Abstract
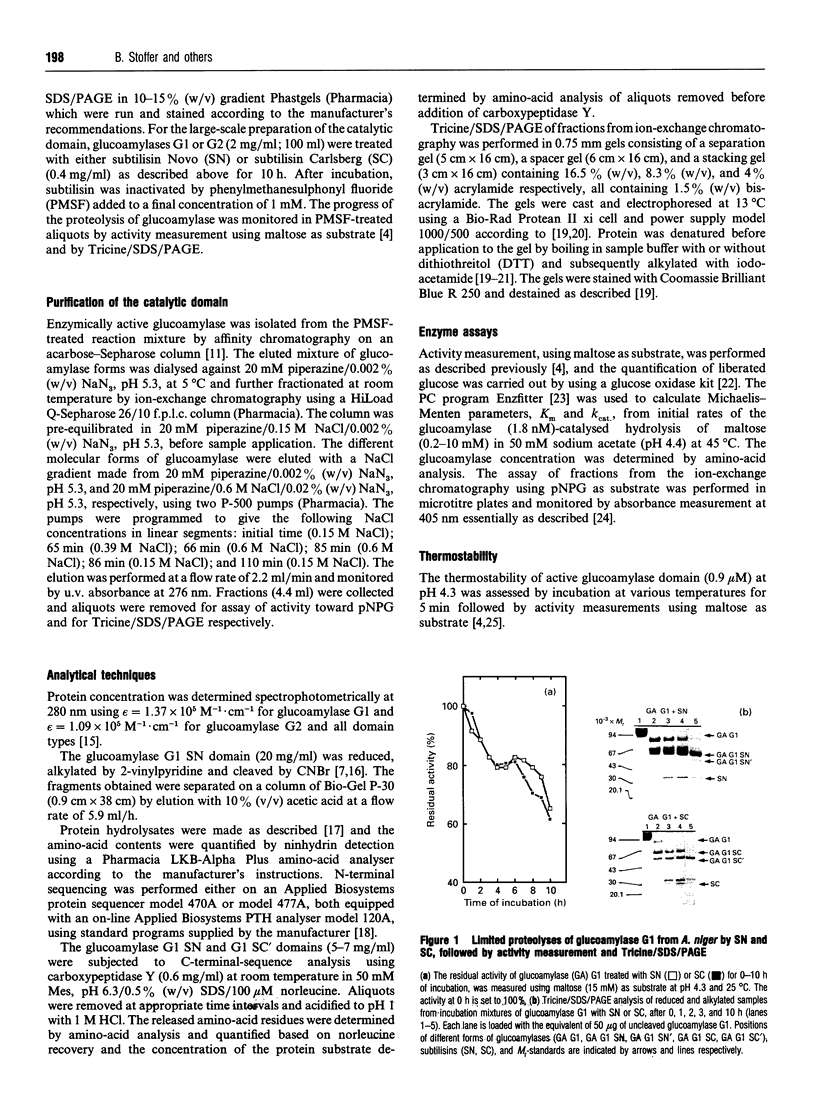
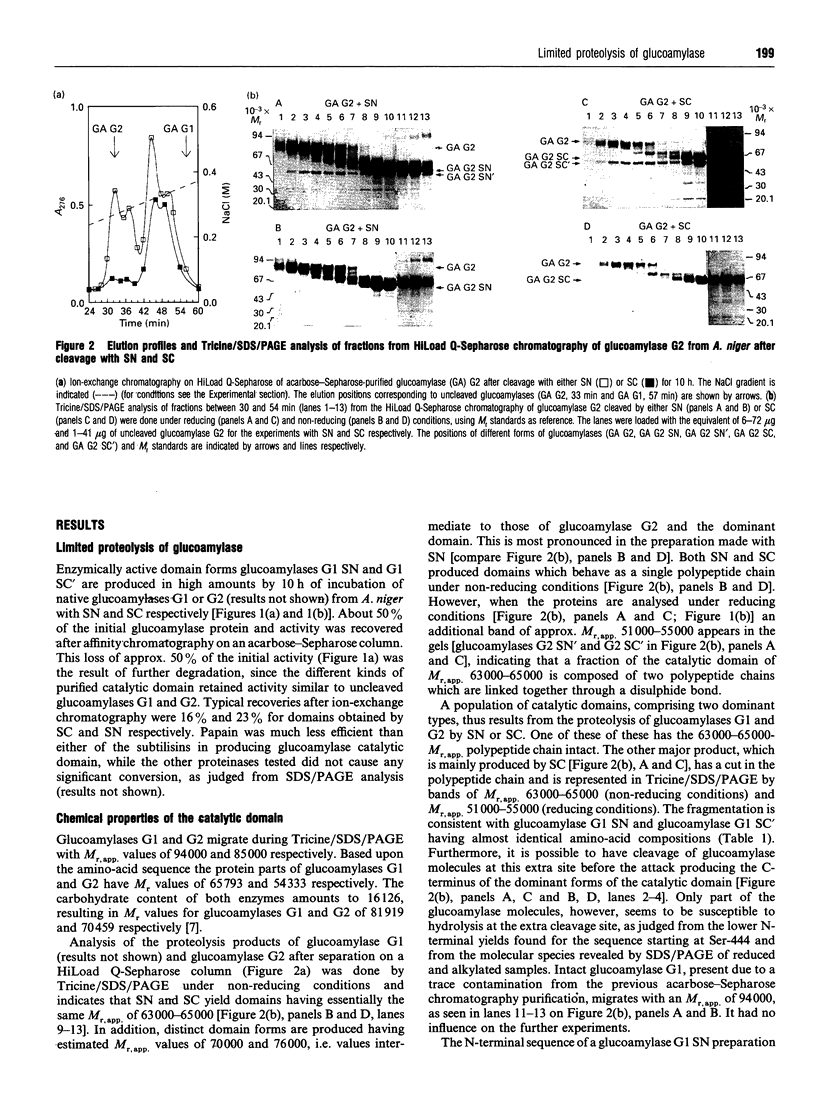
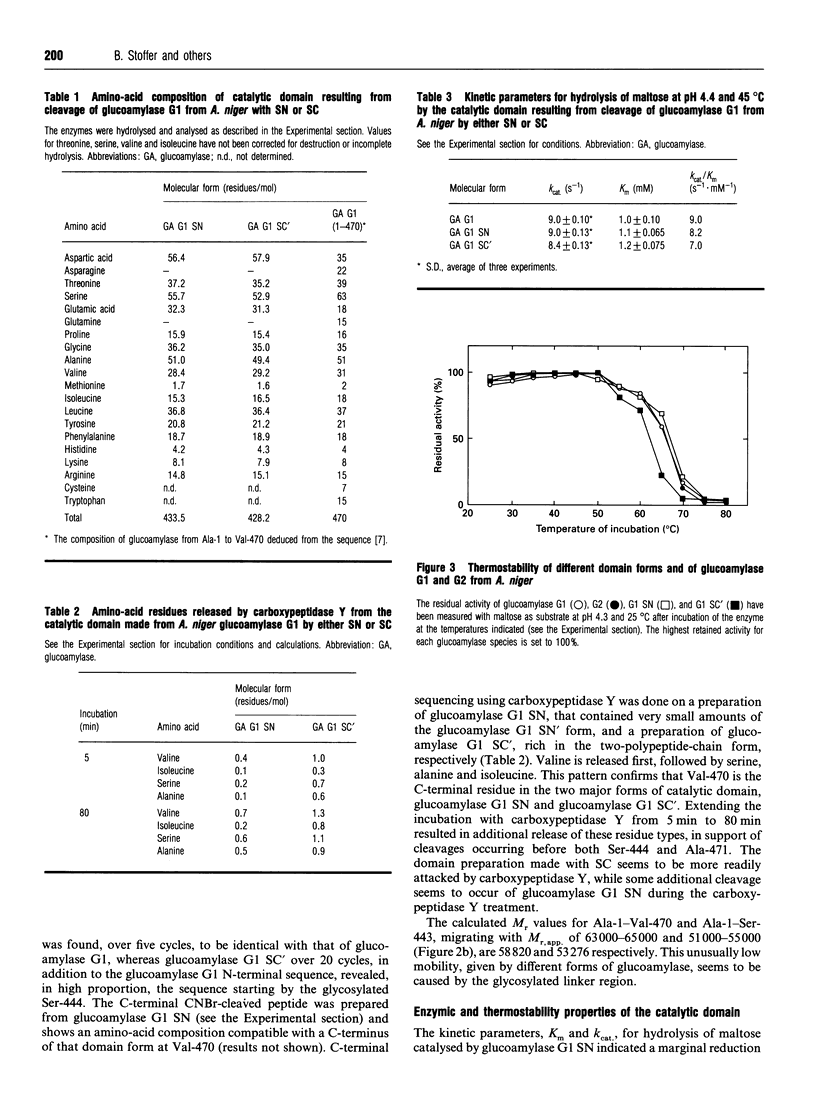
The catalytic domain of glucoamylases G1 and G2 from Aspergillus niger is produced in vitro in high yield by limited proteolysis using either subtilisin Novo or subtilisin Carlsberg. Purification by affinity chromatography on an acarbose-Sepharose column followed by ion-exchange chromatography on HiLoad Q-Sepharose leads to separation of a number of structurally closely related forms of domain. The cleavage occurs primarily between Val-470 and Ala-471 as indicated by C-terminal sequencing, whereas the N-terminus is intact. Subtilisin Carlsberg, in addition, produces a type of domain which is hydrolysed before Ser-444, an O-glycosylated residue. This leaves the fragment Ser-444-Val-470 disulphide-bonded to the large N-terminal part of the catalytic domain. Subtilisin Novo, in contrast, tends to yield a minor fraction of forms extending approx. 30-40 amino-acid residues beyond Val-470. The thermostability is essentially the same for the single-chain catalytic domain and the original glucoamylases G1 and G2, whereas the catalytic domain cut between Ser-443 and Ser-444 is less thermostable. For both types of domain the kinetic parameters, Km and kcat., for hydrolysis of maltose are very close to the values found for glucoamylases G1 and G2.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe J., Nakajima K., Hizukuri S. Alteration of the properties of Aspergillus sp. K-27 glucoamylase on limited proteolysis with subtilisin. Carbohydr Res. 1990 Aug 1;203(1):129–138. doi: 10.1016/0008-6215(90)80052-5. [DOI] [PubMed] [Google Scholar]
- Aleshin A., Golubev A., Firsov L. M., Honzatko R. B. Crystal structure of glucoamylase from Aspergillus awamori var. X100 to 2.2-A resolution. J Biol Chem. 1992 Sep 25;267(27):19291–19298. doi: 10.2210/pdb1gly/pdb. [DOI] [PubMed] [Google Scholar]
- Belshaw N. J., Williamson G. Production and purification of a granular-starch-binding domain of glucoamylase 1 from Aspergillus niger. FEBS Lett. 1990 Sep 3;269(2):350–353. doi: 10.1016/0014-5793(90)81191-p. [DOI] [PubMed] [Google Scholar]
- Evans R., Ford C., Sierks M., Nikolov Z., Svensson B. Activity and thermal stability of genetically truncated forms of Aspergillus glucoamylase. Gene. 1990 Jul 2;91(1):131–134. doi: 10.1016/0378-1119(90)90174-p. [DOI] [PubMed] [Google Scholar]
- Hiromi K., Ohnishi M., Tanaka A. Subsite structure and ligand binding mechanism of glucoamylase. Mol Cell Biochem. 1983;51(1):79–95. doi: 10.1007/BF00215589. [DOI] [PubMed] [Google Scholar]
- Itoh T., Ohtsuki I., Yamashita I., Fukui S. Nucleotide sequence of the glucoamylase gene GLU1 in the yeast Saccharomycopsis fibuligera. J Bacteriol. 1987 Sep;169(9):4171–4176. doi: 10.1128/jb.169.9.4171-4176.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen H. M., MacGregor E. A., Sierks M. R., Svensson B. Comparison of the domain-level organization of starch hydrolases and related enzymes. Biochem J. 1991 Nov 15;280(Pt 1):51–55. doi: 10.1042/bj2800051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada G. Enzymatic studies on a cellulase system of Trichoderma viride. IV. Purification and properties of a less-random type cellulase. J Biochem. 1976 Nov;80(5):913–922. doi: 10.1093/oxfordjournals.jbchem.a131377. [DOI] [PubMed] [Google Scholar]
- Ploug M., Jensen A. L., Barkholt V. Determination of amino acid compositions and NH2-terminal sequences of peptides electroblotted onto PVDF membranes from tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis: application to peptide mapping of human complement component C3. Anal Biochem. 1989 Aug 15;181(1):33–39. doi: 10.1016/0003-2697(89)90390-4. [DOI] [PubMed] [Google Scholar]
- Ploug M., Stoffer B., Jensen A. L. In situ alkylation of cysteine residues in a hydrophobic membrane protein immobilized on polyvinylidene difluoride membranes by electroblotting prior to microsequence and amino acid analysis. Electrophoresis. 1992 Mar;13(3):148–153. doi: 10.1002/elps.1150130130. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Sierks M. R., Ford C., Reilly P. J., Svensson B. Catalytic mechanism of fungal glucoamylase as defined by mutagenesis of Asp176, Glu179 and Glu180 in the enzyme from Aspergillus awamori. Protein Eng. 1990 Jan;3(3):193–198. doi: 10.1093/protein/3.3.193. [DOI] [PubMed] [Google Scholar]
- Sierks M. R., Ford C., Reilly P. J., Svensson B. Site-directed mutagenesis at the active site Trp120 of Aspergillus awamori glucoamylase. Protein Eng. 1989 Aug;2(8):621–625. doi: 10.1093/protein/2.8.621. [DOI] [PubMed] [Google Scholar]
- Svensson B., Clarke A. J., Svendsen I., Møller H. Identification of carboxylic acid residues in glucoamylase G2 from Aspergillus niger that participate in catalysis and substrate binding. Eur J Biochem. 1990 Feb 22;188(1):29–38. doi: 10.1111/j.1432-1033.1990.tb15367.x. [DOI] [PubMed] [Google Scholar]
- Svensson B., Jespersen H., Sierks M. R., MacGregor E. A. Sequence homology between putative raw-starch binding domains from different starch-degrading enzymes. Biochem J. 1989 Nov 15;264(1):309–311. doi: 10.1042/bj2640309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson B., Larsen K., Gunnarsson A. Characterization of a glucoamylase G2 from Aspergillus niger. Eur J Biochem. 1986 Feb 3;154(3):497–502. doi: 10.1111/j.1432-1033.1986.tb09425.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Kato K., Ikegami Y., Irie M. Different behavior towards raw starch of three forms of glucoamylase from a Rhizopus sp. J Biochem. 1985 Sep;98(3):663–671. doi: 10.1093/oxfordjournals.jbchem.a135323. [DOI] [PubMed] [Google Scholar]
- Williamson G., Belshaw N. J., Williamson M. P. O-glycosylation in Aspergillus glucoamylase. Conformation and role in binding. Biochem J. 1992 Mar 1;282(Pt 2):423–428. doi: 10.1042/bj2820423. [DOI] [PMC free article] [PubMed] [Google Scholar]