Abstract
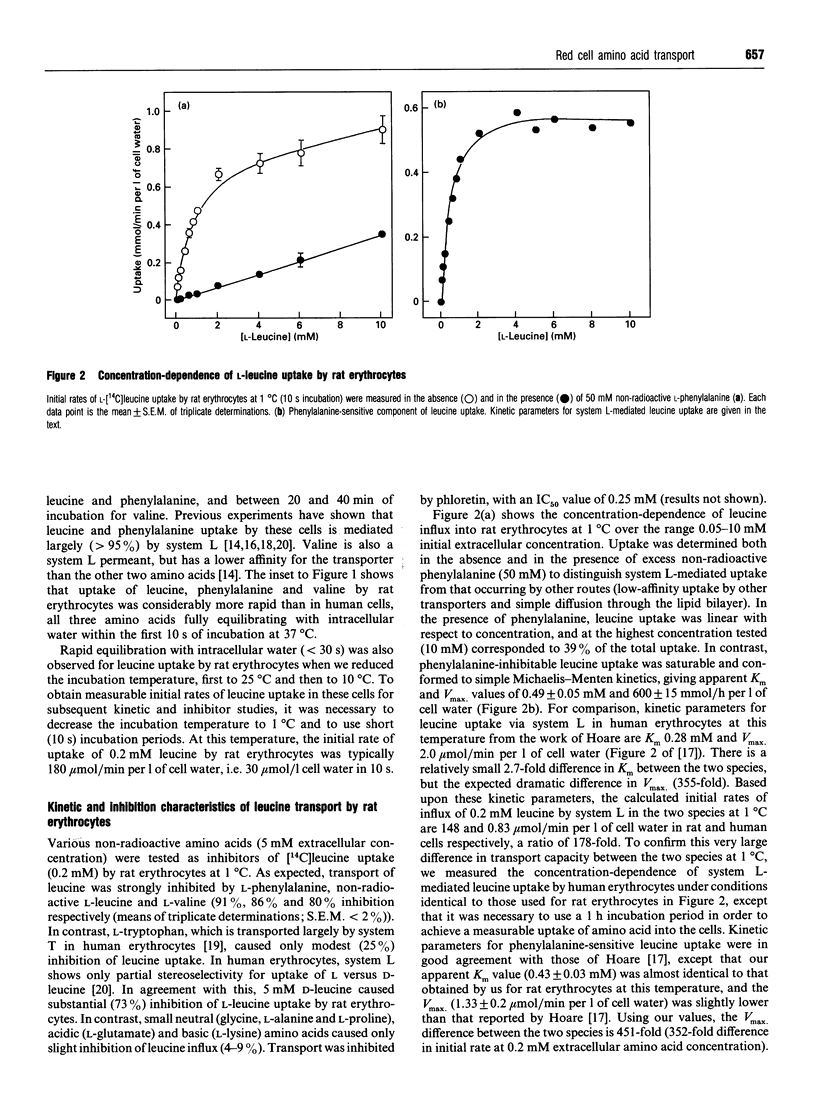
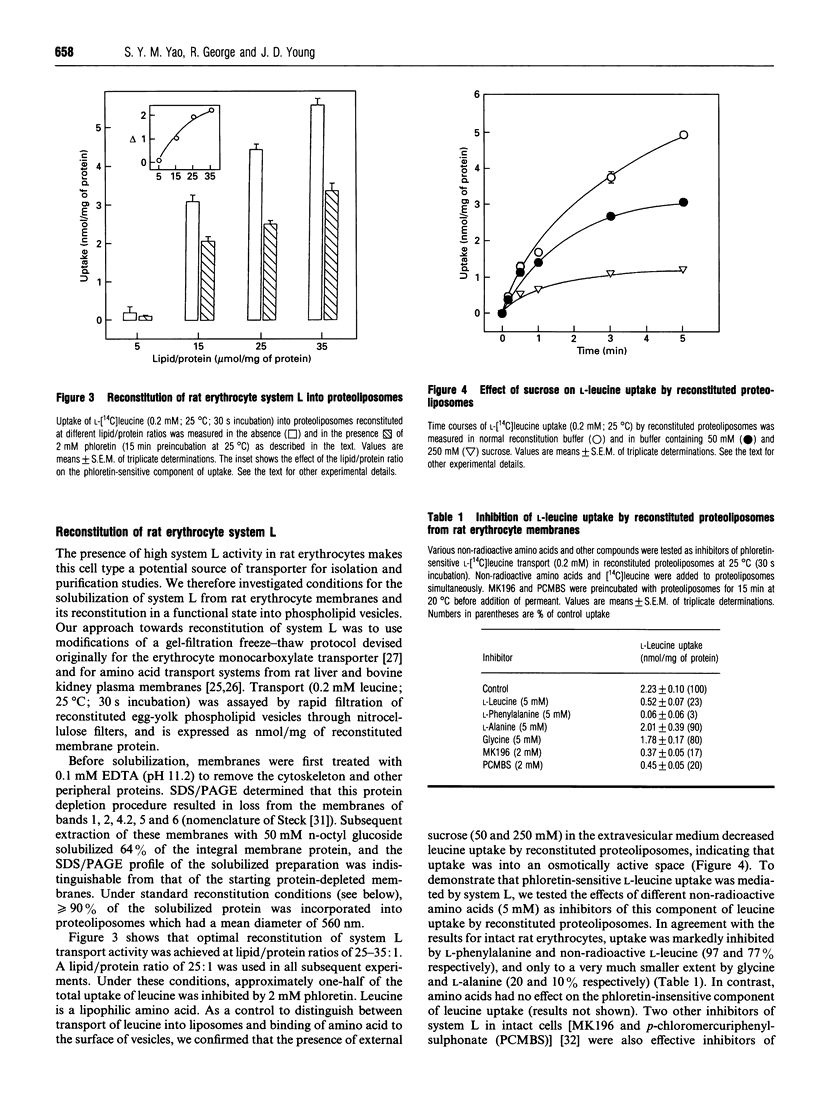
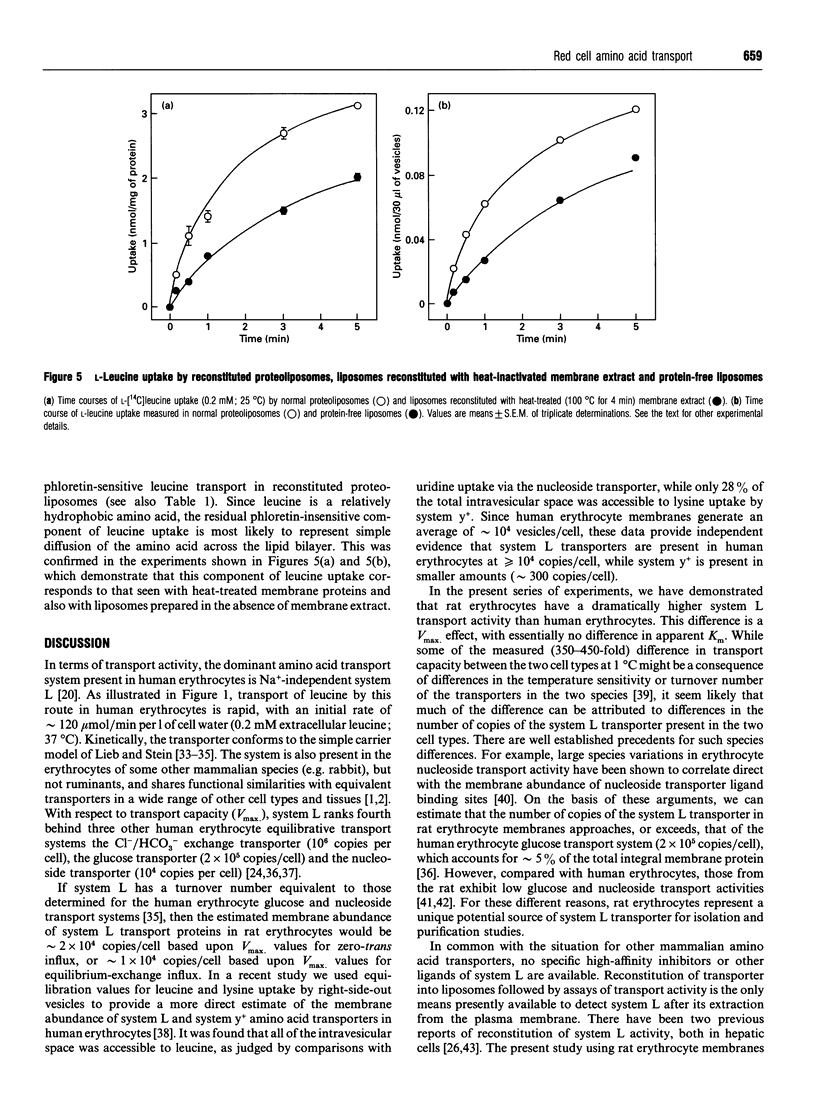
In many cell types, including human erythrocytes, membrane transport of hydrophobic amino acids such as leucine and phenylalanine is mediated primarily by Na(+)-independent system L. In this paper we demonstrate that erythrocytes from the rat have a 400-fold higher system L transport capacity than human erythrocytes. We have exploited this high transport activity to achieve the first successful reconstitution of an erythrocyte amino acid transporter into phospholipid vesicles. Rat erythrocyte membranes were depleted of extrinsic membrane proteins, solubilized in 50 mM n-octyl glucoside and reconstituted into egg-yolk phospholipid vesicles by a gel filtration freeze-thaw protocol. Optimal reconstitution of transport activity occurred at lipid/protein ratios of 25-35:1. At a lipid/protein ratio of 25:1, one-half of the total uptake of L-[14C]leucine (0.2 mM, 25 degrees C) was inhibited by 2 mM phloretin and thus judged to be carrier-mediated. This component of L-leucine uptake was inhibited by non-radioactive L-phenylalanine and L-leucine, and only to a very much weaker extent by glycine and L-alanine. Two other inhibitors of system L in intact cells, MK196 and PCMBS (p-chloromercuriphenylsulphonate), were also effective inhibitors of phloretin-sensitive L-leucine transport in reconstituted proteoliposomes. Phloretin-insensitive uptake of L-leucine in proteoliposomes occurred by simple diffusion across the lipid bilayer.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertran J., Werner A., Moore M. L., Stange G., Markovich D., Biber J., Testar X., Zorzano A., Palacin M., Murer H. Expression cloning of a cDNA from rabbit kidney cortex that induces a single transport system for cystine and dibasic and neutral amino acids. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5601–5605. doi: 10.1073/pnas.89.12.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracy D. S., Schenerman M. A., Kilberg M. S. Solubilization and reconstitution of hepatic System A-mediated amino acid transport. Preparation of proteoliposomes containing glucagon-stimulated transport activity. Biochim Biophys Acta. 1987 May 12;899(1):51–58. doi: 10.1016/0005-2736(87)90238-0. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Exploiting amino acid structure to learn about membrane transport. Adv Enzymol Relat Areas Mol Biol. 1979;49:41–101. doi: 10.1002/9780470122945.ch2. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Organic ion transport during seven decades. The amino acids. Biochim Biophys Acta. 1984 Sep 3;779(3):255–269. doi: 10.1016/0304-4157(84)90012-1. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M. ON THE MECHANISM OF ACTION OF PHOSPHOLIPASE A. Biochem J. 1963 Sep;88:414–423. doi: 10.1042/bj0880414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellory J. C., Willis J. S. Adaptive changes in membrane-transport systems of hibernators. Biochem Soc Trans. 1983 Aug;11(4):330–332. doi: 10.1042/bst0110330. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Levy A. G. Transport of dibasic amino acids by human erythrocytes. Metabolism. 1972 May;21(5):413–431. doi: 10.1016/0026-0495(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Harvey C. M., Ellory J. C. Identification of amino acid transporters in the red blood cell. Methods Enzymol. 1989;173:122–160. doi: 10.1016/s0076-6879(89)73010-x. [DOI] [PubMed] [Google Scholar]
- Hoare D. G. The temperature dependence of the transport of L-leucine in human erythrocytes. J Physiol. 1972 Mar;221(2):331–348. doi: 10.1113/jphysiol.1972.sp009754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare D. G. The transport of L-leucine in human erythrocytes: a new kinetic analysis. J Physiol. 1972 Mar;221(2):311–329. doi: 10.1113/jphysiol.1972.sp009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Nucleoside transport in rat erythrocytes: two components with differences in sensitivity to inhibition by nitrobenzylthioinosine and p-chloromercuriphenyl sulfonate. J Membr Biol. 1986;93(1):1–10. doi: 10.1007/BF01871013. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lieb W. R., Stein W. D. Testing and characterizing the simple carrier. Biochim Biophys Acta. 1974 Dec 10;373(2):178–196. doi: 10.1016/0005-2736(74)90144-8. [DOI] [PubMed] [Google Scholar]
- Lin S., Spudich J. A. Biochemical studies on the mode of action of cytochalasin B. Cytochalasin B binding to red cell membrane in relation to glucose transport. J Biol Chem. 1974 Sep 25;249(18):5778–5783. [PubMed] [Google Scholar]
- Lynch A. M., McGivan J. D. A rapid method for the reconstitution of Na+-dependent neutral amino acid transport from bovine renal brush-border membranes. Biochem J. 1987 Jun 15;244(3):503–508. doi: 10.1042/bj2440503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick J. I., Johnstone R. M. Simple and effective purification of a Na+-dependent amino acid transport system from Ehrlich ascites cell plasma membrane. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7877–7881. doi: 10.1073/pnas.85.21.7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsumoto Y., Mohri T. Reconstitution of the L-leucine-H+ cotransporter of the plasma membrane from Chang liver cells into proteoliposomes. Biochim Biophys Acta. 1991 Jan 30;1061(2):171–174. doi: 10.1016/0005-2736(91)90282-d. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Poole R. C., Halestrap A. P. Reconstitution of the L-lactate carrier from rat and rabbit erythrocyte plasma membranes. Biochem J. 1988 Sep 1;254(2):385–390. doi: 10.1042/bj2540385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A. R., McGivan J. D. A rapid method for the functional reconstitution of amino acid transport systems from rat liver plasma membranes. Partial purification of System A. Biochem J. 1988 Nov 1;255(3):963–969. doi: 10.1042/bj2550963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radian R., Bendahan A., Kanner B. I. Purification and identification of the functional sodium- and chloride-coupled gamma-aminobutyric acid transport glycoprotein from rat brain. J Biol Chem. 1986 Nov 25;261(33):15437–15441. [PubMed] [Google Scholar]
- Rosenberg R. L-Leucine transport in human red blood cells: a detailed kinetic analysis. J Membr Biol. 1981;62(1-2):79–93. doi: 10.1007/BF01870202. [DOI] [PubMed] [Google Scholar]
- Rosenberg R., Young J. D., Ellory J. C. L-Tryptophan transport in human red blood cells. Biochim Biophys Acta. 1980 May 23;598(2):375–384. doi: 10.1016/0005-2736(80)90015-2. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarappoo B. K., Kilberg M. S. Functional reconstitution of the hepatic system N amino acid transport activity. Biochem J. 1991 Feb 15;274(Pt 1):97–101. doi: 10.1042/bj2740097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate S. S., Yan N., Udenfriend S. Expression cloning of a Na(+)-independent neutral amino acid transporter from rat kidney. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):1–5. doi: 10.1073/pnas.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse C. M., Fincham D. A., Ellory J. C., Young J. D. Use of membrane vesicles to estimate the numbers of system y+ and system L amino acid transporters in human erythrocytes. Biochem J. 1991 Jul 15;277(Pt 2):565–568. doi: 10.1042/bj2770565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINTER C. G., CHRISTENSEN H. N. MIGRATION OF AMINO ACIDS ACROSS THE MEMBRANE OF THE HUMAN ERYTHROCYTE. J Biol Chem. 1964 Mar;239:872–878. [PubMed] [Google Scholar]
- Wang H., Kavanaugh M. P., North R. A., Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991 Aug 22;352(6337):729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- Wells R. G., Hediger M. A. Cloning of a rat kidney cDNA that stimulates dibasic and neutral amino acid transport and has sequence similarity to glucosidases. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5596–5600. doi: 10.1073/pnas.89.12.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieth J. O. Bicarbonate exchange through the human red cell membrane determined with [14C] bicarbonate. J Physiol. 1979 Sep;294:521–539. doi: 10.1113/jphysiol.1979.sp012944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolowyk M. W., Young J. D., Ellory J. C. Inhibition of amino acid transport by loop diuretics. Proc West Pharmacol Soc. 1983;26:247–249. [PubMed] [Google Scholar]
- Young J. D., Jarvis S. M. Nucleoside transport in animal cells. Biosci Rep. 1983 Apr;3(4):309–322. doi: 10.1007/BF01122895. [DOI] [PubMed] [Google Scholar]
- Young J. D., Jones S. E., Ellory J. C. Amino acid transport in human and in sheep erythrocytes. Proc R Soc Lond B Biol Sci. 1980 Sep 26;209(1176):355–375. doi: 10.1098/rspb.1980.0100. [DOI] [PubMed] [Google Scholar]


