Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest malignancies and is currently the third leading cause of cancer death. The aggressiveness of PDAC stems from late diagnosis, early metastasis, and poor efficacy of current chemotherapies. Thus, there is an urgent need for effective biomarkers for early detection of PDAC and development of new therapeutic strategies. It has long been known that cellular glycosylation is dysregulated in pancreatic cancer cells, however, tumor-associated glycans and their cognate glycosylating enzymes have received insufficient attention as potential clinical targets. Aberrant glycosylation affects a broad range of pathways that underpin tumor initiation, metastatic progression, and resistance to cancer treatment. One of the prevalent alterations in the cancer glycome is an enrichment in a select group of sialylated glycans including sialylated, branched N-glycans, sialyl Lewis antigens, and sialylated forms of truncated O-glycans such as the sialyl Tn antigen. These modifications affect the activity of numerous cell surface receptors, which collectively impart malignant characteristics typified by enhanced cell proliferation, migration, invasion and apoptosis-resistance. Additionally, sialic acids on tumor cells engage inhibitory Siglec receptors on immune cells to dampen anti-tumor immunity, further promoting cancer progression. The goal of this review is to summarize the predominant changes in sialylation occurring in pancreatic cancer, the biological functions of sialylated glycoproteins in cancer pathogenesis, and the emerging strategies for targeting sialoglycans and Siglec receptors in cancer therapeutics.
1. Introduction
Pancreatic cancer is one of the most aggressive epithelial malignancies. The most common and deadly form, pancreatic ductal adenocarcinoma (PDAC), has a dismal 5-year survival rate of less than 10% (Siegel, Miller, Fuchs, & Jemal, 2021). The high mortality of patients is due to the early invasion of tumor cells into adjacent tissue, early metastasis, and late diagnosis; together these features limit the only potential curative treatment for PDAC, surgical resection (Kabashi, Dedushi, Ramadani, Mucaj, Hoxhaj, & Jerliu, 2016; Rahib, Smith, Aizenberg, Rosenzweig, Fleshman, & Matrisian, 2014). Current treatments including chemotherapies, targeted therapies, and immunotherapies have failed to substantially improve PDAC patient survival, therefore new avenues for treatment must be considered. One of the areas of pancreatic cancer biology that has received limited attention is the role of cell surface glycans in tumor cell behavior. Aberrant glycosylation was one of the earliest observed characteristics of a cancer cell (Almeida & Kolarich, 2016; Helenius & Aebi, 2001; Reis, Osorio, Silva, Gomes, & David, 2010; Rudd, Woods, Wormald, Opdenakker, Downing, & Campbell, 1995; Vajaria & Patel, 2017) and surface glycans have been implicated in chemoresistance, tumor aggressiveness and metastasis (Laubli & Borsig, 2019; Munkley & Elliott, 2016).
Abnormal sialylation is one of the predominant glycan alterations observed during carcinogenesis, and is associated with malignant properties including invasion and metastasis (Shah, Telang, Shah, & Patel, 2008). Sialic acid, a negatively-charged monosaccharide, is added to the non-reducing terminal position of glycoconjugates. Sialic acids are linked through either α2,3 or α2,6 bonds to galactose or an α2,6 bond to N-acetylgalactose (GalNAc). Sialic acid can also be added in an α2,8 linkage to another sialic acid, forming polysialic acid. To date, twenty different sialyltransferases have been identified in humans (Harduin-Lepers, Krzewinski-Recchi, Colomb, Foulquier, Groux-Degroote, & Delannoy, 2012). These enzymes add sialic acid to either glycolipids or to the N- or O-linked sugar chains of glycoproteins. Sialyltransferases are subdivided into four general families: (1) the ST3Gal enzymes, ST3Gal1-6, which add α2,3-linked sialic acid to galactose; (2) ST6Gal1 and 2, which add α2,6-linked sialic acid to galactose; (3) ST6GalNAc1-6, which add α2,6 sialic acid to GalNAc, and (4) ST8Sia1-6, which generate the polysialic acid structure. While α2,8-linked polysialylation is increased in some types of cancer (e.g., neuroblastoma) (Pietrobono & Stecca, 2021; Sato & Kitajima, 2021), malignant epithelial cells more commonly exhibit an enrichment in α2,3 and α2,6 sialic acids (Bellis, Reis, Varki, Kannagi, & Stanley, 2022). The hypersialylation of tumor cells can be caused by the upregulation of select sialyltransferases, increased availability of CMP-sialic acid, or decreased neuraminidase levels in the cell (Bhide & Colley, 2017; Bull, Stoel, den Brok, & Adema, 2014; Rodrigues & Macauley, 2018). An extensive literature has underscored the importance of sialoglycans in fundamental processes such as cell-cell communication, cell-extracellular matrix communication and intracellular signaling induced by cell surface receptors.
Dysregulated sialylation is a hallmark feature of pancreatic and other malignancies. Examples of tumor-associated sialoglycans found on glycoproteins include hypersialylated N-glycans, sialyl Lewis antigens, and sialylated forms of truncated O-glycans (sialyl-Tn and sialyl-T antigens) (Bellis et al., 2022; Christiansen, Chik, Lee, Anugraham, Abrahams, & Packer, 2014; Dall’Olio, Malagolini, Trinchera, & Chiricolo, 2014; Dobie & Skropeta, 2021; Munkley, 2019). Changes in the sialylation of gangliosides are also common in cancer, however these have been described elsewhere (Kannagi, Cai, Huang, Chao, & Sakuma, 2018; Kasprowicz, Sophie, Lagadec, & Delannoy, 2022), and will not be a focus of this review. Sialoglycans play a critical role in regulating tumor cell phenotype by modulating the activity of transmembrane and secreted glycoproteins. Additionally, the hypersialylation of tumor cells can dampen the immune response through sialic acid binding to Siglecs (Sialic acid-binding immunoglobulin-type lectins) on the surface of various immune cells. The presence of sialic acids on tumor cells has been associated with a Siglec-mediated reduction in effector T cells, an increase in regulatory T cells (Perdicchio, Cornelissen, Streng-Ouwehand, Engels, Verstege, & Boon, 2016), and differentiation of monocytes into macrophages with an immunosuppressive phenotype (Rodriguez, Boelaars, Brown, Eveline Li, Kruijssen & Bruijns, 2021). Together these effects facilitate tumor escape from immune surveillance.
The lack of effective treatments for pancreatic cancer underscores the need for a better understanding of the molecular mechanisms that drive cancer initiation and progression. Elucidating the mechanisms by which sialoglycans promote carcinogenesis may reveal important new targets for therapeutic intervention or biomarkers that can be used to track disease advancement or recurrence. In this review, we summarize the changes in sialylation occurring in pancreatic cancer, the functional contribution of aberrantly-sialylated glycoproteins to pathogenesis, and the corresponding role of Siglec receptors in directing immunosuppression in response to hypersialylation.
2. Hypersialylation of N-glycosylated proteins
An increase in highly-sialylated, branched N-glycans (Fig. 1A) is one of the prevalent glycan alterations observed in epithelial cancers including PDAC (Bellis et al., 2022). Hypersialylation of tumor cells has been reported for decades, however more recent glycan profiling studies have provided important insights into the overall structure of tumor sialoglycans. Comprehensive glycomics analyses using mass spectrometry approaches have confirmed that N-glycans expressed by cancer cells carry elevated levels of both α2,3 and α2,6 sialylation. For example, analyses of PDAC patient tissues using MALDI imaging mass spectrometry indicated that α2,3 and α2,6 sialylated N-glycans were enriched in the malignant, compared to healthy, pancreas (McDowell, Klamer, Hall, West, Wisniewski & Powers, 2020). Interestingly, while α2,3 sialylation was more abundant than α2,6 sialylation in cancer tissues, α2,6 sialylation appeared to be more specific for adenocarcinoma cells (McDowell, Klamer, Hall, West, Wisniewski, & Powers, 2020). The N-glycans on serum glycoproteins isolated from PDAC patients also displayed increased levels of sialic acid (Zhao, Qiu, Simeone, & Lubman, 2007), and the ratio of α2,6 sialylation versus α2,3 sialylation on the serum glycoproteins was higher for patient samples than for healthy controls (Vreeker, Hanna-Sawires, Mohammed, Bladergroen, Nicolardi, & Dotz, 2020). Studies of N-glycan composition are reinforced by lectin microarray results (Kurz, Chen, Vucic, Baptiste, Loomis & Agrawal, 2021; Rodriguez, Boelaars, Brown, Eveline Li, Kruijssen, & Bruijns, 2021; Wagatsuma, Nagai-Okatani, Matsuda, Masugi, Imaoka, & Yamazaki, 2020). In a microarray incorporating lectins for numerous glycan structures, an increase in α2,3 and α2,6 sialylation was one of the dominant modifications identified in tissues from PDAC patients as well as the “KC” PDAC mouse model (Kurz, Chen, Vucic, Baptiste, Loomis, & Agrawal, 2021). The KC model, which expresses oncogenic KRas (KRasG12D) (Hingorani, Petricoin, Maitra, Rajapakse, King, & Jacobetz, 2003), recapitulates human PDAC in that more than 90% of PDAC patients have activating mutations in KRas (Wood & Hruban, 2012). These collective studies showing cancer-related enrichment in α2,3 and α2,6 sialylated N-glycans are consistent with a wealth of literature high-lighting functional roles for these sialoglycans in cancer pathogenesis.
Fig. 1. Sialoglycans enriched in tumor cells and their cognate receptors, Siglecs.
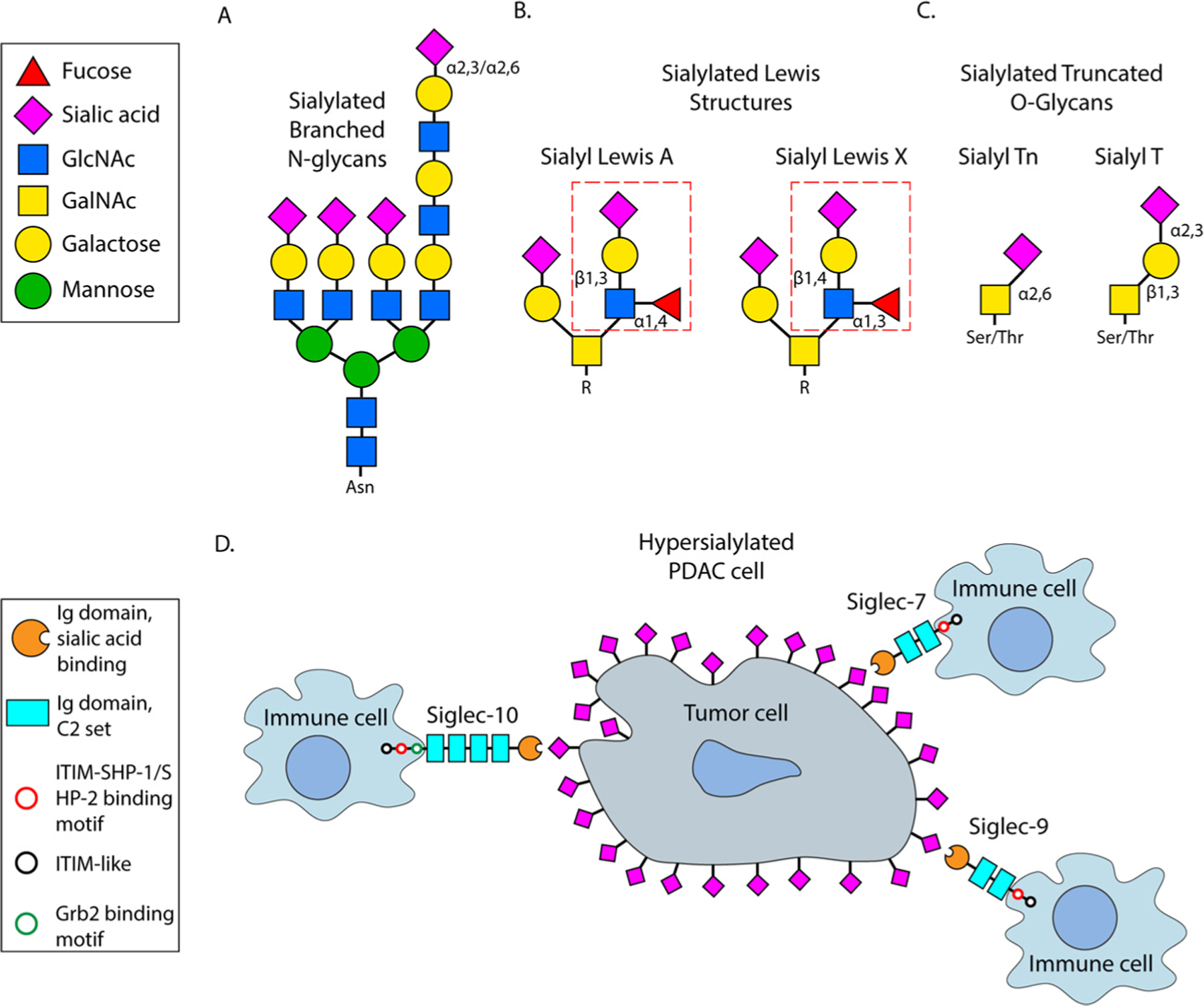
(A) N-linked, branched glycan terminating with either an α2,3 or α2,6 linked sialic acid. (B) Sialylated Lewis structures including sialyl Lewis A (left) and sialyl Lewis X (right). Dashed red box indicates the sialyl Lewis tetrasaccharide. (C) Sialylated forms of truncated O-glycans typified by sialyl Tn (left) and sialyl T (right) antigens. (D) Schematic demonstrating a hypersialylated pancreatic cancer cell and immune cells expressing various inhibitory Siglecs (Siglec-7, Siglec-9, and Siglec-10).
The α2,3-linked sialylation of N-glycans is elaborated by three main sialyltransferases, ST3Gal3, ST3Gal4 and ST6Gal6 (Chung, Yin, Wang, Chuang, Chu, & Betenbaugh, 2015). Of these, ST3Gal3 and ST3Gal4 are reportedly overexpressed in pancreatic cancer (Perez-Garay, Arteta, Llop, Cobler, Pages & Ortiz, 2013; Perez-Garay, Arteta, Pages, de Llorens, de Bolos & Vidal-Vanaclocha, 2010; Rodriguez et al., 2021). The expression of ST3Gal3 and ST3Gal4 is increased by TGFβ (Zhang, Zhang, Holst, Blochl, Madunic, & Wuhrer, 2022), a well-known inducer of epithelial to mesenchymal transition (EMT), as well as pro-inflammatory cytokines found within the tumor microenvironment including TNFα and IL-1β (Bassaganas, Allende, Cobler, Ortiz, Llop, & de Bolos, 2015). The high expression of ST3Gal4 in combination with ST3Gal1 correlates with significantly lower survival rates for PDAC patients (Rodriguez et al., 2021). Heightened activity of ST3Gal3 and ST3Gal4 is associated with enhanced pancreatic cancer cell migration, invasion and metastasis (Bassaganas, Carvalho, Dias, Perez-Garay, Ortiz, & Figueras, 2014; Guerrero, Miro, Wong, Massaguer, Martinez-Bosch, & Llorens, 2020; Perez-Garay, Arteta, Llop, Cobler, Pages, & Ortiz, 2013; Perez-Garay, Arteta, Pages, de Llorens, de Bolos, & Vidal-Vanaclocha, 2010). Some of these behaviors may relate to the increased production of sialyl Lewis X structures, given that sialoglycans generated by ST3Gal3 and ST3Gal4 serve as substrates for subsequent fucosylation (as discussed in the next section). The forced overexpression of ST3Gal3 and ST3Gal4 in MDAPanc-28 pancreatic cancer cells was shown to promote cell adhesion and motility (Perez-Garay et al., 2010, 2013), whereas knockdown of ST3Gal3 or ST3Gal4 in the BxPC-3 and Capan-1 PDAC lines inhibited migratory capacity (Guerrero, Miro, Wong, Massaguer, Martinez-Bosch, & Llorens, 2020). These enzymes were further implicated in metastasis in studies utilizing the splenic injection metastasis model. Mice injected intrasplenically with MDAPanc-28 cells engineered with ST3Gal3 or ST3Gal4 overexpression developed more metastatic tumors in the liver and other organs, and had decreased survival (Perez-Garay et al., 2010, 2013). Surface levels of α2,3 sialylation on tumor cells were similarly correlated with metastasis. N-glycan profiling of four different pancreatic cancer cell lines showed that lines with higher α2,3 sialylation had more metastatic potential compared to cells with low α2,3 sialylation (Holst, Belo, Giovannetti, van Die, & Wuhrer, 2017).
The α2,6 sialic acid linkage on N-glycans is directed primarily by the ST6Gal1 sialyltransferase. ST6Gal2 can also sialylate N-glycans in an α2,6 linkage, however, ST6Gal2 expression is either embryonic or largely confined to the brain after birth (Takashima et al., 2003). ST6Gal1 expression is upregulated in numerous malignancies including pancreatic cancer (Dorsett, Marciel, Hwang, Ankenbauer, Bhalerao, & Bellis, 2021; Garnham, Scott, Livermore & Munkley, 2019; Lu & Gu, 2015). Upregulation occurs, in part, through transcriptional activation of ST6GAL1 by oncogenic forms of ras (Dalziel, Dall’Olio, Mungul, Piller, & Piller, 2004; Seales, Jurado, Singhal, & Bellis, 2003). Increased ST6Gal1-mediated sialylation has widespread effects on tumor cell phenotype. High expression of ST6Gal1 in cancer cells promotes cell migration and invasion (Britain, Bhalerao, Silva, Chakraborty, Buchsbaum, & Crowley, 2021; Hait, Maiti, Wu, Andersen, Hsu, & Wu, 2022; Isaji, Im, Gu, Wang, Hang, & Lu, 2014; Lin, Kemmner, Grigull, & Schlag, 2002; Ranjan & Kalraiya, 2013; Rao, Beggs, Ankenbauer, Hwang, Ma, & Salaita, 2022; Seales, Jurado, Brunson, Wakefield, Frost, & Bellis, 2005; Zhu, Srivatana, Ullah, Gagneja, Berenson, & Lance, 2001) as well as resistance to apoptosis induced by various forms of cell stress including hypoxia and serum growth factor deprivation (Britain et al., 2017; Jones, Dorsett, Hjelmeland, & Bellis, 2018). ST6Gal1 activity also facilitates tumor resistance to chemotherapy and radiotherapy (Chakraborty, Dorsett, Trummell, Yang, Oliver, & Bonner, 2018; Lee, Lee, Bae, & Lee, 2008; Lee, Lee, Seo, Park, & Lee, 2010; Schultz, Holdbrooks, Chakraborty, Grizzle, Landen, & Buchsbaum, 2016; Schultz, Swindall, Wright, Sztul, Landen, & Bellis, 2013; Smithson, Irwin, Williams, Alexander, Smythies, & Nearing, 2022). For instance, high expression of ST6Gal1 in MiaPaCa2 and BxPC3 PDAC cells protects cells from DNA damage induced by gemcitabine (Chakraborty, Dorsett, Trummell, Yang, Oliver, & Bonner, 2018), which is a frontline treatment for pancreatic cancer. One of the mechanisms by which ST6Gal1 enables cells to withstand cytotoxic stimuli may be through promoting cancer stem cell (CSC) characteristics. CSCs are notoriously resistant to apoptosis (Garcia-Mayea, Mir, Masson, Paciucci, & ME, 2020; Najafi et al., 2019; Safa, 2016). Increased ST6Gal1 activity endows cancer cells with all of the key features of a CSC including upregulation of CSC surface markers, enhanced spheroid growth, and increased expression of stem cell transcription factors such as Sox9 (Schultz, Holdbrooks, Chakraborty, Grizzle, Landen, & Buchsbaum, 2016; Swindall, Londoño-Joshi, Schultz, Fineberg, Buchsbaum, & Bellis, 2013). Moreover, knockdown of ST6Gal1 in MiaPaCa2 PDAC cells impairs tumor-initiating potential in in vivo limiting dilution assays (Schultz et al., 2016), the gold standard assay for establishing CSC status. Consistent with a role in imparting stem-like features, ST6Gal1 activity promotes EMT in the Suit2 and S2-LM7AA pancreatic cancer cell lines (Britain, Bhalerao, Silva, Chakraborty, Buchsbaum, & Crowley, 2021). Recent studies in mouse models strongly support a role for ST6Gal1 in pancreatic cancer progression.
In the KC PDAC model, deletion of St6gal1 impeded the formation of early neoplastic lesions known as PanINs (Pancreatic intraepithelial neo-plasias) (Kurz et al., 2021). Additionally, Hsieh et al. reported that ST6Gal1 contributed to the transition between pancreatitis and PDAC development (Hsieh, Shyr, Liao, Chen, Wang, & Lu, 2017). Specifically, the administration of fructose to KC mice with cerulein-induced pancreatitis accelerated invasive PDAC, and ST6Gal1 activity was central to this process.
The mechanism by which enhanced α2,3 and α2,6 sialylation regulates tumor cell behavior depends, in large part, upon sialylation-induced changes in the activity of cell surface receptor glycoproteins. The hypersialylation of N-glycans can influence many aspects of glycoprotein structure and/or localization including conformation, oligomerization and cell surface retention. In turn, altered receptor sialylation modulates intracellular signaling cascades and gene expression. It is noteworthy that receptor tyrosine kinases (RTKs) seem to be particularly affected by modifications in sialylation (Duarte et al., 2022; Gao, Luan, Melamed, & Brockhausen, 2021). Some of the RTKs known to be activated by α2,3 sialylation include the Insulin Receptor and the oncogenic RTKs, MET and RON (Balmana, Diniz, Feijao, Barrias, Mereiter, & Reis, 2020; Gomes, Osorio, Pinto, Campos, Oliveira, & Reis, 2013; Mereiter, Magalhaes, Adamczyk, Jin, Almeida, & Drici, 2016). Reis and colleagues demonstrated that increased α2,3 sialylation of MET resulting from the overexpression of ST3Gal4 stimulated receptor activation, cell invasion and resistance to the tyrosine kinase inhibitor, crizotinib (Balmana et al., 2020; Gomes et al., 2013). RTKs are similarly affected by alterations in α2,6 sialylation. An activating role for ST6Gal1-mediated α2,6 sialylation has been reported for EGFR, MET and ERBB2 (Her2) (Britain et al., 2021; Britain, Holdbrooks, Anderson, Willey, & Bellis, 2018; Liu, Liu, Pan, Huang, Qi, & Li, 2019; Liu, Zhu, Linhai, Song, Gui, & Tan, 2018; Qian, Zhu, Tang, Shen, Ai, & Li, 2009; Rao, Beggs, Ankenbauer, Hwang, Ma, & Salaita, 2022). In pancreatic cancer cells, the α2,6 sialylation of EGFR promoted cell invasiveness and EMT (Britain et al., 2021), and protected against the EGFR inhibitor, gefitinib (Britain et al., 2018). Along with RTKs, the pro-survival function of ST6Gal1 is mediated through the α2,6 sialylation of the TNFR1 and Fas death receptors. The α2,6 sialylation of TNFR1 and Fas blocks ligand-induced internalization of these receptors, thereby preventing apoptotic signaling (Holdbrooks et al., 2018; Swindall & Bellis, 2011). Finally, integrin cell adhesion receptors represent another key target for sialylation by ST6Gal1. Indeed, many of ST6Gal1’s effects on tumor cell migration and invasion are driven by sialylation of the β1 integrin subunit (Christie, Shaikh, Lucas, Lucas, & Bellis, 2008; Hou, Hang, Isaji, Lu, Fukuda, & Gu, 2016; Seales et al., 2005; Shaikh, Seales, Clem, Hennessy, Zhuo, & Bellis, 2008). Taken together, these studies point to the importance of receptor sialylation in tumor cell growth, apoptosis-resistance, migration and invasiveness.
In conjunction with well-established functional roles for receptor sialylation, emerging evidence suggests that the sialylation of N-glycans may be a pivotal determinant for the efficacy of therapeutic monoclonal anti-bodies (Duarte et al., 2022). Duarte et al. showed that the α2,6 sialylation of N-glycans on ERBB2 blocked the binding of Trastuzumab, and it was further suggested that high ST6Gal1 levels may be predictive of a poor patient response to Trastuzumab therapy (Duarte, Rodrigues, Gomes, Hensbergen, Ederveen, & de Ru, 2021). Likewise, the α2,6 sialylation of EGFR protected against cytotoxicity induced by Cetuximab (Rodrigues, Duarte, Gomes, Balmana, Martins, & Hensbergen, 2021). In light of the growing use of tumor-targeting therapeutic antibodies, these studies have significant implications for cancer treatment.
3. Sialyl Lewis antigens
A common feature of pancreatic tumors is an increase in expression of sialyl Lewis antigens (Fig. 1B) (Satomura, Sawabu, Takemori, Ohta, Watanabe, & Okai, 1991; Singh, Pal, Yadav, Tang, Partyka, & Kletter, 2015; Zhang, Yang, Li, Wu, Zhang, & Chen, 2015). Sialyl Lewis structures can be found on N- and O-linked glycans on glycoproteins, or on glycolipids (Trinchera et al., 2017). The Lewis X (LeX) antigen is generated by the addition of an α1,3-linked fucose to GlcNAc on type 2N-acetyllactosamine (LacNAc). The sialyl Lewis X (sLeX) structure contains both an α1,3-linked fucose and an α2,3-linked sialic acid on type 2 LacNAc. Alternatively, α1,4 fucosylation occurs on type 1 LacNAc, leading to Lewis A (LeA). The sialyl LeA (sLeA) tetrasaccharide includes the α1,4-linked fucose and an α2,3-linked sialic acid on type 1 LacNAc. Multiple glycosyltransferases contribute to the synthesis of sialyl Lewis antigens, including various α1,3 fucosyl-transferases and α1,4 fucosyltransferases, which transfer fucose to type 2 and type 1 LacNAcs, respectively. Additionally, several α2,3 sialyltransferases direct the sialylation of type 1 and type 2 LacNAcs. Increased sialyl Lewis expression is one of the main changes to the glycome in pancreatic cancer, and functionally, sialyl Lewis antigens play a seminal role in hematogenous metastasis (Natoni et al., 2016; Satomura, Sawabu, Takemori, Ohta, Watanabe, & Okai, 1991; Sozzani, Arisio, Porpiglia, & Benedetto, 2008). Sialyl Lewis antigens are preferentially expressed on the surface of metastatic adenocarcinoma cells (Hanski, Hanski, Zimmer, Ogorek, Devine, & Riecken, 1995). These structures mediate tumor cell interactions with the vascular endothelium, facilitating tumor cell extravasation and metastasis (Lowe, Stoolman, Nair, Larsen, Berhend, & Marks, 1990; Takada, Ohmori, Yoneda, Tsuyuoka, Hasegawa, & Kiso, 1993; Walz, Aruffo, Kolanus, Bevilacqua, & Seed, 1990).
The sLeA antigen is highly expressed in embryonic tissues, down-regulated in adult tissues, and then re-expressed in malignant lesions (Goonetilleke & Siriwardena, 2007; Lahdenne, Pitkanen, Rajantie, Kuusela, Siimes, & Lanning, 1995). FUT3 is the primary fucosyltransferase involved in generating sLeA, along with the ST3Gal3 sialyltransferase (Dall’Olio et al., 2021; Trinchera et al., 2017). FUT3 overexpression is associated with a poor patient prognosis in breast (do Nascimento et al., 2020) and renal carcinoma (Meng, Xu, Yang, Zhou, Chang, & Shi, 2017) and knockdown of FUT3 inhibits pancreatic cancer cell proliferation and invasion in vivo (Zhan et al., 2018). The sLeA antigen has been identified on a variety of proteins including carcinoembryonic antigen, circulating apo-lipoproteins, and mucins (Tang, Hsueh, Kletter, Bern, & Haab, 2015). The binding of tumor cell sLeA antigens to E-selectin on endothelial cells promotes tumor cell rolling, an event necessary for the subsequent arrest of tumor cells and exit from the vasculature (Kannagi, 2007; Takada, Ohmori, Takahashi, Tsuyuoka, Yago, & Zenita, 1991). Supporting a pro-metastatic role for sLeA, treatment with function-blocking antibodies against sLeA inhibited the development of liver metastases after intraperitoneal transplantation of SW1990 pancreatic cancer cells (Hosono, Narita, Kimura, Sato, Nakashio, & Kasai, 1998). Moreover, high sLeA expression on SUIT2 pancreatic cancer cells increased their adherence to endothelial cells after TNFα stimulation, and the sLeA-expressing SUIT2 cells more readily developed liver metastasis when implanted into nude mice after inflammatory insult (Nozawa, Hirota, Okabe, Shibata, Iwamura, & Haga, 2000). Levels of sLeA have been consistently correlated with metastasis across multiple pancreatic cancer cell lines. In carcinoma cell lines generated from 9 pancreatic cancer patients, the expression of sLeA was significantly associated with metastasis and strikingly, the intensity of surface sLeA on each cell line directly correlated with the number of metastatic colonies in the liver (Kishimoto, Ishikura, Kimura, Takahashi, Kato, & Yoshiki, 1996). Definitive evidence that sLeA antigens play a causal role in pancreatic cancer progression was provided by the Tuveson laboratory. This group generated a genetically-engineered mouse model that expressed sLeA antigens in the pancreas via the transgenic expression of the FUT3 and β3GALT5 glycosyltransferases (Engle, Tiriac, Rivera, Pommier, Whalen & Oni, 2019). Mice expressing sLeA antigens developed severe pancreatitis that, in the presence of oncogenic Kras, led to accelerated pancreatic cancer initiation and reduced survival when compared with mice expressing Kras alone (Engle, Tiriac, Rivera, Pommier, Whalen, & Oni, 2019). In the aggregate, these results implicate the sLeA antigen as an attractive target for immunotherapeutic treatment of PDAC, and fully humanized sLeA antibodies have passed phase 1 A clinical trials for pancreatic cancer (Sawada, Sun, Wu, Hong, Ragupathi, & Livingston, 2011).
The sLeA structure is the epitope of the CA19-9 antigen, which was discovered in 1979 and has been widely used as a clinical biomarker for pancreatic cancer (Herlyn, Sears, Steplewski, & Koprowski, 1982; Herlyn, Steplewski, Herlyn & Koprowski, 1979; Koprowski, Steplewski, Mitchell, Herlyn, Herlyn, & Fuhrer, 1979; Magnani, Brockhaus, Smith, Ginsburg, Blaszczyk, & Mitchell, 1981; Magnani, Nilsson, Brockhaus, Zopf, Steplewski, & Koprowski, 1982; Magnani, Steplewski, Koprowski, & Ginsburg, 1983; Yue, Partyka, Maupin, Hurley, Andrews, & Kaul, 2011). CA19-9 is useful for monitoring patient response to cancer treatment but has limited utility in terms of diagnosis (Galli et al., 2013; Goonetilleke & Siriwardena, 2007; Tempero, Uchida, Takasaki, Burnett, Steplewski, & Pour, 1987; Yue, Maupin, Fallon, Li, Partyka, & Anderson, 2011). However, monitoring CA19-9 antigen on specific proteins, such as mucins, may improve the performance of the CA19-9 assay (Partyka, Maupin, Brand, & Haab, 2012; Tang, Partyka, Hsueh, Sinha, Kletter, & Zeh, 2016; Yue, Maupin, Fallon, Li, Partyka, & Anderson, 2011). CA19-9 antigen has been reported to be carried by MUC1, MUC5AC, and MUC16 in pancreatic cancer (Yue, Goldstein, Hollingsworth, Kaul, Brand, & Haab, 2009; Yue, Maupin, et al., 2011), and the presence of sialyl Lewis epitopes on MUC16 enhances the ability of circulating tumor cells to adhere to E and L-selectins (Chen, Dallas, Balzer, & Konstantopoulos, 2012).
As with sLeA, the sLeX antigen is overexpressed in both PDAC cell lines and tissues (Hosono, Narita, Kimura, Sato, Nakashio, & Kasai, 1998; Kim, Itzkowitz, Yuan, Chung, Satake, & Umeyama, 1988; Peracaula, Tabares, Lopez-Ferrer, Brossmer, de Bolos, & de Llorens, 2005; Satomura et al., 1991; Sinn, Brown, Oberle, & Thompson, 1992). A variety of α1,4 fucosyltransferases can participate in the biosynthesis of sLeX antigens including FUT4,5,6 and 7 (Dall’Olio et al., 2021; Trinchera et al., 2017). Of these, FUT6 is known to be upregulated in pancreatic cancer (Mas, Pasqualini, Caillol, El Battari, Crotte, & Lombardo, 1998). FUT3 can also generate sLeX, as this enzyme has both α1,3 and α1,4 fucosyltransferase activity (Kukowska-Latallo, Larsen, Nair, & Lowe, 1990; Trinchera et al., 2017).
The sialylation of sLeX is mainly elaborated by ST3Gal3,4 or 6 (Carvalho, Harduin-Lepers, Magalhaes, Machado, Mendes, & Costa, 2010). Elevated sLeX levels were documented in approximately 30% of pancreatic cancer tissues (Pour, Tempero, Takasaki, Uchida, Takiyama, & Burnett, 1988) and select proteins with enriched sLeX showed increased expression in PDAC patients compared to healthy controls (Balmana, Sarrats, Llop, Barrabes, Saldova, & Ferri, 2015; Sarrats, Saldova, Pla, Fort, Harvey, & Struwe, 2010). Numerous proteins implicated in pancreatic cancer, including Wnt7b and SPARC, express the sLeX antigen (Rho, Mead, Wright, Brenner, Stave, & Gildersleeve, 2014). Increased expression of sLeX is an efficient marker for predicting the postoperative progression of hepatic metastasis, where PDAC patients with high sLeX have a poor prognosis (Takahashi, Oda, Hasebe, Sasaki, Kinoshita, & Konishi, 2001a, 2001b). In one study, sLeX was found to be upregulated in only 13 of 69 pancreatic cancers, but co-expression of sLeA and sLeX enhanced the ability to differentiate pancreatic cancers from benign pancreatic diseases (Tang, Singh, Partyka, Kletter, Hsueh, & Yadav, 2015).
Interestingly, the expression of sLeX and sLeA antigens may be influenced by the activity of the α1,2 fucosyltransferases, FUT1 and FUT2, which add fucose to galactose (Abrantes, Posada, Guillon, Esteves, & Le Pendu, 2009; Blanas, Sahasrabudhe, Rodriguez, van Kooyk, & van Vliet, 2018). Because FUT1/2 compete with α2,3 sialyltransferases for the galactose on LacNAc, high levels of FUT1/2 can inhibit the production of sialyl Lewis structures (Gorelik, Xu, Henion, Anaraki, & Galili, 1997; Goupille, Hallouin, Meflah, & Le Pendu, 1997; Prieto, Larsen, Cho, Rivera, Shilatifard, & Lowe, 1997). For example, the ectopic expression of FUT1 in pancreatic cancer cells suppressed the expression of sLeA and sLeX, which concomitantly attenuated E selectin-mediated cell adhesion as well as metastatic properties in vivo (Aubert, Panicot, Crotte, Gibier, Lombardo, & Sadoulet, 2000a,2000b). In another study, FUT1 overexpression in pancreatic (BxPC3), hepatic (HepG2), and colon (HT-29) cancer cell lines reduced sLeX, but not sLeA, expression. The FUT1-transduced HT-29 and HepG2 cells, but not BxPC3 cells, failed to bind to E-selectin or to activated endothelial cells (Mathieu, Prorok, Benoliel, Uch, Langlet, & Bongrand, 2004).
The bioavailability of particular sialyltransferases can also regulate sLeA and sLeX expression levels. Peracaula and colleagues examined sialyltransferases in the established PDAC cell lines, Capan-1, MDAPanc-3, MDAPanc-28 and Panc-1 (Perez-Garay et al., 2013). MDAPanc-28 cells had the lowest expression of ST3Gal3 and ST3Gal4 while the other lines had comparable transcript levels of both enzymes. ST3Gal6 was undetectable in all cell lines examined. All cell lines, with the exception of MDAPanc-28, demonstrated high ST3Gal3 and ST3Gal4 expression, which correlated with high α2,3 sialyltransferase activity. Accordingly, greater cell surface levels of sLeA and sLeX were noted in all cell lines except MDAPanc-28. The overexpression of ST3Gal4 in MDAPanc-28 cells resulted in heightened E-selectin-dependent cell adhesion and migration and a heterogeneous increase in sLeX levels. In another study, Capan-1 and MDAPanc-28 cells with forced overexpression of ST3Gal3 displayed upregulated surface sLeX expression and enhanced E-selectin binding capacity and cell migration (Perez-Garay et al., 2010). Conversely, knockdown of ST3Gal3 and ST3Gal4 expression in BxPC3 and Capan-1 cells led to significantly lower levels of sLeX, leading to inefficient binding to E-selectin as well as decreased cell migration and invasion (Guerrero et al., 2020).
Further underscoring the functional importance of sLeA and sLeX antigens, the establishment and growth of metastatic colonies of the human pancreatic carcinoma cell line, PCI-6, were reduced after antibody blockade of sLeA and sLeX (Kawarada, Ishikura, Kishimoto, Kato, Yano, & Kato, 2000). Consistent with this work, Aubert et al. knocked-down FUT3 expression in BxPC3 pancreatic cancer cells and found that this diminished the expression of sLeA and sLeX, and prevented cancer cell adhesion to E-selectin with a resultant decrease in metastasis (Aubert, Panicot-Dubois, Crotte, Sbarra, Lombardo, & Sadoulet, 2000a, 2000b). On the other hand, some data suggest a unique role for Lewis-negative pancreatic tumor cells. In a study including 853 patients with pancreatic cancer, 11.7% of patients were Lewis negative (Liu, Deng, Jin, Gong, Cheng, & Fan, 2020). The Lewis‑negative patients had poorer outcomes and higher metastatic rates than Lewis‑positive patients. Although there is robust evidence for the pro-invasive phenotype conferred by sLeA and sLeX antigen expression, more investigation is needed to elucidate the molecular mechanisms that account for the aggressiveness of Lewis antigen-negative pancreatic cancer.
4. Sialylated forms of truncated O-glycans
Another major alteration in the glycome of neoplastic cells is the expression of immature, truncated O-glycans, exemplified by the Tn, sialyl-Tn (sTn), T and sialyl-T (sT) antigens (Fig. 1C). Truncated O-glycans arise from the disrupted elongation of mucin-type O-glycan chains. Mucin-type O-glycosylation is initiated by a family of N-acetylgalactosaminyltransferases (GALNTs) that transfer GalNAc to the hydroxyl group of serine or threonine residues to generate the Tn antigen (GalNAcα1-Ser/Thr). Galactose is then added to the Tn antigen by the T synthase enzyme (core 1 β1,3 galactosyltransferase, C1GALT1) to produce the T antigen, specifically, the core 1 disaccharide, Galβ1-3GalNAcα1. In normal tissues, the core 1 disaccharide is typically extended by an array of enzymes which together direct the synthesis of elongated, and often branched, O-glycan structures (Magalhaes et al., 2021). The dysregulated synthesis of O-glycans contributes to a number of pathologies including familial tumoral calcinosis (Ichikawa, Guigonis, Imel, Courouble, Heissat, & Henley, 2007; Kato, Jeanneau, Tarp, Benet-Pages, Lorenz-Depiereux, & Bennett, 2006; Topaz, Shurman, Bergman, Indelman, Ratajczak, & Mizrachi, 2004), Tn syndrome (Flores, Lemos, Rema, Taulescu, Seixas, & Reis, 2020; Ju & Cummings, 2005), IgA nephropathy (Allen, Bailey, Brenchley, Buck, Barratt, & Feehally, 2001), high-density lipoprotein metabolism (Kathiresan, Melander, Guiducci, Surti, Burtt, & Rieder, 2008; Wang, Mao, Narimatsu, Ye, Tian, & Goth, 2019) and cancer (Ju, Aryal, Kudelka, Wang, & Cummings, 2014; Kim & Varki, 1997; Kolbl et al., 2015; Springer, 1997; Stowell et al., 2015).
The abnormal expression of immature, truncated O-glycans is a characteristic feature of nearly all epithelial cancers. One of the prime mechanisms underlying the synthesis of truncated O-glycans is a decrease in the activity of the T synthase, which leads to an accumulation in the Tn antigen. The Tn antigen can be sialylated by certain ST6GalNAc enzymes to produce the sTn disaccharide (α2,6 sialylated GalNAc), but cannot be further extended. The T synthase requires a molecular chaperone called COSMC (C1GALT1C1) for proper folding and export to the Golgi (Wang, Ju, Ding, Xia, Wang, & Xia, 2010). In the absence of COSMC, the T synthase is misfolded and targeted for degradation by the proteasome (Ju & Cummings, 2002). Indeed, knockdown of COSMC expression in Panc-1 PDAC cells prevented O-glycan elongation beyond the initial GalNAc addition (Hofmann, Schluter, Lange, Mercanoglu, Ewald & Folster, 2015). The increased levels of Tn antigen in the COSMC knockdown cells were associated with enhanced cell migration and resistance to apoptosis (Hofmann, Schluter, Lange, Mercanoglu, Ewald, & Folster, 2015). In the T3M4 PDAC cell line, genetic deletion of C1GALT1C1 conferred oncogenic characteristics including cell invasiveness, apoptosis-resistance and EMT, along with enhanced tumor growth and invasion in xenograft models (Radhakrishnan, Dabelsteen, Madsen, Francavilla, Kopp & Steentoft, 2014; Thomas, Sagar, Caffrey, Grandgenett, & Radhakrishnan, 2019). A tumor-promoting function for COSMC was further confirmed in a genetically-engineered mouse model. Chugh et al. crossed the COSMC knockout mouse line to the “KPC” PDAC mouse model, which expresses oncogenic KRas (KrasG12D) and mutated p53 (Trp53R172H) (Chugh, Barkeer, Rachagani, Nimmakayala, Perumal & Pothuraju, 2018). KPC mice with COSMC knockout exhibited significantly accelerated PDAC progression, metastasis and mortality. The more advanced malignancy noted in COSMC knock-out mice was attributed, at least in part, to the activation of the ERBB family receptors, EGFR and Her2.
The C1GALT1C1 gene is silenced in many types of cancers including PDAC through hypermethylation (Ju, Lanneau, Gautam, Wang, Xia & Stowell, 2008; Radhakrishnan, Dabelsteen, Madsen, Francavilla, Kopp, & Steentoft, 2014). Radhakrishnan el al. suggested that increased expression of Tn and sTn could be attributed to C1GALT1C1 hypermethylation in 38% of PDAC tumors (Radhakrishnan et al., 2014). Loss-of-function mutations or deletions in C1GALT1C1 constitute another major conduit for increased expression of Tn/sTn (Ju, Lanneau, Gautam, Wang, Xia, & Stowell, 2008). Inactivating mutations in C1GALT1C1 have been documented in many human cancers and are consistently correlated with reduced expression of the T synthase and increased abundance of Tn and sTn (Sun et al., 2018). However, in one study of PDAC patient tissues, no mutations were detected in C1GALT1C1, whereas hypermethylation of C1GALT1C1 was prevalent (Radhakrishnan et al., 2014).
Truncations in O-glycan structure can also occur as a consequence of increased expression of ST6GalNAc1, which appears to be the principal enzyme responsible for generating sTn (Ogawa, Hirohashi, Murai, Nishidate, Okita, & Wang, 2017). The upregulation of ST6GalNAc1 in pancreatic and other cancers has been widely reported (Hruban, Goggins, Parsons, & Kern, 2000; Schuessler, Pintado, Welt, Real, Xu, & Melamed, 1991). Because ST6GalNAc1 and the T synthase both use Tn as a substrate, high levels of ST6GalNAc1 may out-compete the T synthase, resulting in an enrichment in sTn. Tn and sTn antigens are not usually detected in the healthy pancreas, however, these O-glycan structures are markedly upregulated in PDAC tissues (Hofmann et al., 2015; Remmers, Anderson, Linde, DiMaio, Lazenby, & Wandall, 2013; Romer, Aasted, Dabelsteen, Groen, Schnabel & Tan, 2021). The overexpression of Tn and sTn has been associated with enhanced PDAC growth and metastatic dissemination, as well as poor patient prognosis (Burchell et al., 2001; Hofmann et al., 2015; Mereiter, Balmana, Gomes, Magalhaes, & Reis, 2016; Radhakrishnan et al., 2014).
The sTn antigen has served as an important cancer biomarker for decades (Munkley, 2016). Elevated levels of sTn are detectable in tissue sections and sera from PDAC patients (Motoo, Kawakami, Watanabe, Satomura, Ohta, & Okai, 1991; Nanashima, Yamaguchi, Nakagoe, Matsuo, Sumida, & Tsuji, 1999; Thomas et al., 2019) and have both diagnostic and prognostic significance. Mucins are major carriers of sTn, and much of the sTn present in serum is present on shed forms of mucins such as MUC1 and MUC16 (Aithal, Rauth, Kshirsagar, Shah, Lakshmanan, & Junker, 2018; Chen, Zhang, Zhang, Zhu, Ko, & Yung, 2021; Rajesh, Sagar, Rathinavel, Chemparathy, Peng, & Yeh, 2022). In tandem with biomarker function, sTn is receiving increasing attention as a therapeutic target. Humanized antibodies are in development for use in antibody-drug conjugate treatments and other types of immunotherapy (Eavarone, Al-Alem, Lugovskoy, Prendergast, Nazer, & Stein, 2018; Loureiro, Sousa, Ferreira, Chai, Lima, & Pereira, 2018; Prendergast, Galvao da Silva, Eavarone, Ghaderi, Zhang, & Brady, 2017). Additionally, sTn is currently being investigated as a target molecule for vaccines (Ibrahim, Murray, Zhou, Mittendorf, Sample, & Tautchin, 2013) and CAR-T cell therapies (Abrantes, Duarte, Gomes, Walchli, & Reis, 2022; Loureiro, Feldmann, Bergmann, Koristka, Berndt, & Arndt, 2018).
Similar to Tn and sTn, the T antigen and sT antigen are frequently enriched in cancer cells (Cervoni, Cheng, Stackhouse, Heimburg-Molinaro, & Cummings, 2020; Munkley & Elliott, 2016). The sT antigen is elaborated primarily by the ST3Gal1 enzyme, which adds sialic acid in an α2,3 linkage to the galactose of the T antigen. Both ST3Gal1 and the sT antigen are overexpressed in an array of cancers, including pancreatic cancer (Hugonnet, Singh, Haas, & von Gunten, 2021; Rodriguez et al., 2021). However, the T antigen is also expressed in the healthy pancreas (albeit at lower levels) (Doi, Ino, Angata, Shimada, Narimatsu, & Hiraoka, 2020; Osako, Yonezawa, Siddiki, Huang, Ho, & Kim, 1993), which limits its usefulness as a cancer-specific biomarker. Furthermore, the expression of the T antigen in PDAC tissues is much lower than that of the Tn antigen (Chugh, Barkeer, Rachagani, Nimmakayala, Perumal, & Pothuraju, 2018).
Truncated O-glycans are frequently expressed in early stage, pre-malignant lesions that harbor potential to develop into adenocarcinomas (Burchell et al., 2001; Freitas, Campos, Gomes, Pinto, Macedo, & Matos, 2019; Ju et al., 2014; Ju, Wang, Aryal, Lehoux, Ding, & Kudelka, 2013; Julien et al., 2012; Radhakrishnan et al., 2014). Intriguingly, the expression of Tn and sTn is often reduced with increasing tumor progression (Romer, Aasted, Dabelsteen, Groen, Schnabel, & Tan, 2021). It has been suggested that the expression of Tn/sTn and T/sT antigens may be inversely correlated. Tn/sTn antigens are upregulated in premalignant cells, promoting early events in tumorigenesis, whereas the expression levels of T-synthase, and thus T antigen, appear to increase with tumor progression (Bergstrom, Liu, Zhao, Gao, Wu, & Song, 2016; Gao, Bergstrom, Fu, Xie, Chen, & Xia, 2016). Therefore, Tn and sTn may be useful biomarkers or therapeutic targets for early stages in neoplasia, while targeting T and sT antigen may be more effective in established tumors.
5. Activation of Siglec receptors by tumor sialoglycans
The sialylation of surface receptors has profound effects on tumor cell signaling and phenotype, however tumor sialylation also plays a major role in regulating the behavior of immune cells within the tumor microenvironment. Sialic acids present on the surface of tumor cells serve as ligands for the Siglec family of mammalian lectins (Fig. 1D) (Varki & Angata, 2006). Hypersialylated N-glycans, sialyl Lewis structures, and sTn antigens all serve as important ligands for Siglecs (Gonzalez-Gil & Schnaar, 2021). Siglecs are expressed by a diversity of immune cells including tumor-infiltrating T cells, B cells, NK cells, dendritic cells, and macrophages (Crocker & Varki, 2001; Duan & Paulson, 2020; Gonzalez-Gil & Schnaar, 2021; Varki & Angata, 2006). Siglecs are categorized into two subtypes according to sequence homology, CD33-related Siglecs that show high sequence identity (50–99%) and the others (Siglec-1, Siglec-2, Siglec-4, and Siglec-15) sharing 25%–30% sequence identity (Gonzalez-Gil & Schnaar, 2021; Laubli, Kawanishi, George Vazhappilly, Matar, Merheb, & Sarwar Siddiqui, 2021; Lim et al., 2021). Fourteen Siglecs have been identified in humans (Gonzalez-Gil & Schnaar, 2021). Thirteen of these are found on overlapping immune cell types, and one (Siglec-4) on myelinated neurons. Structurally, Siglecs have an extra-cellular N-terminal V-set immunoglobulin (Ig) domain with a conserved sialic acid-binding site, followed by varying numbers of C2-set Ig-like domains (Crocker, Clark, Filbin, Gordon, Jones, & Kehrl, 1998; Crocker et al., 2007; Duan & Paulson, 2020; Gonzalez-Gil & Schnaar, 2021). The extracellular domain is connected to a single membrane-spanning domain and a cytosolic domain containing multiple regulatory motifs. Based on the intracellular motifs, Siglecs can exert activating or inhibitory effects to modulate the immune system. Siglecs that carry intracellular immunoreceptor tyrosine-based inhibitory (ITIM) motifs are major drivers of inhibitory immune responses (Gonzalez-Gil & Schnaar, 2021; Jiang, Qi, Kang, & Wang, 2022). Immune cells infiltrating into the tumor bind to tumor sialic acids through their surface Siglecs; this interaction consequently suppresses the immune response leading to tumor escape from immune surveillance (Hudak et al., 2014; Laubli, Pearce, Schwarz, Siddiqui, Deng, & Stanczak, 2014). Hence, Siglecs serve as immune checkpoint molecules much like the well-known immunotherapy targets, programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) (Fraschilla & Pillai, 2017; Macauley, Kawasaki, Peng, Wang, He, & Arlian, 2015; Sharma & Allison, 2015). There is currently growing interest in exploiting the sialoglycan-Siglec axis as a novel avenue for immune checkpoint therapy (Adams, Stanczak, von Gunten, & Laubli, 2018; Bull, Heise, Adema, & Boltje, 2016; Daly et al., 2019), and several Siglec inhibitors have entered into clinical trials.
PDAC has an aggressive and complex tumor microenvironment (Dougan, 2017; Rodriguez et al., 2021) and anti-PD1 immunotherapy shows little effectiveness for treatment of this malignancy (Dougan, 2017; Feng, Xiong, Cao, Yang, Zheng, & Song, 2017). Recently, Rodriguez et al. uncovered a role for the inhibitory Siglecs, Siglec-7 and Siglec-9, in PDAC progression (Rodriguez et al., 2021). It was demonstrated that the engagement of Siglec-7 and Siglec-9 on monocytic cells with sialic acids on PDAC cells induced the conversion of monocytes into immunosuppressive macrophages within the microenvironment. In this same report, the Siglec-dependent reprogramming of monocytes was associated with poor patient outcomes. This work established Siglec activation as a key mechanism underlying PDAC-mediated immunosuppression, and also identified Siglecs as promising new immunotherapy targets that could potentially serve as alternatives to PD1-targeting treatments. Notably, CAR-T cell therapies have been developed that incorporate Siglec-7 or Siglec-9 to enable targeting of sialic acid-expressing tumor cells. Siglec-7/9-based CAR-T cell therapy is currently being tested for potential treatment of melanoma (Meril, Harush, Reboh, Matikhina, Barliya, & Cohen, 2020), however there is hope that this strategy may have efficacy in PDAC. Humanized monoclonal antibodies against Siglecs also hold promise for treatments utilizing antibody-drug conjugates (Lim et al., 2021; Smith & Bertozzi, 2021).
A role for Siglec-4 (also called Myelin Associated Glycoprotein or MAG) has been reported in the recurrence of PDAC after surgical resection (Lim et al., 2021; Swanson, McDermott, Singh, Eggers, Crocker, & Hollingsworth, 2007). Siglec-4 is mainly expressed in myelinated neurons and auto-antibodies against Siglec-4 have been observed in several neuropathic syndromes (Dougan, 2017). Apart from the brain, an interaction between Siglec-4 and MUC1 expressed on tumor cells is involved in pancreatic cancer perineural invasion (Swanson et al., 2007). Perineural invasion is a common pathologic manifestation in PDAC, whereby cancer cells invade the endoneurium of pancreatic nerves. This process leads to significant lower back pain in PDAC patients and is a source of local recurrence after the resection of primary tumor masses (Liu, Ma, Xu, Lei, Li, & Wang, 2012; Swanson et al., 2007; Takahashi, Hasebe, Oda, Sasaki, Kinoshita, & Konishi, 2001a, 2001b). Swanson et al. showed that Siglec-4 binds to pancreatic cancer cells in a sialic acid–dependent manner and interacts with the MUC1 adhesive mucin (Swanson et al., 2007). MUC1 is overexpressed and aberrantly glycosylated in PDAC and is responsible for the adhesion of PDAC cells to Siglec-4-expressing pancreatic nerves. Future studies of the Siglec-4 and MUC1 interaction could provide new avenues for developing treatments for pancreatic cancer pain and recurrence.
More recently, Siglec-15 has been implicated in pancreatic cancer progression and prognosis. High Siglec-15 mRNA expression was associated with worse overall survival of PDAC patients (Li, Huang, Chen, Yao, Ke, & He, 2020). Siglec-15 has also been reported to promote PDAC immune evasion. Li et al. showed that sialic acids on PDAC cells interacted with Siglec-15 to potentiate the immunosuppressive properties of tumor-associated macrophages within the PDAC microenvironment (Li, Jin, Li, Ye, Li, & Jiang, 2022). However, a separate study suggested that Siglec-15 was associated with favorable patient outcomes (Chen, Mo, Zhang, Ma, Lu, & Yu, 2022). In this latter report, Siglec-15 was primarily expressed by moderate to well-differentiated tumors, and was found to be a good prognostic indicator for PDAC. Further studies will be needed to resolve this discrepancy. Like Siglec-15, Siglec-10 has emerged as a potential mediator of immunosuppression in PDAC. In this case, Siglec-10 appears to exert its biological effects by binding to the CD24 surface receptor (Yin & Gao, 2020). The binding of Siglec-10 to sialylated forms of CD24 expressed by pancreatic cancer cells facilitated tumor cell escape from immune recognition. CD24 is a well-known marker for cancer stem cells, and is a major player in the malignant behavior of pancreatic cancer (Ikenaga, Ohuchida, Mizumoto, Yu, Kayashima, & Hayashi, 2010). The identification of a Siglec-10-CD24 interaction highlights a new glycosylation-dependent mechanism by which CD24 may promote tumor progression.
6. Concluding remarks
Despite advances in our understanding of the molecular mechanisms underlying pancreatic cancer, little progress has been made in the treatment of this cancer and the 5-year survival rate remains dismal. Although it is well-recognized that large scale changes in the glycome occur during pancreatic cancer, this knowledge has not been effectively utilized to develop new therapeutics to combat this disease. In this review, we have summarized the evidence supporting functional roles for tumor-associated sialoglycans in pancreatic cancer, with a particular focus on hypersialylated N-glycans, sialyl Lewis antigens, and sialylated, truncated O-glycans. These sialylated structures have a significant impact on tumor cell phenotype, and also contribute to immune suppression through the engagement of Siglec receptors. Although studies of tumor glycosylation have historically lagged behind other areas of cancer research, recent developments in the field are illuminating the enormous potential for glycans, including sialoglycans, to serve as cancer biomarkers and clinical targets. Targeting sialoglycans offers a novel approach for treating pancreatic cancer, providing an alternative to current therapeutic modalities which remain largely ineffective.
Acknowledgments
This study was supported by National Institutes of Health grants R01 CA225177 (SLB) and U01 CA233581, Alliance of Glycobiologists for Cancer Research (SLB). MPM was supported by National Institutes of Health grant F32 CA264906.
References
- Abrantes J, Posada D, Guillon P, Esteves PJ, & Le Pendu J (2009). Widespread gene conversion of alpha-2-fucosyltransferase genes in mammals. Journal of Molecular Evolution, 69(1), 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrantes R, Duarte HO, Gomes C, Walchli S, & Reis CA (2022). CAR-Ts: New perspectives in cancer therapy. FEBS Letters, 596(4), 403–416. [DOI] [PubMed] [Google Scholar]
- Adams OJ, Stanczak MA, von Gunten S, & Laubli H (2018). Targeting sialic acid-Siglec interactions to reverse immune suppression in cancer. Glycobiology, 28(9), 640–647. [DOI] [PubMed] [Google Scholar]
- Aithal A, Rauth S, Kshirsagar P, Shah A, Lakshmanan I, Junker WM, et al. (2018). MUC16 as a novel target for cancer therapy. Expert Opinion on Therapeutic Targets, 22(8), 675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AC, Bailey EM, Brenchley PE, Buck KS, Barratt J, & Feehally J (2001). Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: Observations in three patients. Kidney International, 60(3), 969–973. [DOI] [PubMed] [Google Scholar]
- Almeida A, & Kolarich D (2016). The promise of protein glycosylation for personalised medicine. Biochimica et Biophysica Acta, 1860(8), 1583–1595. [DOI] [PubMed] [Google Scholar]
- Aubert M, Panicot L, Crotte C, Gibier P, Lombardo D, Sadoulet MO, et al. (2000a). Restoration of alpha(1,2) fucosyltransferase activity decreases adhesive and metastatic properties of human pancreatic cancer cells. Cancer Research, 60(5), 1449–1456. [PubMed] [Google Scholar]
- Aubert M, Panicot-Dubois L, Crotte C, Sbarra V, Lombardo D, Sadoulet MO, et al. (2000b). Peritoneal colonization by human pancreatic cancer cells is inhibited by antisense FUT3 sequence. International Journal of Cancer, 88(4), 558–565. [DOI] [PubMed] [Google Scholar]
- Balmana M, Diniz F, Feijao T, Barrias CC, Mereiter S, & Reis CA (2020). Analysis of the effect of increased alpha2,3-sialylation on RTK activation in MKN45 gastric cancer spheroids treated with crizotinib. International Journal of Molecular Sciences, 21(3), 722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmana M, Sarrats A, Llop E, Barrabes S, Saldova R, Ferri MJ, et al. (2015). Identification of potential pancreatic cancer serum markers: Increased sialyl-Lewis X on ceruloplasmin. Clinica Chimica Acta; International Journal of Clinical Chemistry, 442, 56–62. [DOI] [PubMed] [Google Scholar]
- Bassaganas S, Allende H, Cobler L, Ortiz MR, Llop E, de Bolos C, et al. (2015). Inflammatory cytokines regulate the expression of glycosyltransferases involved in the biosynthesis of tumor-associated sialylated glycans in pancreatic cancer cell lines. Cytokine, 75(1), 197–206. [DOI] [PubMed] [Google Scholar]
- Bassaganas S, Carvalho S, Dias AM, Perez-Garay M, Ortiz MR, Figueras J, et al. (2014). Pancreatic cancer cell glycosylation regulates cell adhesion and invasion through the modulation of alpha2beta1 integrin and E-cadherin function. PLoS One, 9(5), e98595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellis SL, Reis CA, Varki A, Kannagi R, & Stanley P (2022). Glycosylation changes in cancer. In Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Kinoshita T, Mohnen D, Packer NH, Prestegard JH, Schnaar RL, & Seeberger PH (Eds.). Essentials of Glycobiology (Vol. Essentials of Glycobiology) Cold Spring Harbor Laboratory Press; 10.1101/glycobiology.3e.033 [DOI] [PubMed] [Google Scholar]
- Bergstrom K, Liu X, Zhao Y, Gao N, Wu Q, Song K, et al. (2016). Defective intestinal mucin-type O-glycosylation causes spontaneous colitis-associated cancer in mice. Gastroenterology, 151(1), 152–164 e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhide GP, & Colley KJ (2017). Sialylation of N-glycans: Mechanism, cellular compartmentalization and function. Histochemistry and Cell Biology, 147(2), 149–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanas A, Sahasrabudhe NM, Rodriguez E, van Kooyk Y, & van Vliet SJ (2018). Fucosylated antigens in cancer: An alliance toward tumor progression, metastasis, and resistance to chemotherapy. Frontiers in Oncology, 8, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britain CM, Bhalerao N, Silva AD, Chakraborty A, Buchsbaum DJ, Crowley MR, et al. (2021). Glycosyltransferase ST6Gal-I promotes the epithelial to mesenchymal transition in pancreatic cancer cells. The Journal of Biological Chemistry, 296, 100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britain CM, Dorsett KA, & Bellis SL (2017). Glycosyltransferase ST6Gal-I protects tumor cells against serum growth factor withdrawal by enhancing survival signaling and proliferative potential. The Journal of Biological Chemistry, 292(11), 4663–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britain CM, Holdbrooks AT, Anderson JC, Willey CD, & Bellis SL (2018). Sialylation of EGFR by the ST6Gal-I sialyltransferase promotes EGFR activation and resistance to gefitinib-mediated cell death. Journal of Ovarian Research, 11(1), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull C, Heise T, Adema GJ, & Boltje TJ (2016). Sialic acid mimetics to target the sialic acid-siglec axis. Trends in Biochemical Sciences, 41(6), 519–531. [DOI] [PubMed] [Google Scholar]
- Bull C, Stoel MA, den Brok MH, & Adema GJ (2014). Sialic acids sweeten a tumor’s life. Cancer Research, 74(12), 3199–3204. [DOI] [PubMed] [Google Scholar]
- Burchell JM, Mungul A, & Taylor-Papadimitriou J (2001). O-linked glycosylation in the mammary gland: Changes that occur during malignancy. Journal of Mammary Gland Biology and Neoplasia, 6(3), 355–364. [DOI] [PubMed] [Google Scholar]
- Carvalho AS, Harduin-Lepers A, Magalhaes A, Machado E, Mendes N, Costa LT, et al. (2010). Differential expression of alpha-2,3-sialyltransferases and alpha-1,3/4-fucosyl-transferases regulates the levels of sialyl Lewis a and sialyl Lewis x in gastrointestinal carcinoma cells. The International Journal of Biochemistry G Cell Biology, 42(1), 80–89. [DOI] [PubMed] [Google Scholar]
- Cervoni GE, Cheng JJ, Stackhouse KA, Heimburg-Molinaro J, & Cummings RD (2020). O-glycan recognition and function in mice and human cancers. The Biochemical Journal, 477(8), 1541–1564. [DOI] [PubMed] [Google Scholar]
- Chakraborty A, Dorsett KA, Trummell HQ, Yang ES, Oliver PG, Bonner JA, et al. (2018). ST6Gal-I sialyltransferase promotes chemoresistance in pancreatic ductal adenocarcinoma by abrogating gemcitabine-mediated DNA damage. The Journal of Biological Chemistry, 293(3), 984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Dallas MR, Balzer EM, & Konstantopoulos K (2012). Mucin 16 is a functional selectin ligand on pancreatic cancer cells. The FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 26(3), 1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhang Z, Zhang S, Zhu P, Ko JK, & Yung KK (2021). MUC1: Structure, function, and clinic application in epithelial cancers. International Journal of Molecular Sciences, 22(12), 6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Mo S, Zhang Y, Ma H, Lu Z, Yu S, et al. (2022). Analysis of a novel immune checkpoint, Siglec-15, in pancreatic ductal adenocarcinoma. The Journal of Pathology: Clinical Research, 8(3), 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen MN, Chik J, Lee L, Anugraham M, Abrahams JL, & Packer NH (2014). Cell surface protein glycosylation in cancer. Proteomics, 14(4–5), 525–546. [DOI] [PubMed] [Google Scholar]
- Christie DR, Shaikh FM, Lucas J. A. t., Lucas JA 3rd, & Bellis SL (2008). ST6Gal-I expression in ovarian cancer cells promotes an invasive phenotype by altering integrin glycosylation and function. Journal of Ovarian Research, 1(1), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh S, Barkeer S, Rachagani S, Nimmakayala RK, Perumal N, Pothuraju R, et al. (2018). Disruption of C1galt1 gene promotes development and metastasis of pancreatic adenocarcinomas in mice. Gastroenterology, 155(5), 1608–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Yin B, Wang Q, Chuang KY, Chu JH, & Betenbaugh MJ (2015). Assessment of the coordinated role of ST3GAL3, ST3GAL4 and ST3GAL6 on the alpha2,3 sialylation linkage of mammalian glycoproteins. Biochemical and Biophysical Research Communications, 463(3), 211–215. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Clark EA, Filbin M, Gordon S, Jones Y, Kehrl JH, et al. (1998). Siglecs: A family of sialic-acid binding lectins. Glycobiology, 8(2), v. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Paulson JC, & Varki A (2007). Siglecs and their roles in the immune system. Nature Reviews. Immunology, 7(4), 255–266. [DOI] [PubMed] [Google Scholar]
- Crocker PR, & Varki A (2001). Siglecs, sialic acids and innate immunity. Trends in Immunology, 22(6), 337–342. [DOI] [PubMed] [Google Scholar]
- Dall’Olio F, Malagolini N, Trinchera M, & Chiricolo M (2014). Sialosignaling: Sialyltransferases as engines of self-fueling loops in cancer progression. Biochimica et Biophysica Acta, 1840(9), 2752–2764. [DOI] [PubMed] [Google Scholar]
- Dall’Olio F, Pucci M, & Malagolini N (2021). The cancer-associated antigens Sialyl Lewis (a/x) and Sd(a): Two opposite faces of terminal glycosylation. Cancers (Basel), 13(21), 5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J, Carlsten M, & O’Dwyer M (2019). Sugar free: Novel immunotherapeutic approaches targeting siglecs and sialic acids to enhance natural killer cell cytotoxicity against cancer. Frontiers in Immunology, 10, 1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel M, Dall’Olio F, Mungul A, Piller V, & Piller F (2004). Ras oncogene induces beta-galactoside alpha2,6-sialyltransferase (ST6Gal I) via a RalGEF-mediated signal to its housekeeping promoter. European Journal of Biochemistry, 271(18), 3623–3634. [DOI] [PubMed] [Google Scholar]
- Dobie C, & Skropeta D (2021). Insights into the role of sialylation in cancer progression and metastasis. British Journal of Cancer, 124(1), 76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi N, Ino Y, Angata K, Shimada K, Narimatsu H, & Hiraoka N (2020). Clinicopathological significance of core 3 O-glycan synthetic enzyme, beta1,3-N-acetylglucosaminyltransferase 6 in pancreatic ductal adenocarcinoma. PLoS One, 15(11), e0242851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsett KA, Marciel MP, Hwang J, Ankenbauer KE, Bhalerao N, & Bellis SL (2021). Regulation of ST6GAL1 sialyltransferase expression in cancer cells. Glycobiology, 31(5), 530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan SK (2017). The pancreatic cancer microenvironment. Cancer Journal (Sudbury, Mass.), 23(6), 321–325. [DOI] [PubMed] [Google Scholar]
- Duan S, & Paulson JC (2020). Siglecs as immune cell checkpoints in disease. Annual Review of Immunology, 38, 365–395. [DOI] [PubMed] [Google Scholar]
- Duarte HO, Reis CA, & Gomes J (2022). Insights on ErbB glycosylation – contributions to precision oncology. Trends Cancer, 8(6), 448–455. [DOI] [PubMed] [Google Scholar]
- Duarte HO, Rodrigues JG, Gomes C, Hensbergen PJ, Ederveen ALH, de Ru AH, et al. (2021). ST6Gal1 targets the ectodomain of ErbB2 in a site-specific manner and regulates gastric cancer cell sensitivity to trastuzumab. Oncogene, 40(21), 3719–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eavarone DA, Al-Alem L, Lugovskoy A, Prendergast JM, Nazer RI, Stein JN, et al. (2018). Humanized anti-Sialyl-Tn antibodies for the treatment of ovarian carcinoma. PLoS One, 13(7), e0201314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle DD, Tiriac H, Rivera KD, Pommier A, Whalen S, Oni TE, et al. (2019). The glycan CA19-9 promotes pancreatitis and pancreatic cancer in mice. Science, 364(6446), 1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, Xiong G, Cao Z, Yang G, Zheng S, Song X, et al. (2017). PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Letters, 407, 57–65. [DOI] [PubMed] [Google Scholar]
- Flores AR, Lemos I, Rema A, Taulescu M, Seixas F, Reis CA, et al. (2020). Tn and Sialyl-Tn antigens in canine gastric tissues. Veterinary and Comparative Oncology, 18(4), 615–625. [DOI] [PubMed] [Google Scholar]
- Fraschilla I, & Pillai S (2017). Viewing Siglecs through the lens of tumor immunology. Immunological Reviews, 276(1), 178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas D, Campos D, Gomes J, Pinto F, Macedo JA, Matos R, et al. (2019). O-glycans truncation modulates gastric cancer cell signaling and transcription leading to a more aggressive phenotype. EBioMedicine, 40, 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli C, Basso D, & Plebani M (2013). CA 19–9: Handle with care. Clinical Chemistry and Laboratory Medicine, 51(7), 1369–1383. [DOI] [PubMed] [Google Scholar]
- Gao N, Bergstrom K, Fu J, Xie B, Chen W, & Xia L (2016). Loss of intestinal O-glycans promotes spontaneous duodenal tumors. American Journal of Physiology, 311(1), G74–G83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Luan X, Melamed J, & Brockhausen I (2021). Role of glycans on key cell surface receptors that regulate cell proliferation and cell death. Cells, 10(5), 1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mayea Y, Mir C, Masson F, Paciucci R, & ME LL (2020). Insights into new mechanisms and models of cancer stem cell multidrug resistance. Seminars in Cancer Biology, 60, 166–180. [DOI] [PubMed] [Google Scholar]
- Garnham R, Scott E, Livermore KE, & Munkley J (2019). ST6GAL1: A key player in cancer. Oncology Letters, 18(2), 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes C, Osorio H, Pinto MT, Campos D, Oliveira MJ, & Reis CA (2013). Expression of ST3GAL4 leads to SLe(x) expression and induces c-Met activation and an invasive phenotype in gastric carcinoma cells. PLoS One, 8(6), e66737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gil A, & Schnaar RL (2021). Siglec ligands. Cells, 10(5), 1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonetilleke KS, & Siriwardena AK (2007). Systematic review of carbohydrate antigen (CA 19–9) as a biochemical marker in the diagnosis of pancreatic cancer. European Journal of Surgical Oncology, 33(3), 266–270. [DOI] [PubMed] [Google Scholar]
- Gorelik E, Xu F, Henion T, Anaraki F, & Galili U (1997). Reduction of metastatic properties of BL6 melanoma cells expressing terminal fucose(alpha)1–2-galactose after alpha1,2-fucosyltransferase cDNA transfection. Cancer Research, 57(2), 332–336. [PubMed] [Google Scholar]
- Goupille C, Hallouin F, Meflah K, & Le Pendu J (1997). Increase of rat colon carcinoma cells tumorigenicity by alpha(1–2) fucosyltransferase gene transfection. Glycobiology, 7(2), 221–229. [DOI] [PubMed] [Google Scholar]
- Guerrero PE, Miro L, Wong BS, Massaguer A, Martinez-Bosch N, Llorens R, et al. (2020). Knockdown of alpha2,3-sialyltransferases impairs pancreatic cancer cell migration, invasion and E-selectin-dependent adhesion. International Journal of Molecular Sciences, 21(17), 6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Maiti A, Wu R, Andersen VL, Hsu CC, Wu Y, et al. (2022). Extracellular sialyltransferase st6gal1 in breast tumor cell growth and invasiveness. Cancer Gene Therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski C, Hanski ML, Zimmer T, Ogorek D, Devine P, & Riecken EO (1995). Characterization of the major sialyl-Lex-positive mucins present in colon, colon carcinoma, and sera of patients with colorectal cancer. Cancer Research, 55(4), 928–933. [PubMed] [Google Scholar]
- Harduin-Lepers A, Krzewinski-Recchi MA, Colomb F, Foulquier F, Groux-Degroote S, & Delannoy P (2012). Sialyltransferases functions in cancers. Frontiers in Bioscience (Elite Ed), 4, 499–515. [DOI] [PubMed] [Google Scholar]
- Helenius A, & Aebi M (2001). Intracellular functions of N-linked glycans. Science, 291(5512), 2364–2369. [DOI] [PubMed] [Google Scholar]
- Herlyn M, Sears HF, Steplewski Z, & Koprowski H (1982). Monoclonal antibody detection of a circulating tumor-associated antigen. I. Presence of antigen in sera of patients with colorectal, gastric, and pancreatic carcinoma. Journal of Clinical Immunology, 2(2), 135–140. [DOI] [PubMed] [Google Scholar]
- Herlyn M, Steplewski Z, Herlyn D, & Koprowski H (1979). Colorectal carcinoma-specific antigen: Detection by means of monoclonal antibodies. Proceedings of the National Academy of Sciences of the United States of America, 76(3), 1438–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. (2003). Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell, 4(6), 437–450. [DOI] [PubMed] [Google Scholar]
- Hofmann BT, Schluter L, Lange P, Mercanoglu B, Ewald F, Folster A, et al. (2015). COSMC knockdown mediated aberrant O-glycosylation promotes oncogenic properties in pancreatic cancer. Molecular Cancer, 14, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdbrooks AT, Britain CM, & Bellis SL (2018). ST6Gal-I sialyltransferase promotes tumor necrosis factor (TNF)-mediated cancer cell survival via sialylation of the TNF receptor 1 (TNFR1) death receptor. The Journal of Biological Chemistry, 293(5), 1610–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst S, Belo AI, Giovannetti E, van Die I, & Wuhrer M (2017). Profiling of different pancreatic cancer cells used as models for metastatic behaviour shows large variation in their N-glycosylation. Scientific Reports, 7(1), 16623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosono J, Narita T, Kimura N, Sato M, Nakashio T, Kasai Y, et al. (1998). Involvement of adhesion molecules in metastasis of SW1990, human pancreatic cancer cells. Journal of Surgical Oncology, 67(2), 77–84. [DOI] [PubMed] [Google Scholar]
- Hou S, Hang Q, Isaji T, Lu J, Fukuda T, & Gu J (2016). Importance of membrane-proximal N-glycosylation on integrin beta1 in its activation and complex formation. The FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 30(12), 4120–4131. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Goggins M, Parsons J, & Kern SE (2000). Progression model for pancreatic cancer. Clinical Cancer Research, 6(8), 2969–2972. [PubMed] [Google Scholar]
- Hsieh CC, Shyr YM, Liao WY, Chen TH, Wang SE, Lu PC, et al. (2017). Elevation of beta-galactoside alpha2,6-sialyltransferase 1 in a fructoseresponsive manner promotes pancreatic cancer metastasis. Oncotarget, 8(5), 7691–7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak JE, Canham SM, & Bertozzi CR (2014). Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nature Chemical Biology, 10(1), 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugonnet M, Singh P, Haas Q, & von Gunten S (2021). The distinct roles of sialyltransferases in cancer biology and onco-immunology. Frontiers in Immunology, 12, 799861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim NK, Murray JL, Zhou D, Mittendorf EA, Sample D, Tautchin M, et al. (2013). Survival advantage in patients with metastatic breast cancer receiving endocrine therapy plus sialyl Tn-KLH vaccine: Post hoc analysis of a large randomized trial. Journal of Cancer, 4(7), 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa S, Guigonis V, Imel EA, Courouble M, Heissat S, Henley JD, et al. (2007). Novel GALNT3 mutations causing hyperostosis-hyperphosphatemia syndrome result in low intact fibroblast growth factor 23 concentrations. The Journal of Clinical Endocrinology and Metabolism, 92(5), 1943–1947. [DOI] [PubMed] [Google Scholar]
- Ikenaga N, Ohuchida K, Mizumoto K, Yu J, Kayashima T, Hayashi A, et al. (2010). Characterization of CD24 expression in intraductal papillary mucinous neoplasms and ductal carcinoma of the pancreas. Human Pathology, 41(10), 1466–1474. [DOI] [PubMed] [Google Scholar]
- Isaji T, Im S, Gu W, Wang Y, Hang Q, Lu J, et al. (2014). An oncogenic protein Golgi phosphoprotein 3 up-regulates cell migration via sialylation. The Journal of Biological Chemistry, 289(30), 20694–20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang KY, Qi LL, Kang FB, & Wang L (2022). The intriguing roles of Siglec family members in the tumor microenvironment. Biomarker Research, 10(1), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RB, Dorsett KA, Hjelmeland AB, & Bellis SL (2018). The ST6Gal-I sialyltransferase protects tumor cells against hypoxia by enhancing HIF-1alpha signaling. The Journal of Biological Chemistry, 293(15), 5659–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T, Aryal RP, Kudelka MR, Wang Y, & Cummings RD (2014). The Cosmc connection to the Tn antigen in cancer. Cancer Biomarkers: Section A of Disease Markers, 14(1), 63–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T, & Cummings RD (2002). A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proceedings of the National Academy of Sciences of the United States of America, 99(26), 16613–16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T, & Cummings RD (2005). Protein glycosylation: Chaperone mutation in Tn syndrome. Nature, 437(7063), 1252. [DOI] [PubMed] [Google Scholar]
- Ju T, Lanneau GS, Gautam T, Wang Y, Xia B, Stowell SR, et al. (2008). Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Research, 68(6), 1636–1646. [DOI] [PubMed] [Google Scholar]
- Ju T, Wang Y, Aryal RP, Lehoux SD, Ding X, Kudelka MR, et al. (2013). Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers. Proteomics. Clinical Applications, 7(9–10), 618–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien S, Videira PA, & Delannoy P (2012). Sialyl-tn in cancer: (How) did we miss the target? Biomolecules, 2(4), 435–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashi S, Dedushi K, Ramadani N, Mucaj S, Hoxhaj A, & Jerliu N (2016). Pancreatic carcinoma: The disease that kills. World Journal of Oncology, 7(1), 13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R (2007). Carbohydrate antigen sialyl Lewis a—Its pathophysiological significance and induction mechanism in cancer progression. Chang Gung Medical Journal, 30(3), 189–209. [PubMed] [Google Scholar]
- Kannagi R, Cai BH, Huang HC, Chao CC, & Sakuma K (2018). Gangliosides and tumors. Methods in Molecular Biology, 1804, 143–171. [DOI] [PubMed] [Google Scholar]
- Kasprowicz A, Sophie GD, Lagadec C, & Delannoy P (2022). Role of GD3 synthase ST8Sia I in cancers. Cancers (Basel), 14(5), 1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, et al. (2008). Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nature Genetics, 40(2), 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Jeanneau C, Tarp MA, Benet-Pages A, Lorenz-Depiereux B, Bennett EP, et al. (2006). Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. The Journal of Biological Chemistry, 281(27), 18370–18377. [DOI] [PubMed] [Google Scholar]
- Kawarada Y, Ishikura H, Kishimoto T, Kato H, Yano T, Kato H, et al. (2000). The role of sialylated Lewis antigens on hematogenous metastases of human pancreas carcinoma cell lines in vivo. Pathology, Research and Practice, 196(4), 259–263. [DOI] [PubMed] [Google Scholar]
- Kim YJ, & Varki A (1997). Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconjugate Journal, 14(5), 569–576. [DOI] [PubMed] [Google Scholar]
- Kim YS, Itzkowitz SH, Yuan M, Chung Y, Satake K, Umeyama K, et al. (1988). Lex and Ley antigen expression in human pancreatic cancer. Cancer Research, 48(2), 475–482. [PubMed] [Google Scholar]
- Kishimoto T, Ishikura H, Kimura C, Takahashi T, Kato H, & Yoshiki T (1996). Phenotypes correlating to metastatic properties of pancreas adenocarcinoma in vivo: The importance of surface sialyl Lewis(a) antigen. International Journal of Cancer, 69(4), 290–294. [DOI] [PubMed] [Google Scholar]
- Kolbl AC, Andergassen U, & Jeschke U (2015). The role of glycosylation in breast cancer metastasis and cancer control. Frontiers in Oncology, 5, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, & Fuhrer P (1979). Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genetics, 5(6), 957–971. [DOI] [PubMed] [Google Scholar]
- Kukowska-Latallo JF, Larsen RD, Nair RP, & Lowe JB (1990). A cloned human cDNA determines expression of a mouse stage-specific embryonic antigen and the Lewis blood group alpha(1,3/1,4)fucosyltransferase. Genes G Development, 4(8), 1288–1303. [DOI] [PubMed] [Google Scholar]
- Kurz E, Chen S, Vucic E, Baptiste G, Loomis C, Agrawal P, et al. (2021). Integrated systems analysis of the murine and human pancreatic cancer glycomes reveals a tumor-promoting role for ST6GAL1. Molecular G Cellular Proteomics, 20, 100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahdenne P, Pitkanen S, Rajantie J, Kuusela P, Siimes MA, Lanning M, et al. (1995). Tumor markers CA 125 and CA 19–9 in cord blood and during infancy: Developmental changes and use in pediatric germ cell tumors. Pediatric Research, 38(5), 797–801. [DOI] [PubMed] [Google Scholar]
- Laubli H, & Borsig L (2019). Altered cell adhesion and glycosylation promote cancer immune suppression and metastasis. Frontiers in Immunology, 10, 2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubli H, Kawanishi K, George Vazhappilly C, Matar R, Merheb M, & Sarwar Siddiqui S (2021). Tools to study and target the Siglec-sialic acid axis in cancer. The FEBS Journal, 288(21), 6206–6225. [DOI] [PubMed] [Google Scholar]
- Laubli H, Pearce OM, Schwarz F, Siddiqui SS, Deng L, Stanczak MA, et al. (2014). Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proceedings of the National Academy of Sciences of the United States of America, 111(39), 14211–14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Lee HJ, Bae S, & Lee YS (2008). Protein sialylation by sialyltransferase involves radiation resistance. Molecular Cancer Research, 6(8), 1316–1325. [DOI] [PubMed] [Google Scholar]
- Lee M, Lee HJ, Seo WD, Park KH, & Lee YS (2010). Sialylation of integrin beta1 is involved in radiation-induced adhesion and migration in human colon cancer cells. International Journal of Radiation Oncology, Biology, Physics, 76(5), 1528–1536. [DOI] [PubMed] [Google Scholar]
- Li QT, Huang ZZ, Chen YB, Yao HY, Ke ZH, He XX, et al. (2020). Integrative analysis of siglec-15 mRNA in human cancers based on data mining. Journal of Cancer, 11(9), 2453–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TJ, Jin KZ, Li H, Ye LY, Li PC, Jiang B, et al. (2022). SIGLEC15 amplifies immunosuppressive properties of tumor-associated macrophages in pancreatic cancer. Cancer Letters, 530, 142–155. [DOI] [PubMed] [Google Scholar]
- Lim J, Sari-Ak D, & Bagga T (2021). Siglecs as therapeutic targets in cancer. Biology (Basel), 10(11), 1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Kemmner W, Grigull S, & Schlag PM (2002). Cell surface alpha 2,6 sialylation affects adhesion of breast carcinoma cells. Experimental Cell Research, 276(1), 101–110. [DOI] [PubMed] [Google Scholar]
- Liu B, Liu Q, Pan S, Huang Y, Qi Y, Li S, et al. (2019). The HOTAIR/miR-214/ST6GAL1 crosstalk modulates colorectal cancer procession through mediating sialylated c-Met via JAK2/STAT3 cascade. Journal of Experimental G Clinical Cancer Research, 38(1), 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Deng S, Jin K, Gong Y, Cheng H, Fan Z, et al. (2020). Lewis anti-gennegative pancreatic cancer: An aggressive subgroup. International Journal of Oncology, 56(4), 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Ma Q, Xu Q, Lei J, Li X, Wang Z, et al. (2012). Therapeutic potential of perineural invasion, hypoxia and desmoplasia in pancreatic cancer. Current Pharmaceutical Design, 18(17), 2395–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Zhu M, Linhai Y, Song Y, Gui X, Tan G, et al. (2018). Increasing HER2 α2,6 sialylation facilitates gastric cancer progression and resistance via the Akt and ERK pathways. Oncology Reports, 40(5), 2997–3005. [DOI] [PubMed] [Google Scholar]
- Loureiro LR, Feldmann A, Bergmann R, Koristka S, Berndt N, Arndt C, et al. (2018). Development of a novel target module redirecting UniCAR T cells to Sialyl Tn-expressing tumor cells. Blood Cancer Journal, 8(9), 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro LR, Sousa DP, Ferreira D, Chai W, Lima L, Pereira C, et al. (2018). Novel monoclonal antibody L2A5 specifically targeting sialyl-Tn and short glycans terminated by alpha-2–6 sialic acids. Scientific Reports, 8(1), 12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe JB, Stoolman LM, Nair RP, Larsen RD, Berhend TL, & Marks RM (1990). ELAM-1—dependent cell adhesion to vascular endothelium determined by a transfected human fucosyltransferase cDNA. Cell, 63(3), 475–484. [DOI] [PubMed] [Google Scholar]
- Lu J, & Gu J (2015). Significance of beta-galactoside alpha2,6 sialyltranferase 1 in cancers. Molecules, 20(5), 7509–7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley MS, Kawasaki N, Peng W, Wang SH, He Y, Arlian BM, et al. (2015). Unmasking of CD22 co-receptor on germinal center B-cells occurs by alter-native mechanisms in mouse and man. The Journal of Biological Chemistry, 290(50), 30066–30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes A, Duarte HO, & Reis CA (2021). The role of O-glycosylation in human disease. Molecular Aspects of Medicine, 79, 100964. [DOI] [PubMed] [Google Scholar]
- Magnani JL, Brockhaus M, Smith DF, Ginsburg V, Blaszczyk M, Mitchell KF, et al. (1981). A monosialoganglioside is a monoclonal antibody-defined antigen of colon carcinoma. Science, 212(4490), 55–56. [DOI] [PubMed] [Google Scholar]
- Magnani JL, Nilsson B, Brockhaus M, Zopf D, Steplewski Z, Koprowski H, et al. (1982). A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. The Journal of Biological Chemistry, 257(23), 14365–14369. [PubMed] [Google Scholar]
- Magnani JL, Steplewski Z, Koprowski H, & Ginsburg V (1983). Identification of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal anti-body 19–9 in the sera of patients as a mucin. Cancer Research, 43(11), 5489–5492. [PubMed] [Google Scholar]
- Mas E, Pasqualini E, Caillol N, El Battari A, Crotte C, Lombardo D, et al. (1998). Fucosyltransferase activities in human pancreatic tissue: comparative study between cancer tissues and established tumoral cell lines. Glycobiology, 8(6), 605–613. [DOI] [PubMed] [Google Scholar]
- Mathieu S, Prorok M, Benoliel AM, Uch R, Langlet C, Bongrand P, et al. (2004). Transgene expression of alpha(1,2)-fucosyltransferase-I (FUT1) in tumor cells selectively inhibits sialyl-Lewis x expression and binding to E-selectin without affecting synthesis of sialyl-Lewis a or binding to P-selectin. The American Journal of Pathology, 164(2), 371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell CT, Klamer Z, Hall J, West CA, Wisniewski L, Powers TW, et al. (2020). Imaging mass spectrometry and lectin analysis of N-linked glycans in carbohydrate antigen-defined pancreatic cancer tissues. Molecular G Cellular Proteomics, 20, 100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Xu L, Yang Y, Zhou L, Chang Y, Shi T, et al. (2017). High expression of FUT3 is linked to poor prognosis in clear cell renal cell carcinoma. Oncotarget, 8(37), 61036–61047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereiter S, Balmana M, Gomes J, Magalhaes A, & Reis CA (2016). Glycomic approaches for the discovery of targets in gastrointestinal cancer. Frontiers in Oncology, 6, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereiter S, Magalhaes A, Adamczyk B, Jin C, Almeida A, Drici L, et al. (2016). Glycomic analysis of gastric carcinoma cells discloses glycans as modulators of RON receptor tyrosine kinase activation in cancer. Biochimica et Biophysica Acta, 1860(8), 1795–1808. [DOI] [PubMed] [Google Scholar]
- Meril S, Harush O, Reboh Y, Matikhina T, Barliya T, & Cohen CJ (2020). Targeting glycosylated antigens on cancer cells using siglec-7/9-based CAR T-cells. Molecular Carcinogenesis, 59(7), 713–723. [DOI] [PubMed] [Google Scholar]
- Motoo Y, Kawakami H, Watanabe H, Satomura Y, Ohta H, Okai T, et al. (1991). Serum sialyl-Tn antigen levels in patients with digestive cancers. Oncology, 48(4), 321–326. [DOI] [PubMed] [Google Scholar]
- Munkley J (2016). The role of sialyl-Tn in cancer. International Journal of Molecular Sciences, 17(3), 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkley J (2019). The glycosylation landscape of pancreatic cancer. Oncology Letters, 17(3), 2569–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkley J, & Elliott DJ (2016). Hallmarks of glycosylation in cancer. Oncotarget, 7(23), 35478–35489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi M, Mortezaee K, & Majidpoor J (2019). Cancer stem cell (CSC) resistance drivers. Life Sciences, 234, 116781. [DOI] [PubMed] [Google Scholar]
- Nanashima A, Yamaguchi H, Nakagoe T, Matsuo S, Sumida Y, Tsuji T, et al. (1999). High serum concentrations of sialyl Tn antigen in carcinomas of the biliary tract and pancreas. Journal of Hepato-Biliary-Pancreatic Surgery, 6(4), 391–395. [DOI] [PubMed] [Google Scholar]
- do Nascimento JCF, Beltrao EIC, & Rocha CRC (2020). High FUT3 expression is a marker of lower overall survival of breast cancer patients. Glycoconjugate Journal, 37(2), 263–275. [DOI] [PubMed] [Google Scholar]
- Natoni A, Macauley MS, & O’Dwyer ME (2016). Targeting selectins and their ligands in cancer. Frontiers in Oncology, 6, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa F, Hirota M, Okabe A, Shibata M, Iwamura T, Haga Y, et al. (2000). Tumor necrosis factor alpha acts on cultured human vascular endothelial cells to increase the adhesion of pancreatic cancer cells. Pancreas, 21(4), 392–398. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Hirohashi Y, Murai A, Nishidate T, Okita K, Wang L, et al. (2017). ST6GALNAC1 plays important roles in enhancing cancer stem phenotypes of colorectal cancer via the Akt pathway. Oncotarget, 8(68), 112550–112564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osako M, Yonezawa S, Siddiki B, Huang J, Ho JJ, Kim YS, et al. (1993). Immunohistochemical study of mucin carbohydrates and core proteins in human pan-creatic tumors. Cancer, 71(7), 2191–2199. [DOI] [PubMed] [Google Scholar]
- Partyka K, Maupin KA, Brand RE, & Haab BB (2012). Diverse monoclonal antibodies against the CA 19–9 antigen show variation in binding specificity with consequences for clinical interpretation. Proteomics, 12(13), 2212–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracaula R, Tabares G, Lopez-Ferrer A, Brossmer R, de Bolos C, & de Llorens R (2005). Role of sialyltransferases involved in the biosynthesis of Lewis antigens in human pancreatic tumour cells. Glycoconjugate Journal, 22(3), 135–144. [DOI] [PubMed] [Google Scholar]
- Perdicchio M, Cornelissen LA, Streng-Ouwehand I, Engels S, Verstege MI, Boon L, et al. (2016). Tumor sialylation impedes T cell mediated anti-tumor responses while promoting tumor associated-regulatory T cells. Oncotarget, 7(8), 8771–8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garay M, Arteta B, Llop E, Cobler L, Pages L, Ortiz R, et al. (2013). alpha2,3-Sialyltransferase ST3Gal IV promotes migration and metastasis in pancreatic adenocarcinoma cells and tends to be highly expressed in pancreatic adenocarcinoma tissues. The International Journal of Biochemistry G Cell Biology, 45(8), 1748–1757. [DOI] [PubMed] [Google Scholar]
- Perez-Garay M, Arteta B, Pages L, de Llorens R, de Bolos C, Vidal-Vanaclocha F, et al. (2010). alpha2,3-sialyltransferase ST3Gal III modulates pancreatic cancer cell motility and adhesion in vitro and enhances its metastatic potential in vivo. PLoS One, 5(9), e12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobono S, & Stecca B (2021). Aberrant sialylation in cancer: Biomarker and potential target for therapeutic intervention? Cancers (Basel), 13(9), 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pour PM, Tempero MM, Takasaki H, Uchida E, Takiyama Y, Burnett DA, et al. (1988). Expression of blood group-related antigens ABH, Lewis A, Lewis B, Lewis X, Lewis Y, and CA 19–9 in pancreatic cancer cells in comparison with the patient’s blood group type. Cancer Research, 48(19), 5422–5426. [PubMed] [Google Scholar]
- Prendergast JM, Galvao da Silva AP, Eavarone DA, Ghaderi D, Zhang M, Brady D, et al. (2017). Novel anti-Sialyl-Tn monoclonal antibodies and antibody-drug conjugates demonstrate tumor specificity and anti-tumor activity. mAbs, 9(4), 615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto PA, Larsen RD, Cho M, Rivera HN, Shilatifard A, Lowe JB, et al. (1997). Expression of human H-type alpha1,2-fucosyltransferase encoding for blood group H(O) antigen in Chinese hamster ovary cells. Evidence for preferential fucosylation and truncation of polylactosamine sequences. The Journal of Biological Chemistry, 272(4), 2089–2097. [DOI] [PubMed] [Google Scholar]
- Qian J, Zhu CH, Tang S, Shen AJ, Ai J, Li J, et al. (2009). alpha2,6-Hyposialylation of c-Met abolishes cell motility of ST6Gal-I-knockdown HCT116 cells. Acta Pharmacologica Sinica, 30(7), 1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan P, Dabelsteen S, Madsen FB, Francavilla C, Kopp KL, Steentoft C, et al. (2014). Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proceedings of the National Academy of Sciences of the United States of America, 111(39), E4066–E4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, & Matrisian LM (2014). Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Research, 74(11), 2913–2921. [DOI] [PubMed] [Google Scholar]
- Rajesh C, Sagar S, Rathinavel AK, Chemparathy DT, Peng XL, Yeh JJ, et al. (2022). Truncated O-glycan-bearing MUC16 enhances pancreatic cancer cells aggressiveness via alpha4beta1 integrin complexes and FAK signaling. International Journal of Molecular Sciences, 23(10), 5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan A, & Kalraiya RD (2013). alpha2,6 Sialylation associated with increased beta 1,6-branched N-oligosaccharides influences cellular adhesion and invasion. Journal of Biosciences, 38(5), 867–876. [DOI] [PubMed] [Google Scholar]
- Rao TC, Beggs RR, Ankenbauer KE, Hwang J, Ma VP, Salaita K, et al. (2022). ST6Gal-I-mediated sialylation of the epidermal growth factor receptor modulates cell mechanics and enhances invasion. The Journal of Biological Chemistry, 298(4), 101726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis CA, Osorio H, Silva L, Gomes C, & David L (2010). Alterations in glyco-sylation as biomarkers for cancer detection. Journal of Clinical Pathology, 63(4), 322–329. [DOI] [PubMed] [Google Scholar]
- Remmers N, Anderson JM, Linde EM, DiMaio DJ, Lazenby AJ, Wandall HH, et al. (2013). Aberrant expression of mucin core proteins and o-linked glycans associated with progression of pancreatic cancer. Clinical Cancer Research, 19(8), 1981–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho JH, Mead JR, Wright WS, Brenner DE, Stave JW, Gildersleeve JC, et al. (2014). Discovery of sialyl Lewis A and Lewis X modified protein cancer biomarkers using high density antibody arrays. Journal of proteomics, 96, 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues E, & Macauley MS (2018). Hypersialylation in cancer: Modulation of inflammation and therapeutic opportunities. Cancers (Basel), 10(6), 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues JG, Duarte HO, Gomes C, Balmana M, Martins AM, Hensbergen PJ, et al. (2021). Terminal alpha2,6-sialylation of epidermal growth factor receptor modulates antibody therapy response of colorectal cancer cells. Cell Oncology (Dordr), 44(4), 835–850. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, Boelaars K, Brown K, Eveline Li RJ, Kruijssen L, Bruijns SCM, et al. (2021). Sialic acids in pancreatic cancer cells drive tumour-associated macrophage differentiation via the Siglec receptors Siglec-7 and Siglec-9. Nature Communications, 12(1), 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer TB, Aasted MKM, Dabelsteen S, Groen A, Schnabel J, Tan E, et al. (2021). Mapping of truncated O-glycans in cancers of epithelial and non-epithelial origin. British Journal of Cancer, 125(9), 1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd PM, Woods RJ, Wormald MR, Opdenakker G, Downing AK, Campbell ID, et al. (1995). The effects of variable glycosylation on the functional activities of ribonuclease, plasminogen and tissue plasminogen activator. Biochimica et Biophysica Acta, 1248(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Safa AR (2016). Resistance to cell death and its modulation in cancer stem cells. Critical Reviews in Oncogenesis, 21(3–4), 203–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrats A, Saldova R, Pla E, Fort E, Harvey DJ, Struwe WB, et al. (2010). Glycosylation of liver acute-phase proteins in pancreatic cancer and chronic pancreatitis. Proteomics. Clinical Applications, 4(4), 432–448. [DOI] [PubMed] [Google Scholar]
- Sato C, & Kitajima K (2021). Polysialylation and disease. Molecular Aspects of Medicine, 79, 100892. [DOI] [PubMed] [Google Scholar]
- Satomura Y, Sawabu N, Takemori Y, Ohta H, Watanabe H, Okai T, et al. (1991). Expression of various sialylated carbohydrate antigens in malignant and nonmalignant pancreatic tissues. Pancreas, 6(4), 448–458. [DOI] [PubMed] [Google Scholar]
- Sawada R, Sun SM, Wu X, Hong F, Ragupathi G, Livingston PO, et al. (2011). Human monoclonal antibodies to sialyl-Lewis (CA19.9) with potent CDC, ADCC, and antitumor activity. Clinical Cancer Research, 17(5), 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuessler MH, Pintado S, Welt S, Real FX, Xu M, Melamed MR, et al. (1991). Blood group and blood-group-related antigens in normal pancreas and pancreas cancer: enhanced expression of precursor type 1, Tn and sialyl-Tn in pancreas cancer. International Journal of Cancer, 47(2), 180–187. [DOI] [PubMed] [Google Scholar]
- Schultz MJ, Holdbrooks AT, Chakraborty A, Grizzle WE, Landen CN, Buchsbaum DJ, et al. (2016). The tumor-associated glycosyltransferase ST6Gal-I regulates stem cell transcription factors and confers a cancer stem cell phenotype. Cancer Research, 76(13), 3978–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz MJ, Swindall AF, Wright JW, Sztul ES, Landen CN, & Bellis SL (2013). ST6Gal-I sialyltransferase confers cisplatin resistance in ovarian tumor cells. Journal of Ovarian Research, 6(1), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seales EC, Jurado GA, Brunson BA, Wakefield JK, Frost AR, & Bellis SL (2005). Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Research, 65, 4645–4652. [DOI] [PubMed] [Google Scholar]
- Seales EC, Jurado GA, Singhal A, & Bellis SL (2003). Ras oncogene directs expression of a differentially sialylated, functionally altered beta1 integrin. Oncogene, 22(46), 7137–7145. [DOI] [PubMed] [Google Scholar]
- Shah MH, Telang SD, Shah PM, & Patel PS (2008). Tissue and serum alpha 2–3- and alpha 2–6-linkage specific sialylation changes in oral carcinogenesis. Glycoconjugate Journal, 25(3), 279–290. [DOI] [PubMed] [Google Scholar]
- Shaikh FM, Seales EC, Clem WC, Hennessy KM, Zhuo Y, & Bellis SL (2008). Tumor cell migration and invasion are regulated by expression of variant integrin glycoforms. Experimental Cell Research, 314(16), 2941–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, & Allison JP (2015). Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell, 161(2), 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Fuchs HE, & Jemal A (2021). Cancer statistics, 2021. CA: A Cancer Journal for Clinicians, 71(1), 7–33. [DOI] [PubMed] [Google Scholar]
- Singh S, Pal K, Yadav J, Tang H, Partyka K, Kletter D, et al. (2015). Upregulation of glycans containing 3′ fucose in a subset of pancreatic cancers uncovered using fusion-tagged lectins. Journal of Proteome Research, 14(6), 2594–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn HP, Brown SA, Oberle E, & Thompson JS (1992). Analysis of the Lewisx epitope in human pancreas and pancreatic adenocarcinomas. International Journal of Pancreatology, 11(2), 125–135. [DOI] [PubMed] [Google Scholar]
- Smith BAH, & Bertozzi CR (2021). The clinical impact of glycobiology: Targeting selectins, Siglecs and mammalian glycans. Nature Reviews. Drug Discovery, 20(3), 217–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithson M, Irwin R, Williams G, Alexander KL, Smythies LE, Nearing M, et al. (2022). Sialyltransferase ST6GAL-1 mediates resistance to chemoradiation in rectal cancer. The Journal of Biological Chemistry, 298(3), 101594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzani P, Arisio R, Porpiglia M, & Benedetto C (2008). Is Sialyl Lewis x antigen expression a prognostic factor in patients with breast cancer? International Journal of Surgical Pathology, 16(4), 365–374. [DOI] [PubMed] [Google Scholar]
- Springer GF (1997). Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. Journal of Molecular Medicine (Berl), 75(8), 594–602. [DOI] [PubMed] [Google Scholar]
- Stowell SR, Ju T, & Cummings RD (2015). Protein glycosylation in cancer. Annual Review of Pathology, 10, 473–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Ju T, & Cummings RD (2018). Differential expression of Cosmc, T-synthase and mucins in Tn-positive colorectal cancers. BMC Cancer, 18(1), 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson BJ, McDermott KM, Singh PK, Eggers JP, Crocker PR, & Hollingsworth MA (2007). MUC1 is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Research, 67(21), 10222–10229. [DOI] [PubMed] [Google Scholar]
- Swindall AF, & Bellis SL (2011). Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. The Journal of Biological Chemistry, 286(26), 22982–22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindall AF, Londoño-Joshi AI, Schultz MJ, Fineberg N, Buchsbaum DJ, & Bellis SL (2013). ST6Gal-I protein expression is upregulated in human epithelial tumors and correlates with stem cell markers in normal tissues and colon cancer cell lines. Cancer Research, 73(7), 2368–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A, Ohmori K, Takahashi N, Tsuyuoka K, Yago A, Zenita K, et al. (1991). Adhesion of human cancer cells to vascular endothelium mediated by a carbohydrate antigen, sialyl Lewis A. Biochemical and Biophysical Research Communications, 179(2), 713–719. [DOI] [PubMed] [Google Scholar]
- Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, et al. (1993). Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Research, 53(2), 354–361. [PubMed] [Google Scholar]
- Takahashi S, Hasebe T, Oda T, Sasaki S, Kinoshita T, Konishi M, et al. (2001a). Extra-tumor perineural invasion predicts postoperative development of peritoneal dissemination in pancreatic ductal adenocarcinoma. Anticancer Research, 21(2B), 1407–1412. [PubMed] [Google Scholar]
- Takahashi S, Oda T, Hasebe T, Sasaki S, Kinoshita T, Konishi M, et al. (2001b). Overexpression of sialyl Lewis x antigen is associated with formation of extratumoral venous invasion and predicts postoperative development of massive hepatic metastasis in cases with pancreatic ductal adenocarcinoma. Pathobiology, 69(3), 127–135. [DOI] [PubMed] [Google Scholar]
- Takashima S, Tsuji S, & Tsujimoto M (2003). Comparison of the enzymatic properties of mouse beta-galactoside alpha2,6-sialyltransferases, ST6Gal I and II. Journal of Biochemistry, 134(2), 287–296. [DOI] [PubMed] [Google Scholar]
- Tang H, Hsueh P, Kletter D, Bern M, & Haab B (2015). The detection and discovery of glycan motifs in biological samples using lectins and antibodies: New methods and opportunities. Advances in Cancer Research, 126, 167–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Partyka K, Hsueh P, Sinha JY, Kletter D, Zeh H, et al. (2016). Glycans related to the CA19-9 antigen are elevated in distinct subsets of pancreatic cancers and improve diagnostic accuracy over CA19-9. Cellular and Molecular Gastroenterology and Hepatology, 2(2), 201–221 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Singh S, Partyka K, Kletter D, Hsueh P, Yadav J, et al. (2015). Glycan motif profiling reveals plasma sialyl-lewis x elevations in pancreatic cancers that are negative for sialyl-lewis A. Molecular G Cellular Proteomics, 14(5), 1323–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempero MA, Uchida E, Takasaki H, Burnett DA, Steplewski Z, & Pour PM (1987). Relationship of carbohydrate antigen 19–9 and Lewis antigens in pancreatic cancer. Cancer Research, 47(20), 5501–5503. [PubMed] [Google Scholar]
- Thomas D, Sagar S, Caffrey T, Grandgenett PM, & Radhakrishnan P (2019). Truncated O-glycans promote epithelial-to-mesenchymal transition and stemness properties of pancreatic cancer cells. Journal of Cellular and Molecular Medicine, 23(10), 6885–6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, et al. (2004). Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nature Genetics, 36(6), 579–581. [DOI] [PubMed] [Google Scholar]
- Trinchera M, Aronica A, & Dall’Olio F (2017). Selectin ligands Sialyl-Lewis a and Sialyl-Lewis x in gastrointestinal cancers. Biology (Basel), 6(1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajaria BN, & Patel PS (2017). Glycosylation: A hallmark of cancer? Glycoconjugate Journal, 34(2), 147–156. [DOI] [PubMed] [Google Scholar]
- Varki A, & Angata T (2006). Siglecs—The major subfamily of I-type lectins. Glycobiology, 16(1), 1R–27R. [DOI] [PubMed] [Google Scholar]
- Vreeker GCM, Hanna-Sawires RG, Mohammed Y, Bladergroen MR, Nicolardi S, Dotz V, et al. (2020). Serum N-Glycome analysis reveals pancreatic cancer disease signatures. Cancer Medicine, 9(22), 8519–8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagatsuma T, Nagai-Okatani C, Matsuda A, Masugi Y, Imaoka M, Yamazaki K, et al. (2020). Discovery of pancreatic ductal adenocarcinoma-related aberrant glycosylations: A multilateral approach of lectin microarray-based tissue glycomic profiling with public transcriptomic datasets. Frontiers in Oncology, 10, 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz G, Aruffo A, Kolanus W, Bevilacqua M, & Seed B (1990). Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science, 250(4984), 1132–1135. [DOI] [PubMed] [Google Scholar]
- Wang S, Mao Y, Narimatsu Y, Ye Z, Tian W, Goth CK, et al. (2019). Site-specific O-glycosylation of members of the low-density lipoprotein receptor superfamily enhances ligand interactions. The Journal of Biological Chemistry, 294(21), 8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ju T, Ding X, Xia B, Wang W, Xia L, et al. (2010). Cosmc is an essential chaperone for correct protein O-glycosylation. Proceedings of the National Academy of Sciences of the United States of America, 107(20), 9228–9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LD, & Hruban RH (2012). Pathology and molecular genetics of pancreatic neoplasms. Cancer Journal (Sudbury, Mass.), 18(6), 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin SS, & Gao FH (2020). Molecular mechanism of tumor cell immune escape mediated by CD24/Siglec-10. Frontiers in Immunology, 11, 1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue T, Goldstein IJ, Hollingsworth MA, Kaul K, Brand RE, & Haab BB (2009). The prevalence and nature of glycan alterations on specific proteins in pancreatic cancer patients revealed using antibody-lectin sandwich arrays. Molecular G Cellular Proteomics, 8(7), 1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue T, Maupin KA, Fallon B, Li L, Partyka K, Anderson MA, et al. (2011). Enhanced discrimination of malignant from benign pancreatic disease by measuring the CA 19–9 antigen on specific protein carriers. PLoS One, 6(12), e29180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue T, Partyka K, Maupin KA, Hurley M, Andrews P, Kaul K, et al. (2011). Identification of blood-protein carriers of the CA 19–9 antigen and characterization of prevalence in pancreatic diseases. Proteomics, 11(18), 3665–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L, Chen L, & Chen Z (2018). Knockdown of FUT3 disrupts the proliferation, migration, tumorigenesis and TGF-beta induced EMT in pancreatic cancer cells. Oncology Letters, 16(1), 924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang Z, Holst S, Blochl C, Madunic K, Wuhrer M, et al. (2022). Transforming growth factor-beta challenge alters the N-, O-, and glycosphingolipid glycomes in PaTu-S pancreatic adenocarcinoma cells. The Journal of Biological Chemistry, 298(3), 101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yang J, Li H, Wu Y, Zhang H, & Chen W (2015). Tumor markers CA19-9, CA242 and CEA in the diagnosis of pancreatic cancer: A meta-analysis. International Journal of Clinical and Experimental Medicine, 8(7), 11683–11691. [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Qiu W, Simeone DM, & Lubman DM (2007). N-linked glycosylation profiling of pancreatic cancer serum using capillary liquid phase separation coupled with mass spectrometric analysis. Journal of Proteome Research, 6(3), 1126–1138. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Srivatana U, Ullah A, Gagneja H, Berenson CS, & Lance P (2001). Suppression of a sialyltransferase by antisense DNA reduces invasiveness of human colon cancer cells in vitro. Biochimica et Biophysica Acta, 1536(2–3), 148–160. [DOI] [PubMed] [Google Scholar]


