Abstract
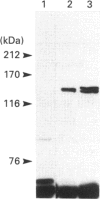
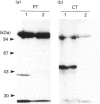
Cells of the osteoblastic cell line MC3T3-E1 were shown to contain at least three phosphatidylinositol-specific phospholipase C (PI-PLC) isoenzymes (PLC-beta, PLC-gamma and PLC-delta) by Western blotting analysis with various anti-PLC antibodies. Stimulation of inositol phosphate production in MC3T3-E1 cells by bradykinin (BK) occurred via a GTP-binding protein. Inositol phosphate formation on stimulation by BK was not affected by pretreatment with pertussis toxin, whereas it was potentiated by cholera toxin pretreatment. Elevation of cellular cyclic AMP levels by brief pretreatment with dibutyryl cyclic AMP or forskolin failed to enhance the BK-mediated generation of inositol phosphates, but long-term preincubation with these agents partially mimicked the action of the cholera toxin. Cholera toxin also caused an increase in BK receptor number. Cycloheximide, a protein biosynthesis inhibitor, prevented the potentiating actions of the cholera toxin and the cyclic AMP-elevating agents on BK-induced inositol phosphate production, and also inhibited the increase in BK receptor number. The specific binding of [3H]BK to the whole MC3T3-E1 cells in the presence or absence of cholera toxin was completely inhibited by the B2 BK receptor antagonist D-Arg[Hyp3,Thi5,8,D-Phe7]BK, but not by the B1 BK receptor agonist des-Arg9-BK. These data suggest that the activation of PI-PLC induced by cholera toxin in BK-stimulated MC3T3-E1 cells was caused by an enhancement of the synthesis of BK receptor protein(s), at least part of which was mediated by a sustained increase in the intracellular level of cyclic AMP.
Full text
PDF


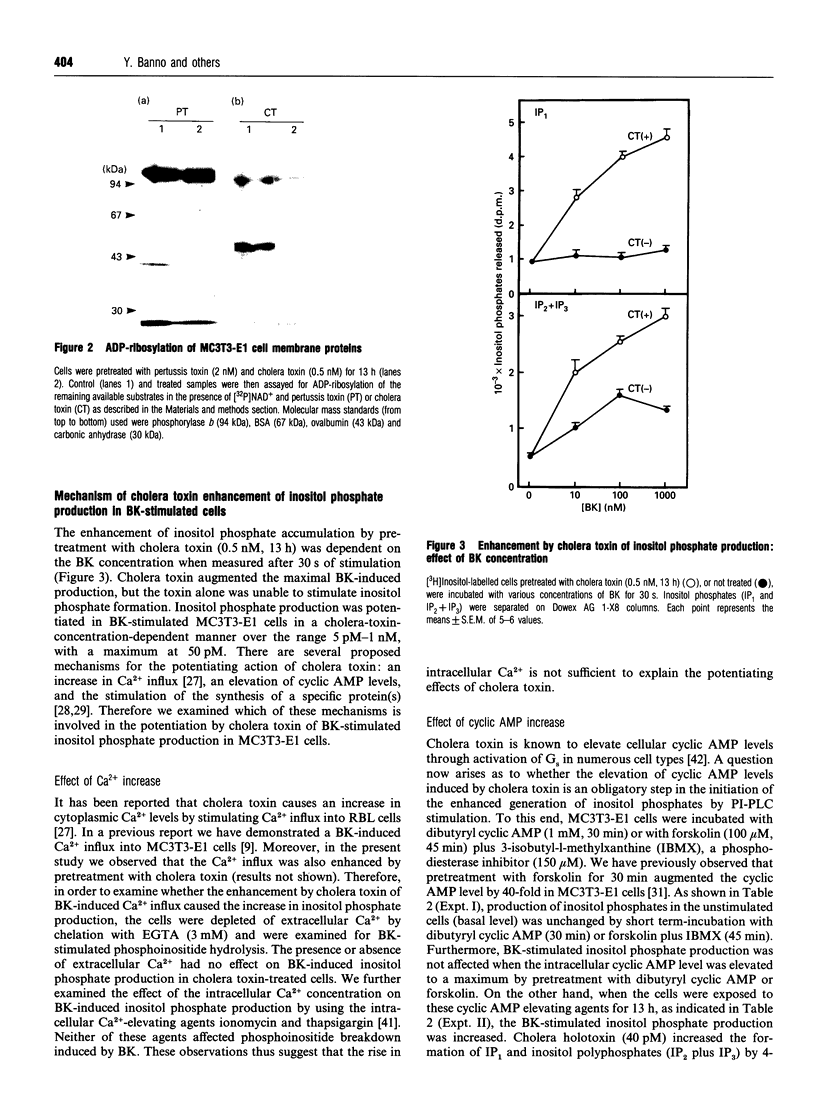
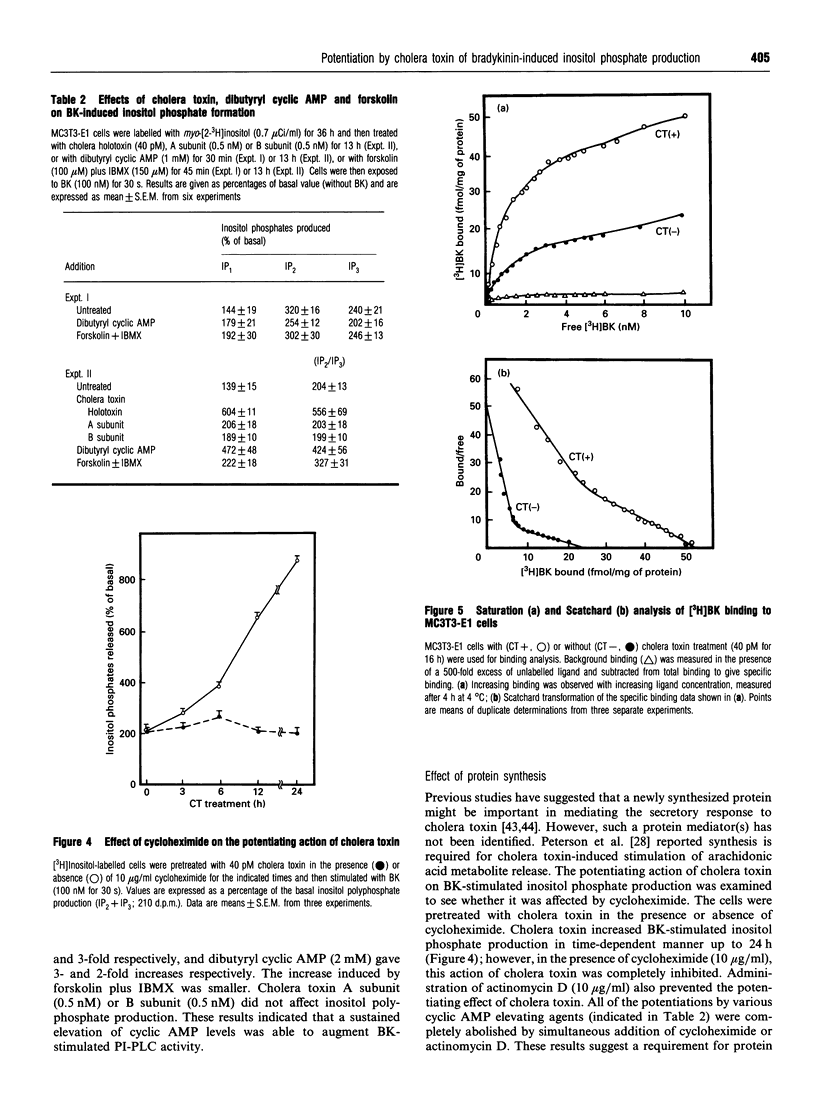


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babich M., King K. L., Nissenson R. A. Thrombin stimulates inositol phosphate production and intracellular free calcium by a pertussis toxin-insensitive mechanism in osteosarcoma cells. Endocrinology. 1990 Feb;126(2):948–954. doi: 10.1210/endo-126-2-948. [DOI] [PubMed] [Google Scholar]
- Banno Y., Nakashima T., Kumada T., Ebisawa K., Nonomura Y., Nozawa Y. Effects of gelsolin on human platelet cytosolic phosphoinositide-phospholipase C isozymes. J Biol Chem. 1992 Apr 5;267(10):6488–6494. [PubMed] [Google Scholar]
- Banno Y., Yada Y., Nozawa Y. Purification and characterization of membrane-bound phospholipase C specific for phosphoinositides from human platelets. J Biol Chem. 1988 Aug 15;263(23):11459–11465. [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini D. R., Nedwin G. E., Bringman T. S., Smith D. D., Mundy G. R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986 Feb 6;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Blank J. L., Ross A. H., Exton J. H. Purification and characterization of two G-proteins that activate the beta 1 isozyme of phosphoinositide-specific phospholipase C. Identification as members of the Gq class. J Biol Chem. 1991 Sep 25;266(27):18206–18216. [PubMed] [Google Scholar]
- Bone E. A., Fretten P., Palmer S., Kirk C. J., Michell R. H. Rapid accumulation of inositol phosphates in isolated rat superior cervical sympathetic ganglia exposed to V1-vasopressin and muscarinic cholinergic stimuli. Biochem J. 1984 Aug 1;221(3):803–811. doi: 10.1042/bj2210803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman F., Morrow B., Kopal M., Bilezikian J. P. Stimulation of inositol phosphate formation in ROS 17/2.8 cell membranes by guanine nucleotide, calcium, and parathyroid hormone. J Bone Miner Res. 1989 Jun;4(3):413–420. doi: 10.1002/jbmr.5650040317. [DOI] [PubMed] [Google Scholar]
- Ely J. A., Ambroz C., Baukal A. J., Christensen S. B., Balla T., Catt K. J. Relationship between agonist- and thapsigargin-sensitive calcium pools in adrenal glomerulosa cells. Thapsigargin-induced Ca2+ mobilization and entry. J Biol Chem. 1991 Oct 5;266(28):18635–18641. [PubMed] [Google Scholar]
- Finkelstein R. A., Jehl J. J., Goth A. Pathogenesis of experimental cholera: choleragen-induced rat foot edema; a method of screening anticholera drugs. Proc Soc Exp Biol Med. 1969 Dec;132(3):835–840. doi: 10.3181/00379727-132-34318. [DOI] [PubMed] [Google Scholar]
- Fu T., Okano Y., Nozawa Y. Bradykinin-induced generation of inositol 1,4,5-trisphosphate in fibroblasts and neuroblastoma cells: effect of pertussis toxin, extracellular calcium, and down-regulation of protein kinase C. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1429–1435. doi: 10.1016/s0006-291x(88)81035-0. [DOI] [PubMed] [Google Scholar]
- Gardner S. D., Milligan G., Rice J. E., Wakelam M. J. The effect of cholera toxin on the inhibition of vasopressin-stimulated inositol phospholipid hydrolysis is a cyclic AMP-mediated event at the level of receptor binding. Biochem J. 1989 May 1;259(3):679–684. doi: 10.1042/bj2590679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Guillon G., Balestre M. N., Lombard C., Rassendren F., Kirk C. J. Influence of bacterial toxins and forskolin upon vasopressin-induced inositol phosphate accumulation in WRK 1 cells. Biochem J. 1989 Jun 15;260(3):665–672. doi: 10.1042/bj2600665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson G. T., Lerner U. Bradykinin stimulates bone resorption and lysosomal-enzyme release in cultured mouse calvaria. Biochem J. 1984 Apr 1;219(1):329–332. doi: 10.1042/bj2190329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutowski S., Smrcka A., Nowak L., Wu D. G., Simon M., Sternweis P. C. Antibodies to the alpha q subfamily of guanine nucleotide-binding regulatory protein alpha subunits attenuate activation of phosphatidylinositol 4,5-bisphosphate hydrolysis by hormones. J Biol Chem. 1991 Oct 25;266(30):20519–20524. [PubMed] [Google Scholar]
- Higashida H., Streaty R. A., Klee W., Nirenberg M. Bradykinin-activated transmembrane signals are coupled via No or Ni to production of inositol 1,4,5-trisphosphate, a second messenger in NG108-15 neuroblastoma-glioma hybrid cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):942–946. doi: 10.1073/pnas.83.4.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden J. B., Shoback D. M., Pattison G., Stobo J. D. Cholera toxin inhibits the T-cell antigen receptor-mediated increases in inositol trisphosphate and cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5673–5677. doi: 10.1073/pnas.83.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Kitazawa T., Somlyo A. V., Somlyo A. P. Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1,4,5-trisphosphate in pharmacomechanical coupling. J Biol Chem. 1989 Oct 25;264(30):17997–18004. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Liebmann C., Offermanns S., Spicher K., Hinsch K. D., Schnittler M., Morgat J. L., Reissmann S., Schultz G., Rosenthal W. A high-affinity bradykinin receptor in membranes from rat myometrium is coupled to pertussis toxin-sensitive G-proteins of the Gi family. Biochem Biophys Res Commun. 1990 Mar 30;167(3):910–917. doi: 10.1016/0006-291x(90)90610-y. [DOI] [PubMed] [Google Scholar]
- Ljunggren O., Johansson H., Ljunghall S., Fredholm B. B., Lerner U. H. Bradykinin induces formation of inositol phosphates and causes an increase in cytoplasmic Ca2+ in the osteoblastic cell line MC3T3-E1. J Bone Miner Res. 1991 May;6(5):443–452. doi: 10.1002/jbmr.5650060504. [DOI] [PubMed] [Google Scholar]
- Ljunggren O., Vavrek R., Stewart J. M., Lerner U. H. Bradykinin-induced burst of prostaglandin formation in osteoblasts is mediated via B2 bradykinin receptors. J Bone Miner Res. 1991 Aug;6(8):807–815. doi: 10.1002/jbmr.5650060805. [DOI] [PubMed] [Google Scholar]
- Lotersztajn S., Pavoine C., Mallat A., Stengel D., Insel P. A., Pecker F. Cholera toxin blocks glucagon-mediated inhibition of the liver plasma membrane (Ca2+-Mg2+)-ATPase. J Biol Chem. 1987 Mar 5;262(7):3114–3117. [PubMed] [Google Scholar]
- Martin T. F., Lewis J. E., Kowalchyk J. A. Phospholipase C-beta 1 is regulated by a pertussis toxin-insensitive G-protein. Biochem J. 1991 Dec 15;280(Pt 3):753–760. doi: 10.1042/bj2800753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. J., Findlay D. M., MacIntyre I., Eisman J. A., Michelangeli V. P., Moseley J. M., Partridge N. C. Calcitonin receptors in a cloned human breast cancer cell line (MCF 7). Biochem Biophys Res Commun. 1980 Sep 16;96(1):150–156. doi: 10.1016/0006-291x(80)91193-6. [DOI] [PubMed] [Google Scholar]
- McCloskey M. A. Cholera toxin potentiates IgE-coupled inositol phospholipid hydrolysis and mediator secretion by RBL-2H3 cells. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7260–7264. doi: 10.1073/pnas.85.19.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Nakamura F., Ogata K., Shiozaki K., Kameyama K., Ohara K., Haga T., Nukada T. Identification of two novel GTP-binding protein alpha-subunits that lack apparent ADP-ribosylation sites for pertussis toxin. J Biol Chem. 1991 Jul 5;266(19):12676–12681. [PubMed] [Google Scholar]
- Okajima F., Katada T., Ui M. Coupling of the guanine nucleotide regulatory protein to chemotactic peptide receptors in neutrophil membranes and its uncoupling by islet-activating protein, pertussis toxin. A possible role of the toxin substrate in Ca2+-mobilizing receptor-mediated signal transduction. J Biol Chem. 1985 Jun 10;260(11):6761–6768. [PubMed] [Google Scholar]
- Olashaw N. E., Pledger W. J. Epidermal growth factor stimulates formation of inositol phosphates in BALB/c/3T3 cells pretreated with cholera toxin and isobutylmethylxanthine. J Biol Chem. 1988 Jan 25;263(3):1111–1114. [PubMed] [Google Scholar]
- Olashaw N. E., Rhee S. G., Pledger W. J. Cyclic AMP agonists induce the phosphorylation of phospholipase C-tau and of a 76 kDa protein co-precipitated by anti-(phospholipase C-tau) monoclonal antibodies in BALB/c-3T3 cells. Relationship to inositol phosphate formation. Biochem J. 1990 Dec 1;272(2):297–303. doi: 10.1042/bj2720297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. W., Berg W. D., Jr, Coppenhaver D. H. Synthesis of protein in intestinal cells exposed to cholera toxin. Proc Soc Exp Biol Med. 1987 Nov;186(2):174–182. doi: 10.3181/00379727-186-42599. [DOI] [PubMed] [Google Scholar]
- Peterson J. W., Reitmeyer J. C., Jackson C. A., Ansari G. A. Protein synthesis is required for cholera toxin-induced stimulation of arachidonic acid metabolism. Biochim Biophys Acta. 1991 Mar 19;1092(1):79–84. doi: 10.1016/0167-4889(91)90179-2. [DOI] [PubMed] [Google Scholar]
- Rhee S. G., Kim H., Suh P. G., Choi W. C. Multiple forms of phosphoinositide-specific phospholipase C and different modes of activation. Biochem Soc Trans. 1991 Apr;19(2):337–341. doi: 10.1042/bst0190337. [DOI] [PubMed] [Google Scholar]
- Roberts R. A., Gullick W. J. Bradykinin receptor number and sensitivity to ligand stimulation of mitogenesis is increased by expression of a mutant ras oncogene. J Cell Sci. 1989 Nov;94(Pt 3):527–535. doi: 10.1242/jcs.94.3.527. [DOI] [PubMed] [Google Scholar]
- Sakai T., Okano Y., Nozawa Y., Oka N. Different protein kinase C isozymes could modulate bradykinin-induced extracellular calcium-dependent and -independent increases in osteoblast-like MC3T3-E1 cells. Cell Calcium. 1992 May;13(5):329–340. doi: 10.1016/0143-4160(92)90068-4. [DOI] [PubMed] [Google Scholar]
- Smrcka A. V., Hepler J. R., Brown K. O., Sternweis P. C. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science. 1991 Feb 15;251(4995):804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- Socorro L., Alexander R. W., Griendling K. K. Cholera toxin modulation of angiotensin II-stimulated inositol phosphate production in cultured vascular smooth muscle cells. Biochem J. 1990 Feb 1;265(3):799–807. doi: 10.1042/bj2650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Lazzaro M., Goad D., Bosma T., Levine L. Tumor necrosis factor-alpha (cachectin) stimulates bone resorption in mouse calvaria via a prostaglandin-mediated mechanism. Endocrinology. 1987 May;120(5):2029–2036. doi: 10.1210/endo-120-5-2029. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Kozawa O., Yoneda M., Oiso Y., Takatsuki K., Asano T., Kato K. Possible coupling of prostaglandin E2 receptor with pertussis toxin-sensitive guanine nucleotide-binding protein in osteoblast-like cells. J Biochem. 1991 Feb;109(2):229–233. [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Nishibe S., Kim J. W., Kim H., Rhee S. G., Carpenter G. Identification of two epidermal growth factor-sensitive tyrosine phosphorylation sites of phospholipase C-gamma in intact HSC-1 cells. J Biol Chem. 1990 Mar 5;265(7):3944–3948. [PubMed] [Google Scholar]
- Wharton W., Leof E., Pledger W. J., O'Keefe E. J. Modulation of the epidermal growth factor receptor by platelet-derived growth factor and choleragen: effects on mitogenesis. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5567–5571. doi: 10.1073/pnas.79.18.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaga F., Hirata M., Koga T. Evidence for coupling of bradykinin receptors to a guanine-nucleotide binding protein to stimulate arachidonate liberation in the osteoblast-like cell line, MC3T3-E1. Biochim Biophys Acta. 1991 Sep 3;1094(2):139–146. doi: 10.1016/0167-4889(91)90001-e. [DOI] [PubMed] [Google Scholar]