Abstract
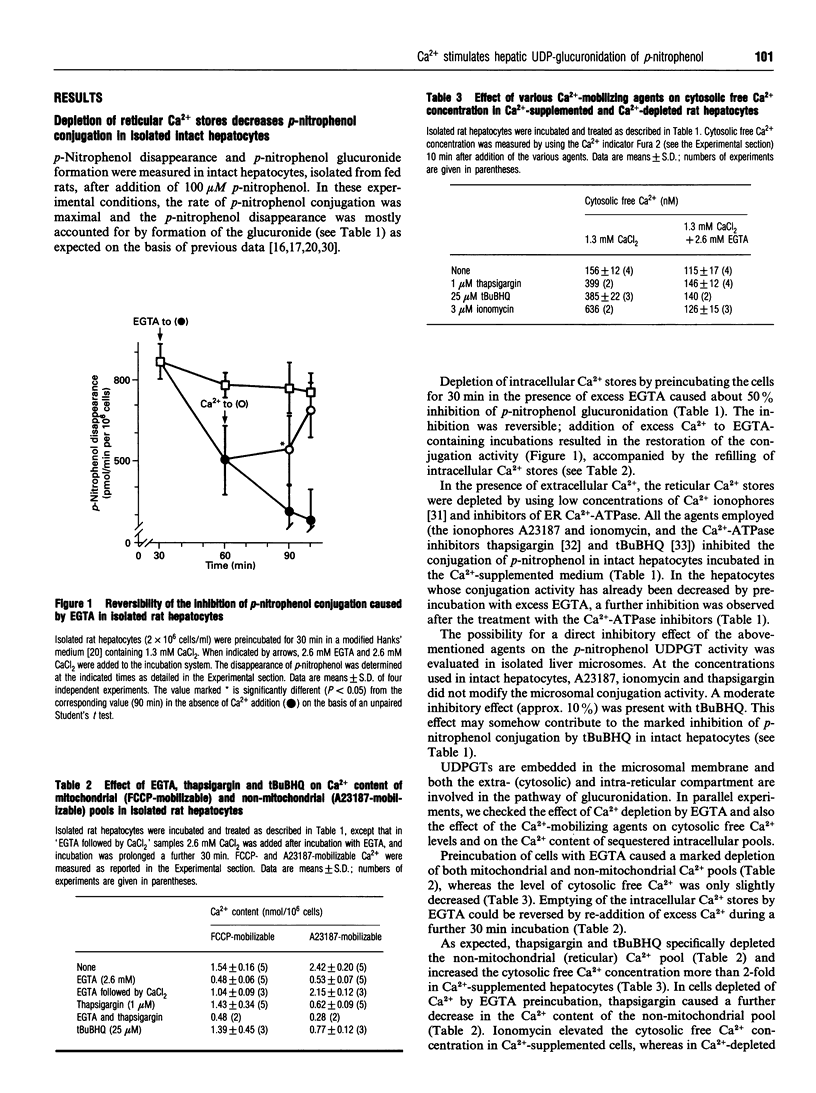
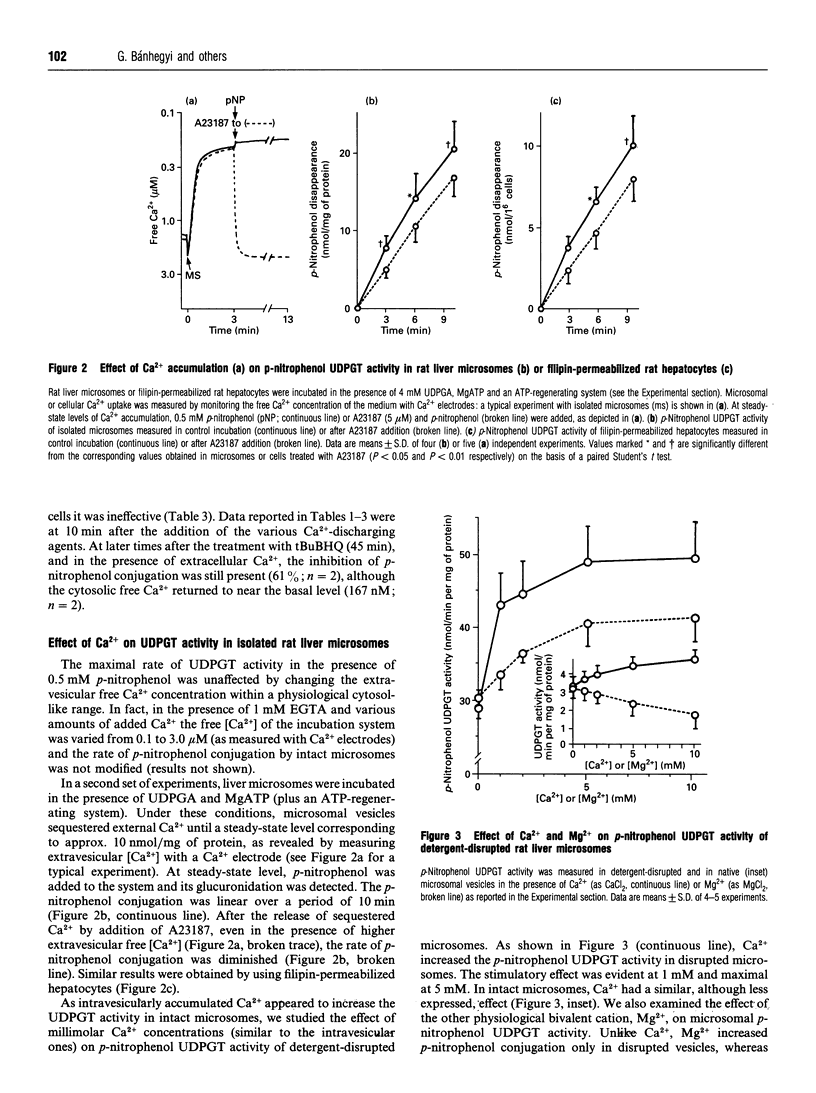
The relationship between the intraluminal Ca2+ content of endoplasmic reticulum and the rate of the glucuronidation of p-nitrophenol was investigated in isolated rat hepatocytes. Different agents which decrease the Ca2+ level in the endoplasmic reticulum [calcium ionophores (A23187, ionomycin) or Ca(2+)-ATPase inhibitors(thapsigargin,2,5-di-(t-butyl)-1,4-benzohydroquinone+ ++)] inhibited the conjugation of p-nitrophenol. Depletion of intracellular Ca2+ stores by preincubation of hepatocytes in the absence of free Ca2+ (in the presence of excess EGTA) also decreased the rate of glucuronidation; Ca2+ re-admission to EGTA-treated hepatocytes restored glucuronidation. In intact liver microsomes the p-nitrophenol UDP-glucuronosyl-transferase activity was not modified by varying the external free Ca2+ concentrations within a cytosol-like range. Emptying of the Ca2+ from the lumen of microsomal vesicles by A23187, after MgATP-stimulated Ca2+ sequestration, decreased the glucuronidation of p-nitrophenol. A similar effect was observed in filipin-permeabilized hepatocytes. In native and in detergent-treated microsomes, Ca2+ (1-10 mM) increased the p-nitrophenol UDP-glucuronosyltransferase activity. It is suggested that the physiological concentration of Ca2+ in the lumen of the endoplasmic reticulum is necessary for the optimal activity of p-nitrophenol UDP-glucuronosyltransferase; the depletion of Ca2+ decreases the activity of the enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Jones D. P., Orrenius S. Effect of calcium ions on ethanol oxidation and drug glucuronidation in isolated hepatocytes. Biochem J. 1979 Dec 15;184(3):709–711. doi: 10.1042/bj1840709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky S. A., Kauffman F. C., Sokolove P. M., Tsukuda T., Thurman R. G. Calcium-mediated inhibition of glucuronide production by epinephrine in the perfused rat liver. J Biol Chem. 1984 Jun 25;259(12):7705–7711. [PubMed] [Google Scholar]
- Beuers U., Pogonka T., Esterline R., Ji S., Jungermann K. Inhibition of para-nitrophenol extraction by stimulation of the hepatic nerves in the perfused rat liver. Toxicol Lett. 1986 Dec;34(2-3):247–252. doi: 10.1016/0378-4274(86)90216-x. [DOI] [PubMed] [Google Scholar]
- Bock K. W., Burchell B., Dutton G. J., Hänninen O., Mulder G. J., Owens I. S., Siest G., Tephly T. R. UDP-glucuronosyltransferase activities. Guidelines for consistent interim terminology and assay conditions. Biochem Pharmacol. 1983 Mar 15;32(6):953–955. doi: 10.1016/0006-2952(83)90610-x. [DOI] [PubMed] [Google Scholar]
- Booth C., Koch G. L. Perturbation of cellular calcium induces secretion of luminal ER proteins. Cell. 1989 Nov 17;59(4):729–737. doi: 10.1016/0092-8674(89)90019-6. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Brostrom M. A. Calcium-dependent regulation of protein synthesis in intact mammalian cells. Annu Rev Physiol. 1990;52:577–590. doi: 10.1146/annurev.ph.52.030190.003045. [DOI] [PubMed] [Google Scholar]
- Burchell B., Weatherill P. 4-Nitrophenol UDPglucuronyltransferase (rat liver). Methods Enzymol. 1981;77:169–177. doi: 10.1016/s0076-6879(81)77022-8. [DOI] [PubMed] [Google Scholar]
- Bànhegyi G., Fulceri R., Bellomo G., Romani A., Pompella A., Benedetti A. Role of a nonmitochondrial Ca2+ pool in the synergistic stimulation by cyclic AMP and vasopressin of Ca2+ uptake in isolated rat hepatocytes. Arch Biochem Biophys. 1991 Jun;287(2):320–328. doi: 10.1016/0003-9861(91)90485-2. [DOI] [PubMed] [Google Scholar]
- Bánhegyi G., Garzó T., Antoni F., Mandl J. Glycogenolysis--and not gluconeogenesis--is the source of UDP-glucuronic acid for glucuronidation. Biochim Biophys Acta. 1988 Dec 15;967(3):429–435. doi: 10.1016/0304-4165(88)90106-7. [DOI] [PubMed] [Google Scholar]
- Bánhegyi G., Garzó T., Mészáros G., Faragó A., Antoni F., Mandl J. Cyclic AMP-dependent phosphorylation in the control of biotransformation in the liver. Biochem Pharmacol. 1988 Mar 1;37(5):849–854. doi: 10.1016/0006-2952(88)90171-2. [DOI] [PubMed] [Google Scholar]
- Bánhegyi G., Puskás R., Garzó T., Antoni F., Mandl J. High amounts of glucose and insulin inhibit p-nitrophenol conjugation in mouse hepatocytes. Biochem Pharmacol. 1991 Aug 22;42(6):1299–1302. doi: 10.1016/0006-2952(91)90269-b. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Czarny M., Sabała P., Ucieklak A., Kaczmarek L., Barańska J. Inhibition of phosphatidylserine synthesis by glutamate, acetylcholine, thapsigargin and ionophore A23187 in glioma C6 cells. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1582–1587. doi: 10.1016/s0006-291x(05)81588-8. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. On the role of the calcium transport cycle in heart and other mammalian mitochondria. FEBS Lett. 1980 Sep 22;119(1):1–8. doi: 10.1016/0014-5793(80)80986-0. [DOI] [PubMed] [Google Scholar]
- Fasolato C., Zottini M., Clementi E., Zacchetti D., Meldolesi J., Pozzan T. Intracellular Ca2+ pools in PC12 cells. Three intracellular pools are distinguished by their turnover and mechanisms of Ca2+ accumulation, storage, and release. J Biol Chem. 1991 Oct 25;266(30):20159–20167. [PubMed] [Google Scholar]
- Fulceri R., Bellomo G., Gamberucci A., Benedetti A. Liver glucose-6-phosphatase activity is not modulated by physiological intracellular Ca2+ concentrations. Biochem J. 1991 May 1;275(Pt 3):805–807. doi: 10.1042/bj2750805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulceri R., Bellomo G., Mirabelli F., Gamberucci A., Benedetti A. Measurement of mitochondrial and non-mitochondrial Ca2+ in isolated intact hepatocytes: a critical re-evaluation of the use of mitochondrial inhibitors. Cell Calcium. 1991 Jun;12(6):431–439. doi: 10.1016/0143-4160(91)90069-q. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Bian J. H., Short A. D., Rybak S. L., Gill D. L. Persistent intracellular calcium pool depletion by thapsigargin and its influence on cell growth. J Biol Chem. 1991 Dec 25;266(36):24690–24697. [PubMed] [Google Scholar]
- Hauser S. C., Ziurys J. C., Gollan J. L. A membrane transporter mediates access of uridine 5'-diphosphoglucuronic acid from the cytosol into the endoplasmic reticulum of rat hepatocytes: implications for glucuronidation reactions. Biochim Biophys Acta. 1988 Nov 17;967(2):149–157. doi: 10.1016/0304-4165(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Rodrigues L. M., Whitton P. D. Rapid stimulation by vasopressin, oxytocin and angiotensin II of glycogen degradation in hepatocyte suspensions. Biochem J. 1978 May 15;172(2):311–317. doi: 10.1042/bj1720311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne V., Söling H. D. Guanosine 5'-triphosphate releases calcium from rat liver and guinea pig parotid gland endoplasmic reticulum independently of inositol 1,4,5-trisphosphate. FEBS Lett. 1986 Jul 7;202(2):267–273. doi: 10.1016/0014-5793(86)80699-8. [DOI] [PubMed] [Google Scholar]
- ISSELBACHER K. J., CHRABAS M. F., QUINN R. C. The solubilization and partial purification of a glucuronyl transferase from rabbit liver microsomes. J Biol Chem. 1962 Oct;237:3033–3036. [PubMed] [Google Scholar]
- Jansen P. L., Mulder G. J., Burchell B., Bock K. W. New developments in glucuronidation research: report of a workshop on "glucuronidation, its role in health and disease". Hepatology. 1992 Mar;15(3):532–544. doi: 10.1002/hep.1840150328. [DOI] [PubMed] [Google Scholar]
- Jorgenson R. A., Nordlie R. C. Multifunctional glucose-6-phosphatase studied in permeable isolated hepatocytes. J Biol Chem. 1980 Jun 25;255(12):5907–5915. [PubMed] [Google Scholar]
- Kalapos M. P., Garzó T., Bánhegyi G., Mucha I., Antoni F., Mandl J. Inhibitory effect of A 23187 on protein synthesis in isolated murine hepatocytes. Acta Biochim Biophys Hung. 1987;22(1):59–66. [PubMed] [Google Scholar]
- Kass G. E., Duddy S. K., Moore G. A., Orrenius S. 2,5-Di-(tert-butyl)-1,4-benzohydroquinone rapidly elevates cytosolic Ca2+ concentration by mobilizing the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool. J Biol Chem. 1989 Sep 15;264(26):15192–15198. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Llopis J., Kass G. E., Duddy S. K., Farell G. C., Gahm A., Orrenius S. Mobilization of the hormone-sensitive calcium pool increases hepatocyte tight junctional permeability in the perfused rat liver. FEBS Lett. 1991 Mar 11;280(1):84–86. doi: 10.1016/0014-5793(91)80209-l. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Wikström L. Calcium is required for folding of newly made subunits of the asialoglycoprotein receptor within the endoplasmic reticulum. J Biol Chem. 1992 Jun 25;267(18):12753–12760. [PubMed] [Google Scholar]
- Lukács G. L., Kapus A., Fonyó A. Parallel measurement of oxoglutarate dehydrogenase activity and matrix free Ca2+ in fura-2-loaded heart mitochondria. FEBS Lett. 1988 Feb 29;229(1):219–223. doi: 10.1016/0014-5793(88)80831-7. [DOI] [PubMed] [Google Scholar]
- Mulder G. J. Glucuronidation and its role in regulation of biological activity of drugs. Annu Rev Pharmacol Toxicol. 1992;32:25–49. doi: 10.1146/annurev.pa.32.040192.000325. [DOI] [PubMed] [Google Scholar]
- Mulder G. J. The heterogeneity of uridine diphosphate glucuronyltransferase from rat liver. Biochem J. 1971 Nov;125(1):9–15. doi: 10.1042/bj1250009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke L. A., Kauffman F. C., Evans R. K., Belinsky S. A., Thurman R. G. p-Nitrophenol conjugation in perfused livers from normal and phenobarbital-treated rats: influence of nutritional state. Res Commun Chem Pathol Pharmacol. 1979 Jan;23(1):185–193. [PubMed] [Google Scholar]
- STOREY I. D. SOME DIFFERENCES IN THE CONJUGATION OF O-AMINOPHENOL AND P-NITROPHENOL BY THE URIDINE DIPHOSPHATE TRANSGLUCURONYLASE OF MOUSE-LIVER HOMOGENATES. Biochem J. 1965 Apr;95:209–214. doi: 10.1042/bj0950209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J. F. The involvement of calcium in transport of secretory proteins from the endoplasmic reticulum. Cell. 1990 Apr 20;61(2):197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- Schöllhammer I., Poll D. S., Bickel M. H. Liver microsomal beta-glucuronidase and UDP-glucuronyltransferase. Enzyme. 1975;20(5):269–276. doi: 10.1159/000458949. [DOI] [PubMed] [Google Scholar]
- Shipley L. A., Eacho P. I., Sweeny D. J., Weiner M. Inhibition of glucuronidation and sulfation by dibutyryl cyclic AMP in isolated rat hepatocytes. Drug Metab Dispos. 1986 Sep-Oct;14(5):526–531. [PubMed] [Google Scholar]
- Thastrup O. Role of Ca2(+)-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2(+)-ATPase inhibitor, thapsigargin. Agents Actions. 1990 Jan;29(1-2):8–15. doi: 10.1007/BF01964706. [DOI] [PubMed] [Google Scholar]
- Tomlinson G. A., Yaffe S. J. The formation of bilirubin and p-nitrophenyl glucuronides by rabbit liver. Biochem J. 1966 May;99(2):507–512. doi: 10.1042/bj0990507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Ca2+-selective electrodes: a novel PVC-gelled neutral carrier mixture compared with other currently available sensors. J Neurosci Methods. 1981 Jun;4(1):73–86. doi: 10.1016/0165-0270(81)90020-0. [DOI] [PubMed] [Google Scholar]
- Waddell I. D., Gibb L., Burchell A. Calcium activates glucose-6-phosphatase in intact rat hepatic microsomes. Biochem J. 1990 Apr 15;267(2):549–551. doi: 10.1042/bj2670549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T., Kane L. P., Carson G. R., Terhorst C. Depletion of cellular calcium accelerates protein degradation in the endoplasmic reticulum. J Biol Chem. 1991 Mar 5;266(7):4500–4507. [PubMed] [Google Scholar]
- Yost G. S., Finley B. L. Ethanol as an inducer of UDP-glucuronyltransferase: a comparison with phenobarbital and 3-methylcholanthrene induction in rabbit hepatic microsomes. Biochem Biophys Res Commun. 1983 Feb 28;111(1):219–223. doi: 10.1016/s0006-291x(83)80139-9. [DOI] [PubMed] [Google Scholar]
- Zakim D., Dannenberg A. J. How does the microsomal membrane regulate UDP-glucuronosyltransferases? Biochem Pharmacol. 1992 Apr 1;43(7):1385–1393. doi: 10.1016/0006-2952(92)90192-l. [DOI] [PubMed] [Google Scholar]
- Zakim D., Goldenberg J., Vessey D. A. Effects of metals on the properties of hepatic microsomal uridine diphosphate glucuronyltransferase. Biochemistry. 1973 Oct 9;12(21):4068–4074. doi: 10.1021/bi00745a007. [DOI] [PubMed] [Google Scholar]


