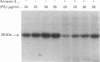
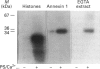
Abstract
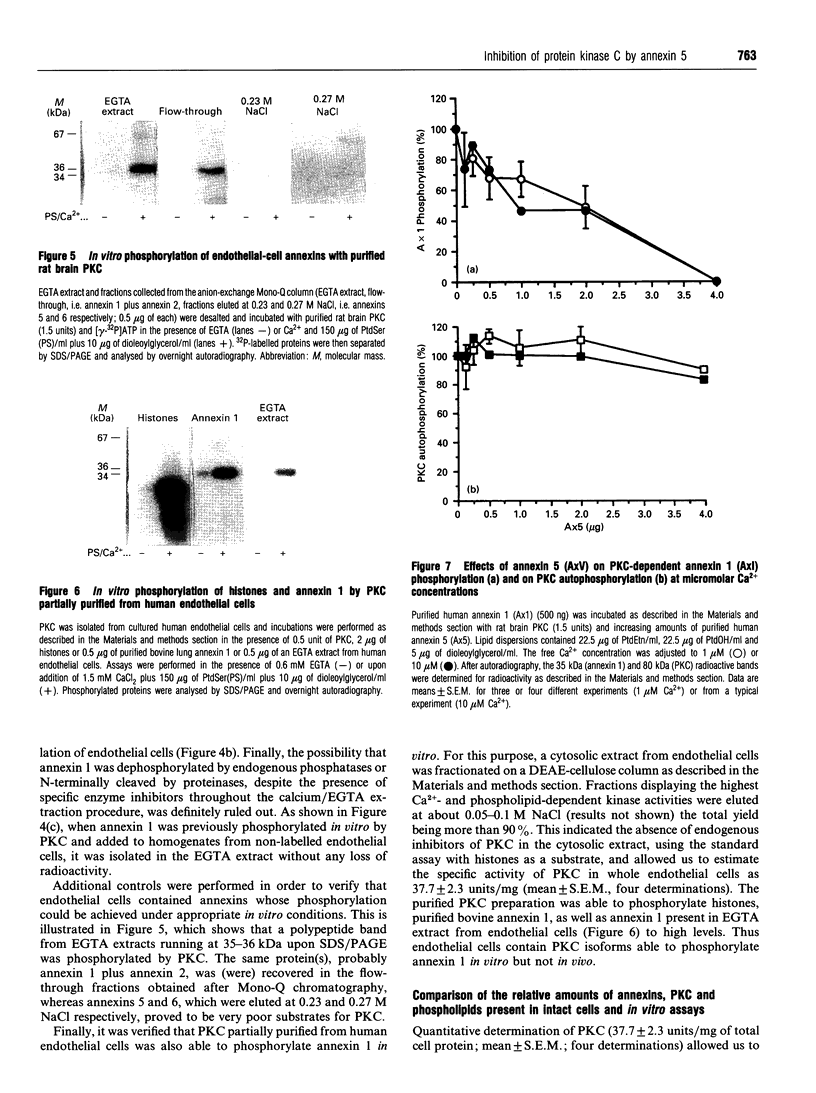
In vitro phosphorylation of annexin 1 by purified rat brain protein kinase C (PKC) has been studied in the presence of annexin 5, which is not a substrate for PKC. Annexin 5 promoted a dose-dependent inhibition of annexin 1 phosphorylation, which could be overcome by increasing the concentration of phosphatidylserine (PtdSer). In addition, a close relationship was found between the amount of PtdSer uncovered by annexin 5 and the residual phosphorylation of annexin 1. These data fit with the 'surface depletion model' explaining the antiphospholipase activity of annexins. In order to check the possibility that the in vitro effect of annexin 5 could be of some physiological relevance, annexins 1, 2, and 5, as well as the light chain of calpactin 1 (p11), have been quantified in human endothelial cells by measuring the radioactivity bound to the proteins after Western blotting with specific antibodies and 125I-labelled secondary antibody. Our data indicate that annexins 1 and 5, PKC and PtdSer are present in human endothelial cells in relative amounts very similar to those used in vitro under conditions permitting the detection of the inhibitory effect of annexin 5. Since annexin 1 remained refractory to PKC-dependent phosphorylation in intact cells, we suggest that annexin 5 might exert its inhibitory effect towards PKC in vivo, provided that its binding to phospholipids can occur at physiological (micromolar) concentrations of Ca2+. This was previously shown to occur in vitro using phosphatidylethanolamine/phosphatidic acid vesicles [Blackwood and Ernst (1990) Biochem. J. 266, 195-200]. Using identical assay conditions, which also allowed expression of PKC activity, annexin 5 again inhibited annexin 1 phosphorylation without interfering with PKC autophosphorylation. These data suggest that annexins 1 and 5 might interact with each other on the lipid surface, resulting in a specific inhibition of annexin 1 phosphorylation by PKC. Whether a similar mechanism also occurs in vivo remains to be determined.
Full text
PDF

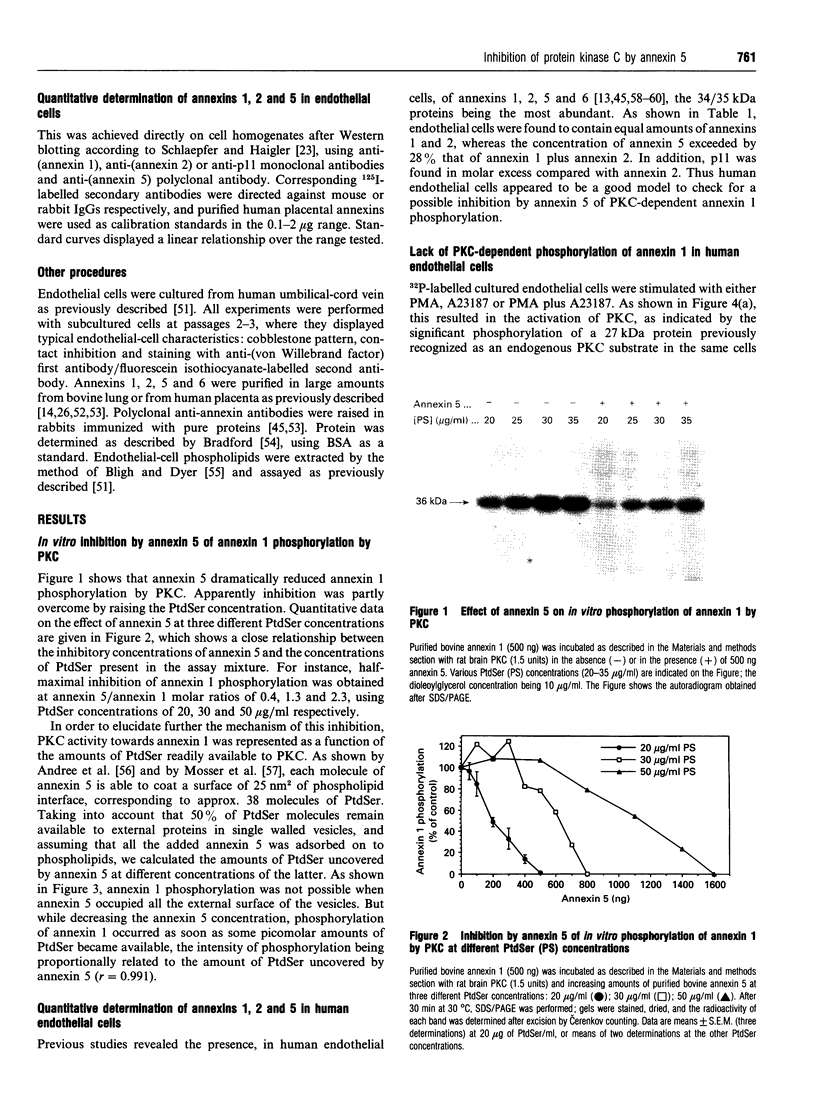
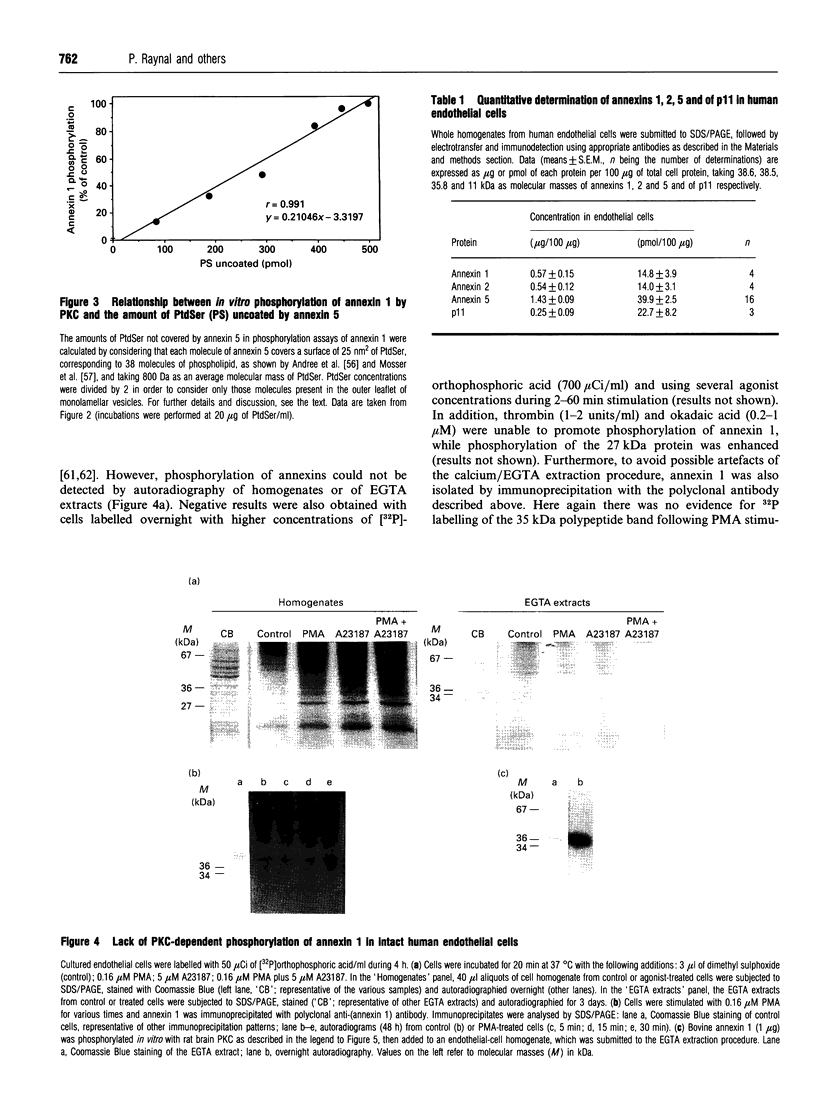


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarsman A. J., Mynbeek G., van den Bosch H., Rothhut B., Prieur B., Comera C., Jordan L., Russo-Marie F. Lipocortin inhibition of extracellular and intracellular phospholipases A2 is substrate concentration dependent. FEBS Lett. 1987 Jul 13;219(1):176–180. doi: 10.1016/0014-5793(87)81212-7. [DOI] [PubMed] [Google Scholar]
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989 Jul 27;340(6231):313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Andree H. A., Reutelingsperger C. P., Hauptmann R., Hemker H. C., Hermens W. T., Willems G. M. Binding of vascular anticoagulant alpha (VAC alpha) to planar phospholipid bilayers. J Biol Chem. 1990 Mar 25;265(9):4923–4928. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bienkowski M. J., Petro M. A., Robinson L. J. Inhibition of thromboxane A synthesis in U937 cells by glucocorticoids. Lack of evidence for lipocortin 1 as the second messenger. J Biol Chem. 1989 Apr 15;264(11):6536–6544. [PubMed] [Google Scholar]
- Blackwood R. A., Ernst J. D. Characterization of Ca2(+)-dependent phospholipid binding, vesicle aggregation and membrane fusion by annexins. Biochem J. 1990 Feb 15;266(1):195–200. doi: 10.1042/bj2660195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Browning J., Pepinsky B. B., Wallner B., Flower R. J., Peers S. H. Too soon for consensus? Nature. 1990 Jul 26;346(6282):324–324. doi: 10.1038/346324a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Fabiato A., Leslie B. A., Putney J. W., Jr Calcium pools in saponin-permeabilized guinea pig hepatocytes. J Biol Chem. 1983 Dec 25;258(24):15336–15345. [PubMed] [Google Scholar]
- Burns A. L., Magendzo K., Shirvan A., Srivastava M., Rojas E., Alijani M. R., Pollard H. B. Calcium channel activity of purified human synexin and structure of the human synexin gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3798–3802. doi: 10.1073/pnas.86.10.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chap H., Comfurius P., Bevers E. M., Fauvel J., Vicendo P., Douste-Blazy L., Zwaal R. F. Potential anticoagulant activity of lipocortins and other calcium/phospholipid binding proteins. Biochem Biophys Res Commun. 1988 Feb 15;150(3):972–978. doi: 10.1016/0006-291x(88)90724-3. [DOI] [PubMed] [Google Scholar]
- Christmas P., Callaway J., Fallon J., Jones J., Haigler H. T. Selective secretion of annexin 1, a protein without a signal sequence, by the human prostate gland. J Biol Chem. 1991 Feb 5;266(4):2499–2507. [PubMed] [Google Scholar]
- Crumpton M. J., Dedman J. R. Protein terminology tangle. Nature. 1990 May 17;345(6272):212–212. doi: 10.1038/345212a0. [DOI] [PubMed] [Google Scholar]
- Darbon J. M., Tournier J. F., Tauber J. P., Bayard F. Possible role of protein phosphorylation in the mitogenic effect of high density lipoproteins on cultured vascular endothelial cells. J Biol Chem. 1986 Jun 15;261(17):8002–8008. [PubMed] [Google Scholar]
- Davidson F. F., Dennis E. A., Powell M., Glenney J. R., Jr Inhibition of phospholipase A2 by "lipocortins" and calpactins. An effect of binding to substrate phospholipids. J Biol Chem. 1987 Feb 5;262(4):1698–1705. [PubMed] [Google Scholar]
- De B. K., Misono K. S., Lukas T. J., Mroczkowski B., Cohen S. A calcium-dependent 35-kilodalton substrate for epidermal growth factor receptor/kinase isolated from normal tissue. J Biol Chem. 1986 Oct 15;261(29):13784–13792. [PubMed] [Google Scholar]
- Díaz-Muñoz M., Hamilton S. L., Kaetzel M. A., Hazarika P., Dedman J. R. Modulation of Ca2+ release channel activity from sarcoplasmic reticulum by annexin VI (67-kDa calcimedin). J Biol Chem. 1990 Sep 15;265(26):15894–15899. [PubMed] [Google Scholar]
- Edashige K., Utsumi T., Sato E. F., Ide A., Kasai M., Utsumi K. Requirement of protein association with membranes for phosphorylation by protein kinase C. Arch Biochem Biophys. 1992 Jul;296(1):296–301. doi: 10.1016/0003-9861(92)90575-h. [DOI] [PubMed] [Google Scholar]
- Edashige K., Utsumi T., Utsumi K. Inhibition of 12-O-tetradecanoyl phorbol-13-acetate promoted tumorigenesis by cepharanthine, a biscoclaurine alkaloid, in relation to the inhibitory effect on protein kinase C. Biochem Pharmacol. 1991 Jan 1;41(1):71–78. doi: 10.1016/0006-2952(91)90012-t. [DOI] [PubMed] [Google Scholar]
- Fauvel J., Vicendo P., Roques V., Ragab-Thomas J., Granier C., Vilgrain I., Chambaz E., Rochat H., Chap H., Douste-Blazy L. Isolation of two 67 kDa calcium-binding proteins from pig lung differing in affinity for phospholipids and in anti-phospholipase A2 activity. FEBS Lett. 1987 Sep 14;221(2):397–402. doi: 10.1016/0014-5793(87)80963-8. [DOI] [PubMed] [Google Scholar]
- Fava R. A., Cohen S. Isolation of a calcium-dependent 35-kilodalton substrate for the epidermal growth factor receptor/kinase from A-431 cells. J Biol Chem. 1984 Feb 25;259(4):2636–2645. [PubMed] [Google Scholar]
- Flaherty M. J., West S., Heimark R. L., Fujikawa K., Tait J. F. Placental anticoagulant protein-I: measurement in extracellular fluids and cells of the hemostatic system. J Lab Clin Med. 1990 Feb;115(2):174–181. [PubMed] [Google Scholar]
- Funakoshi T., Hendrickson L. E., McMullen B. A., Fujikawa K. Primary structure of human placental anticoagulant protein. Biochemistry. 1987 Dec 15;26(25):8087–8092. doi: 10.1021/bi00399a011. [DOI] [PubMed] [Google Scholar]
- Gassama-Diagne A., Fauvel J., Chap H. Calcium-independent phospholipases from guinea pig digestive tract as probes to study the mechanism of lipocortin. J Biol Chem. 1990 Mar 15;265(8):4309–4314. [PubMed] [Google Scholar]
- Geisow M. J., Fritsche U., Hexham J. M., Dash B., Johnson T. A consensus amino-acid sequence repeat in Torpedo and mammalian Ca2+-dependent membrane-binding proteins. Nature. 1986 Apr 17;320(6063):636–638. doi: 10.1038/320636a0. [DOI] [PubMed] [Google Scholar]
- Gerke V., Weber K. Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J. 1984 Jan;3(1):227–233. doi: 10.1002/j.1460-2075.1984.tb01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Tack B. F. Amino-terminal sequence of p36 and associated p10: identification of the site of tyrosine phosphorylation and homology with S-100. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7884–7888. doi: 10.1073/pnas.82.23.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Tack B., Powell M. A. Calpactins: two distinct Ca++-regulated phospholipid- and actin-binding proteins isolated from lung and placenta. J Cell Biol. 1987 Mar;104(3):503–511. doi: 10.1083/jcb.104.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Woodgett J. R., Isacke C. M., Hunter T. The protein-tyrosine kinase substrate p36 is also a substrate for protein kinase C in vitro and in vivo. Mol Cell Biol. 1986 Jul;6(7):2738–2744. doi: 10.1128/mcb.6.7.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann R., Maurer-Fogy I., Krystek E., Bodo G., Andree H., Reutelingsperger C. P. Vascular anticoagulant beta: a novel human Ca2+/phospholipid binding protein that inhibits coagulation and phospholipase A2 activity. Its molecular cloning, expression and comparison with VAC-alpha. Eur J Biochem. 1989 Oct 20;185(1):63–71. doi: 10.1111/j.1432-1033.1989.tb15082.x. [DOI] [PubMed] [Google Scholar]
- Hullin F., Raynal P., Ragab-Thomas J. M., Fauvel J., Chap H. Effect of dexamethasone on prostaglandin synthesis and on lipocortin status in human endothelial cells. Inhibition of prostaglandin I2 synthesis occurring without alteration of arachidonic acid liberation and of lipocortin synthesis. J Biol Chem. 1989 Feb 25;264(6):3506–3513. [PubMed] [Google Scholar]
- Isacke C. M., Lindberg R. A., Hunter T. Synthesis of p36 and p35 is increased when U-937 cells differentiate in culture but expression is not inducible by glucocorticoids. Mol Cell Biol. 1989 Jan;9(1):232–240. doi: 10.1128/mcb.9.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal H. K., Chaney W. G., Anderson C. W., Davis R. G., Vishwanatha J. K. The protein-tyrosine kinase substrate, calpactin I heavy chain (p36), is part of the primer recognition protein complex that interacts with DNA polymerase alpha. J Biol Chem. 1991 Mar 15;266(8):5169–5176. [PubMed] [Google Scholar]
- Johnsson N., Nguyen Van P., Söling H. D., Weber K. Functionally distinct serine phosphorylation sites of p36, the cellular substrate of retroviral protein kinase; differential inhibition of reassociation with p11. EMBO J. 1986 Dec 20;5(13):3455–3460. doi: 10.1002/j.1460-2075.1986.tb04669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keutzer J. C., Hirschhorn R. R. The growth-regulated gene 1B6 is identified as the heavy chain of calpactin I. Exp Cell Res. 1990 May;188(1):153–159. doi: 10.1016/0014-4827(90)90291-h. [DOI] [PubMed] [Google Scholar]
- Klee C. B. Ca2+-dependent phospholipid- (and membrane-) binding proteins. Biochemistry. 1988 Sep 6;27(18):6645–6653. doi: 10.1021/bi00418a001. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M. H., Bell R. M. Phospholipid functional groups involved in protein kinase C activation, phorbol ester binding, and binding to mixed micelles. J Biol Chem. 1989 Sep 5;264(25):14797–14805. [PubMed] [Google Scholar]
- Lee M. H., Bell R. M. Supplementation of the phosphatidyl-L-serine requirement of protein kinase C with nonactivating phospholipids. Biochemistry. 1992 Jun 9;31(22):5176–5182. doi: 10.1021/bi00137a013. [DOI] [PubMed] [Google Scholar]
- Machoczek K., Fischer M., Söling H. D. Lipocortin I and lipocortin II inhibit phosphoinositide- and polyphosphoinositide-specific phospholipase C. The effect results from interaction with the substrates. FEBS Lett. 1989 Jul 17;251(1-2):207–212. doi: 10.1016/0014-5793(89)81456-5. [DOI] [PubMed] [Google Scholar]
- Maurer-Fogy I., Reutelingsperger C. P., Pieters J., Bodo G., Stratowa C., Hauptmann R. Cloning and expression of cDNA for human vascular anticoagulant, a Ca2+-dependent phospholipid-binding protein. Eur J Biochem. 1988 Jul 1;174(4):585–592. doi: 10.1111/j.1432-1033.1988.tb14139.x. [DOI] [PubMed] [Google Scholar]
- Michener M. L., Dawson W. B., Creutz C. E. Phosphorylation of a chromaffin granule-binding protein in stimulated chromaffin cells. J Biol Chem. 1986 May 15;261(14):6548–6555. [PubMed] [Google Scholar]
- Miele L., Cordella-Miele E., Facchiano A., Mukherjee A. B. Novel anti-inflammatory peptides from the region of highest similarity between uteroglobin and lipocortin I. Nature. 1988 Oct 20;335(6192):726–730. doi: 10.1038/335726a0. [DOI] [PubMed] [Google Scholar]
- Mosser G., Ravanat C., Freyssinet J. M., Brisson A. Sub-domain structure of lipid-bound annexin-V resolved by electron image analysis. J Mol Biol. 1991 Jan 20;217(2):241–245. doi: 10.1016/0022-2836(91)90538-h. [DOI] [PubMed] [Google Scholar]
- Patte C., Rothhut B., Russo-Marie F., Blanquet P. R. Possible involvement of a lipocortin in the initiation of DNA synthesis by human endothelial cells. Exp Cell Res. 1991 Nov;197(1):12–20. doi: 10.1016/0014-4827(91)90474-9. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Sinclair L. K., Chow E. P., O'Brine-Greco B. A dimeric form of lipocortin-1 in human placenta. Biochem J. 1989 Oct 1;263(1):97–103. doi: 10.1042/bj2630097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Tizard R., Mattaliano R. J., Sinclair L. K., Miller G. T., Browning J. L., Chow E. P., Burne C., Huang K. S., Pratt D. Five distinct calcium and phospholipid binding proteins share homology with lipocortin I. J Biol Chem. 1988 Aug 5;263(22):10799–10811. [PubMed] [Google Scholar]
- Pfäffle M., Ruggiero F., Hofmann H., Fernández M. P., Selmin O., Yamada Y., Garrone R., von der Mark K. Biosynthesis, secretion and extracellular localization of anchorin CII, a collagen-binding protein of the calpactin family. EMBO J. 1988 Aug;7(8):2335–2342. doi: 10.1002/j.1460-2075.1988.tb03077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynal P., van Bergen en Henegouwen P. M., Hullin F., Ragab-Thomas J. M., Fauvel J., Verkleij A., Chap H. Morphological and biochemical evidence for partial nuclear localization of annexin 1 in endothelial cells. Biochem Biophys Res Commun. 1992 Jul 15;186(1):432–439. doi: 10.1016/s0006-291x(05)80826-5. [DOI] [PubMed] [Google Scholar]
- Reutelingsperger C. P., Hornstra G., Hemker H. C. Isolation and partial purification of a novel anticoagulant from arteries of human umbilical cord. Eur J Biochem. 1985 Sep 16;151(3):625–629. doi: 10.1111/j.1432-1033.1985.tb09150.x. [DOI] [PubMed] [Google Scholar]
- Ross T. S., Tait J. F., Majerus P. W. Identity of inositol 1,2-cyclic phosphate 2-phosphohydrolase with lipocortin III. Science. 1990 May 4;248(4955):605–607. doi: 10.1126/science.2159184. [DOI] [PubMed] [Google Scholar]
- Sahal D., Fujita-Yamaguchi Y. Protein kinase assay by paper-trichloroacetic acid method: high performance using phosphocellulose paper and washing an ensemble of samples on flat sheets. Anal Biochem. 1987 Nov 15;167(1):23–30. doi: 10.1016/0003-2697(87)90129-1. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D. D., Haigler H. T. Expression of annexins as a function of cellular growth state. J Cell Biol. 1990 Jul;111(1):229–238. doi: 10.1083/jcb.111.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer D. D., Haigler H. T. In vitro protein kinase C phosphorylation sites of placental lipocortin. Biochemistry. 1988 Jun 14;27(12):4253–4258. doi: 10.1021/bi00412a008. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D. D., Jones J., Haigler H. T. Inhibition of protein kinase C by annexin V. Biochemistry. 1992 Feb 18;31(6):1886–1891. doi: 10.1021/bi00121a043. [DOI] [PubMed] [Google Scholar]
- Shibata S., Sato H., Maki M. Calphobindins (placental annexins) inhibit protein kinase C. J Biochem. 1992 Oct;112(4):552–556. doi: 10.1093/oxfordjournals.jbchem.a123937. [DOI] [PubMed] [Google Scholar]
- Stratton K. R., Worley P. F., Huganir R. L., Baraban J. M. Muscarinic agonists and phorbol esters increase tyrosine phosphorylation of a 40-kilodalton protein in hippocampal slices. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2498–2501. doi: 10.1073/pnas.86.7.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. M., Hullin F., Chap H., Douste-Blazy L. Phosphatidylcholine is the major phospholipid providing arachidonic acid for prostacyclin synthesis in thrombin-stimulated human endothelial cells. Thromb Res. 1984 Apr 15;34(2):117–123. doi: 10.1016/0049-3848(84)90068-9. [DOI] [PubMed] [Google Scholar]
- Varticovski L., Chahwala S. B., Whitman M., Cantley L., Schindler D., Chow E. P., Sinclair L. K., Pepinsky R. B. Location of sites in human lipocortin I that are phosphorylated by protein tyrosine kinases and protein kinases A and C. Biochemistry. 1988 May 17;27(10):3682–3690. doi: 10.1021/bi00410a024. [DOI] [PubMed] [Google Scholar]
- Wice B. M., Gordon J. I. A strategy for isolation of cDNAs encoding proteins affecting human intestinal epithelial cell growth and differentiation: characterization of a novel gut-specific N-myristoylated annexin. J Cell Biol. 1992 Jan;116(2):405–422. doi: 10.1083/jcb.116.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijkander J., Sundler R. A role for protein kinase C-mediated phosphorylation in the mobilization of arachidonic acid in mouse macrophages. Biochim Biophys Acta. 1989 Jan 17;1010(1):78–87. doi: 10.1016/0167-4889(89)90187-0. [DOI] [PubMed] [Google Scholar]
- William F., Haigler H. T., Kraft A. S. Lack of phosphorylation of lipocortin I in A431 epidermoid carcinoma cells treated with phorbol esters. Biochem Biophys Res Commun. 1989 Apr 28;160(2):474–479. doi: 10.1016/0006-291x(89)92457-1. [DOI] [PubMed] [Google Scholar]
- William F., Mroczkowski B., Cohen S., Kraft A. S. Differentiation of HL-60 cells is associated with an increase in the 35-kDa protein lipocortin I. J Cell Physiol. 1988 Dec;137(3):402–410. doi: 10.1002/jcp.1041370303. [DOI] [PubMed] [Google Scholar]
- Wirl G., Schwartz-Albiez R. Collagen-binding proteins of mammary epithelial cells are related to Ca2(+)- and phospholipid-binding annexins. J Cell Physiol. 1990 Sep;144(3):511–522. doi: 10.1002/jcp.1041440320. [DOI] [PubMed] [Google Scholar]
- Wooten M. W., Vandenplas M., Nel A. E. Rapid purification of protein kinase C from rat brain. A novel method employing protamine-agarose affinity column chromatography. Eur J Biochem. 1987 Apr 15;164(2):461–467. doi: 10.1111/j.1432-1033.1987.tb11079.x. [DOI] [PubMed] [Google Scholar]
- Zaks W. J., Creutz C. E. Ca(2+)-dependent annexin self-association on membrane surfaces. Biochemistry. 1991 Oct 8;30(40):9607–9615. doi: 10.1021/bi00104a007. [DOI] [PubMed] [Google Scholar]
- Zokas L., Glenney J. R., Jr The calpactin light chain is tightly linked to the cytoskeletal form of calpactin I: studies using monoclonal antibodies to calpactin subunits. J Cell Biol. 1987 Nov;105(5):2111–2121. doi: 10.1083/jcb.105.5.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]