Abstract
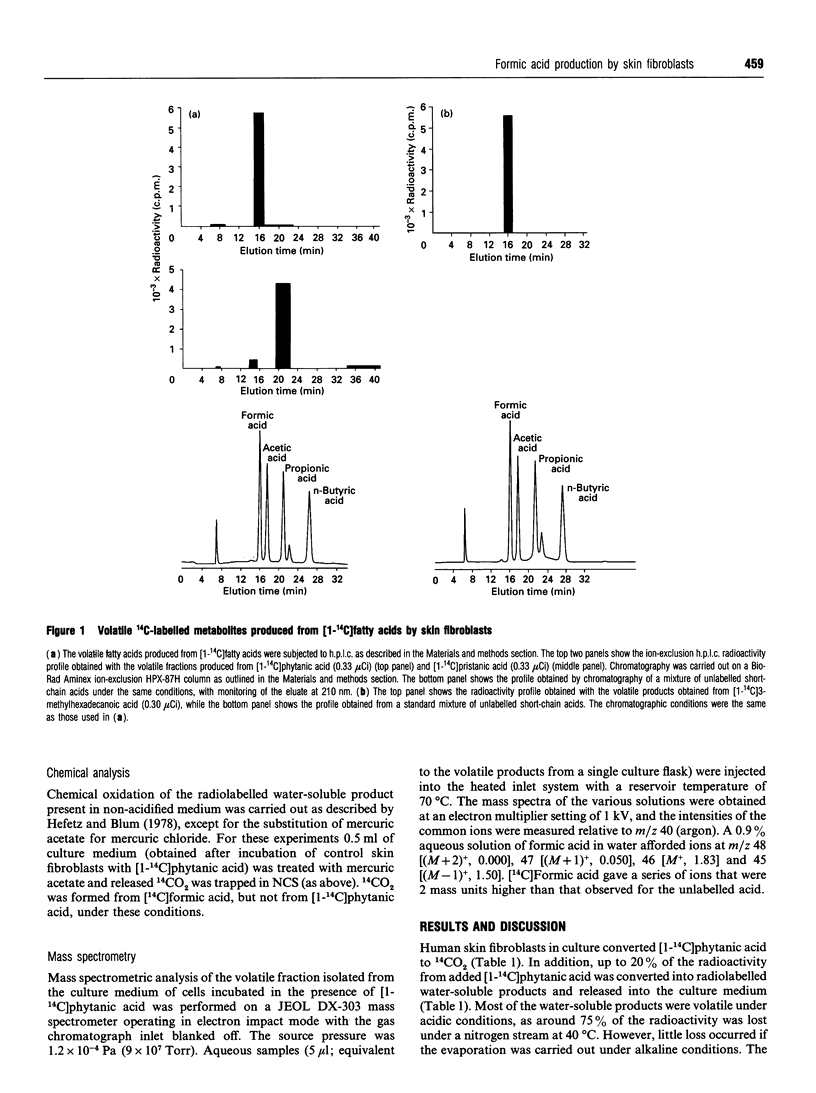
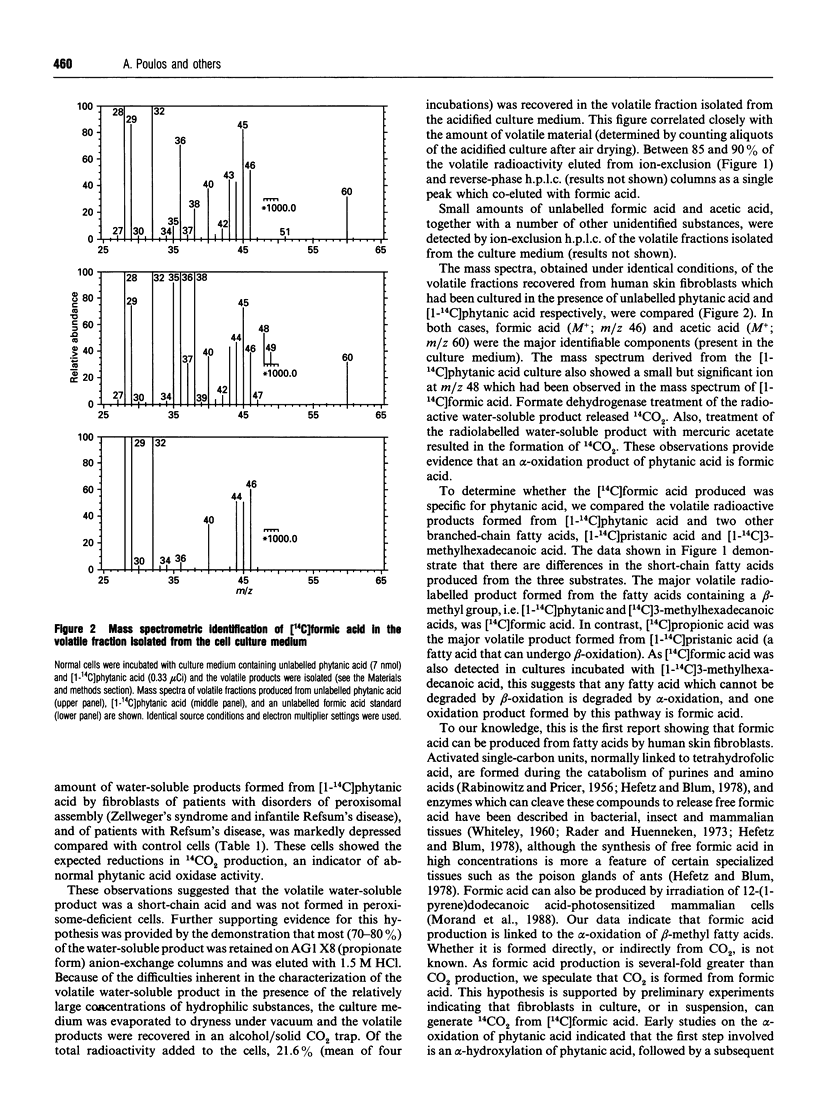
Human skin fibroblasts in culture can oxidize beta-methyl fatty acids, such as phytanic acid and 3-methylhexadecanoic acid, to CO2 and water-soluble products. The latter are released largely into the culture medium. The major water-soluble product formed from [1-14C]phytanic and [1-14C]3-methylhexadecanoic acids is [14C]formic acid. As phytanic acid and 3-methylhexadecanoic acids contain beta-methyl groups and theoretically cannot be degraded by beta-oxidation, we postulate that formic acid is formed from fatty acids by alpha-oxidation. The marked reduction in formic acid production from beta-methyl fatty acids in peroxisome-deficient skin fibroblasts suggests that peroxisomes are involved in the generation of C1 units.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Buchanan D. N., Thoene J. G. Dual-column high-performance liquid chromatographic urinary organic acid profiling. Anal Biochem. 1982 Jul 15;124(1):108–116. doi: 10.1016/0003-2697(82)90227-5. [DOI] [PubMed] [Google Scholar]
- Hefetz A., Blum M. S. Biosynthesis of formic acid by the poison glands of formicine ants. Biochim Biophys Acta. 1978 Nov 1;543(4):484–496. doi: 10.1016/0304-4165(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Herndon J. H., Jr, Steinberg D., Uhlendorf B. W., Fales H. M. Refsum's disease: characterization of the enzyme defect in cell culture. J Clin Invest. 1969 Jun;48(6):1017–1032. doi: 10.1172/JCI106058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand O. H., Zoeller R. A., Raetz C. R. Disappearance of plasmalogens from membranes of animal cells subjected to photosensitized oxidation. J Biol Chem. 1988 Aug 15;263(23):11597–11606. [PubMed] [Google Scholar]
- Poulos A. Diagnosis of Refsum's disease using [1-14C]phytanic acid as substrate. Clin Genet. 1981 Oct;20(4):247–253. doi: 10.1111/j.1399-0004.1981.tb01029.x. [DOI] [PubMed] [Google Scholar]
- Poulos A., Sharp P., Fellenberg A. J., Johnson D. W. Accumulation of pristanic acid (2, 6, 10, 14 tetramethylpentadecanoic acid) in the plasma of patients with generalised peroxisomal dysfunction. Eur J Pediatr. 1988 Feb;147(2):143–147. doi: 10.1007/BF00442211. [DOI] [PubMed] [Google Scholar]
- Poulos A., Singh H., Paton B., Sharp P., Derwas N. Accumulation and defective beta-oxidation of very long chain fatty acids in Zellweger's syndrome, adrenoleukodystrophy and Refsum's disease variants. Clin Genet. 1986 May;29(5):397–408. doi: 10.1111/j.1399-0004.1986.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Poulos A., van Crugten C., Sharp P., Carey W. F., Robertson E., Becroft D. M., Saudubray J. M., Poll-The B. T., Christensen E., Brandt N. Prenatal diagnosis of Zellweger syndrome and related disorders: impaired degradation of phytanic acid. Eur J Pediatr. 1986 Dec;145(6):507–510. doi: 10.1007/BF02429053. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ J. C., PRICER W. E., Jr Purine fermentation by Clostridium cylindrosporum. IV. 4-Ureido-5-imidazolecarboxylic acid. J Biol Chem. 1956 Jan;218(1):189–199. [PubMed] [Google Scholar]
- Robertson E. F., Poulos A., Sharp P., Manson J., Wise G., Jaunzems A., Carter R. Treatment of infantile phytanic acid storage disease: clinical, biochemical and ultrastructural findings in two children treated for 2 years. Eur J Pediatr. 1988 Feb;147(2):133–142. doi: 10.1007/BF00442210. [DOI] [PubMed] [Google Scholar]
- Schulz H. Beta oxidation of fatty acids. Biochim Biophys Acta. 1991 Jan 28;1081(2):109–120. doi: 10.1016/0005-2760(91)90015-a. [DOI] [PubMed] [Google Scholar]
- Singh H., Poulos A. A comparative study of stearic and lignoceric acid oxidation by human skin fibroblasts. Arch Biochem Biophys. 1986 Oct;250(1):171–179. doi: 10.1016/0003-9861(86)90714-9. [DOI] [PubMed] [Google Scholar]
- Singh H., Usher S., Johnson D., Poulos A. A comparative study of straight chain and branched chain fatty acid oxidation in skin fibroblasts from patients with peroxisomal disorders. J Lipid Res. 1990 Feb;31(2):217–225. [PubMed] [Google Scholar]
- Skjeldal O. H., Stokke O. Evidence against alpha-hydroxyphytanic acid as an intermediate in the metabolism of phytanic acid. Scand J Clin Lab Invest. 1988 Feb;48(1):97–102. doi: 10.3109/00365518809085400. [DOI] [PubMed] [Google Scholar]
- Skjeldal O. H., Stokke O., Norseth J., Lie S. O. Phytanic acid oxidase activity in cultured skin fibroblasts. Diagnostic usefulness and limitations. Scand J Clin Lab Invest. 1986 May;46(3):283–287. doi: 10.3109/00365518609083671. [DOI] [PubMed] [Google Scholar]
- Skjeldal O. H., Stokke O. The subcellular localization of phytanic acid oxidase in rat liver. Biochim Biophys Acta. 1987 Sep 4;921(1):38–42. doi: 10.1016/0005-2760(87)90167-6. [DOI] [PubMed] [Google Scholar]
- Street J. M., Johnson D. W., Singh H., Poulos A. Metabolism of saturated and polyunsaturated fatty acids by normal and Zellweger syndrome skin fibroblasts. Biochem J. 1989 Jun 15;260(3):647–655. doi: 10.1042/bj2600647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street J. M., Singh H., Poulos A. Metabolism of saturated and polyunsaturated very-long-chain fatty acids in fibroblasts from patients with defects in peroxisomal beta-oxidation. Biochem J. 1990 Aug 1;269(3):671–677. doi: 10.1042/bj2690671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders R. J., van Roermund C. W., Jakobs C., ten Brink H. J. Identification of pristanoyl-CoA oxidase and phytanic acid decarboxylation in peroxisomes and mitochondria from human liver: implications for Zellweger syndrome. J Inherit Metab Dis. 1991;14(3):349–352. doi: 10.1007/BF01811700. [DOI] [PubMed] [Google Scholar]
- Watkins P. A., Mihalik S. J. Mitochondrial oxidation of phytanic acid in human and monkey liver: implication that Refsum's disease is not a peroxisomal disorder. Biochem Biophys Res Commun. 1990 Mar 16;167(2):580–586. doi: 10.1016/0006-291x(90)92064-7. [DOI] [PubMed] [Google Scholar]


