Abstract
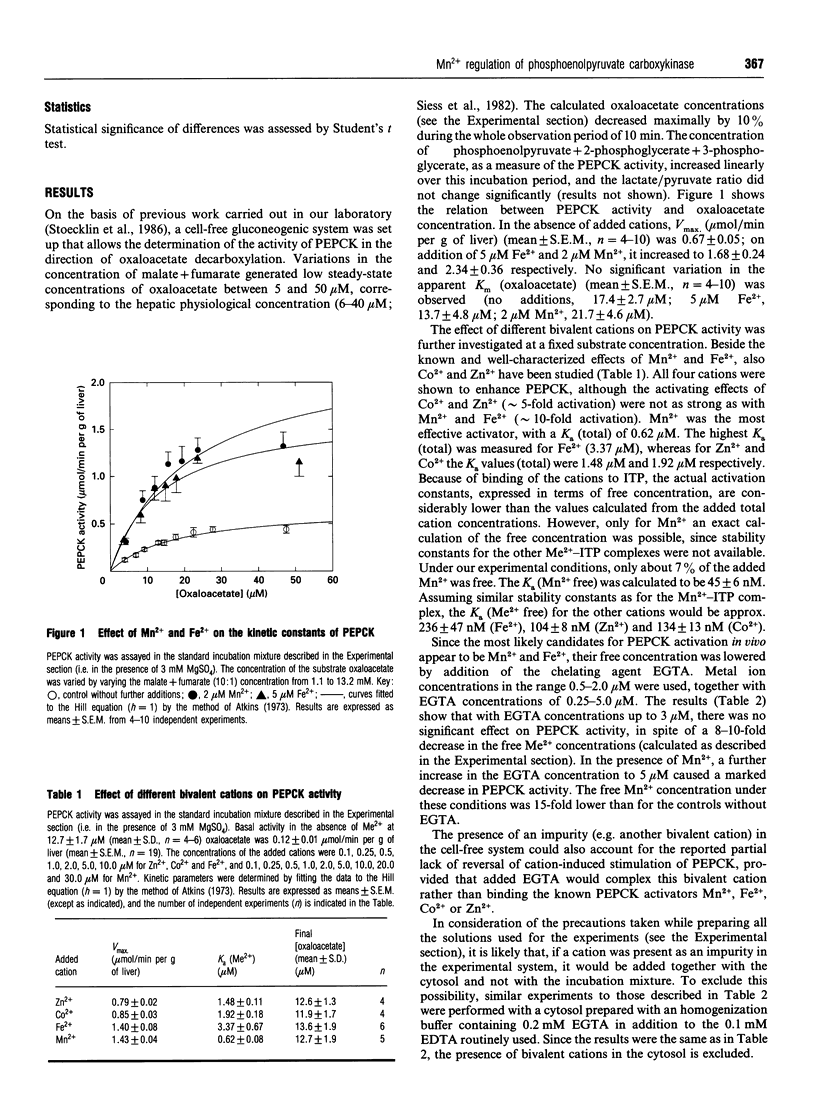
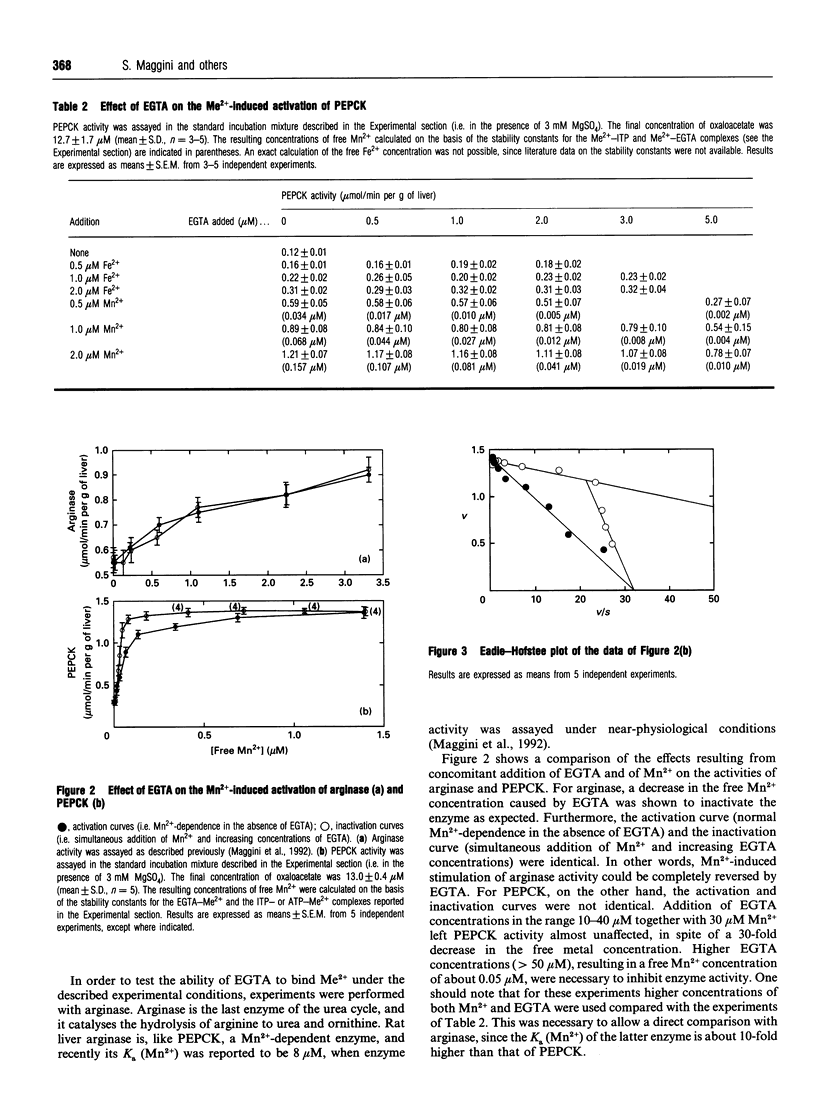
A cytosolic cell-free system prepared from rat liver was used to study the effect of bivalent cations on the activity of the gluconeogenic enzyme phosphoenolpyruvate carboxykinase (PEPCK). Steady-state concentrations of oxaloacetate in the range 5-50 microM were generated from increasing concentrations of malate+fumarate (10:1); 2 mM ITP and 3 mM Mg2+ were added as cofactors. Micromolar concentrations of Mn2+, Fe2+ and, to a lesser extent, of Zn2+ and Co2+ were shown to stimulate PEPCK activity. Vmax. (mumol/min per g of liver) increased from 0.67 to 1.68 on addition of 5 microM Fe2+ and to 2.34 with 2 microM Mn2+, whereas no significant effect on the Km for oxaloacetate was observed. The apparent K(a) values (total) were 0.62 microM for Mn2+, 1.48 microM for Zn2+, 1.92 microM for Co2+ and 3.37 microM for Fe2+, being 2-8-fold lower than the corresponding published values. Variations of the free Mn2+ concentration were obtained (a) by increasing the Mn2+ concentration (i.e. activation curve) and (b) by simultaneous addition of Mn2+ and increasing concentrations of the chelating agent EGTA (i.e. inactivation curve). Different results were obtained for the activation and inactivation curves. The inactivation curve showed that PEPCK activity was almost unaffected by variations of the free Mn2+ concentration over the range 0.05-0.15 microM. Under comparable experimental conditions, rat liver arginase (another Mn(2+)-dependent enzyme) was completely inactivated. From kinetic evidence, the existence of two distinct molecular forms of cytosolic rat liver PEPCK with different Mn2+ affinities is postulated. Considering the high affinity of PEPCK for Mn2+ and its relative insensitivity to changes in the free Mn2+ concentration, it seems rather unlikely that changes in the free cation concentration play a major role in regulating PEPCK activity in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash D. E., Schramm V. L. Determination of free and bound manganese(II) in hepatocytes from fed and fasted rats. J Biol Chem. 1982 Aug 25;257(16):9261–9264. [PubMed] [Google Scholar]
- Atkins G. L. A simple digital-computer program for estimating the parameters of the hill equation. Eur J Biochem. 1973 Feb 15;33(1):175–180. doi: 10.1111/j.1432-1033.1973.tb02667.x. [DOI] [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. Purification of phosphoenolpyruvate carboxykinase from the cytosol fraction of rat liver and the immunochemical demonstration of differences between this enzyme and the mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1969 Oct 25;244(20):5625–5630. [PubMed] [Google Scholar]
- Bartfai T. Preparation of metal-chelate complexes and the design of steady-state kinetic experiments involving metal nucleotide complexes. Adv Cyclic Nucleotide Res. 1979;10:219–242. [PubMed] [Google Scholar]
- Beale E. G., Chrapkiewicz N. B., Scoble H. A., Metz R. J., Quick D. P., Noble R. L., Donelson J. E., Biemann K., Granner D. K. Rat hepatic cytosolic phosphoenolpyruvate carboxykinase (GTP). Structures of the protein, messenger RNA, and gene. J Biol Chem. 1985 Sep 5;260(19):10748–10760. [PubMed] [Google Scholar]
- Bentle L. A., Lardy H. A. Interaction of anions and divalent metal ions with phosphoenolpyruvate carboxykinase. J Biol Chem. 1976 May 25;251(10):2916–2921. [PubMed] [Google Scholar]
- Bentle L. A., Lardy H. A. P-enolpyruvate carboxykinase ferroactivator. Purification and some properties. J Biol Chem. 1977 Feb 25;252(4):1431–1440. [PubMed] [Google Scholar]
- Bentle L. A., Snoke R. E., Lardy H. A. A protein factor required for activation of phosphoenolpyruvate carboxykinase by ferrous ions. J Biol Chem. 1976 May 25;251(10):2922–2928. [PubMed] [Google Scholar]
- Brand I. A., Heinickel A. Key enzymes of carbohydrate metabolism as targets of the 11.5-kDa Zn(2+)-binding protein (parathymosin). J Biol Chem. 1991 Nov 5;266(31):20984–20989. [PubMed] [Google Scholar]
- Brinkworth R. I., Hanson R. W., Fullin F. A., Schramm V. L. Mn2+-sensitive and -insensitive forms of phosphoenolpyruvate carboxykinase (GTP). J Biol Chem. 1981 Nov 10;256(21):10795–10802. [PubMed] [Google Scholar]
- Colombo G., Carlson G. M., Lardy H. A. Phosphoenolpyruvate carboxykinase (guanosine triphosphate) from rat liver cytosol. Separation of homogeneous forms of the enzyme with high and low activity by chromatography on agarose-hexane-guanosine triphosphate. Biochemistry. 1978 Dec 12;17(25):5321–5329. doi: 10.1021/bi00618a001. [DOI] [PubMed] [Google Scholar]
- Colombo G., Lardy H. A. Phosphoenolpyruvate carboxykinase (guanosine 5'-triphosphate) from rat liver cytosol. Divalent cation involvement in the decarboxylation reactions. Biochemistry. 1981 May 12;20(10):2758–2767. doi: 10.1021/bi00513a009. [DOI] [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., BUECHER T. [On the metabolite content and the metabolite concentration in the liver of the rat]. Biochem Z. 1959;332:18–46. [PubMed] [Google Scholar]
- Höppner W., Beckert L., Buck F., Seitz H. J. Is the p29 protein involved in the rapid regulation of phosphoenolpyruvate carboxykinase (GTP)? J Biol Chem. 1991 Sep 15;266(26):17257–17260. [PubMed] [Google Scholar]
- Lardy H., Hughes P. E. Regulation of gluconeogenesis at phosphoenolpyruvate carboxykinase. Curr Top Cell Regul. 1984;24:171–179. doi: 10.1016/b978-0-12-152824-9.50023-x. [DOI] [PubMed] [Google Scholar]
- Lee M. H., Hebda C. A., Nowak T. The role of cations in avian liver phosphoenolpyruvate carboxykinase catalysis. Activation and regulation. J Biol Chem. 1981 Dec 25;256(24):12793–12801. [PubMed] [Google Scholar]
- Maggini S., Stoecklin-Tschan F. B., Mörikofer-Zwez S., Walter P. New kinetic parameters for rat liver arginase measured at near-physiological steady-state concentrations of arginine and Mn2+. Biochem J. 1992 May 1;283(Pt 3):653–660. doi: 10.1042/bj2830653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlman M. A., Walter P., Lardy H. A. Paths of carbon in gluconeogenesis and lipogenesis. VII. The synthesis of precursors for gluconeogenesis from pyruvate and bicarbonate by rat kidney mitochondria. J Biol Chem. 1967 Oct 25;242(20):4594–4602. [PubMed] [Google Scholar]
- Merryfield M. L., Kramp D. C., Lardy H. A. Purification and characterization of a rat liver ferroactivator with catalase activity. J Biol Chem. 1982 Apr 25;257(8):4646–4654. [PubMed] [Google Scholar]
- Mörikofer-Zwez S. Fructose 1,6-bisphosphatase in rat liver cytosol: interactions between the effects of K+, Zn2+, Mn2+, and fructose 2,6-bisphosphate as measured in a steady-state assay. Arch Biochem Biophys. 1983 Jun;223(2):572–583. doi: 10.1016/0003-9861(83)90622-7. [DOI] [PubMed] [Google Scholar]
- Mörikofer-Zwez S., Stoecklin F. B., Walter P. In vitro formation of glucose 6-phosphate from 3-phosphoglycerate by rat liver cytosol. Hoppe Seylers Z Physiol Chem. 1981 Jan;362(1):47–57. doi: 10.1515/bchm2.1981.362.1.47. [DOI] [PubMed] [Google Scholar]
- NORDLIE R. C., LARDY H. A. Mammalian liver phosphoneolpyruvate carboxykinase activities. J Biol Chem. 1963 Jul;238:2259–2263. [PubMed] [Google Scholar]
- Punekar N. S., Lardy H. A. Phosphoenolpyruvate carboxykinase ferroactivator 1. Mechanism of action and identity with glutathione peroxidase. J Biol Chem. 1987 May 15;262(14):6714–6719. [PubMed] [Google Scholar]
- Richardson D. R., Baker E. Intermediate steps in cellular iron uptake from transferrin. Detection of a cytoplasmic pool of iron, free of transferrin. J Biol Chem. 1992 Oct 25;267(30):21384–21389. [PubMed] [Google Scholar]
- Schramm V. L., Fullin F. A., Zimmerman M. D. Kinetic studies of the interaction of substrates, Mn2+, and Mg2+ with the Mn2+-sensitive and -insensitive forms of phosphoenolpyruvate carboxykinase. J Biol Chem. 1981 Nov 10;256(21):10803–10808. [PubMed] [Google Scholar]
- Snoke R. E., Johnston J. B., Lardy H. A. Response of phosphopyruvate carboxylase to tryptophan metabolites and metal ions. Eur J Biochem. 1971 Dec;24(2):342–346. doi: 10.1111/j.1432-1033.1971.tb19692.x. [DOI] [PubMed] [Google Scholar]
- Stoecklin F. B., Mörikofer-Zwez S., Walter P. Formation of hexose 6-phosphates from lactate + pyruvate + glutamate by a cell-free system from rat liver. Biochem J. 1986 May 15;236(1):61–70. doi: 10.1042/bj2360061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titheradge M. A., Picking R. A., Haynes R. C., Jr Physiological concentrations of 2-oxoglutarate regulate the activity of phosphoenolpyruvate carboxykinase in liver. Biochem J. 1992 Aug 1;285(Pt 3):767–771. doi: 10.1042/bj2850767. [DOI] [PMC free article] [PubMed] [Google Scholar]


