Abstract
Background
The triglyceride-glucose (TyG) index and estimated glucose disposal rate (eGDR), which are calculated using different parameters, are widely used as markers of insulin resistance and are associated with cardiovascular diseases and prognosis. However, whether they have an additive effect on the risk of mortality remains unclear. This study aimed to explore whether the combined assessment of the TyG index and eGDR improved the prediction of long-term mortality in individuals with and without diabetes.
Methods
In this cross-sectional and cohort study, data were derived from the National Health and Nutrition Examination Survey (NHANES) 2001–2018, and death record information was obtained from the National Death Index. The associations of the TyG index and eGDR with all-cause and cardiovascular mortality were determined by multivariate Cox regression analysis and restricted cubic splines.
Results
Among the 17,787 individuals included in the analysis, there were 1946 (10.9%) all-cause deaths and 649 (3.6%) cardiovascular deaths during a median follow-up of 8.92 years. In individuals with diabetes, the restricted cubic spline curves for the associations of the TyG index and eGDR with mortality followed a J-shape and an L-shape, respectively. The risk of mortality significantly increased after the TyG index was > 9.04 (all-cause mortality) or > 9.30 (cardiovascular mortality), and after eGDR was < 4 mg/kg/min (both all-cause and cardiovascular mortality). In individuals without diabetes, the association between eGDR and mortality followed a negative linear relationship. However, there was no association between the TyG index and mortality. Compared with individuals in the low TyG and high eGDR group, those in the high TyG and low eGDR group (TyG > 9.04 and eGDR < 4) showed the highest risk for all-cause mortality (hazard ratio [HR] = 1.592, 95% confidence interval [CI] 1.284–1.975) and cardiovascular mortality (HR = 1.683, 95% CI 1.179-2.400) in the overall population. Similar results were observed in individuals with and without diabetes.
Conclusions
There was a potential additive effect of the TyG index and eGDR on the risk of long-term mortality in individuals with and without diabetes, which provided additional information for prognostic prediction and contributed to improving risk stratification.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02396-8.
Keywords: Triglyceride-glucose index, Estimated glucose disposal rate, Insulin resistance, NHANES, Mortality
Introduction
Insulin resistance refers to the reduced efficiency of insulin in promoting glucose uptake and utilization for various reasons, leading to the appearance of metabolic abnormalities [1]. It is recognized as an important risk factor for multiple metabolic diseases, including type 2 diabetes, hypertension, dyslipidemia, and obesity, and is a distinctive feature of metabolic syndrome [2]. Furthermore, insulin resistance is also an independent risk factor for cardiovascular diseases and adverse cardiovascular outcomes [3–5]. The Insulin Resistance Intervention after Stroke trial has shown that the use of pioglitazone, which improves insulin sensitivity, can reduce the risk of adverse cardiovascular outcomes [6]. Therefore, early identification of high-risk patients with insulin resistance and active treatment to improve insulin sensitivity may help prevent cardiovascular diseases and improve patient prognosis.
The gold standard for quantifying insulin sensitivity is the glucose disposal rate obtained from the hyperinsulinemic-euglycemic clamp test [7, 8]. However, this test is invasive and expensive, hindering its application in routine clinical practice. The homeostatic model assessment of insulin resistance (HOMA-IR) has been used as an alternative to the hyperinsulinemic-euglycemic clamp test [9]. As HOMA-IR is calculated using fasting glucose and fasting insulin [9], its value can be influenced by insulin use, particularly for patients with diabetes. In addition to elevated fasting glucose and insulin levels, insulin resistance is also associated with dyslipidemia, central obesity, and hypertension [2]. Therefore, various insulin resistance indices based on commonly available laboratory measurements and anthropometric parameters, including the triglyceride-glucose (TyG) index [10], TyG-derived parameters [11], estimated glucose disposal rate (eGDR) [12, 13], and other markers [14–17] have been proposed.
The TyG index is a widely recognized non-invasive, simple indicator of insulin resistance [10]. Its associations with cardiovascular diseases and prognosis have been extensively validated in different populations [18–21]. However, while evaluating insulin resistance markers that influence cardiovascular outcomes independent of traditional cardiovascular risk factors, some patients who do not have elevated levels of the TyG index remain at high risk of adverse cardiovascular outcomes. Previous studies have mainly focused on comparing the predictive value of different insulin resistance indices on prognosis [22–24], but have not explored whether different insulin resistance indices have an additive effect on prognosis and whether this effect differs between individuals with and without diabetes. This may contribute to improving risk stratification and guide optimal therapy. To accomplish this goal, we analyzed data from the National Health and Nutrition Examination Survey (NHANES), aiming to determine whether combining the TyG index with other insulin resistance indices has an additive effect on long-term mortality in individuals with and without diabetes.
Methods
Study population and design
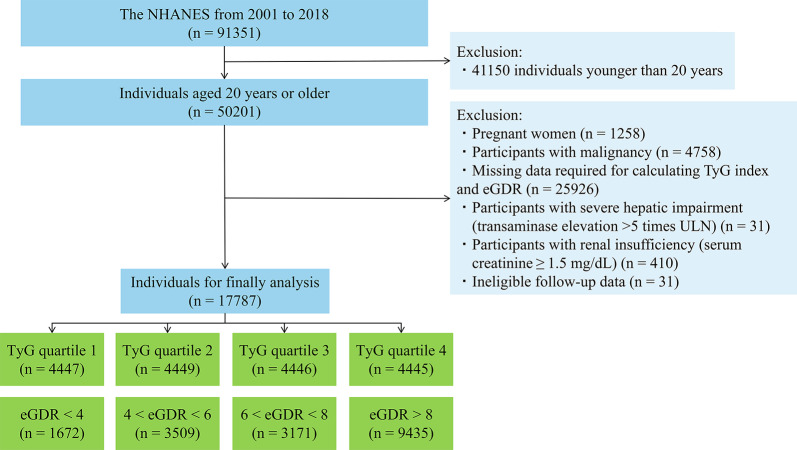
The NHANES, conducted by the Centers for Disease Control and Prevention National Center for Health Statistics, is a nationally representative, cross-sectional health survey that aims to assess the health and nutritional status of both adults and children. Participants were selected through a complex, stratified, multistage probability cluster sampling design to be representative of the non-institutionalized, civilian population of the United States. The Ethics Review Committee of the National Center for Health Statistics approved the NHANES study protocol, and all participants provided written informed consent before participation. This study used data from 9 cycles of the NHANES from 2001 to 2018, which were available at https://www.cdc.gov/nchs/nhanes/index.htm. The exclusion criteria were as follows: (1) participants aged less than 20 years (n = 41150); (2) pregnant women (n = 1258); (3) participants with malignancy (n = 4758); (4) participants with missing data required for calculating the TyG index and eGDR (n = 25926); (5) participants with severe hepatic impairment (transaminase elevation > 5 times the upper limit of normal) (n = 31); (6) participants with renal insufficiency (serum creatinine ≥ 1.5 mg/dL) (n = 410); and (7) participants with ineligible follow-up data (n = 31). Finally, 17,787 participants were included in the final analysis. The flowchart for participant selection is shown in Fig. 1.
Fig. 1.
Flow chart of participant recruitment and screening. Abbreviations: eGDR, estimated glucose disposal rate; NHANES, National Health and Nutrition Examination Survey; TyG, triglyceride-glucose; ULN, upper limit of normal
Insulin resistance indices
The formulas for calculating diverse insulin resistance indices were as follows:
TyG index = Ln [fasting triglyceride (mg/dL) × fasting glucose (mg/dL)/2] [10];
TyG-BMI index = TyG index × BMI [11];
TyG-WC index = TyG index × waist circumference (cm) [11];
TyG-WHtR index = TyG index × waist circumference (cm)/height (cm) [11];
eGDR = 21.158 - (0.09 × waist circumference [cm]) − (3.407 × hypertension [yes 1 or no 0]) − (0.551 × glycated hemoglobin A1c [HbA1c] [%]) [12, 13];
HOMA-IR = (fasting glucose [mmol/L] × fasting insulin [µU/mL])/22.5 [9].
TG/HDL-C = fasting triglyceride (mg/dL)/fasting high-density lipoprotein cholesterol (HDL-C) (mg/dL) [14].
Metabolic score for insulin resistance (METS-IR) = Ln (2 × fasting glucose [mg/dL] + fasting triglyceride [mg/dL]) × BMI/Ln (fasting HDL-C [mg/dL]) [15].
Visceral adiposity index (VAI) = male: (waist circumference [cm]/[39.68 + 1.88 × BMI]) × (fasting triglyceride [mmol/L]/1.03) × (1.31/fasting HDL-C [mmol/L]); female: (waist circumference [cm]/[36.58 + 1.89 × BMI]) × (fasting triglyceride [mmol/L]/0.81) × (1.52/fasting HDL-C [mmol/L]) [16].
Lipid accumulation product (LAP) = male: (waist circumference [cm] − 65) × fasting triglyceride (mmol/L); female: (waist circumference [cm] − 58) × fasting triglyceride (mmol/L) [17].
Fasting glucose, fasting triglyceride, fasting HDL-C, and fasting insulin levels were measured after an overnight fast of at least 8 h.
Definitions of covariates
Standardized questionnaires were used to obtain demographic data (age, gender, ethnicity, education level, and family poverty-to-income ratio), health-related behavior data (smoking status and alcohol consumption), medical condition data (diabetes, hypertension, and cardiovascular diseases), and medication data (antidiabetic agents, antihypertensive agents, and antihyperlipidemic agents). Physical examination and laboratory measurements were performed by trained medical professionals in mobile examination centers. Ethnicity was classified as Mexican Hispanics, non-Mexican Hispanics, non-Hispanic White, non-Hispanic Black, or others. Education level was categorized as less than high school, high school or equivalent, or college or above. Smoking status was recorded as never (smoked < 100 cigarettes in their lifetime), former (smoked ≥ 100 cigarettes in their lifetime and currently not smoking), or current (smoked ≥ 100 cigarettes in their lifetime and currently smoking). Diabetes was defined as the presence of at least 1 of the following conditions: (1) fasting glucose ≥ 126 mg/dL; (2) random glucose ≥ 200 mg/dL; (3) 2 h oral glucose tolerance test glucose ≥ 200 mg/dL; (4) HbA1c ≥ 6.5%; (5) self-reported physician diagnosis of diabetes; or (6) use of antidiabetic medications. Blood pressure was measured after 5 min of seated rest, and the average of the first three consecutive measurements was used to calculate the mean systolic and diastolic blood pressure. Hypertension was defined as the presence of at least 1 of the following conditions: (1) systolic blood pressure ≥ 140 mmHg; (2) diastolic blood pressure ≥ 90 mmHg; (3) self-reported physician diagnosis of hypertension; or (4) use of antihypertensive medications. Antidiabetic agents included insulin and other antidiabetic agents (such as metformin, sulfonylureas, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, alpha-glucosidase inhibitors, glucagon-like peptide-1 receptor agonists, and sodium-glucose cotransporter-2 inhibitors). Antihyperlipidemic agents included statins, ezetimibe, and fibrates. Cardiovascular diseases were defined by positive answers to the following questions: “Has a doctor or other health professional ever told you that you had congestive heart failure/coronary heart disease/angina pectoris/myocardial infarction/stroke?” during an individual interview. The estimated glomerular filtration rate (eGFR) was calculated using the modified Modification of Diet in Renal Disease equation [25].
Study endpoint
The endpoints of the present study were all-cause mortality and cardiovascular mortality. To determine the death status of each individual during follow-up, the NHANES public-use mortality file through December 31, 2019, which was linked to the National Death Index with a probabilistic matching algorithm, was used. The causes of death were determined according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes. Cardiovascular mortality was defined as death from heart diseases (ICD-10 codes I00-I09, I11, I13, and I20-I51) or cerebrovascular diseases (ICD-10 codes I60-I69).
Statistical analysis
All analyses were performed using R statistical software (R Foundation for Statistical Computing, Vienna, Austria, version 4.3.1) and were weighted according to sample weights from the National Center for Health Statistics to account for the complex, multistage probability sampling design. As there is no recommended cutoff value of the TyG index for diagnosing insulin resistance, the study population was categorized into four groups based on the quartiles of the TyG index to compare the baseline characteristics of the participants. In addition, the cutoff values of eGDR (< 4, 4–6, 6–8, and ≥ 8 mg/kg/min) were recommended by previous studies demonstrating a significant difference in mortality among these categories in individuals with type 1 diabetes and individuals with type 2 diabetes [13, 26]. Survey data are presented as weighted means with standard errors for continuous variables and unweighted frequencies but weighted proportions for categorical variables. Comparisons among groups were conducted using one-way analyses of variance for continuous variables and chi-square tests for categorical variables.
Receiver operating characteristic (ROC) curves were constructed, and Harrell’s C-index was applied to compare the predictive value of different insulin resistance indices for long-term mortality. In addition, covariate-adjusted receiver operating characteristic curve analysis was performed to adjust for the effect of diabetes on mortality by using the “ROCnReg” package [27]. The associations of the TyG index and eGDR with mortality were analyzed by Kaplan-Meier and Cox regression models. Statistically significant variables in univariate analyses and potentially clinically relevant variables were incorporated into multivariate analyses. Model 1 was adjusted for age, gender, race, and body mass index. Model 2 was adjusted for Model 1 plus smoking status, alcohol consumption, educational level, family poverty-to-income ratio, cardiovascular diseases, antidiabetic agents, antihypertensive agents, antihyperlipidemic agents, eGFR, HDL-C, and low-density lipoprotein cholesterol (LDL-C). The potential dose-response relationships between insulin resistance indices and long-term mortality were determined by restricted cubic spline regressions with 4 knots. The additive effect of the TyG index and eGDR on long-term mortality was explored in various subgroups. In the subgroup analysis, abdominal obesity was defined as a waist circumference > 102 cm for males and > 88 cm for females [28]. Significance was set at p < 0.05 (*), p < 0.01 (†), and p < 0.001 (‡).
Results
Baseline characteristics
The baseline characteristics of the participants stratified by quartiles of the TyG index are shown in Table 1. Among a total of 17,787 participants, 49.7% (weighted) were male and the mean age was 45 ± 16 years. There were 3159 (12.7%, weighted) individuals who had diabetes. Individuals with higher quartiles of the TyG index were more likely to be older, male, current smokers, had lower education levels, and tended to have diabetes, hypertension, and cardiovascular diseases than those with lower quartiles of the TyG index. For individuals with higher quartiles of the TyG index, there were higher levels of BMI, waist circumference, white blood cells, hemoglobin, platelet, fasting glucose, fasting triglyceride, and LDL-C but lower levels of eGFR, HDL-C, and eGDR compared with those with lower quartiles of the TyG index. The baseline characteristics of the participants stratified by the recommended cutoff values of the eGDR are shown in Supplementary Table 1.
Table 1.
Baseline characteristics of study individuals according to quartiles of the TyG index
| aVariables | Overall (n = 17787) |
TyG Q1 < 8.17 (n = 4447) |
TyG Q2 8.17–8.59 (n = 4449) |
TyG Q3 8.59–9.04 (n = 4446) |
TyG Q4 > 9.04 (n = 4445) |
p value |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, years | 45 ± 16 | 40 ± 15 | 45 ± 16 | 47 ± 16 | 49 ± 15 | < 0.001 |
| Age ≥ 70 years, n (%) | 2350 (8.1) | 341 (4.5) | 586 (8.0) | 733 (10.6) | 690 (9.8) | < 0.001 |
| Gender, n (%) | < 0.001 | |||||
| Male | 8842 (49.7) | 1746 (39.0) | 2146 (47.9) | 2339 (53.3) | 2611 (60.1) | |
| Female | 8945 (50.3) | 2701 (61.0) | 2303 (52.1) | 2107 (46.7) | 1834 (39.9) | |
| Ethnicity, n (%) | < 0.001 | |||||
| Mexican Hispanics | 3200 (8.9) | 533 (6.6) | 724 (8.4) | 886 (9.8) | 1057 (11.1) | |
| Non-Mexican Hispanics | 1618 (5.5) | 326 (5.2) | 375 (5.2) | 472 (6.0) | 445 (5.8) | |
| Non-Hispanic White | 7532 (66.7) | 1682 (62.7) | 1894 (67.2) | 1965 (68.5) | 1991 (69.1) | |
| Non-Hispanic Black | 3597 (11.3) | 1405 (17.9) | 1007 (12.3) | 662 (7.8) | 523 (6.4) | |
| Other Race | 1840 (7.5) | 501 (7.6) | 449 (6.8) | 461 (8.0) | 429 (7.6) | |
| Education level, n (%) | < 0.001 | |||||
| Less than high school | 4672 (16.8) | 827 (12.2) | 1092 (16.0) | 1294 (19.0) | 1459 (20.5) | |
| High school or equivalent | 4061 (23.7) | 938 (20.5) | 1024 (23.7) | 1029 (24.3) | 1070 (26.8) | |
| College or above | 9037 (59.5) | 2678 (67.3) | 2332 (60.2) | 2117 (56.7) | 1910 (52.7) | |
| Family PIR, mean ± SE | 2.98 ± 1.63 | 3.03 ± 1.63 | 3.02 ± 1.64 | 2.92 ± 1.63 | 2.93 ± 1.62 | 0.075 |
| Health-related behaviors | ||||||
| Smoking, n (%) | < 0.001 | |||||
| Current | 3892 (21.6) | 797 (17.4) | 1030 (22.2) | 982 (22.4) | 1083 (24.9) | |
| Former | 4159 (23.9) | 816 (19.2) | 948 (22.9) | 1111 (25.3) | 1284 (28.7) | |
| Never | 9720 (54.5) | 2831 (63.4) | 2465 (54.8) | 2348 (52.2) | 2076 (46.4) | |
| Alcohol consumption, n (%) | 12,296 (78.6) | 3036 (79.2) | 3077 (79.0) | 3105 (79.2) | 3078 (77.1) | 0.207 |
| Physical examination | ||||||
| BMI, kg/m2 | 28.79 ± 6.70 | 26.14 ± 6.16 | 28.15 ± 6.31 | 29.82 ± 6.58 | 31.49 ± 6.60 | < 0.001 |
| Waist circumstance, cm | 98.4 ± 16.4 | 90.0 ± 14.8 | 96.5 ± 15.3 | 101.5 ± 15.2 | 106.9 ± 15.4 | < 0.001 |
| SBP, mmHg | 121 ± 17 | 116 ± 15 | 120 ± 16 | 123 ± 16 | 127 ± 17 | < 0.001 |
| DBP, mmHg | 70 ± 12 | 68 ± 11 | 70 ± 11 | 71 ± 12 | 73 ± 12 | < 0.001 |
| Medical conditions | ||||||
| Diabetes, n (%) | 3159 (12.7) | 219 (3.3) | 421 (6.5) | 758 (12.0) | 1761 (31.2) | < 0.001 |
| Hypertension, n (%) | 7011 (34.2) | 1110 (19.8) | 1608 (30.4) | 1921 (39.5) | 2372 (49.6) | < 0.001 |
| Cardiovascular diseases, n (%) | 1591 (7.0) | 224 (4.0) | 360 (5.8) | 436 (7.9) | 571 (10.8) | < 0.001 |
| Medications | ||||||
| Antidiabetic agents, n (%) | 1806 (7.2) | 129 (1.9) | 221 (3.2) | 427 (6.4) | 1029 (18.7) | < 0.001 |
| Insulin, n (%) | 493 (1.7) | 42 (0.6) | 55 (0.9) | 111 (1.6) | 285 (4.1) | < 0.001 |
| Other antidiabetic agents, n (%) | 1589 (6.5) | 107 (1.6) | 196 (2.7) | 377 (5.6) | 909 (17.3) | < 0.001 |
| Antihypertensive agents, n (%) | 4261 (20.2) | 599 (10.1) | 961 (17.8) | 1154 (22.9) | 1547 (31.8) | < 0.001 |
| Antihyperlipidemic agents, n (%) | 2852 (14.5) | 346 (6.9) | 570 (11.3) | 801 (16.7) | 1135 (24.4) | < 0.001 |
| Laboratory measurements | ||||||
| White blood cells, 109/L | 6.8 ± 2.0 | 6.2 ± 1.9 | 6.7 ± 1.9 | 7.0 ± 2.0 | 7.4 ± 2.0 | < 0.001 |
| Hemoglobin, g/dL | 14.5 ± 1.5 | 14.0 ± 1.4 | 14.4 ± 1.4 | 14.6 ± 1.4 | 14.9 ± 1.4 | < 0.001 |
| Platelet, 109/L | 251 ± 65 | 242 ± 61 | 252 ± 65 | 255 ± 66 | 255 ± 67 | < 0.001 |
| ALT, U/L | 25 ± 16 | 21 ± 12 | 24 ± 14 | 27 ± 16 | 31 ± 19 | < 0.001 |
| AST, U/L | 25 ± 12 | 23 ± 11 | 24 ± 11 | 25 ± 11 | 27 ± 12 | < 0.001 |
| Serum creatinine, mg/dL | 0.86 ± 0.18 | 0.83 ± 0.18 | 0.86 ± 0.18 | 0.87 ± 0.19 | 0.88 ± 0.19 | < 0.001 |
| eGFR, mL/min/1.73m2 | 96.3 ± 22.6 | 100.4 ± 22.5 | 95.8 ± 21.5 | 94.7 ± 22.6 | 93.9 ± 23.2 | < 0.001 |
| Fasting glucose, mg/dL | 104.5 ± 28.7 | 93.7 ± 10.1 | 98.8 ± 12.8 | 103.9 ± 17.4 | 123.9 ± 49.1 | < 0.001 |
| HbA1c, % | 5.6 ± 0.9 | 5.3 ± 0.4 | 5.4 ± 0.5 | 5.5 ± 0.6 | 6.1 ± 1.5 | < 0.001 |
| Fasting triglyceride, mg/dL | 129 ± 116 | 56 ± 14 | 90 ± 14 | 130 ± 22 | 253 ± 183 | < 0.001 |
| HDL-C, mg/dL | 54 ± 16 | 63 ± 17 | 56 ± 15 | 51 ± 13 | 43 ± 11 | < 0.001 |
| LDL-C, mg/dL | 115 ± 35 | 101 ± 29 | 117 ± 32 | 123 ± 35 | 122 ± 40 | < 0.001 |
| eGDR, mg/kg/min | 8.07 ± 2.68 | 9.48 ± 2.20 | 8.46 ± 2.42 | 7.63 ± 2.49 | 6.50 ± 2.71 | < 0.001 |
aSurvey data were presented as weighted means ± standard errors for continuous variables, and unweighted frequencies (weighted proportions) for categorical variables
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; eGDR, estimated glucose disposal rate; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin A1c; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; PIR, poverty-to-income ratio; SBP, systolic blood pressure; SE, standard errors; TyG, triglyceride-glucose
ROC curve analysis of the insulin resistance indices for mortality
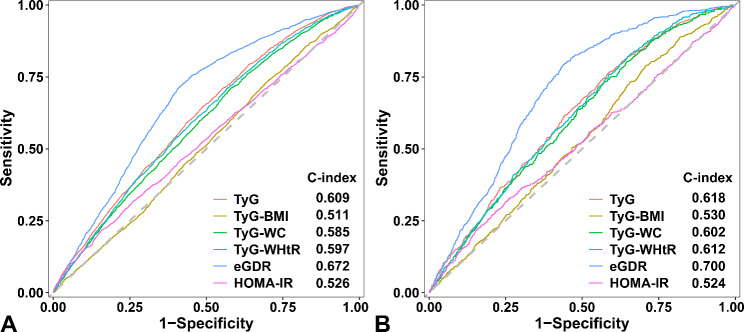
The prognostic performance of the TyG index and eGDR was significantly greater than that of the other indices (Fig. 2 and Supplementary Fig. 1). The TyG index showed a greater Harrell’s C-index for predicting all-cause mortality than the other TyG-derived parameters, including the TyG-BMI index (0.609 vs. 0.511, p < 0.001), the TyG-WC index (0.609 vs. 0.585, p < 0.001), and the TyG-WHtR index (0.609 vs. 0.597, p = 0.046) (Fig. 2A). In addition, the predictive value of the TyG index for cardiovascular mortality was greater than that of the TyG-BMI index (0.618 vs. 0.530, p < 0.001), but was similar to the TyG-WC index (0.618 vs. 0.602, p = 0.116) and the TyG-WHtR index (0.618 vs. 0.612, p = 0.630) (Fig. 2B). The prognostic performance of the TyG index (all-cause mortality: 0.609 vs. 0.526, p < 0.001; cardiovascular mortality: 0.618 vs. 0.524, p < 0.001) and eGDR (all-cause mortality: 0.672 vs. 0.526, p < 0.001; cardiovascular mortality: 0.700 vs. 0.524, p < 0.001) was also significantly greater than that of the HOMA-IR (Fig. 2).
Fig. 2.
Receiver operating characteristic curves of the insulin resistance indices for (A) all-cause mortality and (B) cardiovascular mortality. Abbreviations: eGDR, estimated glucose disposal rate; HOMA-IR, homeostatic model assessment of insulin resistance; TyG, triglyceride-glucose; TyG-BMI, triglyceride glucose-body mass index; TyG-WC, triglyceride glucose-waist circumference; TyG-WHtR, triglyceride glucose-waist to height ratio
Furthermore, we used ROC curve analysis to compare the predictive value of the TyG index, eGDR, and other insulin resistance indices for mortality and found that the prognostic performance of the TyG index and eGDR was significantly greater than that of the other indices (Supplementary Fig. 1).
Given the results above, we sought to explore the potential additive effect of the TyG index and eGDR, which incorporate different parameters in their formulas, on long-term mortality.
Kaplan-Meier survival curves of the TyG index and eGDR for mortality
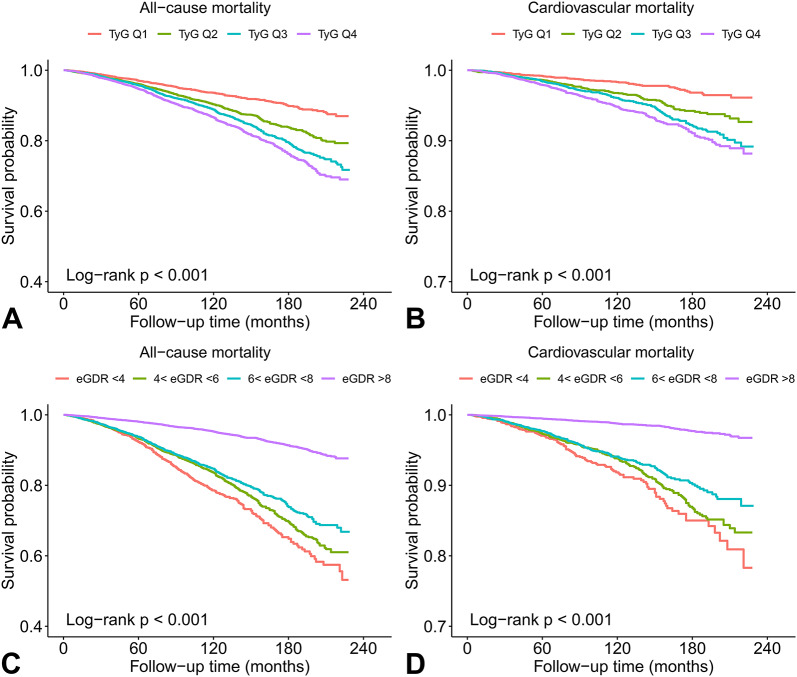
During a median follow-up period of 8.92 years, 1946 (10.9%) all-cause deaths and 649 (3.6%) cardiovascular deaths occurred. Kaplan-Meier survival curves of the TyG index and eGDR for long-term mortality are plotted in Fig. 3. In the overall population, the mortality rates increased with increasing quartiles of the TyG index and decreasing levels of the eGDR (all log-rank p < 0.001).
Fig. 3.
Kaplan-Meier survival curves showing associations of the TyG index (A and B) and eGDR (C and D) with long-term mortality. Abbreviations: eGDR, estimated glucose disposal rate; TyG, triglyceride-glucose
The association between the TyG index and mortality in individuals with and without diabetes
When analyzed as a continuous variable, the TyG index was independently associated with all-cause mortality and cardiovascular mortality in the fully adjusted model in the overall population (all-cause mortality: hazard ratio [HR] = 1.148 per-1 unit increment, 95% confidence interval [CI] 1.037–1.272; cardiovascular mortality: HR = 1.242 per-1 unit increment, 95% CI 1.041–1.482) and individuals with diabetes (all-cause mortality: HR = 1.289 per-1 unit increment, 95% CI 1.099–1.511; cardiovascular mortality: HR = 1.396 per-1 unit increment, 95% CI 1.069–1.822). However, this was not the case for individuals without diabetes (all-cause mortality: HR = 0.973 per-1 unit increment, 95% CI 0.845–1.121; cardiovascular mortality: HR = 1.076 per-1 unit increment, 95% CI 0.837–1.382). When analyzed as a categorical variable, the TyG index was not significantly associated with mortality regardless of whether individuals had diabetes. This result suggested a potential nonlinear relationship between the TyG index and long-term mortality (Supplementary Table 2).
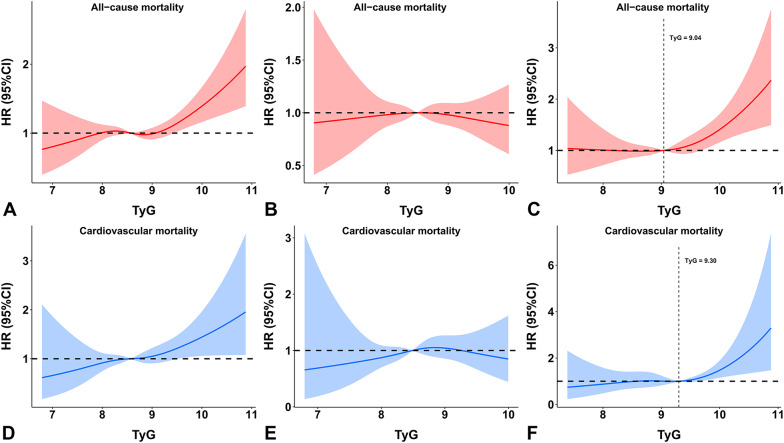
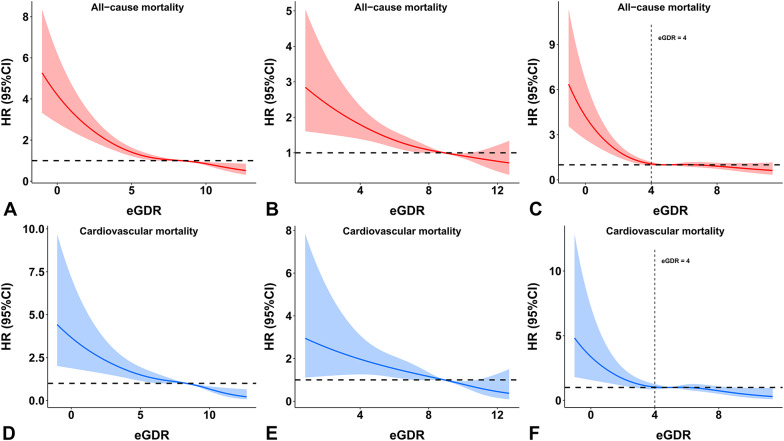
According to the restricted cubic spline analyses, the relationship between the TyG index and mortality followed a J-shape in individuals with diabetes. The risk of all-cause mortality and cardiovascular mortality significantly increased when the TyG index was greater than 9.04 and 9.30, respectively (Fig. 4C and F). However, in individuals without diabetes, the HR of 1 was completely covered by the 95% confidence intervals of the restricted cubic spline curves, showing that the association between the TyG index and mortality was not statistically significant (Fig. 4B and E).
Fig. 4.
Dose-response relationship between the TyG index and mortality in the (A and D) overall population, (B and E) individuals without diabetes, and (C and F) individuals with diabetes according to multivariable-adjusted restricted cubic spline analysis. Adjusted for Model 2 in the Cox regression analysis. Abbreviations: CI, confidence interval; HR, hazard ratio; TyG, triglyceride-glucose
The association between eGDR and mortality in individuals with and without diabetes
When eGDR was analyzed as a continuous variable, it was significantly associated with all-cause mortality and cardiovascular mortality in the fully adjusted model in individuals with diabetes (all-cause mortality: HR = 0.883 per-1 unit increment, 95% CI 0.833–0.936; cardiovascular mortality: HR = 0.857 per-1 unit increment, 95% CI 0.777–0.946) and without diabetes (all-cause mortality: HR = 0.900 per-1 unit increment, 95% CI 0.863–0.939; cardiovascular mortality: HR = 0.854 per-1 unit increment, 95% CI 0.790–0.923). The results did not change when eGDR was analyzed as a categorical variable (Supplementary Table 3).
According to the restricted cubic spline analyses, the association between eGDR and mortality followed an L-shape in individuals with diabetes. The risk of mortality significantly increased when eGDR was lower than 4 mg/kg/min (Fig. 5C and F). In individuals without diabetes, there was a negative linear relationship between eGDR and mortality (Fig. 5B and E).
Fig. 5.
Dose-response relationship between eGDR and mortality in the (A and D) overall population, (B and E) individuals without diabetes, and (C and F) individuals with diabetes according to multivariable-adjusted restricted cubic spline analysis. Adjusted for Model 2 in the Cox regression analysis. Abbreviations: CI, confidence interval; eGDR, estimated glucose disposal rate; HR, hazard ratio
The association between the combination of the TyG index and eGDR and mortality in individuals with and without diabetes
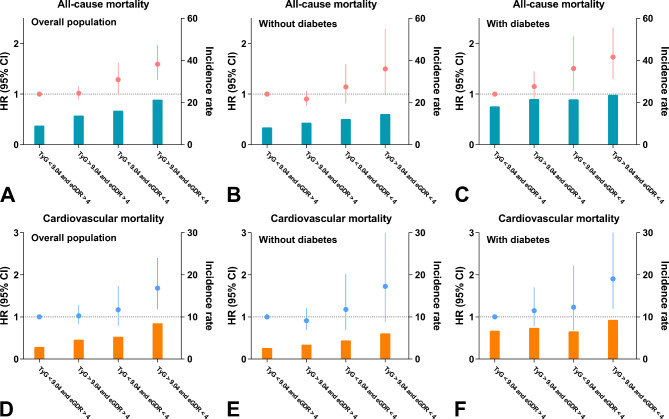
In the overall population, individuals in the high TyG and low eGDR group (TyG > 9.04 and eGDR < 4) showed the highest risk of all-cause mortality (mortality rate: 21.4 vs. 9.1%; HR = 1.592, 95% CI 1.284–1.975) and cardiovascular mortality (mortality rate: 8.5 vs. 2.9%; HR = 1.683, 95% CI 1.179–2.400) compared with individuals in the low TyG and high eGDR group (TyG < 9.04 and eGDR > 4). Similar results were observed in individuals with diabetes. In individuals without diabetes, these associations were marginally significant after adjustment (all-cause mortality: HR = 1.499, 95% CI 0.982–2.288, p = 0.061; cardiovascular mortality: HR = 1.725, 95% CI 0.872–3.414, p = 0.117) (Fig. 6, Supplementary Tables 4 and 5).
Fig. 6.
Association between the combination of the TyG index and eGDR and long-term mortality in the (A and D) overall population, (B and E) individuals without diabetes, and (C and F) individuals with diabetes. Adjusted for Model 2 in the Cox regression analysis. Abbreviations: CI, confidence interval; eGDR, estimated glucose disposal rate; HR, hazard ratio; TyG, triglyceride-glucose
Furthermore, investigating the interaction of insulin resistance, diabetes, and gender differences in cardiovascular prognosis contributes to the development of sex-specific risk stratification [29]. Therefore, we stratified our study population by gender and diabetes status and found that in both males and females with diabetes, an additive effect of the TyG index and eGDR on all-cause mortality was observed. In females without diabetes, the additive effect on cardiovascular mortality was also determined. A similar trend was observed for all-cause mortality in females without diabetes (p = 0.077) and for cardiovascular mortality in both males (p = 0.098) and females with diabetes (p = 0.076), whereas this was not the case for males without diabetes (Tables 2 and 3).
Table 2.
The association of the combination of the TyG index and eGDR with all-cause mortality in males and females with and without diabetes
| Variable | Group | |||
|---|---|---|---|---|
| Low TyG and high eGDR (TyG < 9.04 and eGDR > 4) |
High TyG and high eGDR (TyG > 9.04 and eGDR > 4) |
Low TyG and low eGDR (TyG < 9.04 and eGDR < 4) |
High TyG and low eGDR (TyG > 9.04 and eGDR < 4) |
|
| Males without diabetes | ||||
| Unadjusted | 1.000 |
0.865 (0.719–1.042) |
2.395 (1.714–3.346)‡ |
1.437 (0.919–2.247) |
| Model 1 | 1.000 |
1.020 (0.841–1.238) |
2.001 (1.356–2.952)‡ |
1.624 (0.989–2.667) |
| Model 2 | 1.000 |
0.948 (0.761–1.181) |
1.185 (0.770–1.825) |
1.383 (0.800-2.393) |
| Males with diabetes | ||||
| Unadjusted | 1.000 |
0.961 (0.746–1.238) |
1.344 (0.945–1.914) |
1.255 (0.954–1.650) |
| Model 1 | 1.000 |
1.154 (0.889–1.498) |
1.608 (1.068–2.421)* |
1.663 (1.208–2.289)† |
| Model 2 | 1.000 |
1.179 (0.870–1.597) |
1.332 (0.851–2.083) |
1.556 (1.057–2.289)* |
| Females without diabetes | ||||
| Unadjusted | 1.000 |
1.432 (1.163–1.762)‡ |
1.320 (0.834–2.090) |
2.056 (1.130–3.741)* |
| Model 1 | 1.000 |
0.912 (0.735–1.131) |
1.316 (0.786–2.203) |
1.997 (1.046–3.813)* |
| Model 2 | 1.000 |
0.833 (0.652–1.065) |
1.205 (0.693–2.097) |
1.853 (0.935–3.674) |
| Females with diabetes | ||||
| Unadjusted | 1.000 |
0.928 (0.696–1.239) |
0.935 (0.610–1.433) |
1.183 (0.852–1.645) |
| Model 1 | 1.000 |
1.024 (0.760–1.381) |
1.633 (0.993–2.684) |
1.954 (1.342–2.845)‡ |
| Model 2 | 1.000 |
1.124 (0.772–1.636) |
1.771 (0.987–3.180) |
2.033 (1.281–3.226)† |
Model 1 adjusted for age, gender, race, and body mass index
Model 2 adjusted for model 1 plus smoking status, alcohol consumption, educational level, family poverty-income ratio, cardiovascular diseases, antidiabetic agents, antihypertensive agents, antihyperlipidemic agents, estimated the glomerular filtration rate, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol
Abbreviations: eGDR, estimated glucose disposal rate; TyG, triglyceride-glucose index
*p < 0.05, †p < 0.01, ‡p < 0.001
Table 3.
The association of the combination of the TyG index and eGDR with cardiovascular mortality in males and females with and without diabetes
| Variable | Group | |||
|---|---|---|---|---|
| Low TyG and high eGDR (TyG < 9.04 and eGDR > 4) |
High TyG and high eGDR (TyG > 9.04 and eGDR > 4) |
Low TyG and low eGDR (TyG < 9.04 and eGDR < 4) |
High TyG and low eGDR (TyG > 9.04 and eGDR < 4) |
|
| Males without diabetes | ||||
| Unadjusted | 1.000 |
0.841 (0.610–1.158) |
2.512 (1.428–4.419)† |
1.680 (0.827–3.413) |
| Model 1 | 1.000 |
0.929 (0.664–1.298) |
1.496 (0.786–2.849) |
1.334 (0.602–2.955) |
| Model 2 | 1.000 |
0.849 (0.580–1.241) |
0.918 (0.453–1.862) |
1.057 (0.421–2.657) |
| Males with diabetes | ||||
| Unadjusted | 1.000 |
0.928 (0.614–1.403) |
0.889 (0.458–1.727) |
1.250 (0.803–1.944) |
| Model 1 | 1.000 |
1.146 (0.746–1.758) |
1.230 (0.585–2.588) |
1.890 (1.122–3.185)* |
| Model 2 | 1.000 |
1.200 (0.728–1.979) |
1.146 (0.521–2.521) |
1.751 (0.901–3.404) |
| Females without diabetes | ||||
| Unadjusted | 1.000 |
1.596 (1.096–2.323)* |
1.720 (0.803–3.684) |
3.287 (1.344–8.037)† |
| Model 1 | 1.000 |
1.026 (0.694–1.517) |
1.756 (0.751–4.102) |
3.317 (1.210–9.092)* |
| Model 2 | 1.000 |
0.925 (0.596–1.435) |
1.899 (0.788–4.580) |
4.048 (1.428–11.479)† |
| Females with diabetes | ||||
| Unadjusted | 1.000 |
0.804 (0.487–1.326) |
1.031 (0.516–2.059) |
1.366 (0.803–2.323) |
| Model 1 | 1.000 |
0.947 (0.560–1.603) |
1.263 (0.562–2.840) |
1.999 (1.088–3.671)* |
| Model 2 | 1.000 |
0.955 (0.498–1.831) |
1.254 (0.490–3.207) |
1.933 (0.933–4.002) |
Model 1 adjusted for age, gender, race, and body mass index
Model 2 adjusted for model 1 plus smoking status, alcohol consumption, educational level, family poverty-income ratio, cardiovascular diseases, antidiabetic agents, antihypertensive agents, antihyperlipidemic agents, estimated the glomerular filtration rate, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol
Abbreviations: eGDR, estimated glucose disposal rate; TyG, triglyceride-glucose index
*p < 0.05, †p < 0.01, ‡p < 0.001
According to the ROC curve analyses, the combination of the TyG index and eGDR showed a higher predictive value for both all-cause mortality and cardiovascular mortality than the TyG index (all-cause mortality: 0.678 vs. 0.609, p < 0.001; cardiovascular mortality: 0.704 vs. 0.618, p < 0.001) or eGDR alone (all-cause mortality: 0.678 vs. 0.672, p < 0.001; cardiovascular mortality: 0.704 vs. 0.700, p = 0.038) (Table 4). Adding the TyG index and eGDR to the traditional risk factor model simultaneously further improved the prognostic prediction (Table 5). In the covariate-adjusted receiver operating characteristic curve analysis, similar results were determined after adjusting for diabetes (Tables 4 and 5).
Table 4.
The prognostic value of the TyG index, eGDR, and their combination for mortality
| Harrell’s C-index | p value | aAdjusted Harrell’s C-index | |
|---|---|---|---|
| All-cause mortality | |||
| TyG + eGDR vs. TyG | 0.678 (0.671–0.684) vs. 0.609 (0.601–0.616) | < 0.001 | 0.620 (0.607–0.634) vs. 0.558 (0.545–0.571) |
| TyG + eGDR vs. eGDR | 0.678 (0.671–0.684) vs. 0.672 (0.665–0.679) | < 0.001 | 0.620 (0.607–0.634) vs. 0.615 (0.602–0.629) |
| Cardiovascular mortality | |||
| TyG + eGDR vs. TyG | 0.704 (0.698–0.711) vs. 0.618 (0.611–0.625) | < 0.001 | 0.635 (0.612–0.656) vs. 0.559 (0.537–0.583) |
| TyG + eGDR vs. eGDR | 0.704 (0.698–0.711) vs. 0.700 (0.694–0.707) | 0.038 | 0.635 (0.612–0.656) vs. 0.630 (0.608–0.652) |
Abbreviations: eGDR, estimated glucose disposal rate; TyG, triglyceride-glucose
aCovariate-adjusted receiver operating characteristic curve analysis was adjusted for diabetes
Table 5.
The incremental effects of incorporating the TyG index and eGDR to a traditional risk model on prognostic prediction
| Harrell’s C-index | p value | aAdjusted Harrell’s C-index | |
|---|---|---|---|
| All-cause mortality | |||
| *Model | 0.872 (0.866–0.877) | Ref. | 0.847 (0.835–0.857) |
| Model + TyG | 0.874 (0.869–0.880) | < 0.001 | 0.851 (0.839–0.863) |
| Model + eGDR | 0.874 (0.869–0.880) | < 0.001 | 0.850 (0.839–0.861) |
| Model + TyG + eGDR | 0.876 (0.871–0.881) | < 0.001 | 0.853 (0.841–0.863) |
| Cardiovascular mortality | |||
| Model | 0.872 (0.867–0.878) | Ref. | 0.842 (0.822–0.862) |
| Model + TyG | 0.876 (0.870–0.881) | 0.003 | 0.846 (0.828–0.865) |
| Model + eGDR | 0.876 (0.871–0.881) | 0.005 | 0.846 (0.825–0.865) |
| Model + TyG + eGDR | 0.878 (0.873–0.883) | < 0.001 | 0.848 (0.828–0.868) |
Abbreviations: eGDR, estimated glucose disposal rate; TyG, triglyceride-glucose
*The model incorporates age, gender, race, body mass index, smoking status, alcohol consumption, educational level, family poverty-income ratio, cardiovascular diseases, antidiabetic agents, antihypertensive agents, antihyperlipidemic agents, estimated glomerular filtration rate, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol
aCovariate-adjusted receiver operating characteristic curve analysis was adjusted for diabetes
Subgroup analysis of the association between the combination of the TyG index and eGDR and mortality
In our study population, 1937 individuals had information on diabetes duration. The median duration of diabetes was 8.0 years. In individuals with diabetes duration < 10 years, the risk of all-cause mortality was highest in the group of high TyG and low eGDR (TyG > 9.04 and eGDR < 4) (p = 0.009). A similar trend was observed for all-cause mortality in individuals with diabetes duration ≥ 10 years (p = 0.068) and for cardiovascular mortality in individuals with diabetes duration < 10 years (p = 0.087) or ≥ 10 years (p = 0.214), although they did not reach statistical significance (Table 6). This also confirmed that the combination of the TyG index and eGDR had an additive effect on prognosis in individuals with diabetes.
Table 6.
The association of the combination of the TyG index and eGDR with mortality in individuals with diabetes duration < 10 years and ≥ 10 years
| Variable | Group | |||
|---|---|---|---|---|
| Low TyG and high eGDR (TyG < 9.04 and eGDR > 4) |
High TyG and high eGDR (TyG > 9.04 and eGDR > 4) |
Low TyG and low eGDR (TyG < 9.04 and eGDR < 4) |
High TyG and low eGDR (TyG > 9.04 and eGDR < 4) |
|
| All-cause mortality | ||||
| Diabetes duration < 10 years | ||||
| Unadjusted | 1.000 |
1.197 (0.826–1.733) |
1.494 (0.905–2.466) |
1.466 (0.978–2.197) |
| Model 1 | 1.000 |
1.448 (0.984–2.131) |
1.712 (0.952–3.080) |
2.285 (1.439–3.630)‡ |
| Model 2 | 1.000 |
1.563 (0.991–2.467) |
1.585 (0.820–3.064) |
2.073 (1.197–3.588)† |
| Diabetes duration ≥ 10 years | ||||
| Unadjusted | 1.000 |
0.885 (0.630–1.244) |
1.170 (0.761–1.799) |
1.064 (0.758–1.493) |
| Model 1 | 1.000 |
0.903 (0.636–1.282) |
1.483 (0.906–2.427) |
1.400 (0.945–2.075) |
| Model 2 | 1.000 |
0.976 (0.630–1.512) |
1.467 (0.838–2.568) |
1.596 (0.966–2.636) |
| Cardiovascular mortality | ||||
| Diabetes duration < 10 years | ||||
| Unadjusted | 1.000 |
0.850 (0.470–1.537) |
1.285 (0.588–2.809) |
1.147 (0.605–2.175) |
| Model 1 | 1.000 |
1.148 (0.614–2.147) |
1.512 (0.596–3.834) |
1.808 (0.860–3.803) |
| Model 2 | 1.000 |
1.714 (0.784–3.746) |
1.722 (0.595–4.984) |
2.252 (0.888–5.714) |
| Diabetes duration ≥ 10 years | ||||
| Unadjusted | 1.000 |
1.020 (0.604–1.720) |
0.876 (0.412–1.864) |
1.143 (0.673–1.939) |
| Model 1 | 1.000 |
1.027 (0.598–1.764) |
1.156 (0.498–2.684) |
1.650 (0.891–3.057) |
| Model 2 | 1.000 |
1.013 (0.515–1.994) |
1.151 (0.441-3.000) |
1.663 (0.745–3.712) |
Model 1 adjusted for age, gender, race, and body mass index
Model 2 adjusted for model 1 plus smoking status, alcohol consumption, educational level, family poverty-income ratio, cardiovascular diseases, antidiabetic agents, antihypertensive agents, antihyperlipidemic agents, estimated the glomerular filtration rate, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol
Abbreviations: eGDR, estimated glucose disposal rate; TyG, triglyceride-glucose index
*p < 0.05, †p < 0.01, ‡p < 0.001
For all-cause mortality, similar results were observed within subgroups of ages (Supplementary Fig. 2A and B), gender (Supplementary Fig. 3A and B), smoking status (former/never vs. current) (Supplementary Fig. 4A and B), and renal function (eGFR < 90 vs. eGFR ≥ 90 mL/min/1.73m2) (Supplementary Fig. 5A and B). For cardiovascular mortality, this phenomenon was also determined among individuals < 70 years (Supplementary Fig. 2C), females (Supplementary Fig. 3C), former/never smokers (Supplementary Fig. 4C), and individuals with an eGFR < 90 mL/min/1.73m2 (Supplementary Fig. 5C). In individuals ≥ 70 years (Supplementary Fig. 2D) and males (Supplementary Fig. 3D), similar trends were observed, although they did not reach statistical significance. However, this was not the case for current smokers (Supplementary Fig. 4D) or individuals with an eGFR ≥ 90 mL/min/1.73m2 (Supplementary Fig. 5D).
In individuals without a history of cardiovascular diseases (Supplementary Fig. 6A and C), as well as individuals with a BMI ≥ 30 kg/m2 (Supplementary Fig. 7B and D), with abdominal obesity (Supplementary Fig. 8B and D), or with hypertension (Supplementary Fig. 9B and D), the additive effect of the TyG index and eGDR on both all-cause mortality and cardiovascular mortality was discovered. A similar trend was observed for all-cause mortality in individuals with a history of cardiovascular diseases (Supplementary Fig. 6B) and for cardiovascular mortality in individuals with a history of cardiovascular diseases (Supplementary Fig. 6D) or with a BMI < 30 kg/m2 (Supplementary Fig. 7C), even though the result was not statistically significant. However, we did not find this phenomenon for all-cause mortality in individuals with a BMI < 30 kg/m2 (Supplementary Fig. 7A). For individuals without abdominal obesity or without hypertension, we were unable to examine the potential additive effect due to the small number of positive events in TyG < 9.04 and eGDR < 4 group (for individuals without abdominal obesity or without hypertension) and TyG > 9.04 and eGDR < 4 group (for individuals without hypertension) (Supplementary Fig. 8A, 8C, 9A, and 9C).
Discussion
In this study, we explored the associations of the TyG index and eGDR with long-term mortality in individuals with and without diabetes based on the NHANES database, which represents a large representative sample of the non-institutionalized, civilian population of the United States. The main findings of the present study are summarized as follows. (1) In individuals with diabetes, the association between the TyG index and mortality followed a J-shape. However, there was no association between the TyG index and mortality in individuals without diabetes. (2) The association between eGDR and mortality followed an L-shape in individuals with diabetes and a negative linear relationship in individuals without diabetes. (3) There was a potential additive effect of the TyG index and eGDR on long-term mortality in individuals with and without diabetes. The combination of TyG > 9.04 and eGDR < 4 can effectively identify individuals at the highest risk of mortality in the diabetic population. Our findings provide additional evidence for the application of insulin resistance markers in future clinical practice. The combination of the TyG index and eGDR may help improve risk stratification in individuals with and without diabetes.
The TyG index, calculated based on fasting glucose and triglyceride, is often used as a surrogate measure of insulin resistance [10]. Previous studies have demonstrated that a high TyG index is a risk factor for cardiovascular diseases [18, 19]. Furthermore, it was also proved to be an independent predictor of adverse cardiovascular outcomes in patients with and without diabetes. Liu et al. [20] included 19,604 patients with acute ischemic stroke and type 2 diabetes and discovered that the TyG index was significantly associated with ischemic stroke recurrence and all-cause death. Elevated levels of the TyG index were also independently associated with an increased risk of major adverse cardiovascular events in patients without diabetes who underwent coronary artery bypass grafting [21]. Although the predictive value of the TyG index for cardiovascular outcomes has been verified in different populations [20, 21], its formula only incorporates fasting glucose and triglycerides [10], without considering other indicators closely related to insulin resistance, metabolic syndrome, and cardiovascular prognosis, such as central obesity, hypertension, etc. [2]. Thus, using the TyG index alone to predict cardiovascular prognosis may be insufficient.
The eGDR, calculated based on waist circumference, hypertension, and HbA1c, was originally developed as a tool for evaluating the severity of insulin resistance based on individuals with type 1 diabetes [12]. However, several studies have indicated that low levels of eGDR were also associated with impaired fasting glucose and impaired glucose tolerance [30], as well as poor cardiovascular prognosis in individuals without diabetes and those with type 2 diabetes [13, 31, 32]. A retrospective study included non-ST-segment elevation acute coronary syndrome patients without diabetes and demonstrated that low levels of eGDR were a risk factor for major adverse cardio-cerebral events [31]. Zabala et al. [13] revealed that eGDR was associated with stroke and death during follow-up in patients with type 2 diabetes. In a prospective observational analysis with 7.4 years of follow-up, eGDR was a predictor of diabetic kidney disease and all-cause mortality among patients with type 2 diabetes [32].
In the present study, we found that the eGDR was independently associated with both all-cause mortality and cardiovascular mortality in individuals with and without diabetes. However, this was not the case for the TyG index. In individuals with diabetes, there was a J-shaped relationship between the TyG index and mortality, with cutoff values > 9.04 for all-cause mortality and > 9.30 for cardiovascular mortality. However, the TyG index fails to predict the long-term mortality of individuals without diabetes. This phenomenon was in line with a study of patients with triple-vessel coronary disease [33], which concluded that the TyG index was associated with major adverse cardiac events only in patients with diabetes [33]. Another study of patients with coronary heart disease also reported the association between the TyG index and the severity of coronary artery disease in patients with diabetes, whereas no such correlation was observed in patients without diabetes [34]. Therefore, the association between the TyG index and cardiovascular outcomes in individuals without diabetes remains controversial and needs to be further investigated.
Additionally, an additive effect of the TyG index and eGDR on long-term mortality was observed in our study. Combining them might be useful for further risk stratification for certain individuals. This phenomenon was noted for the overall study population as well as for individuals with and without diabetes. Furthermore, based on the results of our subgroup analysis stratified by gender and diabetes status, the TyG index and eGDR should be assessed simultaneously to improve the risk stratification of females as well as males with diabetes, whereas this was not the case for males without diabetes. This suggested that a sex-specific risk stratification is warranted. In addition, there was also a potential additive effect of the TyG index and eGDR on all-cause mortality in all ages, gender, smoking status, renal function, diabetes duration, and history of cardiovascular diseases subgroups as well as in individuals with a BMI ≥ 30 kg/m2, with abdominal obesity, or with hypertension, whereas this was not the case for individuals with a BMI < 30 kg/m2. For cardiovascular mortality, this potential additive effect was also noted in all subgroups of ages, gender, diabetes duration, history of cardiovascular diseases, and BMI as well as for former/never smokers, individuals with an eGFR < 90 mL/min/1.73m2, with abdominal obesity, or with hypertension, whereas this was not the case for current smokers and individuals with an eGFR ≥ 90 mL/min/1.73m2.
Insulin resistance is correlated with vascular and cardiac remodeling [35], impaired fibrinolysis and thrombus formation [36, 37], oxidative stress [38], and increased plaque vulnerability [39], all of which contribute to cardiovascular events. However, the precise mechanisms explaining the additive effect remain unclear. Insulin resistance is closely related to abnormal glucose metabolism, dyslipidemia, hypertension, and central obesity (increased waist circumference) [2], which are included in the formulas of the TyG index and eGDR [10, 12]. However, the comprehensive effect of these components on insulin resistance has not been fully explored. Fasting blood glucose levels primarily reflect hepatic insulin resistance [1]. A decrease in insulin secretion and insulin resistance in the liver causes excess hepatic glucose production in the fasting state, resulting in fasting hyperglycemia [1]. In contrast, fasting triglyceride levels primarily reflect insulin resistance in skeletal muscle and adipose tissue [40, 41]. Skeletal muscle insulin resistance reduces muscle glycogen synthesis and increases hepatic lipogenesis, promoting the development of proatherogenic dyslipidemia (elevated plasma triglyceride levels and decreased HDL-C levels) [40, 41]. In addition to skeletal muscle and liver, adipose tissue represents the third major metabolic-related tissue affected by insulin [1]. Under normal physiological conditions, insulin suppresses adipocyte lipolysis. Hypertriglyceridemia caused by insulin resistance reflects increased lipolysis from adipose tissue, leading to an excessive release of free fatty acids, thereby promoting the development of insulin resistance in skeletal muscle and the liver [1]. In summary, insulin resistance in the liver, skeletal muscle, and adipose tissue has a detrimental impact on glucose homeostasis, which in turn contributes to poor cardiovascular prognosis [1]. In addition, long-term poor glycemic control reflected by high HbA1c levels, is one of the important causes of hepatic insulin resistance [42] and is associated with poor cardiovascular prognosis [43]. Anthropometric markers such as waist circumference reflect central obesity, which is a classic insulin-resistant state and is strongly related to cardiovascular diseases and poor cardiovascular outcomes [44, 45]. Moreover, insulin resistance increases the risk of hypertension [46]. It appears to interact synergistically with hypertension to increase the risk of poor prognosis [47]. Taken together, the combination of the above-mentioned parameters in the TyG index and eGDR might provide a multi-dimensional reflection of insulin resistance. Each of these parameters is an important feature of metabolic syndrome and is an independent predictor of poor cardiovascular prognosis [2]. Therefore, the combination of these parameters may provide a greater predictive value for mortality, which could be one of the reasons for the additive effect. However, as the present study is the first to investigate the combined effects of the TyG index and eGDR to predict long-term mortality, the specific mechanisms of their additive effect on prognosis are currently unclear and need to be further investigated.
Since both the TyG index and eGDR are prognostic predictors independent of conventional cardiovascular risk factors, our findings suggest that evaluating insulin resistance indicators is also important for assessing residual cardiovascular risk, especially in individuals with diabetes. As the TyG index and eGDR are readily available from clinical data, they are economical, simple, and suitable for assessing insulin resistance in large cohorts, thereby improving individual risk stratification and guiding further therapeutic decisions. Future studies should further explore and validate whether treatment strategies based on these biomarkers improve patient outcomes.
Strengths and limitations
Insulin resistance is associated with several traditional cardiovascular risk factors [2]. The interaction between them makes it difficult to assess the independent effect of insulin resistance on cardiovascular outcomes. Studies with larger sample sizes are required to minimize confounding effects. The major strength of our study is the use of a large nationally representative sample of individuals in the United States, helping to eliminate potential confounding effects. In addition, since the impact of insulin resistance on cardiovascular prognosis is a long-term process, it may become more pronounced with a longer follow-up period. This study had a relatively long duration of follow-up (median 8.92 years), contributing to clarifying the long-term prognostic value of the TyG index and eGDR. Furthermore, our study is the first to investigate the simultaneous assessment of two insulin resistance indices to predict long-term mortality, providing new insights and clues for improving individual risk stratification.
Despite the strengths noted above, there are several limitations in our study. First, although the survival status and causes of death were determined through linkage to the National Death Index, the NHANES data were based on a cross-sectional survey; thus, causality could not be ascertained. Second, we were unable to determine the impact of dynamic changes in the TyG index and eGDR on prognosis. Third, since the NHANES only represents the United States population, further multicenter studies are required to validate the external validity of our findings, thereby enhancing the generalizability and clinical applicability of our conclusions. Fourth, the NHANES database does not provide more specific details on cardiovascular mortality (e.g., fatal myocardial infarction or fatal stroke); therefore, they could not be analyzed in our study. Fifth, insulin resistance constitutes a pivotal component of the metabolic syndrome [2, 48]. Non-alcoholic fatty liver disease (NAFLD) represents the hepatic manifestation of metabolic syndrome [49]. With the rising prevalence of insulin resistance and metabolic syndrome, a substantial proportion of the general population is at a high risk of developing NAFLD and NAFLD-associated hepatocellular carcinoma (HCC), ultimately leading to HCC-related mortality [49]. However, these data are not included in the NHANES database, precluding further analysis of the association of insulin resistance markers with HCC and HCC-related mortality in our study. Finally, despite accounting for many important confounders in the multivariate analysis, the impact of unmeasured or unknown confounding factors on prognosis cannot be fully excluded.
Conclusions
For insulin resistance assessment, simultaneous evaluation of the TyG index and eGDR can provide additional information for predicting long-term mortality in individuals with and without diabetes, which can help improve risk stratification and guide the development of more effective therapeutic measures.
Supplementary Information
Acknowledgements
The authors are grateful to the participants of the NHANES database and all research personnel involved.
Abbreviations
- CI
Confidence interval
- eGDR
Estimated glucose disposal rate
- eGFR
Estimated glomerular filtration rate
- HbA1c
Glycated hemoglobin A1c
- HCC
Hepatocellular carcinoma
- HDL-C
High-density lipoprotein cholesterol
- HOMA-IR
Homeostatic model assessment of insulin resistance
- HR
Hazard ratio
- ICD-10
International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- LAP
Lipid accumulation product
- LDL-C
Low-density lipoprotein cholesterol
- METS-IR
Metabolic score for insulin resistance
- NAFLD
Non-alcoholic fatty liver disease
- NHANES
National Health and Nutrition Examination Survey
- ROC
Receiver operating characteristic
- TyG
Triglyceride-glucose
- TyG-BMI
Triglyceride glucose-body mass index
- TyG-WC
Triglyceride glucose-waist circumference
- TyG-WHtR
Triglyceride glucose-waist to height ratio
- VAI
Visceral adiposity index
Author contributions
ZJ, GY, and HH conceptualized and designed this study. HH and XY participated in data extraction, data cleaning, and statistical analysis. CQ, LY, and LX contributed to the data proofreading and data interpretation. MY and DX assisted in figure plotting. HH prepared the initial manuscript draft. GY and HH participated in the critical revision of the manuscript. ZJ supervised the study. All authors participated in editing, reviewing, and approving the final manuscript.
Funding
This study was supported by the National High Level Hospital Clinical Research Funding (No. 2024-NHLHCRF-JBGS-WZ-06 and 2024-NHLHCRF-YS-01), Beijing Research Ward Construction Clinical Research Project (2022-YJXBF-04-03), National Natural Science Foundation of China (No. 82270352), Capital’s Founds for Health Improvement and Research (No. 2022-1-4062), and Chinese Society of Cardiology’s Foundation (No. CSCF2021B02).
Data availability
The data used in the present study were publicly available from the Centers for Disease Control and Prevention at https://www.cdc.gov/nchs/nhanes/index.htm.
Declarations
Ethics approval and consent to participate
The Ethics Review Committee of the National Center for Health Statistics approved the NHANES study protocol, and all participants provided written informed consent before participation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hao-ming He and Ying-ying Xie contributed equally to this work.
Contributor Information
Yan-xiang Gao, Email: gaoyx1980@163.com.
Jin-gang Zheng, Email: mdjingangzheng@yeah.net.
References
- 1.Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–95. 10.2337/db09-9028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. 2019;40(6):1447–67. 10.1210/er.2018-00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, Sowers JR. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metab Clin Exp. 2021;119:154766. 10.1016/j.metabol.2021.154766 [DOI] [PubMed] [Google Scholar]
- 4.Ago T, Matsuo R, Hata J, Wakisaka Y, Kuroda J, Kitazono T, Kamouchi M. Insulin resistance and clinical outcomes after acute ischemic stroke. Neurology. 2018;90(17):e1470–7. 10.1212/WNL.0000000000005358 [DOI] [PubMed] [Google Scholar]
- 5.Wiebe N, Stenvinkel P, Tonelli M. Associations of chronic inflammation, insulin resistance, and severe obesity with mortality, myocardial infarction, Cancer, and Chronic Pulmonary Disease. JAMA Netw open. 2019;2(8):e1910456. 10.1001/jamanetworkopen.2019.10456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321–31. 10.1056/NEJMoa1506930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–223. [DOI] [PubMed] [Google Scholar]
- 8.Elahi D. In praise of the hyperglycemic clamp. A method for assessment of beta-cell sensitivity and insulin resistance. Diabetes Care. 1996;19(3):278–86. 10.2337/diacare.19.3.278 [DOI] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 10.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51. 10.1210/jc.2010-0288 [DOI] [PubMed] [Google Scholar]
- 11.Cui C, Qi Y, Song J, Shang X, Han T, Han N, Yue S, Zha Y, Xu Z, Li J, et al. Comparison of triglyceride glucose index and modified triglyceride glucose indices in prediction of cardiovascular diseases in middle aged and older Chinese adults. Cardiovasc Diabetol. 2024;23(1):185. 10.1186/s12933-024-02278-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000;49(4):626–32. 10.2337/diabetes.49.4.626 [DOI] [PubMed] [Google Scholar]
- 13.Zabala A, Darsalia V, Lind M, Svensson AM, Franzén S, Eliasson B, Patrone C, Jonsson M, Nyström T. Estimated glucose disposal rate and risk of stroke and mortality in type 2 diabetes: a nationwide cohort study. Cardiovasc Diabetol. 2021;20(1):202. 10.1186/s12933-021-01394-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, Simon J, Krauss RM. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96(3):399–404. 10.1016/j.amjcard.2005.03.085 [DOI] [PubMed] [Google Scholar]
- 15.Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, Viveros-Ruiz T, Cruz-Bautista I, Romo-Romo A, Sánchez-Lázaro D, Meza-Oviedo D, Vargas-Vázquez A, Campos OA, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178(5):533–44. 10.1530/EJE-17-0883 [DOI] [PubMed] [Google Scholar]
- 16.Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, Galluzzo A. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–2. 10.2337/dc09-1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahn HS. The lipid accumulation product performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5:26. 10.1186/1471-2261-5-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Chan JSK, Guan B, Peng S, Wu X, Lu X, Zhou J, Hui JMH, Lee YHA, Satti DI, et al. Triglyceride-glucose index and the risk of heart failure: evidence from two large cohorts and a mendelian randomization analysis. Cardiovasc Diabetol. 2022;21(1):229. 10.1186/s12933-022-01658-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin JL, Yang J, Song XJ, Qin X, Chang YJ, Chen X, Liu FH, Li YZ, Xu HL, Wei YF, et al. Triglyceride-glucose index and health outcomes: an umbrella review of systematic reviews with meta-analyses of observational studies. Cardiovasc Diabetol. 2024;23(1):177. 10.1186/s12933-024-02241-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu D, Yang K, Gu H, Li Z, Wang Y, Wang Y. Predictive effect of triglyceride-glucose index on clinical events in patients with acute ischemic stroke and type 2 diabetes mellitus. Cardiovasc Diabetol. 2022;21(1):280. 10.1186/s12933-022-01704-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z, Xie L, Guo D, Chen S, Liu X, Sun X, Wang J, Zhang Y, Liu L, Cui H, et al. Triglyceride-glucose index in the prediction of adverse cardiovascular events in patients without diabetes mellitus after coronary artery bypass grafting: a multicenter retrospective cohort study. Cardiovasc Diabetol. 2023;22(1):230. 10.1186/s12933-023-01969-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rokicka D, Hudzik B, Wróbel M, Stołtny T, Stołtny D, Nowowiejska-Wiewióra A, Rokicka S, Gąsior M, Strojek K. The prognostic impact of insulin resistance surrogates in patients with acute myocardial infarction with and without type 2 diabetes. Cardiovasc Diabetol. 2024;23(1):147. 10.1186/s12933-024-02240-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, He H, Xie Y, Li J, Luo F, Sun Z, Zheng S, Yang F, Li X, Chen X, et al. Non-insulin-based insulin resistance indexes in predicting atrial fibrillation recurrence following ablation: a retrospective study. Cardiovasc Diabetol. 2024;23(1):87. 10.1186/s12933-024-02158-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Zhao L, Lu Y, Meng X, Zhou X. Association between non-insulin-based insulin resistance indices and cardiovascular events in patients undergoing percutaneous coronary intervention: a retrospective study. Cardiovasc Diabetol. 2023;22(1):161. 10.1186/s12933-023-01898-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–47. 10.7326/0003-4819-139-2-200307150-00013 [DOI] [PubMed] [Google Scholar]
- 26.Nyström T, Holzmann MJ, Eliasson B, Svensson AM, Sartipy U. Estimated glucose disposal rate predicts mortality in adults with type 1 diabetes. Diabetes Obes Metab. 2018;20(3):556–63. 10.1111/dom.13110 [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-Álvarez MX, Roca-Pardiñas J, Cadarso-Suárez C, Tahoces PG. Bootstrap-based procedures for inference in nonparametric receiver-operating characteristic curve regression analysis. Stat Methods Med Res. 2018;27(3):740–64. 10.1177/0962280217742542 [DOI] [PubMed] [Google Scholar]
- 28.Ford ES, Maynard LM, Li C. Trends in mean waist circumference and abdominal obesity among US adults, 1999–2012. JAMA. 2014;312(11):1151–3. 10.1001/jama.2014.8362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu YW, Chang CC, Chou RH, Tsai YL, Liu LK, Chen LK, Huang PH, Lin SJ. Gender difference in the association between TyG index and subclinical atherosclerosis: results from the I-Lan Longitudinal Aging Study. Cardiovasc Diabetol. 2021;20(1):206. 10.1186/s12933-021-01391-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cefalo CMA, Riccio A, Succurro E, Marini MA, Fiorentino TV, Rubino M, Perticone M, Sciacqua A, Andreozzi F, Sesti G. Frequency of prediabetes in individuals with increased adiposity and metabolically healthy or unhealthy phenotypes. Diabetes Obes Metab. 2024;26(8):3191–9. 10.1111/dom.15646 [DOI] [PubMed] [Google Scholar]
- 31.Liu C, Liu X, Ma X, Cheng Y, Sun Y, Zhang D, Zhao Q, Zhou Y. Predictive worth of estimated glucose disposal rate: evaluation in patients with non-ST-segment elevation acute coronary syndrome and non-diabetic patients after percutaneous coronary intervention. Diabetol Metab Syndr. 2022;14(1):145. 10.1186/s13098-022-00915-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Penno G, Solini A, Orsi E, Bonora E, Fondelli C, Trevisan R, Vedovato M, Cavalot F, Zerbini G, Lamacchia O, et al. Insulin resistance, diabetic kidney disease, and all-cause mortality in individuals with type 2 diabetes: a prospective cohort study. BMC Med. 2021;19(1):66. 10.1186/s12916-021-01936-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Zhang C, Jiang L, Xu L, Tian J, Zhao X, Wang D, Zhang Y, Sun K, Zhang C, et al. An elevated triglyceride-glucose index predicts adverse outcomes and interacts with the treatment strategy in patients with three-vessel disease. Cardiovasc Diabetol. 2023;22(1):333. 10.1186/s12933-023-02063-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su J, Li Z, Huang M, Wang Y, Yang T, Ma M, Ni T, Pan G, Lai Z, Li C, et al. Triglyceride glucose index for the detection of the severity of coronary artery disease in different glucose metabolic states in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol. 2022;21(1):96. 10.1186/s12933-022-01523-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavalera M, Wang J, Frangogiannis NG. Obesity, metabolic dysfunction, and cardiac fibrosis: pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Translational Research: J Lab Clin Med. 2014;164(4):323–35. 10.1016/j.trsl.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stegenga ME, van der Crabben SN, Levi M, de Vos AF, Tanck MW, Sauerwein HP, van der Poll T. Hyperglycemia stimulates coagulation, whereas hyperinsulinemia impairs fibrinolysis in healthy humans. Diabetes. 2006;55(6):1807–12. 10.2337/db05-1543 [DOI] [PubMed] [Google Scholar]
- 37.He HM, Zheng SW, Xie YY, Wang Z, Jiao SQ, Yang FR, Li XX, Li J, Sun YH. Simultaneous assessment of stress hyperglycemia ratio and glycemic variability to predict mortality in patients with coronary artery disease: a retrospective cohort study from the MIMIC-IV database. Cardiovasc Diabetol. 2024;23(1):61. 10.1186/s12933-024-02146-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park K, Gross M, Lee DH, Holvoet P, Himes JH, Shikany JM, Jacobs DR Jr. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care. 2009;32(7):1302–7. 10.2337/dc09-0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martínez-Hervás S, Vinué A, Núñez L, Andrés-Blasco I, Piqueras L, Real JT, Ascaso JF, Burks DJ, Sanz MJ, González-Navarro H. Insulin resistance aggravates atherosclerosis by reducing vascular smooth muscle cell survival and increasing CX3CL1/CX3CR1 axis. Cardiovascular Res. 2014;103(2):324–36. 10.1093/cvr/cvu115 [DOI] [PubMed] [Google Scholar]
- 40.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA. 2007;104(31):12587–94. 10.1073/pnas.0705408104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131–41. 10.1056/NEJMra1011035 [DOI] [PubMed] [Google Scholar]
- 42.Arslanian S, Heil BV, Kalhan SC. Hepatic insulin action in adolescents with insulin-dependent diabetes mellitus: relationship with long-term glycemic control. Metab Clin Exp. 1993;42(3):283–90. 10.1016/0026-0495(93)90075-Y [DOI] [PubMed] [Google Scholar]
- 43.Sakurai M, Saitoh S, Miura K, Nakagawa H, Ohnishi H, Akasaka H, Kadota A, Kita Y, Hayakawa T, Ohkubo T, et al. HbA1c and the risks for all-cause and cardiovascular mortality in the general Japanese population: NIPPON DATA90. Diabetes Care. 2013;36(11):3759–65. 10.2337/dc12-2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on visceral obesity. Nat Reviews Endocrinol. 2020;16(3):177–89. 10.1038/s41574-019-0310-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117(13):1658–67. 10.1161/CIRCULATIONAHA.107.739714 [DOI] [PubMed] [Google Scholar]
- 46.Rett K, Wicklmayr M, Mehnert H. New aspects of insulin resistance in hypertension. Eur Heart J. 1994;15(Suppl C):78–81. 10.1093/eurheartj/15.suppl_C.78 [DOI] [PubMed] [Google Scholar]
- 47.da Silva AA, do Carmo JM, Li X, Wang Z, Mouton AJ, Hall JE. Role of Hyperinsulinemia and Insulin Resistance in hypertension: metabolic syndrome revisited. Can J Cardiol. 2020;36(5):671–82. 10.1016/j.cjca.2020.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metabolism. 2008;294(1):E15–26. 10.1152/ajpendo.00645.2007 [DOI] [PubMed] [Google Scholar]
- 49.Yu J, Shen J, Sun TT, Zhang X, Wong N. Obesity, insulin resistance, NASH and hepatocellular carcinoma. Sem Cancer Biol. 2013;23(6 Pt B):483–91. 10.1016/j.semcancer.2013.07.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in the present study were publicly available from the Centers for Disease Control and Prevention at https://www.cdc.gov/nchs/nhanes/index.htm.