threonine is probably favoured over serine, because the interaction of its methyl group with Leu206 would restrict its conformational flexibility. Residues larger than Thr or Ser interfere with substrate binding, supporting previous suggestions that correct positioning of the substrate's fumarate moiety is essential for catalysis to occur. The presence of the 280s loop (i.e. a loop formed by residues 270–290) in the ‘open’ conformation suggests that loop closure, thought to be essential for sequestration of the substrate, may be triggered by the formation of the carbanion or aci-carboxylate intermediates, whose charge distribution more closely mimics that of the sulphate ion found in the active-site region of the inactive dδc1. The 280s loop in dδc1 is in the closed conformation.
Keywords: argininosuccinate lyase, catalysis, δ crystallin, enzyme mechanism, mutagenesis
Abbreviations: AS, argininosuccinate; ASL, argininosuccinate lyase; dδc1, duck δ1 crystallin; dδc2, duck δ2 crystallin
INTRODUCTION
δ Crystallin is the major soluble protein in the eye lenses of birds and terrestrial reptiles. This taxon-specific crystallin evolved from the housekeeping enzyme ASL (argininosuccinate lyase) by a process known as gene sharing [1–4]. ASL is a cytosolic enzyme that catalyses the reversible breakdown of AS (argininosuccinate) to arginine and fumarate. Following recruitment, gene duplication occurred, resulting in two proteins, dδc1 (duck δ1 crystallin) and dδc2 (duck δ2 crystallin). The two δ crystallin isoforms exhibit 94% amino acid sequence identity in ducks, and are 69% and 71% identical respectively to human ASL [4,5]. While dδc2 has retained ASL enzymic activity and outside the lens is the duck orthologue of ASL [6,7], dδc1 is not longer catalytically active, presumably the consequence of having acquired a more specialized structural eye lens role [8–10].
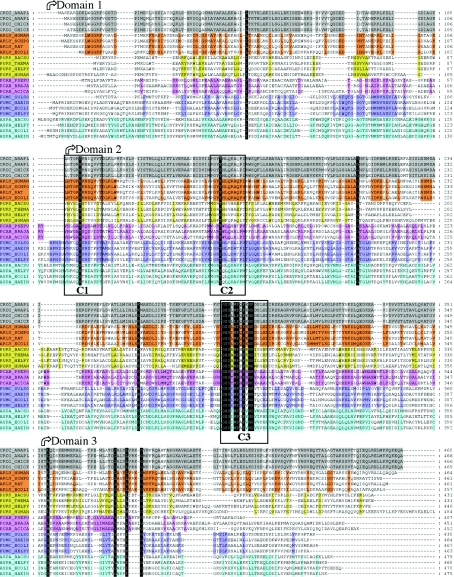
The enzymic mechanism of ASL/dδc2 has to be considered in the context of the superfamily of enzymes to which these proteins belong. Members of the superfamily include class II fumarase [11], aspartase [12], adenylosuccinate lyase [13] and 3-carboxy-cis,cis-muconate lactonizing enzyme [14]. All of these enzymes are active as homotetramers, and catalyse similar acid/base reactions in which a Cα–N or Cα–O bond is cleaved, with the subsequent release of fumarate as one of the products. Although the overall amino acid sequence identity between these proteins is low (20–30%), there are three highly conserved regions (denoted C1–C3 in Figure 1) that are remote from each other in the monomer structure, but which cluster together in the tetramer to form four active sites [15–18], as confirmed by structures of various superfamily members with bound inhibitors, substrates or substrate analogues [19–22].
Figure 1. Sequence alignment showing the conserved residues in the δ crystallin, ASL, adenylosuccinate lyase, 3-carboxy-cis,cis-muconate lactonizing enzyme, fumarase and aspartase families against grey, orange, yellow, purple, blue and cyan backgrounds respectively.
Abbreviations used: CRD1 and CRD2, δ1 and δ2 crystallin respectively; ARLY, ASL; PUR8, adenylosuccinate lyase; FUMC, fumarase C; ASPA, aspartase; PCAB, 3-carboxy-cis,cis-muconate lactonizing enzyme. The other letters represent the species names abbreviated according to the SwissProt nomenclature (e.g. ANAPL corresponds to Anas platyrhynchos, the domestic duck). The delimitation between the three structural domains is shown with arrows, while the location of the three conserved amino acid sequences in the ASL superfamily (C1, C2 and C3) is shown with boxes.
The conservation of residues in the active site, and the similarities of the reactions catalysed and the three-dimensional structures of the proteins, suggest that all members of the superfamily should share a common catalytic mechanism. However, there is currently no agreement regarding the residues involved in acid/base catalysis in the superfamily. Although the strictly conserved residue Lys289 has been proposed to play a crucial role in stabilizing the carbanion intermediate that forms during the reaction mechanism in all superfamily members [15,16], a variety of residues have been suggested to play the role of the acid catalyst [15,21–23]. (Note that the amino acid numbering throughout this paper corresponds to that of dδc2, which has a unique two-residue insertion at amino acid 5, which therefore shifts the numbering by +2 relative to that of dδc1 and ASL). In ASL/dδc2, Ser283, a residue that is strictly conserved across the superfamily, has been proposed to be the acid catalyst [24,25]. His162 or its equivalent residue has been suggested to be the catalytic base for ASL/dδc2, adenylosuccinate lyase and fumarase [15,17,21,26–28]. This histidine is not strictly conserved in other superfamily members, as it is replaced by glutamine in most species of aspartase and by tryptophan, leucine or arginine in 3-carboxy-cis,cis-muconate lactonizing enzyme (Sanger Institute, Protein families database of alignments and HMMs site, Lyase_1 family or PF00206; alignment of 465 sequences at www.sanger.ac.uk/Software/Pfam).
Thr161 has also been hypothesized to play a role in the enzymic mechanism of ASL/dδc2 [24]. This residue, adjacent to His162, is present in region C2 (Figure 1) and is conserved across the superfamily, with the exception of a few species of adenylosuccinate lyase, 3-carboxy-cis,cis-muconate lactonizing enzyme and ASL. In 26 out of 465 sequences in the alignment of the lyase family (PF00206 at www.sanger.ac.uk/Software/Pfam), this threonine is replaced by serine (Figure 1). In the structure of dδc1, Thr161 interacts with both Lys289 and the O-4 of a sulphate ion [24], while in the enzyme–substrate complex containing the dδc2 S283A mutant, Thr161 interacts via a water molecule with Lys289 and is also in close proximity to the Cβ atom and a carboxy group of the fumarate moiety of the AS substrate [25]. These observations, coupled with the lack of conservation of His162 across the superfamily, suggested a role for Thr161 in base catalysis [24]. The role of this residue was probed by site-directed mutagenesis, and kinetic analysis of the mutants found that while the T161S mutation, which mimics the naturally occurring substitution at this position, retained ∼72% catalytic efficiency, the T161A, T161D and T161V mutants were enzymically inactive (Table 1) [25,29]. While the loss of activity in the T161A and T161V mutants re-inforces the importance of a hydroxy group at this position, the effect of the T161D mutation was more difficult to interpret. To examine the role of Thr161 and the effect of the Thr→Ser and Thr→Asp mutations, the structures of the T161S and T161D mutants and of T161D complexed with AS (T161D–AS) have been determined at 2.0 Å resolution.
Table 1. Summary of kinetic parameters for wild-type and mutant dδc2.
The structures presented have been compared with other available ASL/δ crystallin structures, and suggest that a hydroxy group is required at position 161 to help correctly position the side chain of Lys289 and the fumarate moiety of the substrate for catalysis to occur. Threonine is favoured over serine because of the interaction of its methyl group with the neighbouring Leu206. This van der Waals interaction restricts the conformations accessed by the threonine, a restriction that probably improves the catalytic efficiency of the threonine-containing protein. The T161D mutant provides evidence that residues larger than Thr or Ser at position 161 will interfere with substrate binding, and re-inforces previous suggestions that correct positioning of the substrate's fumarate moiety is essential for catalysis to occur. Although initially unexpected, the absence of any significant change in conformation of the 280s loop (i.e. a loop formed by residues 280–290) relative to other ASL/δ2 crystallin structures suggests that loop closure may be triggered by the formation of the enzyme-intermediate whose charge distribution more closely mimics that of the sulphate ion found in the active-site region of the inactive δ1 crystallin. Loop closure is though to be essential for sequestration of the substrate from the solvent during catalysis.
MATERIALS AND METHODS
Site-directed mutagenesis
The construction of pET3d vectors containing the dδc2 gene with the T161D and T161S mutations was described in [25]. The T161A mutant was constructed using the Quick Change Site-Directed Mutagenesis method (Stratagene, La Jolla, CA, U.S.A.). The dδc2 wild-type gene in the pET3d vector served as the template. The oligonucleotides used were 5′-CTTGCCTGGCTACgccCACCTGCAGAAGG and its complement (the codon targeted for mutagenesis is denoted in lower case). DNA sequencing (ACGT Corp., Toronto, Canada) confirmed the presence of the desired mutation.
Expression and purification of Thr161 mutants
For each mutant, the vector was transformed into Escherichia coli BB101 and the protein expressed using a T7 polymerase system. The proteins were expressed and purified on Ni affinity columns as described previously for the C-terminally histidine-tagged wild-type and mutant dδc2 proteins [25,30]. To achieve the desired purity for crystallization, the T161D dδc2 protein was purified further using size-exclusion chromatography. Portions of 500 μl of protein (5 mg/ml) were loaded on a S-200 Sephacryl column of a Pharmacia LKB FPLC system and eluted with 10 mM Tris/HCl, pH 7.5, 1 mM EDTA at a flow rate of 0.5 ml/min. The protein purity of each fraction was assessed using SDS/PAGE, and fractions containing protein of the desired purity were pooled and concentrated prior to use in crystallization trials.
Kinetic characterization of the T161A mutant
T161A dδc2 was assayed for ASL activity by monitoring at 240 nm the production of fumarate at 25 °C (ε=2.44 mM−1·cm−1), as described previously [25]. Stock solutions of disodium AS (Sigma) were prepared in the range 0.2–20 mM. The stock solutions were subsequently diluted so that the final concentration in the reaction mixture (800 μl) was 0.02–2.0 mM. The assay was initially performed by adding 10 μg of protein to start the reaction. To ensure that no ASL activity could be detected at higher protein concentrations, the assay was repeated with 40 and 80 μg of protein.
Crystallization, data collection and structure determination
Rectangular plate-like crystals of T161D and T161S dδc2 were grown at room temperature using the hanging drop vapour diffusion method. The crystals were obtained using 10 mg/ml protein in 10 mM Tris/HCl, pH 7.5, and 1 mM EDTA, and precipitating solutions containing 100 mM Hepes, pH 7.0, 350 mM MgCl2 and 14% (w/v) poly(ethylene glycol) 2000 monomethyl ether for T161D dδc2, and 100 mM Hepes, pH 7.5, 300 mM MgCl2 and 14% (w/v) poly(ethylene glycol) 2000 monomethyl ether for T161S dδc2. Crystals of the inactive T161D dδc2 protein were also soaked for 5 days with 100 mM AS (disodium salt; Sigma). All crystals were soaked prior to flash freezing for 30–60 s in a 15% (v/v) glycerol/mother liquor solution. All data were collected at 100 K at beam line X8C (National Synchrotron Light Source, Brookhaven, NY, U.S.A.) and processed using the DENZO/SCALEPACK software package [31]. Prior to structure determination and refinement, the DREAR program package [32] was used to apply Bayesian statistics to the data to improve the weak data as well as to eliminate negative intensities [33] (Table 2).
Table 2. Data collection and data processing statistics.
Rsym=Σ|I−〈I〉|/ΣI, where I is the measured intensity for symmetry-related reflections and 〈I〉 is the mean intensity for the reflection.
| Parameter | T161S | T161D | T161D–AS |
|---|---|---|---|
| Space group | P21 | P21 | P21 |
| Cell dimensions | a=93.32 Å | a=93.13 Å | a=94.00 Å |
| b=98.87 Å | b=98.49 Å | b=98.81 Å | |
| c=106.10 Å | c=103.47 Å | c=106.39 Å | |
| β=101.53° | β=100.10° | β=101.34° | |
| Molecules/asymmetric unit | 4 | 4 | 4 |
| Resolution limits (Å) | 44.7–2.1 | 43.4–2.0 | 41.8–2.0 |
| Total number of reflections | 870528 | 997947 | 627324 |
| No. of unique reflections | 106162 | 124121 | 128293 |
| Mean redundancy | 8.2 | 8 | 4.9 |
| Completeness (%) | 93.2 (83.8)* | 99.9 (97.8)* | 99.7 (99.4)* |
| Average I/σ(I) | 27 | 23.8 | 18.2 |
| Reflections with I>2σ(I) (%) | 86.9 (74.6)* | 98.1 (94.0)* | 88.1 (67.0)* |
| Rsym | 0.083 (0.472)* | 0.056 (0.121)* | 0.063 (0.20)* |
* Last resolution shell: 2.14–2.1 Å, 2.07–2.0 Å and 2.07–2.0 Å for T161S, T161D and T161D–AS data, respectively.
Because of the ∼4 Å difference in the c-axis dimension of the T161D dδc2 unit cell relative to wild-type dδc2, the T161D dδc2 structure was determined by molecular replacement using the program CNS [34] with a wild-type dδc2 monomer [24] as the search model. The best rotation/translation solution resulted in a residual of 0.341 prior to rigid body refinement. The structures of T161S dδc2 and T161D–AS were solved using the wild-type dδc2 co-ordinates directly. Residuals before rigid body refinement were calculated to be 0.299 and 0.370 for the T161S and T161D–AS structures respectively.
Refinement and model building
The structures were refined using the program CNS [34] with a maximum likelihood target function [35,36], a flat bulk solvent correction [37] and no low resolution or σ(F) cut-off applied to the data. For cross-validation of the model, 10% of the data were randomly selected and used [35]. These data were never used during refinement. Rigid body refinement of the tetramer was followed by rigid body refinement of individual monomers. The monomers were then divided into their three structural domains, and rigid body refinement and B-group refinement of each domain was performed. Each subsequent refinement step consisted of torsion angle simulated annealing [38], grouped and individual B-factor refinement, and the calculation of σA weighted 2Fo-Fc and Fo-Fc electron density maps [39,40]. These maps were used to correct the model by manual rebuilding in Xtalview [41].
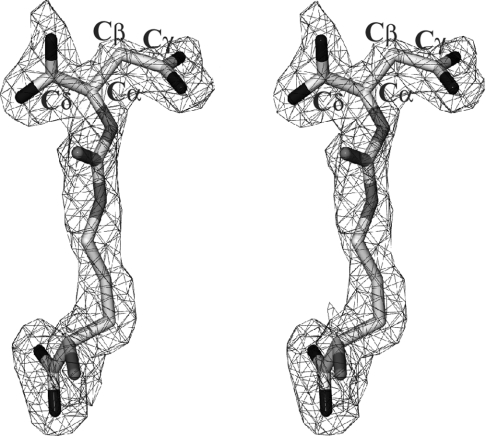
The dδc2 T161D–AS structure exhibited strong σA weighted Fo-Fc electron density in all four active sites (Figure 2), indicating the presence of bound substrate. The AS co-ordinates were obtained from the previously determined dδc2 S283A–AS complex structure [25]. The conformation of the substrate, however, had to be modified to reflect the difference density observed in the current structure. The AS topology and parameter files were generated by combining the parameters generated by XPLO2D [42] and those available for arginine and aspartate in CNS.
Figure 2. Stereo plot of the 2Fo-Fc σA weighted electron density for AS in the T161D–AS structure (contoured at 1σ).
The carbon atoms (light grey) corresponding to the fumarate moiety of the substrate have been labelled. Nitrogen and oxygen atoms are shown in medium and dark shades of grey respectively.
Non-crystallographic symmetry restraints were initially applied and gradually relaxed during the refinement procedure. A cis-peptide was modelled between Ser321 and Thr322 in all monomers. Water molecules with proper hydrogen-bonding co-ordination and electron densities larger than 1σ on 2Fo-Fc and 2.5σ on 2Fo-Fc σA weighted maps were progressively introduced while monitoring the decrease in Rfree. PROCHECK [43] and WHATIF [44] were used to analyse the stereochemistry of the models. The final refinement statistics are presented in Table 3. A total of 893, 925 and 910 water molecules were modelled in the T161S, T161D and T161D–AS structures respectively.
Table 3. Summary of the final model refinement statistics.
Rcryst=Σ (|Fo|−|Fc|)/Σ|Fo|; Rfree=Σ(|Fos|−|Fcs|)/Σ|Fos|, where ‘s’ refers to a subset of data not used in the refinement, representing 10% of the total number of observations. The numbers in parentheses refer to the Rcryst and Rfree in the last resolution shell: 2.14–2.1 Å, 2.07–2.0 Å and 2.07–2.0 Å for the T161S, T161D and T161D–AS structures respectively. R.m.s., root mean square. No residues were found in the disallowed regions of the Ramachandran plots.
| Parameter | T161S | T161D | T161D–AS |
|---|---|---|---|
| Resolution range (Å) | 44.7–2.1 | 43.4–2.0 | 41.8–2.0 |
| Rcryst (%) | 17.8 (21.1) | 17.3 (17.3) | 19.0 (20.9) |
| Rfree (%) | 22.1 (26.5) | 21.3 (23.2) | 22.5 (26.6) |
| No. of reflections used in the refinement | 106162 | 124121 | 128293 |
| No. of reflections used to compute Rfree | 10623 | 12399 | 12767 |
| No. of non-hydrogen atoms | |||
| Protein | 13856 | 13892 | 13833 |
| Solvent | 893 | 925 | 910 |
| AS | 80 | ||
| Mean B factor (Å2) | |||
| Protein | 26 | 20 | 22 |
| Per monomer: A/B/C/D | 25/26/26/25 | 20/20/21/19 | 21/25/21/23 |
| Solvent | 29 | 24 | 26 |
| AS | 25/26/29/31 | ||
| R.m.s. deviation from ideal values | |||
| Bond lengths (Å) | 0.009 | 0.008 | 0.009 |
| Bond angles (°) | 1.3 | 1.2 | 1.3 |
| Dihedral angles (°) | 19.3 | 19.3 | 19.3 |
| Improper angles (°) | 0.93 | 0.86 | 0.9 |
| Residues in most favoured regions of Ramachandran plot (%) | 94.9 | 95.4 | 95.3 |
Amino acid sequence alignments and structural comparisons
The available δ crystallin, ASL, fumarase, aspartase, adenylosuccinate lyase and 3-carboxy-cis,cis-muconate lactonising enzyme sequences were retrieved from the SwissProt data base, and CLUSTAL X [45] was used to perform the multiple sequence alignment. An initial profile was determined by aligning δ crystallin and ASL sequences, and the rest of the sequences were subsequently aligned to this profile. For simplicity, only a few representative sequences of each enzyme in the superfamily are shown in Figure 1. This sequence alignment was further improved based on the structural alignment (see below) of representative structures of dδ1c and dδc2 (PDB codes 1HY0 and 1HY1 respectively), the Q286R ASL mutant (1K62), E. coli fumarase C (1FUO), E. coli aspartase (1JSW) and Thermotoga maritima adenylosuccinate lyase (1C3C).
The structural comparisons were performed using the RIGID option in the program TURBO-FRODO [46]. Structurally equivalent residues were selected and an iterative least-squares fitting procedure was performed prior to calculating the average r.m.s. (root mean square) deviation. For dδc2, the Cα position of residues 120, 122, 126, 128, 130, 180, 184, 188, 190, 245, 247, 250, 254, 300, 302, 304, 308, 310, 340, 342, 348 and 350 of each monomer were used to structurally align the tetramers.
RESULTS
T161A dδc2 mutant
To further probe the necessity for a hydroxy group at position 161 and to examine the effect of removing all side chain substituents, a T161A mutant of dδc2 was constructed. The T161A mutant was found to be catalytically inactive (Table 1), further confirming our hypothesis that a hydroxy group is required for catalysis.
Overall fold and active-site architecture of the T161D and T161S dδc2 mutants
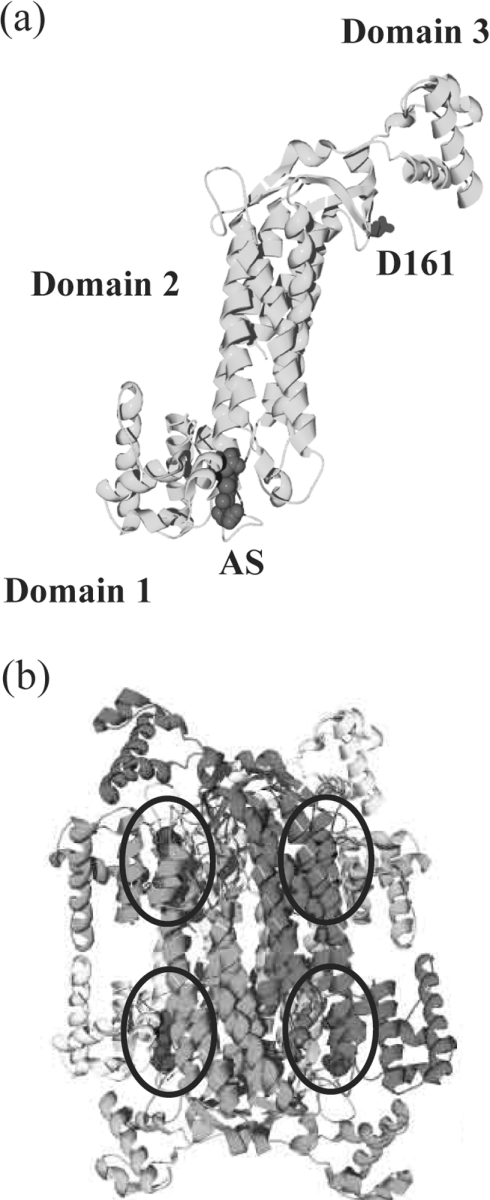
The electron density corresponding to the amino acid at position 161 in the T161S and T161D dδc2 structures (results not shown) confirmed the presence of serine and aspartate side chains respectively, in agreement with the mutagenesis results. The overall structure of the T161D and T161S dδc2 mutants is similar to that described previously for δ crystallin [15,17,19,24,25] and human ASL [18,47] (Figure 3). Variable numbers of residues (18–19) at the N-terminus are missing in all monomers due to the poor quality of the electron density for this region. Additionally, residues 467 and 468 at the C-terminus are also missing in some monomers.
Figure 3. Structure of the T161D monomer and tetramer.
(a) Schematic representation of the T161D–AS monomer (light grey). The side chain of Asp161 (medium grey) is shown in stick representation, with the three structural domains labelled. The AS substrate is rendered as a CPK model in dark grey. (b) The T161D–AS tetramer with each monomer shown in a different shade of grey. The tetramer exhibits D2 or 222 symmetry. The four active sites, formed at the interface of three different monomers, are circled, and the bound AS substrate (dark grey) is shown as depicted in (a).
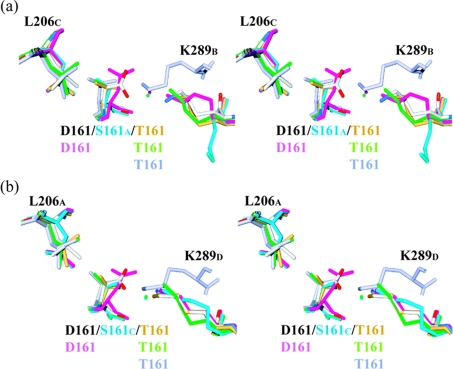
The mutation of Thr161 to Ser or Asp produces very few structural changes in the local environment. This is probably due to the location of residue 161 in the active-site cleft and the limited number of interactions that it makes with other residues and/or water molecules (Figure 4). In wild-type dδc2, the methyl group of Thr161 establishes van der Waals interactions with the side chain of Leu206, while the hydroxy group either has no obvious interacting partners or interacts with Lys289 or a water molecule. The multi-subunit nature of the ASL/dδc crystallin active site means that residues Thr161, Leu206 and Lys289 each belong to different monomers. The conformation of the side chains of Thr161 in wild-type dδc2 in three of the four monomers is similar to that observed in the S283A–AS structure. The presence of bound substrate in the active site of the S283A–AS structure orients all four Thr161 residues so that the threonine's methyl group always interacts with Leu206, while its hydroxy group interacts with Oδ1 of AS (Table 4) and Lys289 via a water molecule (Figure 4).
Figure 4. Stereoview showing interactions between residue 161 and neighbouring Leu206 and Lys289 residues.
Interactions are shown for the T161D–AS (coloured according to atom type), T161D (pink), T161S (blue), S283A–AS (green) and wild-type dδc2 (gold) and dδc1 (blue–grey) structures. The water molecules mediating the interaction between residue 161 and Lys289 are also shown as spheres. Ser161 is illustrated in a conformation that (a) prevents interaction with Lys289 or (b) allows interactions with Lys289. Due to the multi-subunit nature of each active site, the Xi notation represents residue X belonging to monomer i, where i=A, B, C or D.
Table 4. Distances between the fumarate moiety of AS and residue 161 in the S283A–AS, T161S and T161D dδc2 structures.
Distances were determined from the superposition of the S283A–AS with the T161S and T161D structures.
| S283A–AS | T161S | T161D | ||||
|---|---|---|---|---|---|---|
| AS atom | Thr161 | (Å) | Ser161 | (Å) | Asp161 | (Å) |
| Cβ | Oγ1 | 3.5 | Oγ | 3.6 | Oδ1/Oδ2 | 2.1/2.1 |
| Oδ1 | Oγ1 | 3.4 | Oγ | 3.3 | Oδ1/Oδ2 | 2.6/2.6 |
| Oδ2 | Oγ1 | 3.8 | Oγ | 2.7 | Oδ1/Oδ2 | 1.8/3.3 |
In the T161S structure, the side chain of Ser161 is less tightly held in place due to the loss of van der Waals interactions with Leu206 and the absence of bound substrate. Two conformations of Ser161 are observed (Figure 4). In one conformation, the hydroxy group interacts via a water molecule with Lys289, while in the second conformation the hydroxy group is oriented towards Leu206. In this conformation, Lys289 adopts a significantly different conformation than that normally observed (Figure 4a). The water-mediated interaction between Ser161 and Lys289 is only maintained in one of the four active sites (Figure 4b).
In the T161D and T161D–AS structures, the conformation of the bulkier, negatively charged side chains of Asp161 in the four active sites of the unbound structure shows more conformational variation than in the AS-bound structure. In the T161D structure, the negatively charged Asp161 residue interacts with Lys289 via a water molecule (Figure 4). In the T161D–AS structure this interaction is also observed (results not shown), although in two monomers the distance between Asp161 and Lys289 is shortened, allowing direct hydrogen bonding to occur (Figure 4). This shortening is coupled with the ordering of two to three water molecules around the substrate and their interaction with the carboxylate groups of Asp161.
AS binding in the inactive T161D dδc2 mutant
Strong σA weighted difference density in all four active sites (Figure 2) allowed unambiguous modelling of both the arginine and the fumarate moieties of the substrate. The conformation of the bound AS is the same in all four active sites. Residues in domain 1 that co-ordinate the arginine moiety undergo a small (∼2 Å) shift on substrate binding (results not shown). This shift is comparable with the movement seen previously in the S283A–AS [25] and H162N–AS dδc2 structures [19]. No other significant changes in the backbone of the monomers occur on substrate binding. Although the side chain of Asp161 in the T161D–AS structure adopts a different conformation relative to other structures (Figures 4a and 4b), most of the hydrogen-bond interactions between the enzyme and substrate, especially for the arginine moiety, are similar to those found in the S283A–AS and H162–AS enzyme–substrate complexes (Figures 5b–5d). The conformation of the arginine moiety is relatively conserved in the T161D–AS, S283A–AS [25] and H162N–AS dδc2 structures [19] (Figure 5a). It should be noted that, in the H162N–AS complex, the substrate was observed in only one of the four active sites, and that the quality of its electron density was poorer than that observed for either the S283A–AS or the T161D–AS structure (Figure 2). Major differences in the network of interactions occur at the fumarate end of the substrate (Figures 5b–5d), and these are directed related to the flexibility of this part of the substrate and to the differences in the conformation of fumarate moiety observed in the enzyme–substrate complexes determined to date (Figure 5a). As described below, catalysis will be strictly dependent on the correct positioning of the flexible fumarate moiety of the substrate.
Figure 5. Conformation of AS and its interaction with the protein.
(a) Stereo view of AS as observed in the T161D–AS (coloured by atom type), S283A–AS (green) and H162N–AS (orange) structures. For comparison purposes, the sulphate molecule bound in the active-site region of dδc1 (pink) is also shown. (b)–(d) Schematic diagrams of the hydrogen-bonding network (dashed lines) and van der Waals interactions (solid lines) between the AS substrate, water molecules (grey-shaded boxes, denoted with a ‘W’) and active-site residues, as observed in the structures of (b) T161D–AS, (c) S283A–AS and (d) H162N–AS. Only amino acid residues that interact directly with the substrate whose distances are <3.2 Å have been drawn. (e) Stereo view of residues Asp161, His162, Glu296 and the substrate (AS) in the T161D–AS (coloured according to atom type), T161D (pink), T161S (light blue) and wild-type dδ2c (gold) structures. Due to the multi-subunit nature of each active site, the Xi notation represents residue X belonging to monomer i, where i=A, B, C or D.
Conformation of the 280s loop
The conformation of the 280s loop was of particular interest, as residues belonging to the highly conserved C3 sequence (Figure 1) are mapped to this region. The electron density for the 280s loop in the T161S, T161D and T161D–AS structures is in the open conformation, similar to that observed in all other δ2 crystallin/ASL structures determined to date. The only structure where the 280s loop has been observed in a closed conformation is dδc1 [24]. The implications of these observations for the catalytic mechanism are discussed below.
DISCUSSION
Requirement for a small hydroxy-containing residue at position 161
The presence of a strictly conserved small hydroxy group-containing side chain (Thr or Ser) at position 161 in all ASL/δ crystallin superfamily members (Figure 1), and its proximity to the substrate in the S283A–AS dδc2 structure, prompted us to examine the role that this residue may play in catalysis. Site-directed mutagenesis has confirmed the necessity for a hydroxy group at this position, as the T161A, T161V and T161D mutants had no observable enzymic activity, while the T161S mutant had ∼70% catalytic efficiency (Table 1 and [25]). The Thr→Ser substitution does not affect substrate binding, but does decrease the Vmax of the reaction, suggesting that a threonine side chain is better adapted for the subsequent catalytic steps (Table 1 and [25]).
Effect of the Thr161→Ser mutation and reduction in catalytic efficiency
The T161S structure re-inforces the hypothesis that the threonine side chain may be better adapted for catalysis as, unlike Thr161, Ser161 adopts two different side-chain conformations in the T161S structure. The absence of the methyl group eliminates the van der Waals interactions with Leu206 seen in the wild-type dδc2 and other Thr161-containing structures. This interaction orients the threonine so that its hydroxy group always interacts either directly or via a water molecule with Lys289 (Figure 4). In only one active site (active site C) in the T161S structure is the hydroxy group of Ser161 oriented so that it interacts with Lys289 via a water molecule. In the other three active sites, the hydroxy group is oriented towards Leu206, and Lys289 adopts a different conformation (Figure 4a).
The substrate conformation observed in the S283A–AS structure is considered to represent that in the enzyme–substrate complex. In this structure, the mutation does not affect the geometry or the electrostatic environment of the active site [25], as the 280s loop is in the open conformation and >7 Å from the substrate. To understand how the substrate would bind to the T161S mutant, we compared the T161S–AS and S283A–AS structures, and found that active site C of the T161S mutant would be able to bind the substrate in a similar conformation to that observed in the S283A–AS structure. The distances between the Oγ atom of Ser161 and the Oδ1 and Cβ atoms of the fumarate moiety are comparable with those found for Thr161 in the S283A–AS structure (Table 4). The ∼1 Å difference between the hydroxy groups of Ser161 or Thr161 and the Oδ2 atom of AS (Table 4) indicates that a slight re-orientation of the carboxy group of the fumarate and/or of the Ser161 side chain might occur upon substrate binding. In active sites A, B and D, the conformation of the Ser161 side chain would prevent its hydroxy group from interacting with the substrate.
The structure of mutant T161S shows that, in the absence of the methyl group, Ser161 can adopt at least two alternative conformations and that the orientation of Ser161 affects the position of Lys289. Our modelling results also suggest that the T161S mutation does not affect the binding of the arginine moiety of the substrate, but that a small re-orientation of the fumarate moiety and/or serine may occur on substrate binding. Our data also suggest that for catalysis to occur the hydroxy group of Thr161 or Ser161 must interact with Lys289 and Oδ1 of the substrate when it is present, as the absence of such interactions abolishes catalytic activity (Table 1). The reduced catalytic efficiency of the T161S mutant probably results from the conformational flexibility of the Ser161 residue and the necessity for it and Lys289 to re-orientate prior to and/or on substrate binding. A further decrease in catalytic efficiency may result from any small variations in the way in which the fumarate moiety of the substrate is bound.
Effect of the Thr161→Asp mutation
Given the difference in size, it was not unexpected that the conformation of Asp161 would differ from that observed for Thr161 in wild-type dδc2 (Figures 4 and 5). When we examined how substrate might bind to the T161D mutant, the superposition of the T161D and S283A–AS structures suggested that steric clashes would occur between the Oδ1 and Oδ2 atoms of Asp161 and the substrate (Table 4). These potential electrostatic repulsions are relieved in the T161D–AS structure by small conformational changes in the side chain of Asp161 and re-orientation of the substrate (Figures 4 and 5). Examination of other residues implicated in catalysis reveals that although the strong hydrogen bond between the imidazole group of His162 and the Cγ OO− carboxylate of the fumarate moiety (Figure 5e) is maintained in the T161D–AS complex, neither Oδ2 of Asp161 nor Nε2 of His162 is close to the Cβ atom of AS (5.0 Å and 5.7 Å respectively). Neither residue is in a position to abstract the proton from Cβ of the fumarate moiety. The charge relay between His162 and Glu296 is also unaffected by the T161D mutation, which suggests that the intrinsic potential for His162 to abstract a proton is unaltered (Figure 5e). The conformation of the Lys289 side chain in the T161D mutant structures is also similar to that observed in the S281A–AS structure (Figure 4), which would allow this residue to interact with the carbanion intermediate.
Proper co-ordination of the arginine moiety appears to be the driving force for AS binding, as the conformation of this moiety and the interactions that it makes with the protein are conserved in all three enzyme–substrate complexes determined to date (Figures 5b–5d). Catalysis, however, is strictly dependent on the correct positioning of the fumarate moiety, which exhibits considerable conformational variation (Figure 5a). In the T161D mutant, the conformation of the fumarate is influenced by the necessity for the substrate to accommodate the negatively charged aspartate residue. Although flexibility in the fumarate end of the substrate is expected due to the required change in hybridization states of the Cα and Cβ atoms from sp3 to sp2 during the course of the enzymic reaction, the wide range of conformations sampled by this part of the substrate (Figure 5a) was unanticipated. The bulkier, negatively charged Asp161 residue does not appear to impede AS binding, but instead alters the conformation of the fumarate moiety, such that key catalytic residues, for example His162, are prevented from interacting with the substrate.
Combined, the results with the T161S and T161D mutants suggest that the conservation of threonine or serine at this position is the result of two evolutionary pressures. The hydroxy group appears to be required because, when in the correct orientation, it helps to orient not only the strictly conserved Lys289, a residue known to be crucial for catalysis, but also the fumarate moiety of the substrate. Maintenance of a similar size is required, as bulkier residues, while having minimal effects on the local protein environment, would produce significant conformational changes in the substrate, preventing the fumarate moiety from binding to the enzyme in a catalytically competent manner. Our results support the suggestion made previously for Bacillus sp. YM55-1 aspartase [23] that Thr or Ser at position 161 is not involved directly in catalysis, but is involved in binding a carboxy group of the substrate and/or proper positioning the conserved lysine residue.
The charge distribution of the carbanion intermediate triggers closure of the 280s loop
The most conserved amino acid sequence across the ASL/fumarase superfamily (Figure 1) is the highly flexible region called the 280s loop. When electron density permits the modelling of this loop, two distinct conformations have been observed. In T161S, T161D, T161D–AS and other dδc2 structures [15,17,24,25], the 280s loop is in an open conformation, allowing unrestricted access to the active site. However, in the inactive dδc1 structure, the 280s loop is in a closed conformation [24]. The conformational changes observed in dδc1 are the consequence of a sulphate ion bound in the active-site region. The sulphate induces a >8 Å movement in the residues at the tip of the 280s loop and a rigid body motion in domain 3 [24]. These conformational changes shield the substrate from the bulk solvent, a process predicted biochemically for fumarase in 1980 [48,49] and for bovine ASL in 1985 [26].
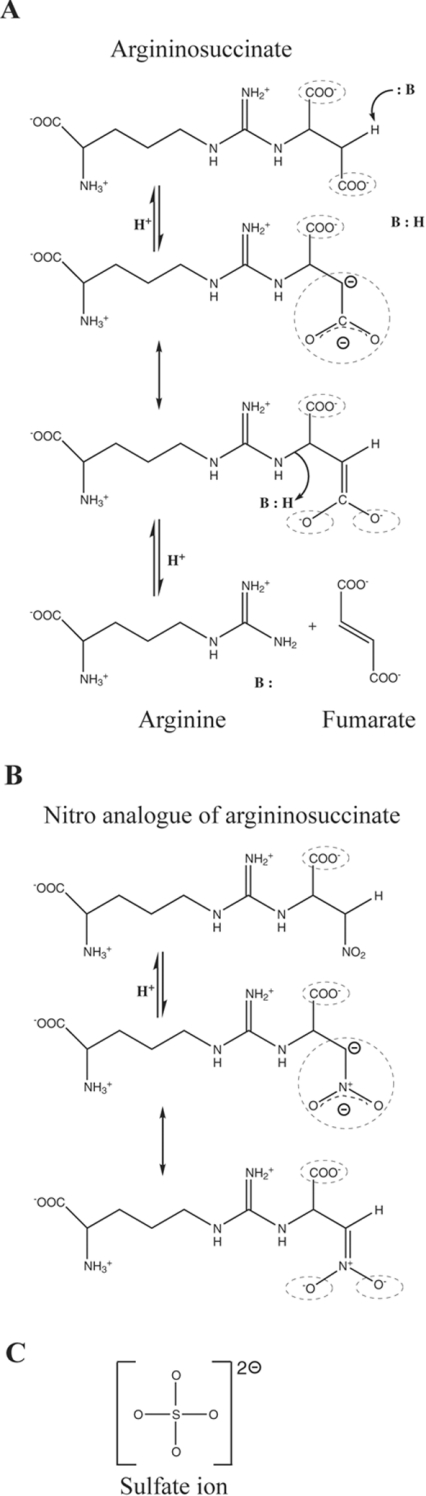
While the sulphate (SO42−) group in the dδc1 structure superimposes with the Cβ–CδOO− end of the fumarate moiety observed in the structure of S283A–AS dδc2 (S–Cβ distance of 0.9 Å), there is a significant difference between the position of the sulphate and the equivalent fumarate group (S–Cβ distance of 2.6 Å) in the T161D–AS structure. Since the substrate in the T161D–AS structure appears to be bound in a non-functional configuration, the following discussion focuses on the substrate conformation observed in the S283A–AS structure. While the sulphate and fumarate moiety of AS each have two formal negative charges, there are significant differences in the distribution of this charge. In AS the two negative charges are quite remote from each other (∼4.5 Å), while in the sulphate ion they are much closer, delocalized over the four oxygen atoms (∼2.4 Å) (Figure 6). The smaller negatively charged sulphate ion is probably a good mimic of the carbanion or aci-carboxylate intermediates that form during the reaction mechanism (Figure 6a). The distance between the Cβ− and the CδOO− groups in the carbanion intermediate would be ∼2.2 Å, similar to the distance between the negative charges of the CδOO2− group in aci-carboxylate form of the intermediate (Figure 6a). The proximity of these negative charges in the intermediates resembles the distance between the partial negative charges on the SO42− group bound in the active-site region of dδc1 (Figure 6c). We propose that, as the charge distribution and the conformation of the fumarate moiety change during the enzymic reaction, the flexible, positively charged side chain of the strictly conserved Lys289 interacts with the negatively charged groups of the intermediates, and in so doing triggers the 280s loop to close over the active site. The proposed role of Lys289 in stabilizing the carbanion intermediate [15] is re-supported by results with the K289R and K289A dδc2 mutants, which are catalytically inactive (M. Tsai, unpublished work), the K289R mutation of E. coli aspartase, which drastically decreased the enzymic activity to only 0.3% of that of the wild-type enzyme [16], and by the structure of dδc1, which shows Nζ of Lys289 interacting with the sulphate [24]. The structural comparisons presented here suggest that the side chain of residue 161 has to be in a conformation that allows it to interact with Lys289, as only in such an orientation is the hydroxy group of Thr or Ser and the Nζ of Lys289 capable of interacting with the substrate. The water molecule that mediates the Thr/Ser161–Lys289 interaction when the 280s loop is in the open conformation is displaced at some point during substrate binding and/or catalysis, to allow Thr161 to hydrogen bond directly with Lys289. The movement of the 280s loop, together with the rigid body motion of domain 3, would sequester the substrate from the solvent and bring Ser283, another residue shown to be important for catalysis [24,25,29], into close proximity with the fumarate moiety (the N1–Cα–CγOO− end). The corresponding loop (denoted the SS-loop) in the thermostable aspartase from Bacillus sp. YM55-1 has been proposed to play the same role [23]. The hypothesis presented here is further supported by the observation that nitro analogues of AS (Figure 6b) are strong competitive inhibitors of bovine ASL, having a Ki 20 times smaller than the Km for the natural substrate [50,51]. As seen in Figure 6, the nitro analogue in the active nitronate form has the same net charge (2−) and is structurally similar to the aci-carboxylate intermediate form of AS. Strong competitive inhibition with the corresponding nitro derivatives has been also reported for adenylosuccinate lyase [52], fumarase and aspartase [48].
Figure 6. Schematic representations of (a) the reaction mechanism of ASL/dδc2, (b) the reaction mechanism with the AS-nitro analogue and (c) the sulphate molecule.
In (a), both the carbanion and aci-carboxylate forms are shown; in (b) both the carbanion and nitronate forms are shown. The charged groups discussed in the text are circled with dashed lines.
Conclusions
In summary, the structure of the dδc2 T161S mutant and the comparison with other δ crystallin structures suggests that Thr and Ser are the only residues that are suitable at position 161, since their hydroxy group is required for the correct positioning of both the side chain of Lys289 and the fumarate moiety of the substrate. Threonine is favoured because the van der Waals interactions of its methyl group with Leu206 restrict its conformational freedom. The substitution of alanine, valine or aspartate for threonine results in an inactive enzyme. While we hypothesize that the T161A and T161V mutants are inactive because they lack a hydroxy group, the T161D and T161D–AS structures suggest that the loss of activity in this mutant is due to the structural rearrangement of the substrate's fumarate moiety, which is required to relieve steric clashes with the aspartate side chain. The flexibility exhibited in the substrate is likely to play an important role during the reaction mechanism, as the substrate has to undergo conformational changes in its transition to arginine and fumarate. The similar charge distribution of the carbanion/aci-carboxylate intermediates and sulphate suggest that it is the formation of the intermediates that most probably triggers the closure of the 280s loop. Loop closure is thought to be essential for sequestration of the substrate from the solvent during catalysis. While the results presented here do not rule out a direct role for Thr/Ser161 in base catalysis, it seems more likely that this residue plays a role in substrate binding.
Acknowledgments
This research is supported by an operating grant from the Canadian Institute for Health Research (CIHR) to P.L.H. and a major facilities access grants from CIHR and the National Science and Engineering Research Council of Canada (NSERC) to operate beamline X8C at the National Synchrotron Light Source (Brookhaven, Long Island, NY, U.S.A.). P.L.H. is the recipient of a CIHR Investigator Award. L.M.S. and M.T. were supported, fully or in part, by the Ontario Student Opportunity Trust Fund, Hospital for Sick Children Foundation Student Scholarship Program.
References
- 1.Piatigorsky J. Lens crystallins and their genes: diversity and tissue-specific expression. FASEB J. 1989;3:1933–1940. doi: 10.1096/fasebj.3.8.2656357. [DOI] [PubMed] [Google Scholar]
- 2.Piatigorsky J., Kantorow M., Gopal-Srivastava R., Tomarev S. I. Recruitment of enzymes and stress proteins as lens crystallins. EXS. 1994;71:241–250. doi: 10.1007/978-3-0348-7330-7_24. [DOI] [PubMed] [Google Scholar]
- 3.Mori M., Matsubasa T., Amaya Y., Takiguchi M. Molecular evolution from argininosuccinate lyase to delta-crystallin. Prog. Clin. Biol. Res. 1990;344:683–699. [PubMed] [Google Scholar]
- 4.Piatigorsky J., O'Brien W. E., Norman B. L., Kalumuck K., Wistow G. J., Borras T., Nickerson J. M., Wawrousek E. F. Gene sharing by delta-crystallin and argininosuccinate lyase. Proc. Natl. Acad. Sci. U.S.A. 1988;85:3479–3483. doi: 10.1073/pnas.85.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Brien W. E., McInnes R., Kalumuck K., Adcock M. Cloning and sequence analysis of cDNA for human argininosuccinate lyase. Proc. Natl. Acad. Sci. U.S.A. 1986;83:7211–7215. doi: 10.1073/pnas.83.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H. J., Chiou S. H., Chang G. G. Biochemical characterization and kinetic analysis of duck delta-crystallin with endogenous argininosuccinate lyase activity. Biochem. J. 1992;283:597–603. doi: 10.1042/bj2830597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiou S. H., Lee H. J., Chu H., Lai T. A., Chang G. G. Screening and kinetic analysis of delta-crystallins with endogenous argininosuccinate lyase activity in the lenses of vertebrates. Biochem. Int. 1991;25:705–713. [PubMed] [Google Scholar]
- 8.Kondoh H., Araki I., Yasuda K., Matsubasa T., Mori M. Expression of the chicken ‘delta 2-crystallin’ gene in mouse cells: evidence for encoding of argininosuccinate lyase. Gene. 1991;99:267–271. doi: 10.1016/0378-1119(91)90137-z. [DOI] [PubMed] [Google Scholar]
- 9.Chiou S. H., Hung C. C., Lin C. W. Biochemical characterization of crystallins from pigeon lenses: structural and sequence analysis of pigeon delta-crystallin. Biochim. Biophys. Acta. 1992;1160:317–324. doi: 10.1016/0167-4838(92)90094-t. [DOI] [PubMed] [Google Scholar]
- 10.Barbosa P., Wistow G. J., Cialkowski M., Piatigorsky J., O'Brien W. E. Expression of duck lens delta-crystallin cDNAs in yeast and bacterial hosts. Delta 2-crystallin is an active argininosuccinate lyase. J. Biol. Chem. 1991;266:22319–22322. [PubMed] [Google Scholar]
- 11.Woods S. A., Schwartzbach S. D., Guest J. R. Two biochemically distinct classes of fumarase in Escherichia coli. Biochim. Biophys. Acta. 1988;954:14–26. doi: 10.1016/0167-4838(88)90050-7. [DOI] [PubMed] [Google Scholar]
- 12.Woods S. A., Miles J. S., Roberts R. E., Guest J. R. Structural and functional relationships between fumarase and aspartase. Nucleotide sequences of the fumarase (fumC) and aspartase (aspA) genes of Escherichia coli K12. Biochem. J. 1986;237:547–557. doi: 10.1042/bj2370547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone R. L., Zalkin H., Dixon J. E. Expression, purification, and kinetic characterization of recombinant human adenylosuccinate lyase. J. Biol. Chem. 1993;268:19710–19716. [PubMed] [Google Scholar]
- 14.Williams S. E., Woolridge E. M., Ransom S. C., Landro J. A., Babbitt P. C., Kozarich J. W. 3-Carboxy-cis,cis-muconate lactonizing enzyme from Pseudomonas putida is homologous to the class II fumarase family: a new reaction in the evolution of a mechanistic motif. Biochemistry. 1992;31:9768–9776. doi: 10.1021/bi00155a033. [DOI] [PubMed] [Google Scholar]
- 15.Simpson A., Bateman O., Driessen H., Lindley P., Moss D., Mylvaganam S., Narebor E., Slingsby C. The structure of avian eye lens delta-crystallin reveals a new fold for a superfamily of oligomeric enzymes. Nat. Struct. Biol. 1994;1:724–734. doi: 10.1038/nsb1094-724. [DOI] [PubMed] [Google Scholar]
- 15a.Erratum. Nat. Struct. Biol. 1994;11:831. [Google Scholar]
- 16.Saribas A. S., Schindler J. F., Viola R. E. Mutagenic investigation of conserved functional amino acids in Escherichia coli L-aspartase. J. Biol. Chem. 1994;269:6313–6319. [PubMed] [Google Scholar]
- 17.Abu-Abed M., Turner M. A., Vallée F., Simpson A., Slingsby C., Howell P. L. Structural comparison of the enzymatically active and inactive forms of delta crystallin and the role of histidine 91. Biochemistry. 1997;36:14012–14022. doi: 10.1021/bi971407s. [DOI] [PubMed] [Google Scholar]
- 18.Turner M. A., Simpson A., McInnes R. R., Howell P. L. Human argininosuccinate lyase: a structural basis for intragenic complementation. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9063–9068. doi: 10.1073/pnas.94.17.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallée F., Turner M. A., Lindley P. L., Howell P. L. Crystal structure of an inactive duck delta II crystallin mutant with bound argininosuccinate. Biochemistry. 1999;38:2425–2434. doi: 10.1021/bi982149h. [DOI] [PubMed] [Google Scholar]
- 20.Weaver T. M., Levitt D. G., Donnelly M. I., Stevens P. P., Banaszak L. J. The multisubunit active site of fumarase C from Escherichia coli. Nat. Struct. Biol. 1995;2:654–662. doi: 10.1038/nsb0895-654. [DOI] [PubMed] [Google Scholar]
- 21.Weaver T., Banaszak L. Crystallographic studies of the catalytic and a second site in fumarase C from Escherichia coli. Biochemistry. 1996;35:13955–13965. doi: 10.1021/bi9614702. [DOI] [PubMed] [Google Scholar]
- 22.Weaver T., Lees M., Zaitsev V., Zaitseva I., Duke E., Lindley P., McSweeny S., Svensson A., Keruchenko J., Keruchenko I., et al. Crystal structures of native and recombinant yeast fumarase. J. Mol. Biol. 1998;280:431–442. doi: 10.1006/jmbi.1998.1862. [DOI] [PubMed] [Google Scholar]
- 23.Fujii T., Sakai H., Kawata Y., Hata Y. Crystal structure of thermostable aspartase from Bacillus sp. YM55-1: structure-based exploration of functional sites in the aspartase family. J. Mol. Biol. 2003;328:635–654. doi: 10.1016/s0022-2836(03)00310-3. [DOI] [PubMed] [Google Scholar]
- 24.Sampaleanu L. M., Vallee F., Slingsby C., Howell P. L. Structural studies of duck delta 1 and delta 2 crystallin suggest conformational changes occur during catalysis. Biochemistry. 2001;40:2732–2742. doi: 10.1021/bi002272k. [DOI] [PubMed] [Google Scholar]
- 25.Sampaleanu L. M., Yu B., Howell P. L. Mutational analysis of duck delta 2 crystallin and the structure of an inactive mutant with bound substrate provide insight into the enzymatic mechanism of argininosuccinate lyase. J. Biol. Chem. 2002;277:4166–4175. doi: 10.1074/jbc.M107465200. [DOI] [PubMed] [Google Scholar]
- 26.Garrard L. J., Bui Q. T., Nygaard R., Raushel F. M. Acid-base catalysis in the argininosuccinate lyase reaction. J. Biol. Chem. 1985;260:5548–5553. [PubMed] [Google Scholar]
- 27.Lee H. J., Chiou S. H., Chang G. G. Inactivation of the endogenous argininosuccinate lyase activity of duck delta-crystallin by modification of an essential histidine residue with diethyl pyrocarbonate. Biochem. J. 1993;293:537–544. doi: 10.1042/bj2930537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patejunas G., Barbosa P., Lacombe M., O'Brien W. E. Exploring the role of histidines in the catalytic activity of duck delta-crystallins using site-directed mutagenesis. Exp. Eye Res. 1995;61:151–154. doi: 10.1016/s0014-4835(05)80034-x. [DOI] [PubMed] [Google Scholar]
- 29.Chakraborty A. R., Davidson A., Howell P. L. Mutational analysis of amino acid residues involved in argininosuccinate lyase activity in duck delta II crystallin. Biochemistry. 1999;38:2435–2443. doi: 10.1021/bi982150g. [DOI] [PubMed] [Google Scholar]
- 30.Sampaleanu L. M., Davidson A. R., Graham C., Wistow G. J., Howell P. L. Domain exchange experiments in duck delta-crystallins: functional and evolutionary implications. Protein Sci. 1999;8:529–537. doi: 10.1110/ps.8.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otwinowski Z., Minor W. Denzo/Scalepack. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 32.Blessing R. H. Data reduction and error analysis for accurate single crystal diffraction intensities. Crystallogr. Rev. 1987;1:3–58. [Google Scholar]
- 33.French S., Wilson K. On the treatment of negative intensity observations. Acta Crystallogr. 1978;A42:517–525. [Google Scholar]
- 34.Brunger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 35.Adams P. D., Pannu N. S., Read R. J., Brunger A. T. Cross-validated maximum likelihood enhances crystallographic simulated annealing refinement. Proc. Natl. Acad. Sci. U.S.A. 1997;94:5018–5023. doi: 10.1073/pnas.94.10.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pannu N. S., Murshudov G. N., Dodson E. J., Read R. J. Incorporation of prior phase information strengthens maximum-likelihood structure refinement. Acta Crystallogr. D Biol. Crystallogr. 1998;54:1285–1294. doi: 10.1107/s0907444998004119. [DOI] [PubMed] [Google Scholar]
- 37.Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]
- 38.Rice L. M., Brunger A. T. Torsion angle dynamics: reduced variable conformational sampling enhances crystallographic structure refinement. Proteins. 1994;19:277–290. doi: 10.1002/prot.340190403. [DOI] [PubMed] [Google Scholar]
- 39.Brunger A. T., Adams P. D., Rice L. M. Recent developments for the efficient crystallographic refinement of macromolecular structures. Curr. Opin. Struct. Biol. 1998;8:606–611. doi: 10.1016/s0959-440x(98)80152-8. [DOI] [PubMed] [Google Scholar]
- 40.Read R. J. Improved Fourier coefficients for maps using phases from partial structures with errors. Acta Crystallogr. A. 1986;42:140–149. [Google Scholar]
- 41.McRee D. E. XtalView/Xfit – A versatile program for manipulating atomic coordinates and electron density. J. Struct. Biol. 1999;125:156–165. doi: 10.1006/jsbi.1999.4094. [DOI] [PubMed] [Google Scholar]
- 42.Kleywegt G. J. CCP4/ESF-EACBM Newsletter on Protein Crystallography. Vol. 31. 1995. Dictionaries for Heteros; pp. 45–50. [Google Scholar]
- 43.Laskowski R. A., MacArthour M. W., Moss D. S., Thornton J. M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993;26:283–291. [Google Scholar]
- 44.Vriend G. WHAT IF: a molecular modeling and drug design program. J. Mol. Graphics. 1990;8:52–56. doi: 10.1016/0263-7855(90)80070-v. [DOI] [PubMed] [Google Scholar]
- 45.Jeanmougin F., Thompson J. D., Gouy M., Higgins D. G., Gibson T. J. Multiple sequence alignment with Clustal X. Trends Biochem. Sci. 1998;23:403–405. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- 46.Roussel A., Cambillau C. Mountain View, CA: Silicon Graphics; 1991. Turbo-Frodo. [Google Scholar]
- 47.Sampaleanu L. M., Vallee F., Thompson G. D., Howell P. L. Three-dimensional structure of the argininosuccinate lyase frequently complementing allele Q286R. Biochemistry. 2001;40:15570–15580. doi: 10.1021/bi011525m. [DOI] [PubMed] [Google Scholar]
- 48.Porter D. J., Bright H. J. 3-Carbanionic substrate analogues bind very tightly to fumarase and aspartase. J. Biol. Chem. 1980;255:4772–4780. [PubMed] [Google Scholar]
- 49.Blanchard J. S., Cleland W. W. Use of isotope effects to deduce the chemical mechanism of fumarase. Biochemistry. 1980;19:4506–4513. doi: 10.1021/bi00560a019. [DOI] [PubMed] [Google Scholar]
- 50.Raushel F. M. Nitro analogs of substrates for argininosuccinate synthetase and argininosuccinate lyase. Arch. Biochem. Biophys. 1984;232:520–525. doi: 10.1016/0003-9861(84)90569-1. [DOI] [PubMed] [Google Scholar]
- 51.Kim S. C., Raushel F. M. Isotopic probes of the argininosuccinate lyase reaction. Biochemistry. 1986;25:4744–4749. doi: 10.1021/bi00365a004. [DOI] [PubMed] [Google Scholar]
- 52.Porter D. J., Rudie N. G., Bright H. J. Nitro analogs of substrates for adenylosuccinate synthetase and adenylosuccinate lyase. Arch. Biochem. Biophys. 1983;225:157–163. doi: 10.1016/0003-9861(83)90019-x. [DOI] [PubMed] [Google Scholar]