Abstract
Two rotavirus vaccines have been licensed in >100 countries worldwide since 2006. As of October 2105, these vaccines have been implemented in the national immunization programs of 79 countries, including 36 low-income countries that are eligible for support for vaccine purchase from Gavi, the Vaccine Alliance. Rotavirus vaccines were initially introduced in Australia and countries of the Americas and Europe after completion of successful clinical trials in these regions, and the impact of routine vaccination in reducing the health burden of severe childhood gastroenteritis in these regions has been well documented. Because of concerns around the performance of orally administered rotavirus vaccines in developing countries, vaccine implementation in these settings only began after additional clinical trials were completed and the World Health Organization issued a global recommendation for use of rotavirus vaccines in 2009. This supplementary issue of Clinical Infectious Diseases includes a collection of articles describing the impact and effectiveness of routine rotavirus vaccination in developing countries that were among the early adopters of rotavirus vaccine. The data highlight the benefits of vaccination and should provide valuable evidence to sustain vaccine use in these countries and encourage other countries to adopt routine rotavirus vaccination to reduce the health burden of severe childhood gastroenteritis.
Keywords: rotavirus, rotavirus vaccines, impact, effectiveness, developing countries
In 2006, pivotal clinical trials of 2 live oral rotavirus vaccines—a pentavalent bovine-human reassortant vaccine (RV5) given in a 3-dose schedule (RotaTeq, Merck & Co), and a monovalent human vaccine (RV1) given in a 2-dose schedule (Rotarix, GSK Biologicals)—demonstrated good efficacy (85%–98%) in preventing severe rotavirus gastroenteritis [1, 2]. In addition, these large trials of 60 000–70 000 infants each were specifically designed to evaluate the risk of intussusception, an uncommon adverse event that had led to the withdrawal of an earlier rotavirus vaccine (RotaShield, Wyeth Lederle) from the United States in 1999; reassuringly, no risk was found with either vaccine [1–6]. The World Health Organization’s (WHO) Strategic Advisory Group of Experts reviewed these data from trials conducted in Europe and the Americas and recommended that rotavirus vaccines be included in the national immunization programs of countries in these regions where vaccine efficacy had been demonstrated [7].
Beginning with the United States in February 2006, many countries in the Americas and Europe, as well as Australia, soon adopted rotavirus vaccines as part of their routine childhood vaccination programs. In many of these countries, the remarkable impact of these vaccines in reducing the burden of severe childhood gastroenteritis has been unequivocally demonstrated. A systematic review of data from 8 countries reported a 49%–89% decline in laboratory-confirmed rotavirus hospitalizations and 17%–55% decline in all-cause gastroenteritis hospitalizations among children aged <5 years within 2 years of vaccine introduction [8].As an unanticipated benefit, in some countries, rotavirus vaccination of young infants has also resulted in the declines in rotavirus disease among children who missed vaccination and among older children and even adults who were not vaccine eligible [9–19]. This phenomenon, known as herd protection, is likely related to reduction in community transmission of rotavirus because vaccination limits the number of children susceptible to rotavirus disease. Most notably, studies from Mexico, Brazil, and Panama showed a reduction in childhood deaths from all-cause diarrhea following vaccine implementation, a key outcome that was not evaluated in clinical trials [20–24].
Despite this impressive success in developed countries, the full impact of rotavirus vaccines remained to be realized in developing countries of Asia and Africa where morbidity and mortality due to rotavirus are greatest. Because of concerns that the performance of orally administered rotavirus vaccines may be diminished in developing countries of Africa and Asia due to possible interference by concurrent enteric infections, greater levels of maternal antibodies, and higher rates of malnutrition and comorbidities, WHO recommended further efficacy testing in these settings prior to issuing a global recommendation for vaccine use [7]. As expected, efficacy trials of both RV5 and RV1 in developing countries showed lower vaccine efficacy (50%–64%) against severe rotavirus gastroenteritis compared with developed countries [25–27]. Notably, despite the diminished efficacy, the public health benefits of vaccination in terms of the numbers of severe rotavirus gastroenteritis episodes prevented for every 100 vaccinated infants were greater in developing compared with developed countries because of the substantially greater baseline rate of severe rotavirus gastroenteritis in developing countries [26]. These considerations led WHO to issue a recommendation for global use of rotavirus vaccines in 2009, particularly in developing countries with high mortality from childhood diarrhea [28–30].
As of September 2015, 79 countries worldwide have implemented rotavirus vaccines in their national immunization programs, including 36 low-income, developing countries that are eligible for support for vaccine purchase from Gavi, the Vaccine Alliance (Figure 1). The global rollout of rotavirus vaccines provides an opportunity to assess the real-world impact of rotavirus vaccination in preventing and reducing the health burden of severe childhood diarrhea in developing countries. Such post–vaccine introduction data are particularly important to generate as (1) vaccine performance in routine programmatic use could differ from the ideal conditions of a clinical trial; (2) widespread vaccine use may result in changes in rotavirus epidemiology (eg, changes in average age of infection and seasonality)that may not be detected in trials; and (3) vaccination may have effects on disease transmission in the community and, thus, may provide indirect benefits to unvaccinated individuals as well (ie, herd immunity). The articles in this supplement describe the effects of rotavirus vaccination in many developing countries in Africa, Eastern Europe/Central Asia, and Latin America that were early adopters of vaccination. The evidence and lessons learned, summarized in this report, will be valuable for these countries to sustain their vaccination programs and will also inform decision making in countries that are considering implementing rotavirus vaccination.
Figure 1.
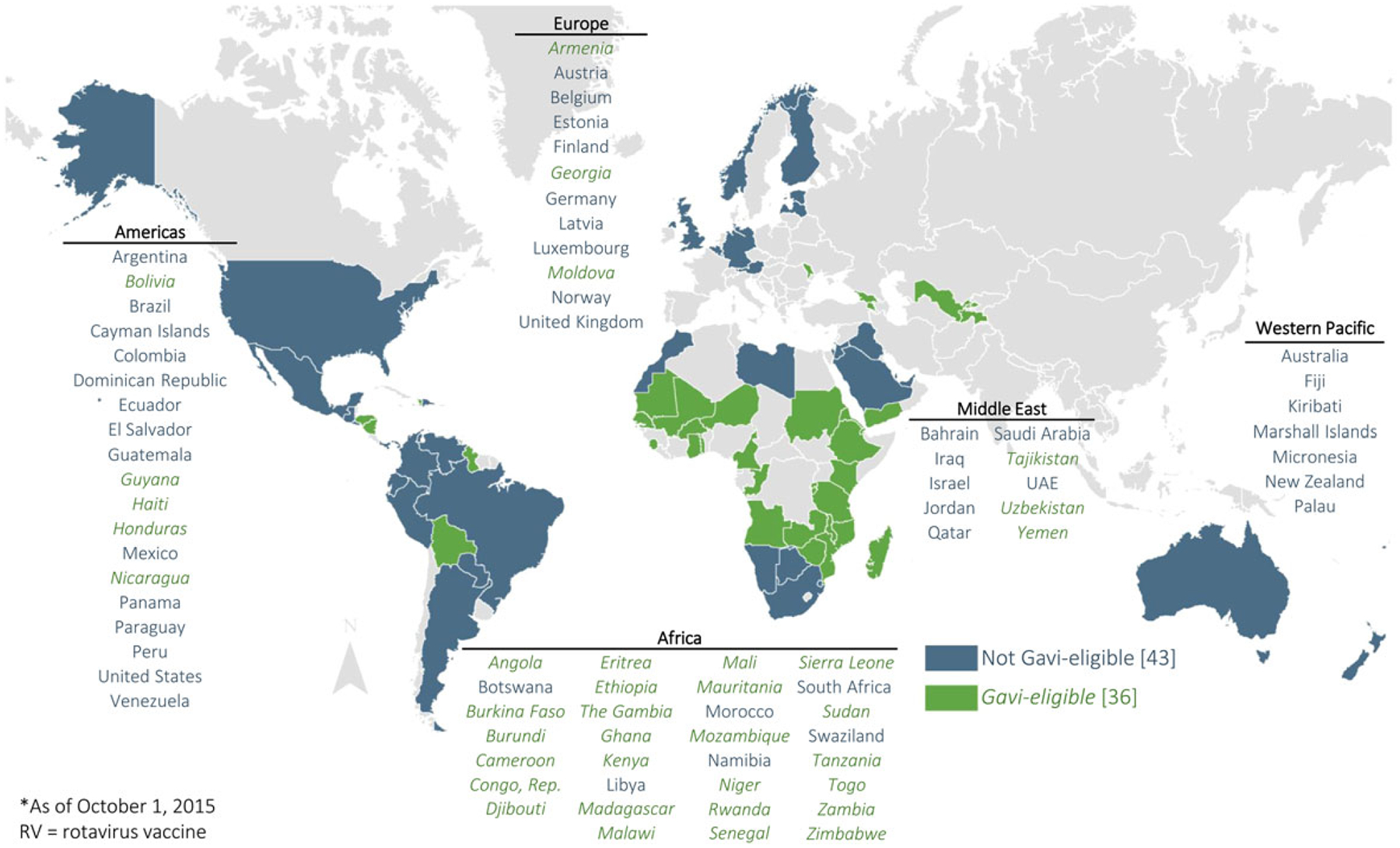
National rotavirus vaccine introduction, by geographic region, as of 1 October 2015. Source: PATH rotavirus vaccine country introduction maps available at http://sites.path.org/rotavirusvaccine/country-introduction-maps-and-spreadsheet/. Abbreviation: UAE, United Arab Emirates.
IMPACT OF ROTAVIRUS VACCINATION IN REDUCING MORBIDITY AND MORTALITY FROM SEVERE DIARRHEA
Perhaps the most convincing and readily interpretable evidence of vaccine impact is the documentation of a decline in the burden of the target disease following vaccine introduction. However, assessing trends in disease before and after vaccine introduction requires cautious interpretation to account for secular trends and other possible factors (eg, changes in surveillance practices or healthcare-seeking behavior) that might be associated with the decline. Several articles in this supplement from African (Botswana, South Africa, Ghana, Togo, Zambia) and East European/Central Asian (Armenia and Moldova) countries show evidence of rapid and substantial declines in severe diarrhea and/or rotavirus disease following vaccine introduction [31–37]. In these evaluations, a role for vaccine in disease reduction is supported by observations such as (1) sharp declines coinciding temporally with the timing of vaccine introduction; (2) greater declines during the months of the year with seasonal peaks of rotavirus disease; and (3) greater initial declines in younger age groups that receive vaccination in the initial years of the vaccination, followed by a progressive decline in older age groups in later years after introduction. Of note, data from Botswana and Zambia showed a decline in in-hospital mortality from diarrhea at sentinel hospitals conducting surveillance [31, 35]. Although some caution in interpretation is warranted given the relatively small number of deaths observed in these studies, these promising data on life-saving benefits of rotavirus vaccination were indicated by findings from Latin American countries that have convincingly shown a decline in diarrhea mortality after rotavirus vaccine implementation. In fact, a report in this supplement found that nationwide diarrhea mortality in Mexican children has been reduced by almost half following rotavirus vaccine implementation, and these declines have been sustained for 7 years after vaccine introduction [38].
VACCINE EFFECTIVENESS IN ROUTINE USE
Observational studies such as those using a case-control design can measure field effectiveness of vaccination in routine programmatic use, proving a measure of vaccine performance under “real world” conditions. These data expand the evidence from clinical trials, as they include groups that may have been excluded from clinical trials (eg, malnourished or immunocompromised children) and children with less rigidly adhered to vaccination schedules (eg, age at administration, interval between doses, the number of doses). Several reports in this supplement provide reassuring evidence that the real-world effectiveness of rotavirus vaccination in developing countries is similar to the vaccine efficacy in prelicensure trials, with a gradient of lower efficacy in countries with greater levels of child mortality [36, 37, 39–45]. Some observations are noteworthy. First, evidence of a decline in effectiveness in the second year of life compared with the first year was seen in some studies but not in others; furthermore, it was encouraging that effectiveness against the most severe rotavirus disease that is likely to be associated with the worst clinical outcomes was well sustained over the first 2 years of life when the vast majority of rotavirus cases occur. Second, evidence of some protection from a partial series of rotavirus vaccine was seen, which is particularly relevant regarding protection against severe rotavirus disease that occurs at a very young age before a child is fully immunized. Finally, both RV5 and RV1 provided protection against a range of circulating rotavirus strains, supporting observations from clinical trials and other postlicensure data that both rotavirus vaccines provide good cross-protection against non-vaccine-type strains.
INDIRECT PROTECTION FROM ROTAVIRUS VACCINATION
Indirect protection (ie, herd immunity) occurs as a result of decreased transmission of the infectious pathogen in the community because of vaccination of a proportion of the population, thereby amplifying the benefits of vaccination among both vaccinated and unvaccinated persons. Indirect protection from rotavirus vaccination has been well documented in developed countries in the Americas and Europe, and in Australia, evident from substantial reductions in disease in age groups who were too old to be vaccinated, including young adults in some settings. However, it was unclear if these observations would extend to developing countries, given differences in population age-group structure and intensity of viral transmission. The reports from Armenia and Moldova in this supplement both demonstrate a decline in severe rotavirus disease among older age groups that were not vaccinated and also greater declines in vaccinated age groups than that expected based on vaccine coverage and effectiveness, indicating evidence of indirect protection [36, 37].However, data from Zambia and South Africa do not indicate any evidence of indirect protection, and thus further evidence is required to understand the extent of herd protection across a range of geographic and socioeconomically diverse settings [32, 35].
THE WAY FORWARD
The early evidence on the real-world impact and effectiveness of rotavirus vaccination in developing countries from the articles in this supplement is encouraging, and provides powerful information to encourage countries to sustain rotavirus vaccine use and to help inform decision making regarding vaccine use in countries that have not yet recommended rotavirus vaccination. However, further monitoring and evidence generation are required to address several key issues (Table 1). First, given the observed variability in vaccine effectiveness across countries, additional evidence should be generated to improve the generalizability of the findings, particularly from challenging settings in the most impoverished countries with the weakest healthcare and immunization programs. Additionally, a better understanding of the extent of herd protection across a range of geographic settings will help to quantify the full impact of a rotavirus vaccination program. Data on the impact and effectiveness of rotavirus vaccines in developing countries can also help drive the research agenda to improve the performance of existing vaccines or develop new vaccines. Second, as the experiences described in these reports are limited to the first 2–3 years after vaccine implementation for most countries, continued monitoring is desirable to assess whether over the long term the observed disease reductions and vaccine effectiveness are sustained and to examine if any changes in disease epidemiology occur, such as shifts in the age distribution of rotavirus cases or the emergence of unusual rotavirus strains due to possible vaccine-driven selection pressure. Third, while changes in the proportion of severe diarrhea attributable to nonrotavirus pathogens are expected to occur with the decline in incidence of rotavirus disease following vaccine implementation, monitoring the incidence of severe diarrheal disease caused by nonrotavirus pathogens before and after rotavirus vaccine implementation would help to assess if vaccination leads to changes in the overall ecology of diarrheal disease. Fourth, to date, no developing country in Asia has implemented routine rotavirus vaccination with either RV5 or RV1; thus, generating evidence from Asian countries as they introduce vaccines is a high priority. Of note, India recently recommended inclusion of an indigenously manufactured rotavirus vaccine (Rotavac) in its national immunization program [46, 47], and Vietnam has also licensed its own rotavirus vaccine (Rotavin), providing initial opportunities to examine the effect of rotavirus vaccination in low-income Asian countries. Fifth, because only 6 of the 36 Gavi-eligible countries that have implemented rotavirus vaccination to date have selected RV5, generation of additional postlicensure data from developing countries for this vaccine in particular should be prioritized. Finally, postlicensure evaluations in developed countries have identified a low risk of intussusception with both RV5 and RV1; however, this risk is exceeded by the marked health benefits of vaccination seen in these countries and has not led to any change in vaccination recommendations from WHO and many national health authorities that have reviewed the evidence [48]. Although the benefits of vaccination are likely to be even more substantial in low-income countries given the greater health burden of rotavirus, efforts should be made to generate information on any intussusception risk associated with vaccination in these settings to allow informed risk-benefit assessments and provide additional confidence in the vaccination program.
Table 1.
Key Priorities for Future Rotavirus Vaccine Monitoring Efforts
|
Abbreviation: RV5, pentavalent bovine-human reassortant vaccine.
Supplement sponsorship.
This article appears as part of the supplement “Health Benefits of Rotavirus Vaccination in Developing Countries,” sponsored by PATH and the CDC Foundation through grants from the Bill and Melinda Gates Foundation and GAVI, the Vaccine Alliance.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of Centers for Disease Control and Prevention (CDC). The views expressed by the authors do not necessarily reflect the views of PATH, the CDC Foundation, the Bill and Melinda Gates Foundation, or GAVI, the Vaccine Alliance.
References
- 1.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23–33. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11–22. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep 1999; 48:1007. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Intussusception among recipients of rotavirus vaccine—United States, 1998–1999. MMWR Morb Mortal Wkly Rep 1999; 48:577–81. [PubMed] [Google Scholar]
- 5.Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med 2001; 344:564–72. [DOI] [PubMed] [Google Scholar]
- 6.Peter G, Myers MG; National Vaccine Advisory Committee, National Vaccine Program Office. Intussusception, rotavirus, and oral vaccines: summary of a workshop. Pediatrics 2002; 110:e67. [DOI] [PubMed] [Google Scholar]
- 7.Conclusions and recommendations from the Immunization Strategic Advisory Group. Wkly Epidemiol Rec 2006; 81:2–11. [PubMed] [Google Scholar]
- 8.Patel MM, Glass R, Desai R, Tate JE, Parashar UD. Fulfilling the promise of rotavirus vaccines: how far have we come since licensure? Lancet Infect Dis 2012; 12:561–70. [DOI] [PubMed] [Google Scholar]
- 9.Yen C, Tate JE, Wenk JD, Harris JM 2nd, Parashar UD. Diarrhea-associated hospitalizations among US children over 2 rotavirus seasons after vaccine introduction. Pediatrics 2011; 127:e9–15. [DOI] [PubMed] [Google Scholar]
- 10.Cortese MM, Tate JE, Simonsen L, Edelman L, Parashar UD. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine up-take from national medical claims databases. Pediatr Infect Dis J 2010; 29:489–94. [DOI] [PubMed] [Google Scholar]
- 11.Lopman BA, Curns AT, Yen C, Parashar UD. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis 2011; 204:980–6. [DOI] [PubMed] [Google Scholar]
- 12.Gastanaduy PA, Curns AT, Parashar UD, Lopman BA. Gastroenteritis hospitalizations in older children and adults in the United States before and after implementation of infant rotavirus vaccination. JAMA 2013; 310:851–3. [DOI] [PubMed] [Google Scholar]
- 13.Paulke-Korinek M, Kundi M, Rendi-Wagner P, et al. Herd immunity after two years of the universal mass vaccination program against rotavirus gastroenteritis in Austria. Vaccine 2011; 29:2791–6. [DOI] [PubMed] [Google Scholar]
- 14.Raes M, Strens D, Vergison A, Verghote M, Standaert B. Reduction in pediatric rotavirus-related hospitalizations after universal rotavirus vaccination in Belgium. Pediatr Infect Dis J 2011; 30:e120–5. [DOI] [PubMed] [Google Scholar]
- 15.Hemming M, Rasanen S, Huhti L, Paloniemi M, Salminen M, Vesikari T. Major reduction of rotavirus, but not norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the National Immunization Programme in Finland. Eur J Pediatr 2013; 172:739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field EJ, Vally H, Grimwood K, Lambert SB. Pentavalent rotavirus vaccine and prevention of gastroenteritis hospitalizations in Australia. Pediatrics 2010; 126: e506–12. [DOI] [PubMed] [Google Scholar]
- 17.Clarke MF, Davidson GP, Gold MS, Marshall HS. Direct and indirect impact on rotavirus positive and all-cause gastroenteritis hospitalisations in South Australian children following the introduction of rotavirus vaccination. Vaccine 2011; 29:4663–7. [DOI] [PubMed] [Google Scholar]
- 18.Yen C, Armero Guardado JA, Alberto P, et al. Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J 2011; 30(1 suppl):S6–10. [DOI] [PubMed] [Google Scholar]
- 19.Safadi MA, Berezin EN, Munford V, et al. Hospital-based surveillance to evaluate the impact of rotavirus vaccination in Sao Paulo, Brazil. Pediatr Infect Dis J 2010; 29:1019–22. [DOI] [PubMed] [Google Scholar]
- 20.do Carmo GM, Yen C, Cortes J, et al. Decline in diarrhea mortality and admissions after routine childhood rotavirus immunization in Brazil: a time-series analysis. PLoS Med 2011; 8:e1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanzieri TM, Linhares AC, Costa I, et al. Impact of rotavirus vaccination on childhood deaths from diarrhea in Brazil. Int J Infect Dis 2011; 15:e206–10. [DOI] [PubMed] [Google Scholar]
- 22.Richardson V, Parashar U, Patel M. Childhood diarrhea deaths after rotavirus vaccination in Mexico. N Engl J Med 2011; 365:772–3. [DOI] [PubMed] [Google Scholar]
- 23.Gastanaduy PA, Sanchez-Uribe E, Esparza-Aguilar M, et al. Effect of rotavirus vaccine on diarrhea mortality in different socioeconomic regions of Mexico. Pediatrics 2013; 131:e1115–20. [DOI] [PubMed] [Google Scholar]
- 24.Bayard V, DeAntonio R, Contreras R, et al. Impact of rotavirus vaccination on childhood gastroenteritis-related mortality and hospital discharges in Panama. Int J Infect Dis 2012; 16:e94–8. [DOI] [PubMed] [Google Scholar]
- 25.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606–14. [DOI] [PubMed] [Google Scholar]
- 26.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289–98. [DOI] [PubMed] [Google Scholar]
- 27.Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615–23. [DOI] [PubMed] [Google Scholar]
- 28.Rotavirus vaccines: an update. Wkly Epidemiol Rec 2009; 84:533–40. [PubMed] [Google Scholar]
- 29.Meeting of the Strategic Advisory Group of Experts on immunization, October 2009—conclusions and recommendations. Wkly Epidemiol Rec 2009; 84: 517–32. [PubMed] [Google Scholar]
- 30.Meeting of the immunization Strategic Advisory Group of Experts, April 2009—conclusions and recommendations. Wkly Epidemiol Rec 2009; 84:220–36. [PubMed] [Google Scholar]
- 31.Enane LA, Gastañaduy PA, Goldfarb DM, et al. Impact of rotavirus vaccination on hospitalizations and deaths from childhood gastroenteritis in Botswana. Clin Infect Dis 2016; 62(suppl 2):S168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groome MJ, Zell ER, Solomon F, et al. Temporal association of rotavirus vaccine introduction and reduction in all-cause childhood diarrheal hospitalizations in South Africa. Clin Infect Dis 2016; 62(suppl 2):S188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armah G, Pringle K, Enweronu-Laryea CC, et al. Impact and effectiveness of monovalent rotavirus vaccine against severe rotavirus diarrhea in Ghana. Clin Infect Dis 2016; 62(suppl 2):S200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsolenyanu E, Mwenda JM, Dagnra A, et al. Early evidence of impact of monovalent rotavirus vaccine in Togo. Clin Infect Dis 2016; 62(suppl 2): S196–9. [DOI] [PubMed] [Google Scholar]
- 35.Mpabalwani EM, Simwaka CJ, Mwenda JM, et al. Impact of rotavirus vaccination on diarrheal hospitalizations in children aged <5 years in Lusaka, Zambia. Clin Infect Dis 2016; 62(suppl 2):S183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahakyan G, Grigoryan S, Wasley A, et al. Impact and effectiveness of monovalent rotavirus vaccine in Armenian children. Clin Infect Dis 2016; 62(suppl 2): S147–54. [DOI] [PubMed] [Google Scholar]
- 37.Gheorghita S, Birca L, Donos S, et al. Impact of rotavirus vaccine introduction and vaccine effectiveness in the republic of Moldova. Clin Infect Dis 2016; 62(suppl 2): S140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sánchez-Uribe E, Esparza-Aguilar M, Parashar UD, Richardson V. Sustained reduction of childhood diarrhea-related mortality and hospitalizations in Mexico after rotavirus vaccine universalization. Clin Infect Dis 2016; 62(suppl 2):S133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pringle KD, Patzi M, Tate JE, et al. Sustained effectiveness of rotavirus vaccine against very severe rotavirus disease through the second year of life, Bolivia 2013–2014. Clin Infect Dis 2016; 62(suppl 2):S115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gastañaduy PA, Contreras-Roldán I, Bernart C, et al. Effectiveness of monovalent and pentavalent rotavirus vaccines in Guatemala. Clin Infect Dis 2016; 62(suppl 2):S121–6. [DOI] [PubMed] [Google Scholar]
- 41.Gastañaduy PA, Steenhoff AP, Mokomane M, et al. Effectiveness of monovalent rotavirus vaccine after programmatic implementation in Botswana: A multisite prospective case-control study. Clin Infect Dis 2016; 62(suppl 2):S161–7. [DOI] [PubMed] [Google Scholar]
- 42.Tate JE, Ngabo F, Donnen P, et al. Effectiveness of pentavalent rotavirus vaccine under conditions of routine use in Rwanda. Clin Infect Dis 2016; 62(suppl 2): S208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bar-Zeev N, Jere KC, Bennett A, et al. Population impact and effectiveness of monovalent rotavirus vaccination in urban Malawian children 3 years after vaccine introduction: ecological and case-control analyses. Clin Infect Dis 2016; 62(suppl 2): S213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leshem E, Givon-Lavi N, Tate JE, et al. Real-world effectiveness of pentavalent rotavirus vaccine among bedouin and Jewish children in Southern Israel. Clin Infect Dis 2016; 62(suppl 2):S155–60. [DOI] [PubMed] [Google Scholar]
- 45.Patel M, Pedreira C, De Oliveira LH, et al. Effectiveness of pentavalent rotavirus vaccine against a diverse range of circulating strains in Nicaragua. Clin Infect Dis 2016; 62(suppl 2):S127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhandari N, Rongsen-Chandola T, Bavdekar A, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet 2014; 383:2136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prime Minister of India Office. Available at: http://pmindia.gov.in/en/news_updates/three-new-vaccines-including-indigenously-developed-rotavirus-vaccine-to-be-provided-to-all-indian-childrenfourth-vaccine-for-adults-to-protect-against-japanese-encephalitis-to-be-introduced-in-high-p/. Accessed 2 October 2015.
- 48.Rotavirus vaccines. WHO position paper—January 2013. Wkly Epidemiol Rec 2013; 88:49–64. [PubMed] [Google Scholar]


