Abstract
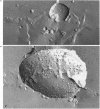
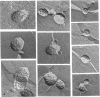
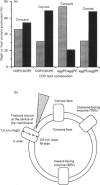
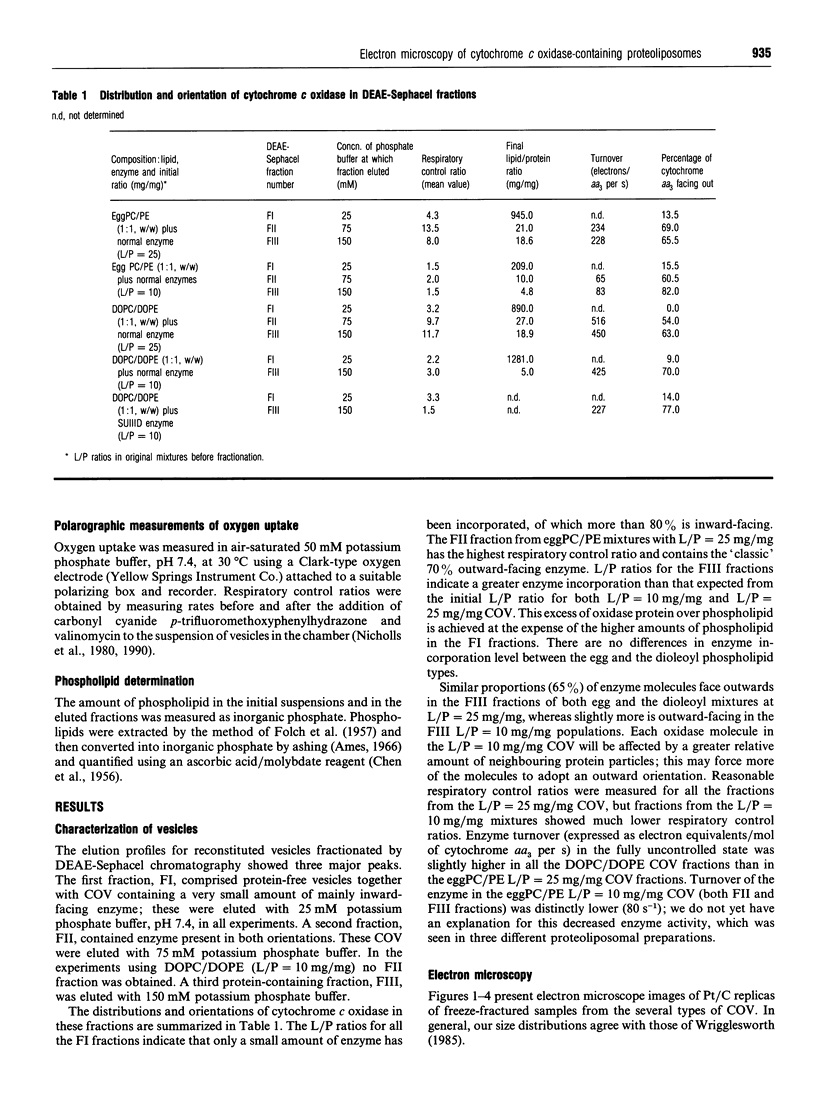
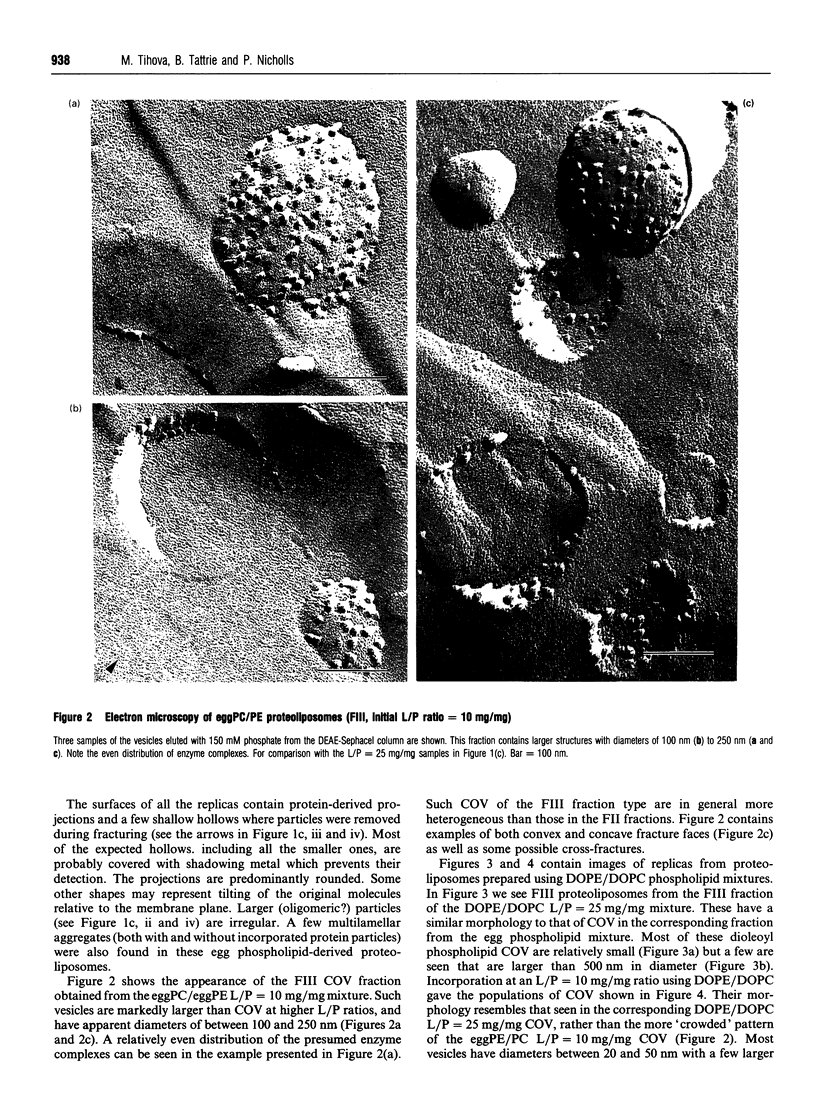
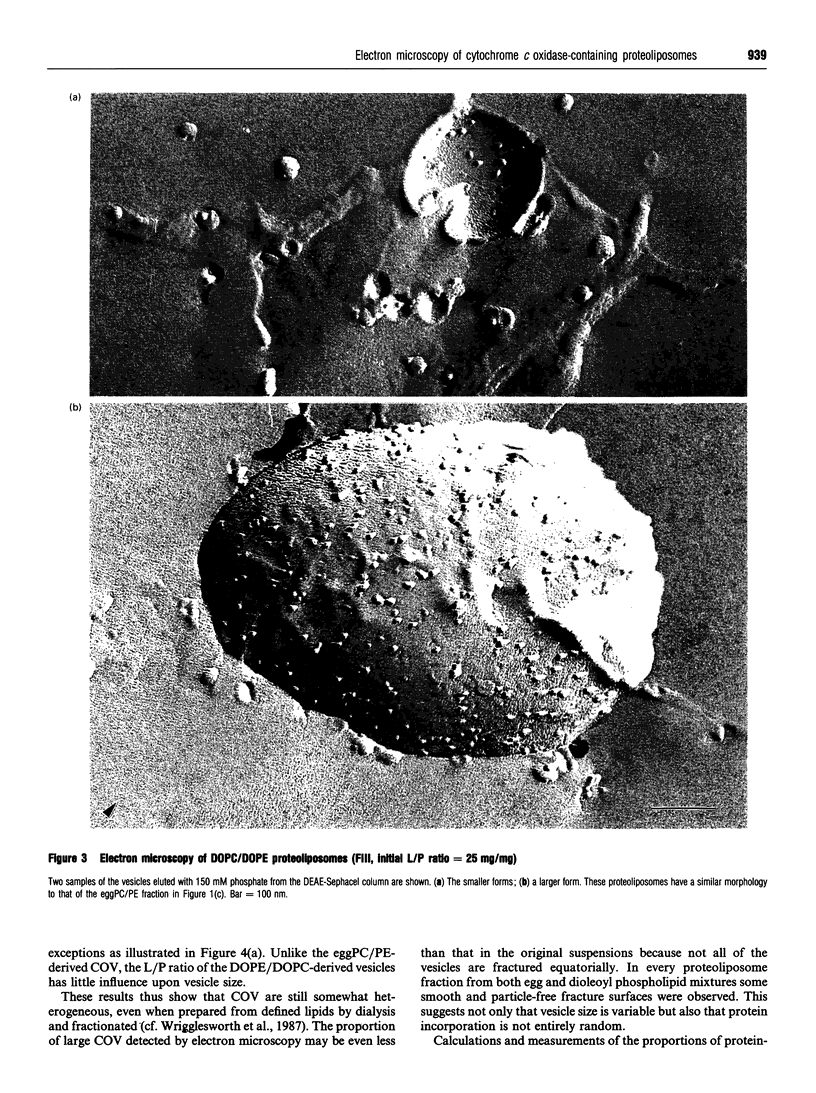
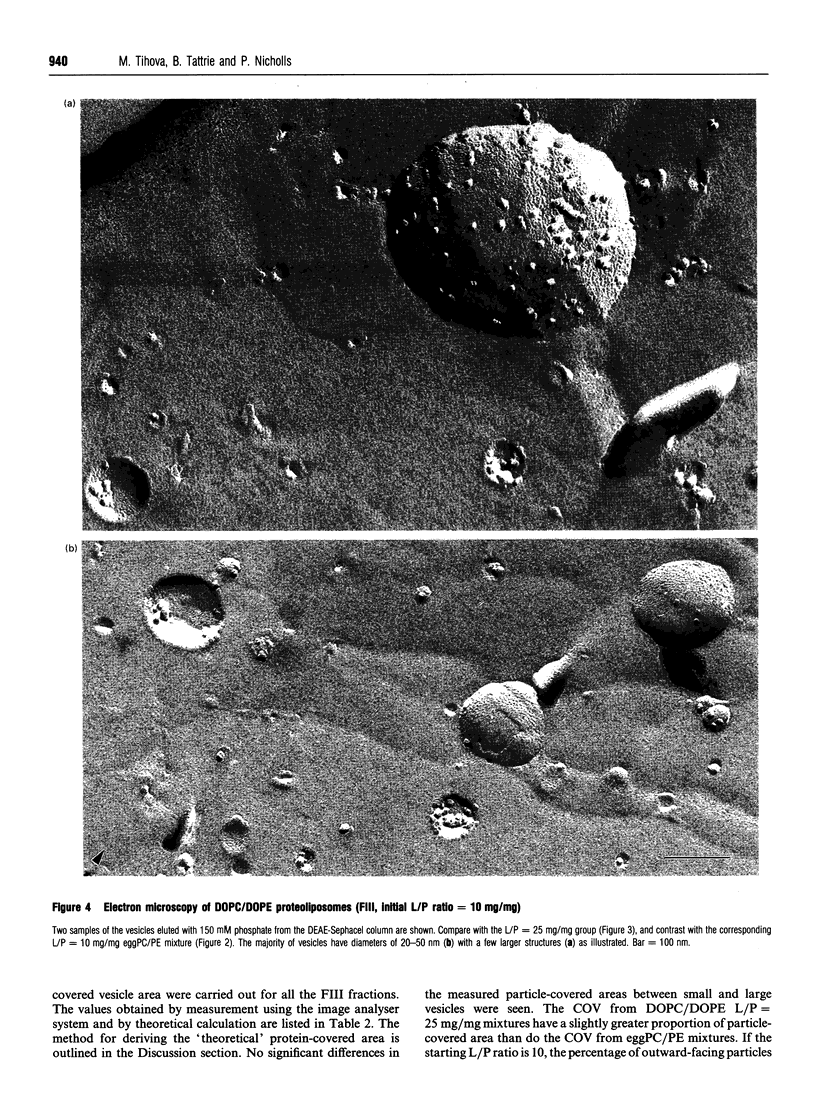
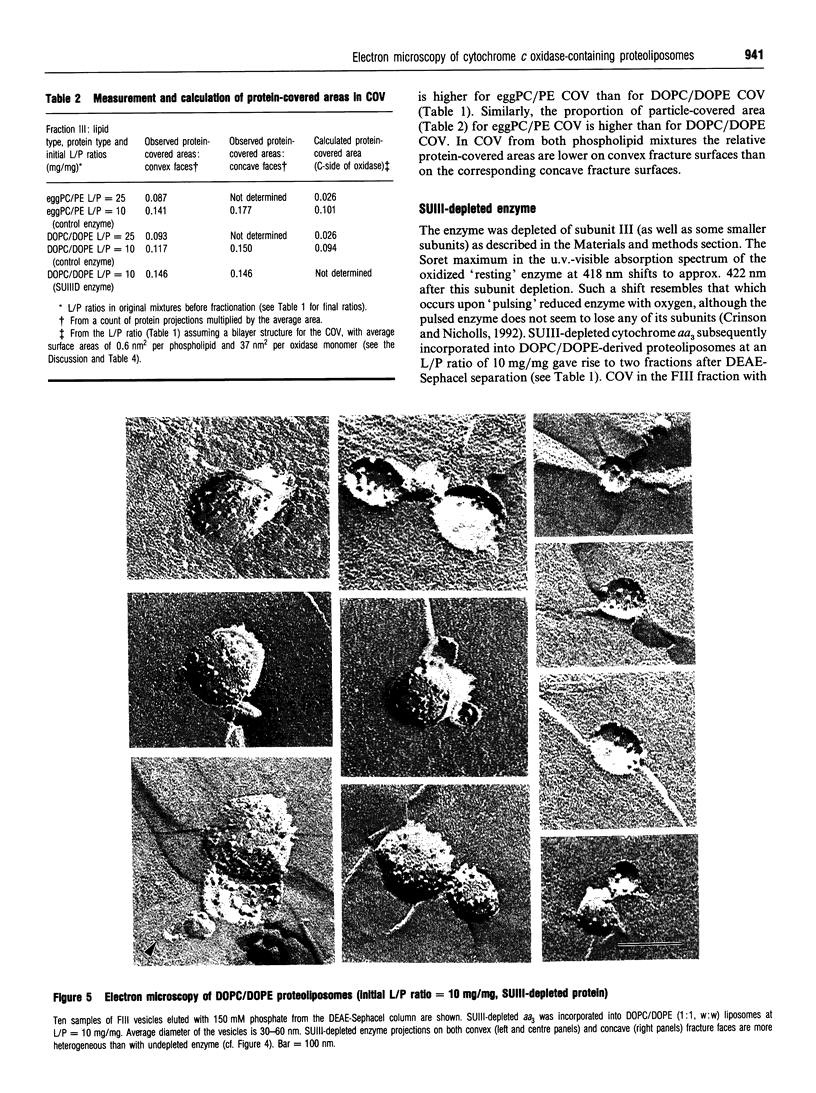
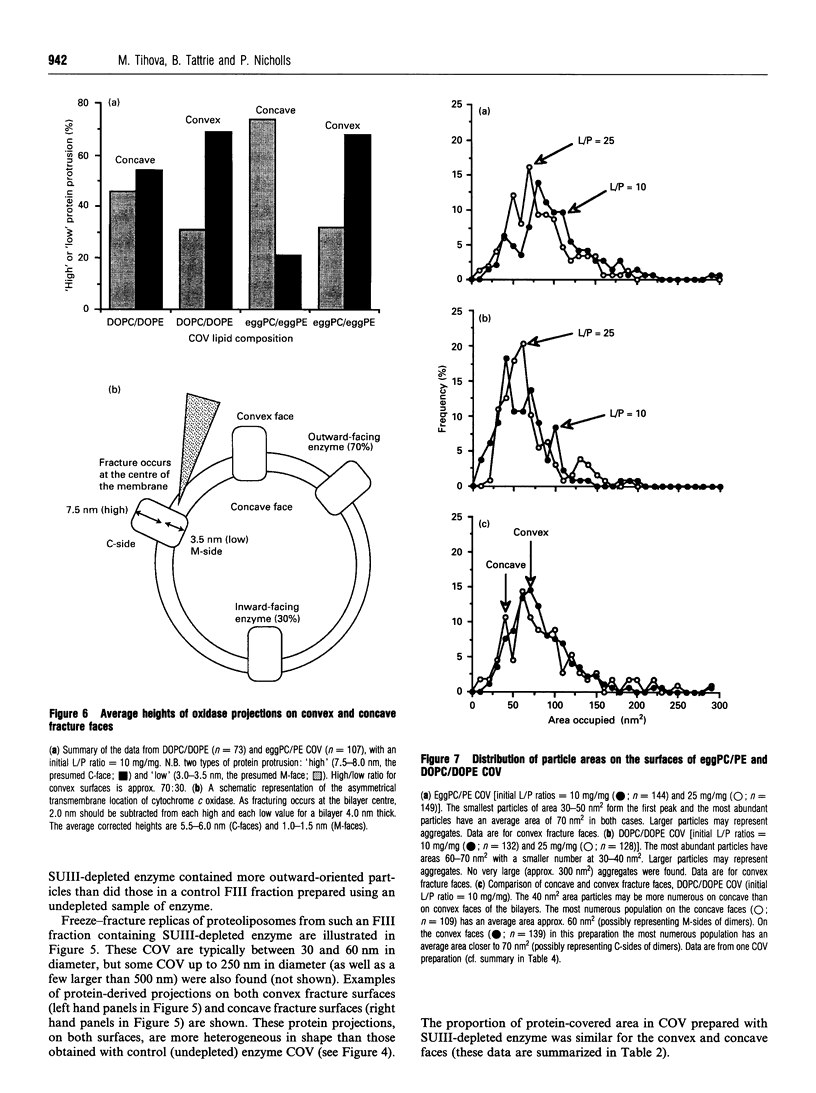
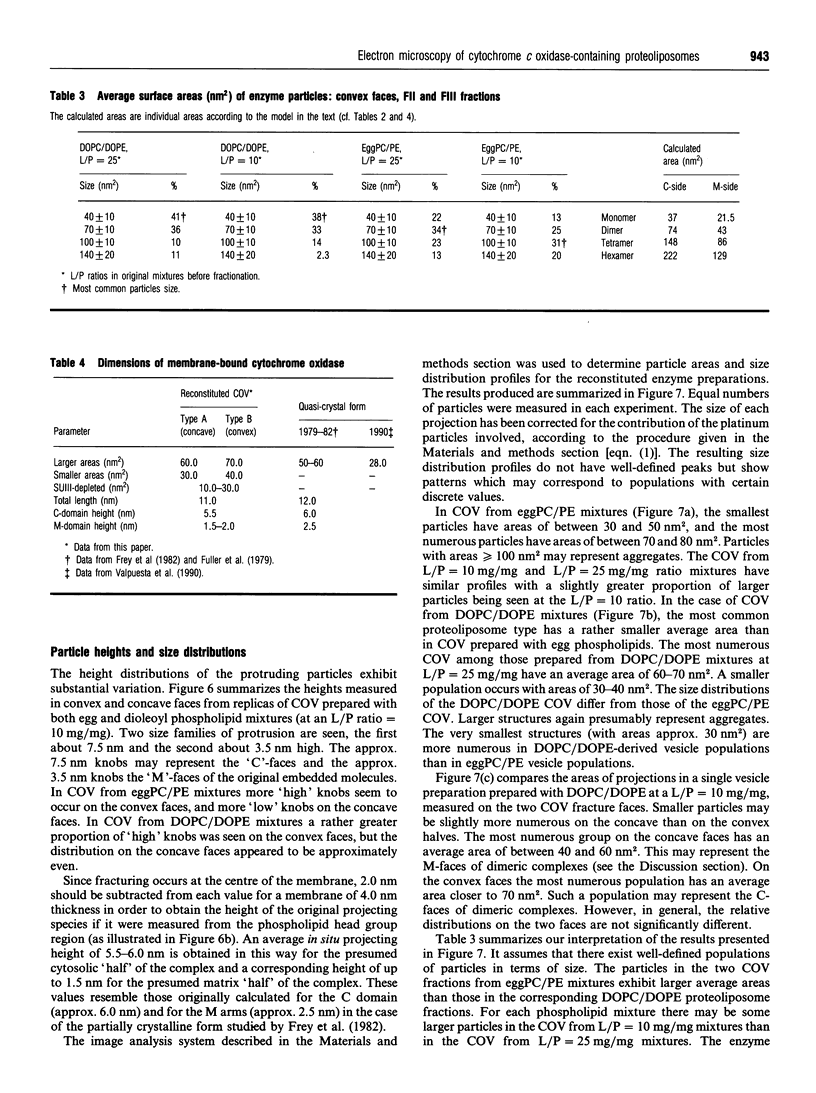
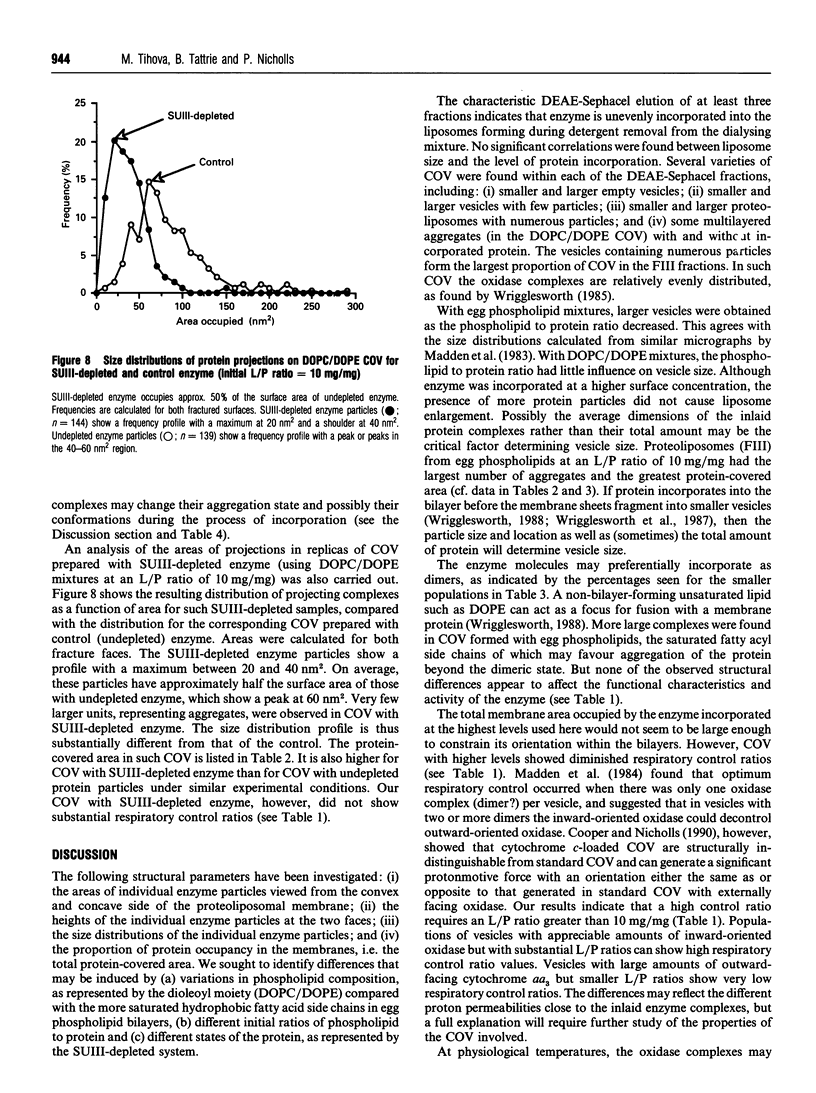
1. Cytochrome c oxidase-containing vesicles were prepared by cholate dialysis using bovine heart cytochrome c oxidase with egg and dioleoylphosphatidylcholine/dioleoylphosphatidylethanolamines (1:1, w/w) at two ratios of phospholipid to protein (25 mg/mg and 10 mg/mg). With each mixture, one or two (FII, FIII) fractions with mostly outward-facing cytochrome aa3 were separated from a fraction (FI) containing mostly inward-facing enzyme and protein-free liposomes by DEAE-Sephacel chromatography. 2. FII and FIII fractions from egg phospholipid mixtures had 60-80% outward-facing enzyme; FII and FIII fractions from dioleoyl phospholipids showed 50-70% outward-facing enzyme. Egg and dioleoyl phospholipid mixtures maintained good respiratory control ratios (8-13) only at the higher lipid/protein ratios. 3. Platinum/carbon replicas of freeze-fractured vesicle surfaces were subjected to image analysis. The results showed two types of membrane projection with average heights of 7.5 nm and 3.5 nm from the fracture plane. The former were more numerous on the convex faces. Calculated areas of the projections indicated the probable presence of both enzyme dimers and higher aggregates. Oxidase dimers may have membrane areas of 70-80 nm2 at the high (7.5 nm) side and 40-50 nm2 on the low (3.5 nm) side. 4. Proteoliposomes prepared with enzyme depleted of subunit III contained predominantly much smaller projecting areas. These probably represent monomers with high side areas of 35-40 nm2 and low side areas of 20-25 nm2. Electron microscopy thus directly confirms the predicted change of aggregation state resulting from subunit depletion. 5. The results are compared with those from two-dimensional crystals. Assuming that the high and low projections are two sides of one family of transmembrane molecules, a total length of 11 nm matches 11-12 nm lengths obtained by crystallography. Our membrane areas match the areas obtained in earlier 'crystal' studies better than the small areas obtained recently by electron cryomicroscopy.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunori M., Antonini G., Malatesta F., Sarti P., Wilson M. T. Cytochrome-c oxidase. Subunit structure and proton pumping. Eur J Biochem. 1987 Nov 16;169(1):1–8. doi: 10.1111/j.1432-1033.1987.tb13572.x. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Malatesta F., Darley-Usmar V. M. Structure of cytochrome c oxidase. Biochim Biophys Acta. 1983 Jul 15;726(2):135–148. doi: 10.1016/0304-4173(83)90003-4. [DOI] [PubMed] [Google Scholar]
- Casey R. P., Ariano B. H., Azzi A. Studies on the transmembrane orientation of cytochrome c oxidase in phospholipid vesicles. Eur J Biochem. 1982 Feb;122(2):313–318. doi: 10.1111/j.1432-1033.1982.tb05882.x. [DOI] [PubMed] [Google Scholar]
- Cooper C. E., Bruce D., Nicholls P. Use of oxonol V as a probe of membrane potential in proteoliposomes containing cytochrome oxidase in the submitochondrial orientation. Biochemistry. 1990 Apr 24;29(16):3859–3865. doi: 10.1021/bi00468a009. [DOI] [PubMed] [Google Scholar]
- Cooper C. E., Nicholls P., Freedman J. A. Cytochrome c oxidase: structure, function, and membrane topology of the polypeptide subunits. Biochem Cell Biol. 1991 Sep;69(9):586–607. doi: 10.1139/o91-089. [DOI] [PubMed] [Google Scholar]
- Cooper C. E., Nicholls P. Structure and vectorial properties of proteoliposomes containing cytochrome oxidase in the submitochondrial orientation. Biochemistry. 1990 Apr 24;29(16):3865–3871. doi: 10.1021/bi00468a010. [DOI] [PubMed] [Google Scholar]
- Costello M. J., Frey T. G. Membranous cytochrome c oxidase. A freeze-fracture electron microscopic analysis. J Mol Biol. 1982 Nov 25;162(1):131–156. doi: 10.1016/0022-2836(82)90165-6. [DOI] [PubMed] [Google Scholar]
- Crinson M., Nicholls P. Routes of electron transfer in beef heart cytochrome c oxidase: is there a unique pathway used by all reductants? Biochem Cell Biol. 1992 May;70(5):301–308. doi: 10.1139/o92-047. [DOI] [PubMed] [Google Scholar]
- Deatherage J. F., Henderson R., Capaldi R. A. Relationship between membrane and cytoplasmic domains in cytochrome c oxidase by electron microscopy in media of different density. J Mol Biol. 1982 Jul 5;158(3):501–514. doi: 10.1016/0022-2836(82)90211-x. [DOI] [PubMed] [Google Scholar]
- Deatherage J. F., Henderson R., Capaldi R. A. Three-dimensional structures of cytochrome c oxidase vesicle crystals in negative stain. J Mol Biol. 1982 Jul 5;158(3):487–499. doi: 10.1016/0022-2836(82)90210-8. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fajer P., Knowles P. F., Marsh D. Rotational motion of yeast cytochrome oxidase in phosphatidylcholine complexes studied by saturation-transfer electron spin resonance. Biochemistry. 1989 Jun 27;28(13):5634–5643. doi: 10.1021/bi00439a045. [DOI] [PubMed] [Google Scholar]
- Falk K. E., Karlsson B. The interaction of protein and lipid in sonicated vesicles of cytochrome c oxidase and phosphatidylcholine studied by 1H NMR spectroscopy. FEBS Lett. 1979 Feb 1;98(1):25–28. doi: 10.1016/0014-5793(79)80143-x. [DOI] [PubMed] [Google Scholar]
- Frey T. G., Costello M. J., Karlsson B., Haselgrove J. C., Leigh J. S. Structure of the cytochrome c oxidase dimer. Electron microscopy of two-dimensional crystals. J Mol Biol. 1982 Nov 25;162(1):113–130. doi: 10.1016/0022-2836(82)90164-4. [DOI] [PubMed] [Google Scholar]
- Fuller S. D., Capaldi R. A., Henderson R. Structure of cytochrome c oxidase in deoxycholate-drived two-dimensional crystals. J Mol Biol. 1979 Oct 25;134(2):305–327. doi: 10.1016/0022-2836(79)90037-8. [DOI] [PubMed] [Google Scholar]
- Gregory L., Ferguson-Miller S. Independent control of respiration in cytochrome c oxidase vesicles by pH and electrical gradients. Biochemistry. 1989 Mar 21;28(6):2655–2662. doi: 10.1021/bi00432a044. [DOI] [PubMed] [Google Scholar]
- Henderson R., Capaldi R. A., Leigh J. S. Arrangement of cytochrome oxidase molecules in two-dimensional vesicle crystals. J Mol Biol. 1977 Jun 5;112(4):631–648. doi: 10.1016/s0022-2836(77)80167-8. [DOI] [PubMed] [Google Scholar]
- Hill B. C., Robinson N. C. Cyanide binding to bovine heart cytochrome c oxidase depleted of subunit III by treatment with lauryl maltoside. J Biol Chem. 1986 Nov 25;261(33):15356–15359. [PubMed] [Google Scholar]
- Krab K., Wikström M. Proton-translocating cytochrome c oxidase in artificial phospholipid vesicles. Biochim Biophys Acta. 1978 Oct 11;504(1):200–214. doi: 10.1016/0005-2728(78)90018-x. [DOI] [PubMed] [Google Scholar]
- Kuboyama M., Yong F. C., King T. E. Studies on cytochrome oxidase. 8. Preparation and some properties of cardiac cytochrome oxidase. J Biol Chem. 1972 Oct 25;247(20):6375–6383. [PubMed] [Google Scholar]
- Longmuir K. J., Capaldi R. A., Dahlquist F. W. Nuclear magnetic resonance studies of lipid-protein interactions. A model of the dynamics and energetics of phosphatidylcholine bilayers that contain cytochrome c oxidase. Biochemistry. 1977 Dec 27;16(26):5746–5755. doi: 10.1021/bi00645a015. [DOI] [PubMed] [Google Scholar]
- Madden T. D., Cullis P. R. Detergent-induced solubilization of cytochrome c oxidase as detected in a novel reconstituted system. J Biol Chem. 1984 Jun 25;259(12):7655–7658. [PubMed] [Google Scholar]
- Madden T. D., Hope M. J., Cullis P. R. Influence of vesicle size and oxidase content on respiratory control in reconstituted cytochrome oxidase vesicles. Biochemistry. 1984 Mar 27;23(7):1413–1418. doi: 10.1021/bi00302a012. [DOI] [PubMed] [Google Scholar]
- Madden T. D., Hope M. J., Cullis P. R. Lipid requirements for coupled cytochrome oxidase vesicles. Biochemistry. 1983 Apr 12;22(8):1970–1974. doi: 10.1021/bi00277a036. [DOI] [PubMed] [Google Scholar]
- Moroney P. M., Scholes T. A., Hinkle P. C. Effect of membrane potential and pH gradient on electron transfer in cytochrome oxidase. Biochemistry. 1984 Oct 9;23(21):4991–4997. doi: 10.1021/bi00316a025. [DOI] [PubMed] [Google Scholar]
- Naqui A., Kumar C., Ching Y. C., Powers L., Chance B. Structure and reactivity of multiple forms of cytochrome oxidase as evaluated by X-ray absorption spectroscopy and kinetics of cyanide binding. Biochemistry. 1984 Dec 4;23(25):6222–6227. doi: 10.1021/bi00320a051. [DOI] [PubMed] [Google Scholar]
- Nałeçz K. A., Bolli R., Ludwig B., Azzi A. The role of subunit III in bovine cytochrome c oxidase. Comparison between native, subunit III-depleted and Paracoccus denitrificans enzymes. Biochim Biophys Acta. 1985 Jul 17;808(2):259–272. doi: 10.1016/0005-2728(85)90008-8. [DOI] [PubMed] [Google Scholar]
- Nicholls P. Control of proteoliposomal cytochrome c oxidase: the partial reactions. Biochem Cell Biol. 1990 Sep;68(9):1135–1141. doi: 10.1139/o90-169. [DOI] [PubMed] [Google Scholar]
- Nicholls P., Cooper C. E., Freedman J. A., Leece B. D. Effects of antibodies to intact cytochrome-c oxidase and its subunit V on the enzymatic activity. Biochem Cell Biol. 1988 Nov;66(11):1218–1225. doi: 10.1139/o88-139. [DOI] [PubMed] [Google Scholar]
- Nicholls P., Cooper C. E., Wrigglesworth J. M. Control of proteoliposomal cytochrome c oxidase: the overall reaction. Biochem Cell Biol. 1990 Sep;68(9):1128–1134. doi: 10.1139/o90-168. [DOI] [PubMed] [Google Scholar]
- Nicholls P., Hildebrandt V. Binding of ligands and spectral shifts in cytochrome c oxidase. Biochem J. 1978 Jul 1;173(1):65–72. doi: 10.1042/bj1730065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls P., Hildebrandt V., Wrigglesworth J. M. Orientation and reactivity of cytochrome aa3 heme groups in proteoliposomes. Arch Biochem Biophys. 1980 Oct 15;204(2):533–543. doi: 10.1016/0003-9861(80)90065-x. [DOI] [PubMed] [Google Scholar]
- Nicholls P., Shaughnessy S. Effects of detergents and cytochrome c binding on scalar and vectorial proton ejection by proteoliposomes containing cytochrome oxidase. Biochem J. 1985 May 15;228(1):201–210. doi: 10.1042/bj2280201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa S., Capitanio N., De Nitto E. Characteristics of the redox-linked proton ejection in beef-heart cytochrome c oxidase reconstituted in liposomes. Eur J Biochem. 1987 May 4;164(3):507–516. doi: 10.1111/j.1432-1033.1987.tb11156.x. [DOI] [PubMed] [Google Scholar]
- Powell G. L., Knowles P. F., Marsh D. Incorporation of cytochrome oxidase into cardiolipin bilayers and induction of nonlamellar phases. Biochemistry. 1990 May 29;29(21):5127–5132. doi: 10.1021/bi00473a018. [DOI] [PubMed] [Google Scholar]
- Racker E. Reconstitution of cytochrome oxidase vesicles and conferral of sensitivity to energy transfer inhibitors. J Membr Biol. 1972 Dec 29;10(3):221–235. doi: 10.1007/BF01867856. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Kachar B., Reese T. S. Dynamic morphology of calcium-induced interactions between phosphatidylserine vesicles. Biophys J. 1985 Apr;47(4):483–489. doi: 10.1016/S0006-3495(85)83941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld A., van Kemenade T. J., Hak T., Verkleij A. J., de Kruijff B. The effect of cytochrome c oxidase on lipid polymorphism of model membranes containing cardiolipin. Eur J Biochem. 1987 Apr 1;164(1):137–140. doi: 10.1111/j.1432-1033.1987.tb11004.x. [DOI] [PubMed] [Google Scholar]
- Rigell C. W., de Saussure C., Freire E. Protein and lipid structural transitions in cytochrome c oxidase-dimyristoylphosphatidylcholine reconstitutions. Biochemistry. 1985 Sep 24;24(20):5638–5646. doi: 10.1021/bi00341a053. [DOI] [PubMed] [Google Scholar]
- Robinson N. C. Specificity and binding affinity of phospholipids to the high-affinity cardiolipin sites of beef heart cytochrome c oxidase. Biochemistry. 1982 Jan 5;21(1):184–188. doi: 10.1021/bi00530a031. [DOI] [PubMed] [Google Scholar]
- Robinson N. C., Talbert L. Triton X-100 induced dissociation of beef heart cytochrome c oxidase into monomers. Biochemistry. 1986 May 6;25(9):2328–2335. doi: 10.1021/bi00357a005. [DOI] [PubMed] [Google Scholar]
- Ruben G. C., Telford J. N. Dimensions of active cytochrome c oxidase in reconstituted liposomes using a gold ball shadow width standard: a freeze-etch electron microscopy study. J Microsc. 1980 Feb;118(2):191–216. doi: 10.1111/j.1365-2818.1980.tb00262.x. [DOI] [PubMed] [Google Scholar]
- Saraste M. Structural features of cytochrome oxidase. Q Rev Biophys. 1990 Nov;23(4):331–366. doi: 10.1017/s0033583500005588. [DOI] [PubMed] [Google Scholar]
- Scotto A. W., Zakim D. Reconstitution of membrane proteins. Spontaneous association of integral membrane proteins with preformed unilamellar lipid bilayers. Biochemistry. 1985 Jul 16;24(15):4066–4075. doi: 10.1021/bi00336a040. [DOI] [PubMed] [Google Scholar]
- Seki S., Oda T. Studies on cytochrome oxidase. II. Ultrastructure of cytochrome oxidase. Arch Biochem Biophys. 1970 May;138(1):122–134. doi: 10.1016/0003-9861(70)90291-2. [DOI] [PubMed] [Google Scholar]
- Valpuesta J. M., Henderson R., Frey T. G. Electron cryo-microscopic analysis of crystalline cytochrome oxidase. J Mol Biol. 1990 Jul 5;214(1):237–251. doi: 10.1016/0022-2836(90)90158-I. [DOI] [PubMed] [Google Scholar]
- Vik S. B., Capaldi R. A. Lipid requirements for cytochrome c oxidase activity. Biochemistry. 1977 Dec 27;16(26):5755–5759. doi: 10.1021/bi00645a016. [DOI] [PubMed] [Google Scholar]
- Wilson K. S., Prochaska L. J. Phospholipid vesicles containing bovine heart mitochondrial cytochrome c oxidase and subunit III-deficient enzyme: analysis of respiratory control and proton translocating activities. Arch Biochem Biophys. 1990 Nov 1;282(2):413–420. doi: 10.1016/0003-9861(90)90137-n. [DOI] [PubMed] [Google Scholar]
- Wrigglesworth J. M., Cooper C. E., Sharpe M. A., Nicholls P. The proteoliposomal steady state. Effect of size, capacitance and membrane permeability on cytochrome-oxidase-induced ion gradients. Biochem J. 1990 Aug 15;270(1):109–118. doi: 10.1042/bj2700109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigglesworth J. M. Incorporation of membrane proteins into liposomal bilayers. Mol Aspects Med. 1988;10(3):223–232. doi: 10.1016/0098-2997(88)90008-8. [DOI] [PubMed] [Google Scholar]
- Wrigglesworth J. M. Quantization of membrane potential generation by cytochrome c oxidase in small vesicles. J Inorg Biochem. 1985 Mar-Apr;23(3-4):311–316. doi: 10.1016/0162-0134(85)85040-6. [DOI] [PubMed] [Google Scholar]
- Wrigglesworth J. M., Wooster M. S., Elsden J., Danneel H. J. Dynamics of proteoliposome formation. Intermediate states during detergent dialysis. Biochem J. 1987 Sep 15;246(3):737–744. doi: 10.1042/bj2460737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Z., Capaldi R. A., Cullis P. R., Madden T. D. Orientation of cytochrome c oxidase molecules in the two populations of reconstituted vesicles resolved by column chromatography on DEAE-Sephacryl. Biochim Biophys Acta. 1985 Jun 26;808(1):209–211. doi: 10.1016/0005-2728(85)90045-3. [DOI] [PubMed] [Google Scholar]